
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 29 September 2022
Sec. Cardio-Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.934214
This article is part of the Research Topic Case Reports in Cardio-Oncology: 2022 View all 39 articles
QT interval prolongation and ventricular arrhythmias (VAs) induced by osimertinib, a third-generation epidermal growth factor receptor tyrosine kinase inhibitor, are life-threatening complications. However, no consensus has been achieved regarding their management. Overdrive pacing has been shown to be effective in shortening the QT interval and terminating torsade de pointes (TdP). Here, we report a case of osimertinib-induced QT prolongation accompanied by frequent VAs and TdP. Osimertinib was immediately discontinued after it was identified as the etiology for QT prolongation and VAs. A temporary pacemaker and overdrive pacing were used after other anti-arrhythmia treatments had failed and successfully shortened the QTc interval and terminated VAs. Repeated Holter monitoring at 1 week showed no remaining VAs or TdP, and the pacemaker was removed. Routine electrocardiography (ECG) surveillance was conducted afterward, and three- and 6-month follow-ups showed good recovery and normal ECG results. Vigilance is required for rare vital arrhythmias in patients taking osimertinib, and ECG surveillance should be conducted.
Epidermal growth factor receptor (EGFR) mutation is one of the most common oncogenic drivers in non-small cell lung cancer (NSCLC). Osimertinib, the third-generation EGFR tyrosine kinase inhibitor (TKI), has substantially improved treatment efficacy for NSCLC with EGFR mutations (1). However, while remaining low in incidence, cardiotoxicities related to EGFR-TKIs, such as congestive heart failure, QT interval prolongation, and ventricular arrhythmias (VAs) have become a safety concern (2), as they are life-threatening complications.
No consensus has been achieved for the management of these cardiotoxicities (2, 3). Overdrive pacing, or pacing with a higher heart rate, has been shown to be effective in shortening QT intervals and terminating torsade de pointes (TdP) (4). However, overdrive pacing in the acute management of osimertinib-induced VAs has rarely been reported in the literature. Here, we report a case of osimertinib-induced QT prolongation, frequent VAs, and TdP for which a temporary pacemaker and overdrive pacing were used. Serial electrocardiography (ECG) and Holter monitoring results during hospitalization and follow-ups confirmed the in-hospital and long-term efficacy and safety of these treatments.
A 60-year-old woman was admitted to our hospital with palpitations and an onset of syncope. The patient had experienced palpitations 3 months previously while working and one episode of syncope later at home. The patient had regained consciousness after 10 seconds but took no action and sought no treatment. Two days preceding admission, the palpitations had become more frequent, and the patient reported feeling dizzy on several occasions. The symptoms were not related to exercise or emotional changes. Seventeen months previously, the patient had been diagnosed with peripheral lung adenocarcinoma and associated brain and bone metastases (Figure 1A). Genomic analysis had indicated EGFR gene mutations, and she had therefore been treated with the EGFR TKI osimertinib (80 mg, QD). The patient had no history of hypertension, diabetes or related family history. There was also no record of previous use of anti-arrhythmic agents.
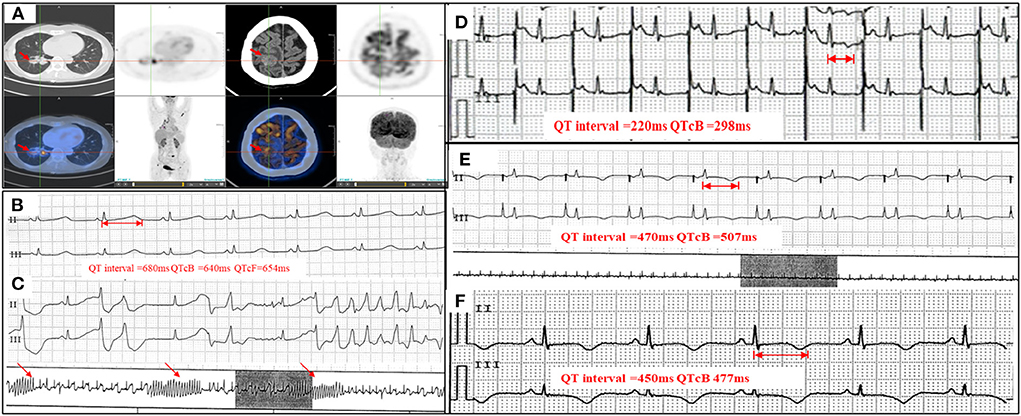
Figure 1. PET-CT, serial Holter monitoring, and ECG. (A) PET-CT showing peripheral pulmonary carcinoma and brain metastasis (arrowheads); (B) Holter monitoring on admission showing prolonged QTc interval (QTcB 640 ms); (C) Holter monitoring on admission showing frequent VTs and TdP (arrowheads); (D) ECG with a temporary pacemaker and overdrive pacing at 110 bpm (QTcB 298 ms); (E) Holter monitoring with a temporary pacemaker and pacing at 70 bpm (QTcB 507 ms); (F) ECG at discharge showing near-normal QTc interval (QTcB 477 ms). VT, ventricular tachycardia; TdP, torsade de pointes; QTcB, QTc interval calculated with Bazett formula; PET-CT, positron emission tomography – computed tomography; ECG, electrocardiogram.
The patient presented with tachycardia (122 bpm) and hypotension (81/68 mmHg) on physical examination. Electrocardiography (ECG) on admission showed prolonged QTc intervals (QTcB 577 ms) and frequent ventricular premature complexes (VPCs). Holter analysis also showed prolonged QTc intervals (longest QTcB 640 ms, Figure 1B), frequent VPCs, and ventricular tachycardias (VTs) (122 episodes/20 h, including multiple TdP) (Figure 1C). Echocardiography showed no structural or functional abnormalities. Laboratory assessments showed normal electrolyte concentrations (K+ 3.9 mmol/L, Ca2+ 2.50 mmol/L, Mg2+ 0.93 mmol/L) and negative cardiac biomarkers. SCN5A and KCH2 mutations were not detected during genetic screening. An ECG that had been conducted prior to osimertinib treatment showed normal QTc intervals (QTcB 417 ms, Supplementary Figure 1A). After ruling out QT prolongation caused by myocardial ischemia or other QT-prolongation drugs, the patient was diagnosed with osimertinib-induced QT prolongation, VAs, and TdP (Probable causality, World Health Organization-Uppsala Monitoring Center [WHO-UMC] causality assessment scale).
Osimertinib was immediately discontinued after it was identified as the etiology for QT prolongation and VAs. Anti-arrhythmia treatments (intravenous magnesium, potassium magnesium aspartate, and oral propranolol) were administered; however, they did not relieve the patient's symptoms. No defibrillation treatment was delivered for a stable hemodynamic status.
After a temporary pacemaker was implanted with the pacing lead placed at the patient's right atrium, overdrive pacing successfully shortened the QTc interval and terminated the VAs. The initial pacing rate of 110 bpm was gradually reduced to 60-−70 bpm within 1 week, and the QTc interval was shortened to 298–507 msec (Figures 1D,E). Repeat Holter monitoring at 1 week showed no VPCs or VTs. The pacemaker was then removed and following consultation with a hematologist, osimertinib was replaced by gefitinib (250 mg QD). At discharge, the patient's symptoms were relieved and her ECG showed normal results (QTc 477 ms) (Figure 1F, Supplementary Figure 1B). Routine ECG surveillance was conducted. Three- (Supplementary Figure 1C) and 6-month follow-ups showed good recovery and normal ECG results (Table 1).
This study reported a case of osimertinib-induced QT prolongation accompanied by frequent VAs and TdP in a patient being treated for NSCLC.
EGFR mutation is one of the most common oncogenic drivers in NSCLC. As such, EGFR-TKIs (including gefitinib, erlotinib, and osimertinib, etc.) are used to inhibit EGFR tyrosine kinase and have enhanced the treatment for NSCLC over the past two decades (5). In particular, osimertinib, a third-generation EGFR-TKI, has been shown to increase treatment efficacy even when compared with that of first- or second-generation EGFR TKIs (6). Osimertinib has thus become the first-line treatment for advanced EGFR-mutant NSCLC patients, especially for those with brain metastases or acquired T790M resistance mutation (1).
Despite their low incidence, cardiotoxicities including congestive heart failure, QT prolongation, and vital arrhythmias have become a safety concern for patients taking EGFR TKIs. Osimertinib-induced QT prolongation was first reported during the phase I trials for the drug (7), after which analyses in two phase III randomized controlled trials also confirmed that osimertinib notably increased the risk of cardiac toxicities, with a risk ratio of 2.62 for QT prolongation (8). The initial FDA risk-benefit assessment reported a low incidence (0.7%) of osimertinib-induced substantial QTc prolongation (QTc ≥ 500 msec), with no QTc-related VAs reported (9). Further, when Anand et al. reviewed the pharmacovigilance database of the FDA Adverse Events Reporting System (FAERS), and compared the cardiotoxicities of different EGFR-TKIs, a total of 315 cardiac adverse events (AE) were noted. Cardiac failure and QT prolongation were the cardiotoxicities most commonly caused by osimertinib. Of patients treated with osimertinib, 33/2,454 (1.3%) developed QT prolongation at a median time of 23 days. A comparison with first- and second-generation EGFR-TKIs has shown that osimertinib is more likely than the others to induce QT prolongation (reported odds ratio 6.6) (2). In a recent retrospective cohort study, Kunimasa et al. compared QT intervals in 72 patients with serial ECGs before and after osimertinib administration and found that QTc intervals were prolonged by approximately 20 ms over a median time of 116 days. However, no fatal arrhythmias were reported in this study (10).
In addition to QT prolongation, VT or TdP were also reported in a limited number of cases taking osimertinib (Table 2) (11–14). This indicates that osimertinib-induced vital arrhythmias are probably underestimated owing to the limitations of retrospective studies and the reporting system. Physicians should be vigilant to the occurrence of these rare vital arrhythmias in patients on osimertinib and conduct ECG surveillance for these patients.
However, the mechanism of osimertinib-induced cardiotoxicity is still unclear (15). In the preliminary IC50 inhibition in-vitro cell test, osimertinib showed weak inhibition of the cardiac potassium channel Kv11.1, which may be a potential mechanism of osimertinib-induced QT prolongation (16). However, further basic research is required for full clarification of the underlying mechanism.
As the treatment of VTs is based on the determination of their etiology, owing to the lack of understanding of the mechanism behind osimertinib-related arrhythmias, there has been no consensus for appropriate management. Magnesium supplementation, cardioversion, and β-blockers are generally used in the management of long QT syndrome (LQTS) related VT and Tdp (4). In the limited cases of osimertinib-induced VAs, point-of-care monitoring-guided magnesium supplementation, cardioversion, and antiarrhythmic drugs have been reportedly used (Table 2) (11–14); however, in this case, all treatment failed to improve this patient's symptoms. Although implantable cardioverter defibrillator (ICD) implantation is suggested in high-risk LQTS patients, it would have certainly led to frequent shocks for this particular patient, and was therefore deemed unsuitable. Additionally, the presence of polymorphic VT and TdP, indicated the ineligibility for radiofrequency ablation. Lastly, left cardiac sympathetic denervation (LCSD) is regarded as a bail-out strategy in the case that other treatments should fail.
Medically (isoprenaline infusion) or electrically (override pacing) speed up the heart can both help to decrease the QTc interval and terminate TdP temporarily. The efficacy of override pacing in comparison with isoprenaline is uncertain due to the lack of randomized comparison evidence (17). However, override pacing would be a better option when the risk of TdP may persist over a more extended period, such as a long-acting drug. As the mean elimination half-life time of osimertinib is 48–59.7 h theoretically (18, 19), temporary pacemaker implantation and overdrive pacing can help to shorten the QTc interval and increase survival during this life-threatening time period.
On the other hand, osimertinib was replaced with gefitinib for chemotherapy after the occurrence of this life-threatening complication. No disease progression or TdP recurrence has been detected and favorable recovery has been archived in the follow-ups.
Osimertinib-induced QT interval prolongation and VAs are underestimated in NSCLC patients, and no consensus has been achieved on standard treatment. This case showed that ECG and Holter monitoring should be performed periodically in patients on osimertinib treatment. Temporary pacemaker implantation and overdrive pacing may be considered a safe and effective treatment for the acute management of osimertinib-induced VAs.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Written informed consent was obtained from the participant for the publication of this case report.
YZ and BD identified the case. XW and YP conducted the literature search and prepared the first draft of the manuscript. KN and PY provided critical revision for the manuscript. All authors contributed to the articles and approved the submitted version.
This research was supported by grants from Jilin Provincial Science and Technology Department International Cooperation Project (No. 20210402016GH) and the National Natural Science Foundation of China (No. 82100337).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.934214/full#supplementary-material
Supplementary Figure 1. Serial 12 leads ECGs before osimertinib treatment and in the recovery stage. (A) 12 leads ECG four months before osimertinib treatment showing normal QTc interval (QTcB 417 ms); (B) 12 leads ECG at discharge showing near-normal QTc interval (QTcB 477 ms); (C) 12 leads ECG at three month's follow-up showing near-normal QTc interval (QTcB 468 ms); QTcB, QTc interval calculated with Bazett formula.
1. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, egfr-mutated advanced nsclc. N Engl J Med. (2020) 382:41–50. doi: 10.1056/NEJMoa1913662
2. Anand K, Ensor J, Trachtenberg B, Bernicker EH. Osimertinib-induced cardiotoxicity. JACC: CardioOncology. (2019) 1:172–8. doi: 10.1016/j.jaccao.2019.10.006
3. Salem J-E, Nguyen LS, Moslehi JJ, Ederhy S, Lebrun-Vignes B, Roden DM, et al. Anticancer drug-induced life-threatening ventricular arrhythmias: a world health organization pharmacovigilance study. Eur Heart J. (2021) 42:3915–28. doi: 10.1093/eurheartj/ehab362
4. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 Aha/Acc/Hrs guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. (2018) 138:e272–391. doi: 10.1016/j.hrthm.2017.10.036
5. Kim C, Liu SV. First-line egfr tki therapy in non-small-cell lung cancer: looking back before leaping forward. Ann Oncol. (2019) 30:1852–5. doi: 10.1093/annonc/mdz415
6. Wu L, Ke L, Zhang Z, Yu J, Meng X. Development of egfr tkis and options to manage resistance of third-generation egfr tki osimertinib: conventional ways and immune checkpoint inhibitors. Front Oncol. (2020) 10:602762. doi: 10.3389/fonc.2020.602762
7. Jänne PA, Yang JC-H, Kim D-W, Planchard D, Ohe Y, Ramalingam SS, et al. Azd9291 in egfr inhibitor–resistant non–small-cell lung cancer. NEJM. (2015) 372:1689–99. doi: 10.1056/NEJMoa1411817
8. Thein KZ, Swarup S, Ball S, Quirch M, Vorakunthada Y, Htwe KK, et al. Incidence of cardiac toxicities in patients with advanced non-small cell lung cancer treated with osimertinib: a combined analysis of two phase iii randomized controlled trials. Ann Oncol. (2018) 29:viii500. doi: 10.1093/annonc/mdy292.011
9. Odogwu L, Mathieu L, Goldberg KB, Blumenthal GM, Larkins E, Fiero MH, et al. Fda benefit-risk assessment of osimertinib for the treatment of metastatic non-small cell lung cancer harboring epidermal growth factor receptor T790m mutation. Oncologist. (2018) 23:353–9. doi: 10.1634/theoncologist.2017-0425
10. Kunimasa K, Kamada R, Oka T, Oboshi M, Kimura M, Inoue T, et al. Cardiac adverse events in egfr-mutated non-small cell lung cancer treated with osimertinib. JACC CardioOncol. (2020) 2:1–10. doi: 10.1016/j.jaccao.2020.02.003
11. Matsuura C, Kato T, Koyama K. Successful management of refractory torsades de pointes due to drug-induced long qt syndrome guided by point-of-care monitoring of ionized magnesium. Cureus. (2021) 13:e13939. doi: 10.7759/cureus.13939
12. Ikebe S, Amiya R, Minami S, Ihara S, Higuchi Y, Komuta K. Osimertinib-induced cardiac failure with qt prolongation and torsade de pointes in a patient with advanced pulmonary adenocarcinoma. Int Cancer Confer J. (2021) 10:68–71. doi: 10.1007/s13691-020-00450-2
13. Bian S, Tang X, Lei W. A case of torsades de pointes induced by the third-generation egfr-tki, osimertinib combined with moxifloxacin. BMC Pulm Med. (2020) 20:181. doi: 10.1186/s12890-020-01217-4
14. Kaira K, Ogiwara Y, Naruse I. Occurrence of ventricular fibrillation in a patient with lung cancer receiving osimertinib. J Thorac Oncol. (2020) 15:e54–e5. doi: 10.1016/j.jtho.2019.11.029
15. Ewer MS, Tekumalla SH, Walding A, Atuah KN. Cardiac safety of osimertinib: a review of data. J Clin Oncol. (2021) 39:328–37. doi: 10.1200/JCO.20.01171
16. Desai MY, Windecker S, Lancellotti P, Bax JJ, Griffin BP, Cahlon O, et al. Prevention, diagnosis, and management of radiation-associated cardiac disease: jacc scientific expert panel. J Am Coll Cardiol. (2019) 74:905–27. doi: 10.1016/j.jacc.2019.07.006
17. Thomas SH, Behr ER. Pharmacological treatment of acquired qt prolongation and torsades de pointes. Br J Clin Pharmacol. (2016) 81:420–7. doi: 10.1111/bcp.12726
18. Gao X, Le X, Costa DB. The safety and efficacy of osimertinib for the treatment of egfr t790m mutation positive non-small-cell lung cancer. Expert Rev Anticancer Ther. (2016) 16:383–90. doi: 10.1586/14737140.2016.1162103
Keywords: osimertinib, QTc interval prolongation, ventricular tachycardia, temporary pacemaker, torsade de pointes (TdPs)
Citation: Zhang Y, Wang X, Pan Y, Du B, Nanthakumar K and Yang P (2022) Overdrive pacing in the acute management of osimertinib-induced ventricular arrhythmias: A case report and literature review. Front. Cardiovasc. Med. 9:934214. doi: 10.3389/fcvm.2022.934214
Received: 02 May 2022; Accepted: 13 September 2022;
Published: 29 September 2022.
Edited by:
Carlo Gabriele Tocchetti, University of Naples Federico II, ItalyReviewed by:
Weichieh Lee, Chi Mei Medical Center, TaiwanCopyright © 2022 Zhang, Wang, Pan, Du, Nanthakumar and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beibei Du, YmVpYmVpZHUyMDEyQGpsdS5lZHUuY24=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.