
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 28 October 2022
Sec. Cardiovascular Therapeutics
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.932054
Objective: Current guidelines recommend potent P2Y12 inhibitors such as ticagrelor over clopidogrel as part of the dual antiplatelet therapy (DAPT) after ST-segment elevation myocardial infarction (STEMI), irrespective of final management strategy. The aim of this multicenter prospective cohort study was to examine the efficacy and safety of bivalirudin with background ticagrelor and aspirin therapy in patients with STEMI undergoing primary percutaneous coronary intervention (PPCI).
Methods: A total of 800 patients with STEMI who were undergoing PPCI and receiving treatment with aspirin and ticagrelor from three Hospitals between April 2019 and September 2021 were included in this study. The patients were assigned, according to the perioperative anticoagulant, to the bivalirudin group (n = 456) or the heparin group (n = 344). In this study, the primary endpoint was 30-day net adverse clinical events (NACEs), a composite of major adverse cardiac or cerebral events (MACCEs, a composite of cardiac death, recurrent myocardial infarction, ischemia-driven target vessel revascularization, or stroke), or any bleeding as defined by the Bleeding Academic Research Consortium (BARC) definition (grades 1–5).
Results: The patients were followed up for 30 days after PPCI. The incidence of NACE was significantly lower in the bivalirudin group than in the heparin group (11.2 vs. 16.0%, P = 0.042), and this significance was mainly a consequence of the reduction in BARC 1 bleeding events in the bivalirudin group compared to the heparin group (3.2 vs. 7.1%, P = 0.010). Results from multivariate Cox regression analysis showed that bivalirudin significantly reduced 30-day NACE (HR: 0.676, 95% CI: 0.462–0.990, P = 0.042) and BARC1 bleeding events (HR: 0.429, 95% CI: 0.222–0.830, P = 0.010). No significant between-group differences were observed for MACCE, all-cause mortality, cardiac death, recurrent myocardial infarction, stroke, target vessel revascularization, stent thrombosis, and BARC2-5 bleeding events at 30 days.
Conclusion: In patients with STEMI who were undergoing primary PCI and receiving treatment with aspirin and ticagrelor, bivalirudin was associated with decreased rates in NACE and minimal bleeding events without significant differences in the rates of MACCE or stent thrombosis when compared with heparin. Nevertheless, large randomized trials are warranted to confirm these observations.
Clinical trial registration: The trial was registered at the Chinese Clinical Trial Registry (ChiCTR, http://www.chictr.org.cn; identifier [ChiCTR1900022529]). Registered on 15 April 2019. Registration title: Effect of bivalirudin combined with ticagrelor in patients with ST-segment elevation myocardial infarction during primary percutaneous coronary intervention.
The prognosis for patients with ST-segment elevation myocardial infarction (STEMI) is improved with the use of primary percutaneous coronary intervention (PPCI). Anticoagulation with bivalirudin or heparin, in combination with antiplatelet agents such as aspirin, P2Y12 inhibitors, and glycoprotein IIb/IIIa inhibitors, is essential to prevent adverse ischemic events, especially stent thrombosis and reinfarction during and after primary PCI in patients with STEMI (1).
Current guidelines recommend potent P2Y12 inhibitors such as ticagrelor over clopidogrel as part of the dual antiplatelet therapy (DAPT) after STEMI (because prasugrel is not listed in China, ticagrelor was selected as potent P2Y12 inhibitors in this study), irrespective of final management strategy (2, 3). In the PLATO trial (4), ticagrelor significantly reduced the rate of composite ischemic endpoints after STEMI compared with clopidogrel. However, the ischemic benefit came with an increased risk of bleeding, which has been shown to adversely affect prognosis (5–7). Choosing the best procedural anticoagulation regimen to balance the risks of ischemia and bleeding during primary PCI is essential to optimize outcomes (8).
Bivalirudin and heparin are the two anticoagulant drugs most commonly used during PCI (9). Several trials reported a reduced bleeding risk with bivalirudin vs. heparin with or without glycoprotein IIb/IIIa inhibition in patients undergoing primary PCI for STEMI (10–12). Bivalirudin may mitigate any increase in bleeding seen with ticagrelor (13). This suggests that bivalirudin may be a better choice of anticoagulant than heparin in patients with STEMI treated with ticagrelor in this setting. However, the efficacy and safety of bivalirudin combined with ticagrelor during primary PCI have not been established in patients with STEMI (8). Therefore, this multicenter prospective cohort study was designed to compare the perioperative efficacy and safety of bivalirudin and heparin on the basis of antiplatelet therapy of ticagrelor combined with aspirin in patients with STEMI undergoing primary PCI.
A total of 800 patients with STEMI who were undergoing PPCI and receiving treatment with aspirin and ticagrelor from three hospitals (The First Affiliated Hospital of the University of Science and Technology of China, The First People's Hospital of Hefei City, and The Second Affiliated Hospital of Anhui Medical University) in China between April 2019 and September 2021 were included in this study (Figure 1).
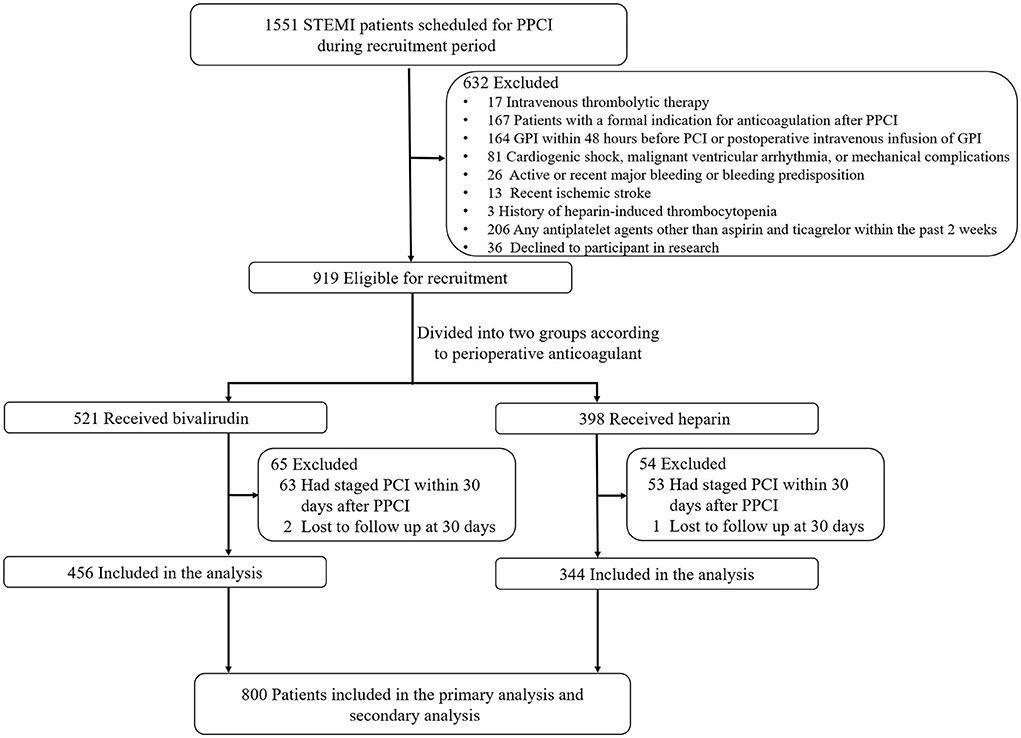
Figure 1. Flow diagram of the study. PPCI, primary percutaneous coronary intervention; GPI, glycoprotein IIb/IIIa inhibitors.
Inclusion criteria: (1) use of antiplatelet agents (aspirin concomitant with ticagrelor, loading or maintenance dose) before PPCI; (2) STEMI with PPCI of culprit lesion; (3) no revascularization for other non-culprit target vessels within 30 days after PPCI; (4) ability to understand and to comply with the study protocol; and (5) signed informed consent form.
Exclusion criteria: (1) intravenous thrombolytic therapy; (2) patients with a formal indication for anticoagulation after PPCI (e.g., atrial fibrillation, left ventricular thrombus, intra-aortic balloon pump, pulmonary embolism, mechanical heart valve); (3) glycoprotein IIb/IIIa inhibitor (GPI) within 48 h before PCI or postoperative intravenous infusion of GPI; (4) cardiogenic shock, malignant ventricular arrhythmia, or mechanical complications; (5) severe hematologic disease or history of intracerebral mass, aneurysm, arteriovenous malformation, recent (<6 months) ischemic stroke, recent (<6 months) intracranial hemorrhage or, gastrointestinal or genitourinary bleeding within the past 2 weeks; (6) history of heparin-induced thrombocytopenia; (7) suspected acute aortic dissection (AAD); (8) known allergy to any study drug; (9) patients with any indication for chronic anticoagulation; (10) bivalirudin and heparin were both used during the peri-procedural period of PCI; (11) any antiplatelet agents other than aspirin and ticagrelor within the past 2 weeks; and (12) current participation in an investigational drug or device trial. GPI use before catheterization or intended use during PCI was contraindicated; however, bail-out use of GPI was allowed and recorded.
This study was approved by the ethics committee at the First Affiliated Hospital of the University of Science and Technology in China (2019-ky03, Supplementary Figure 1). Oral informed consent was obtained from all participants before recruitment, and written informed consent was obtained within 24 h after recruitment.
For eligible subjects, the information on baseline characteristics (gender, age, coronary risk factors, laboratory tests), clinical medications, and detailed PPCI data were collected by designated study staff. A follow-up telephone contact or office visit 30 days after the emergency PCI was conducted for all subjects. The primary endpoint was 30-day net adverse clinical events (NACEs), a composite of major adverse cardiac or cerebral events (MACCEs, a composite of cardiac death, recurrent myocardial infarction, ischemia-driven target vessel revascularization, or stroke) or any bleeding as defined by the Bleeding Academic Research Consortium (BARC) (14) definition (grades 1–5). Major secondary endpoints were MACCE, any bleeding, and certain or probable stent thrombosis at 30 days as defined by the Academic Research Consortium (ARC) (15).
Subjects took a single dose of aspirin (100–300 mg) and ticagrelor (180 mg) before PPCI. Interventional procedures were performed according to standard techniques and the choice between bivalirudin and heparin was at the operators' discretion. Bivalirudin (250 mg/vial, Salubris Pharmaceutical Co, Shenzhen, China) could be started immediately as a bolus of 0.75 mg/kg followed immediately by an infusion of 1.75 mg/kg/h during angiography, and continued during PPCI. ACT was recommended monitoring at 5 min after the first bolus of bivalirudin. If the ACT is <225 seconds, an additional bolus of 0.3 mg/kg was given. The sheath has been removed usually at the end of the procedure. At 30 min after the end of PCI, the physicians decided whether or not to administer bivalirudin (1.75 mg/kg, iv) as needed for no more than 4 h (from the start of this dose). Patients in the heparin group were given a loading dose (100 U/kg) of heparin. ACT was monitored 5 min after the initial dose, and a heparin 20 U/kg bolus was given if ACT was <225 s. After PPCI, the patients were instructed to take aspirin (100 mg, qd) and ticagrelor (90 mg, bid). The use of aspirin lifelong was advised and ticagrelor was prescribed for 12 months. Unless contraindicated, long-term lipid-lowering therapy was recommended. The use of other medications (e.g., beta-blockers, angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers) was left to the discretion of the treating physicians.
Hypertension was defined by an SBP >140 mmHg or a DBP >90 mmHg. Anemia was defined as hemoglobin <120 g/L for men or hemoglobin <110 g/L for women. Cardiac death was defined as any death with a clear relationship to cardiac factors (e.g., myocardial infarction, heart failure, fatal arrhythmia), death of unknown cause, and all procedure-related death, including concurrent treatment-related death. The definition of myocardial infarction was based on the European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Fourth universal definition of myocardial infarction criteria (16). Ischemia-driven target vessel revascularization (TVR) was defined as second PCI or coronary artery bypass grafting (CABG) due to re-stenosis of the target lesion or any part of the same major vessel. Stroke was defined as the presence of a new focal neurologic deficit thought to be vascular in origin, with signs or symptoms lasting more than 24 h or 24 h because of pharmacologic or non-pharmacologic intervention. The preoperative estimated glomerular filtration rate (eGFR) was calculated from serum creatinine (sCr) concentrations using the modified glomerular filtration rate estimating equation for Chinese population: eGFR (ml/min/1.73 m2) = 175 × (sCr)-1.234 × (age)-0.179 × (0.79 if patient is a female subject) (17).
A multivariable Cox regression model was used to analyze the effect of anticoagulants (bivalirudin vs. heparin) on the primary endpoint (NACE). According to the BRIGHT study (12), the estimated incidence of NACE at 30 days of follow-up was 10%. The variable of interest was X1 (perioperative anticoagulant, i.e., bivalirudin vs. heparin), and the estimated log hazard ratio lnΔ = 0.53. With a two-sided significance level of 0.05 and statistical power of 80%, the calculation process of the required sample size is as follows: ① estimate the standard deviation of X1 and obtain σ = 0.6; ② perform multiple linear regression analysis on X1 and other covariates and obtain R2 = 0.0137. Using the PASS 11 software (Supplementary Figure 2), the above parameters were included in the calculation, and the sample size was 787 subjects (≈800 total subjects).
Continuous variables were demonstrated as mean ± standard deviation (SD) or median value [interquartile range (IQR)]. Frequency and proportion were presented for categorical variables. For group comparisons, Pearson's chi-square test or Fisher's exact test was used for categorical variables when appropriate. Student's unpaired t-test or the Mann–Whitney rank-sum test was used for continuous variables when appropriate. For each primary and secondary endpoint, Kaplan–Meier methods were used to estimate 30-day event rates for each group, and comparisons between the two study groups were performed using the log-rank test. A multivariable Cox regression model for clinical endpoints was performed, correcting for those clinical variables significantly different between groups, with a final adjustment model including age, gender, smoking status, eGFR, previous myocardial infarction, and anemia. SPSS v24.0 was used for statistical analysis. All tests were two-sided, and P < 0.05 was considered to be statistically significant.
As shown in Tables 1, 2, the bivalirudin group was older, with a higher prevalence of anemia and lower prevalence of male subjects, prior or current smoking, and previous MI than the heparin group. There were no significant differences in perioperative procedures and medications between the two groups. GPIs were administered in 11.8 and 12.4% of patients in the bivalirudin and UFH groups, respectively. In the bivalirudin group, all patients received a post-procedure infusion of the 1.75 mg/kg/h bivalirudin PCI dose for a median duration of 180 min (IQR, 125–240 min).
During the 30-day follow-up after PCI, a total of 106 NACE (13.2%) occurred:52 NACE (11.2%) in the bivalirudin group and 51 NACE (16.0%) in the heparin group (see Figure 2 and Table 3). The difference was significant between the two groups (P = 0.042). Multivariate Cox regression analysis showed that the incidence of NACE was lower in the bivalirudin group than in the heparin group (P = 0.042). The composite of MACCE or bleeding requiring medical intervention (BARC 2–5 bleeding events) did not differ significantly between the groups (Table 3).
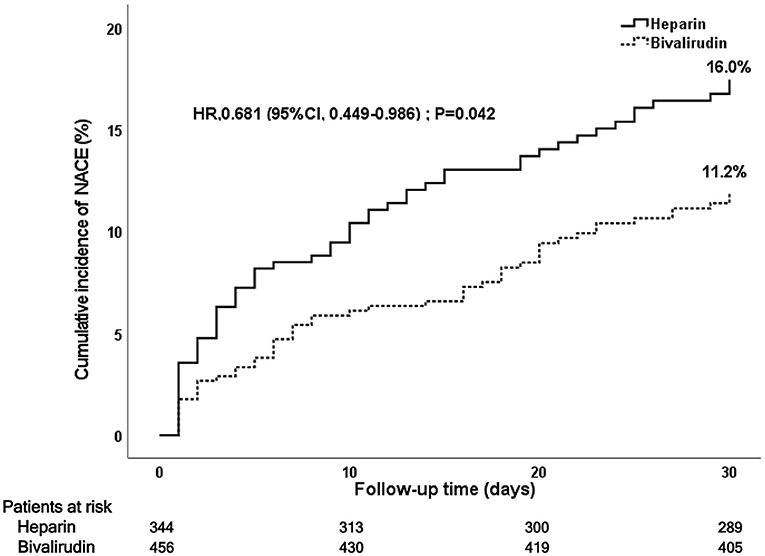
Figure 2. Kaplan–Meier curve of post-PCI 30-day NACE. PCI, percutaneous coronary intervention; NACE, net adverse clinical events.
During the 30-day follow-up after PCI, a total of 42 MACCE occurred: 23 MACCE (5.0%) in the bivalirudin group and 19 MACCE (5.5%) in the heparin group (see Figure 3 and Table 3). The difference did not reach statistical significance (P = 0.767). Multivariate Cox regression analysis showed no significant between-group difference in the incidence of MACCE (P = 0.597). No significant difference was observed in cardiac death (4.0 vs. 3.2%), recurrent myocardial infarction (0.5 vs. 0.9%), stroke (0.5 vs. 0.6%), target vessel revascularization (0.7 vs. 1.2%), or stent thrombosis (0.2 vs. 0.3%) between the bivalirudin group and the heparin group (P > 0.05).
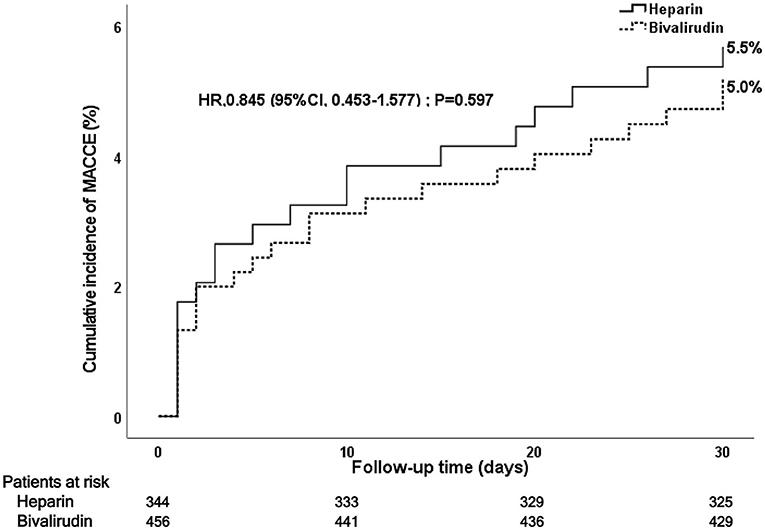
Figure 3. Kaplan–Meier curve of post-PCI 30-day MACCE. PCI, percutaneous coronary intervention; MACCE, major adverse cardiac and cerebrovascular events.
During the 30-day follow-up after PCI, bivalirudin was associated with a lower rate of bleeding events at 30 days (6.7 vs. 11.5%, P = 0.016) than UFH (see Figure 4 and Table 3). This significance was mainly a consequence of the reduction in BARC 1 bleeding events in the bivalirudin group compared to the UFH group (3.2 vs. 7.1%, P = 0.010). Multivariate Cox regression analysis also showed that the rates of all bleeding events and BARC1 bleeding events were significantly lower in the bivalirudin group than in the heparin group. No significant difference was observed in BARC 2–5 bleeding events (3.6 vs. 4.4%), BARC 2 bleeding events (2.2 vs. 3.6%), and BARC 3–5 bleeding events (1.3 vs. 0.9%) between the bivalirudin group and heparin group (P > 0.05).
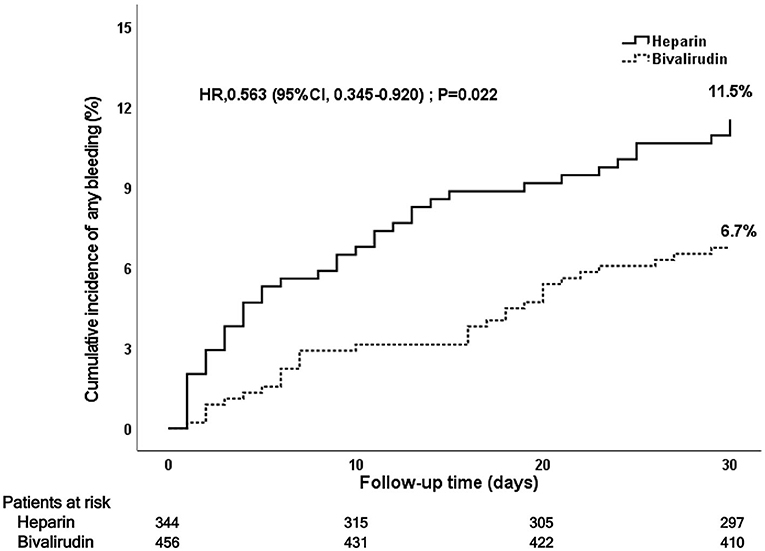
Figure 4. Kaplan–Meier curve of post-PCI 30-day bleeding events. PCI, percutaneous coronary intervention.
In this prospective cohort, multicenter trial, we explored the effectiveness and safety of bivalirudin in patients with STEMI who were undergoing primary PCI and receiving treatment with aspirin and ticagrelor. The main findings were as follows: (1) bivalirudin was associated with a significantly lower rate of NACE than heparin at 30 days after PCI, which was mainly derived from a significant reduction of minimal bleeding, but not major bleeding. (2) Bivalirudin therapy showed a similar risk of causing ischemic events, including ST, compared to heparin.
Administration of the optimal peri-procedural antithrombotic regimen during PCI is essential to balance the risk of bleeding and ischemia. In previous studies comparing bivalirudin with heparin, including the HORIZONS-AMI (10) and BRIGHT (12) trials, bivalirudin has been associated with reduced bleeding but increased early stent thrombosis, a risk that can be mitigated by a prolonged infusion. It is noteworthy that clopidogrel was the default P2Y12 inhibitor used in those trials instead of ticagrelor or prasugrel. Potent P2Y12 inhibitors, such as ticagrelor, have been strongly recommended over clopidogrel to reduce the risk of ischemic recurrences (4). The effective ischemic protection offered by stronger P2Y12 inhibition is potentially counterbalanced by an increased risk of bleeding (4, 18). At variance, the multicenter VALIDATE-SWEDEHEART trial (19), which enrolled patients with STEMI undergoing urgent PCI with potent P2Y12 inhibitors (about 81% of the patients received ticagrelor) and without planned GPI, found similar cardiovascular outcomes and bleeding risk between groups. Moreover, in the VALIDATE-SWEDEHEART trial (19), the rate of stent thrombosis was lower in the bivalirudin group—a finding that might be attributed to the prolonged bivalirudin infusion. Notably, around one-third of patients in the bivalirudin group were given heparin right before the procedure, which introduces imbalances in treatment effects. Thus, even after VALIDATE-SWEDEHEART trial (19), there is no definitive answer to the question of whether to use bivalirudin or heparin during PCI in the current era (8).
Our study has represented contemporary PCI practice (e.g., predominantly performed via radial access, use of potent P2Y12 inhibitor, and limited use of GPI), which made our findings more valid. Both before and after correcting the baseline of the two groups, we found that peri-procedural use of bivalirudin during PCI in patients with STEMI treated with potent P2Y12 inhibitors showed a lower incidence of NACE events compared with heparin, mainly due to the reduced risk of minimal bleeding events (BARC1 bleeding events). The risk for BARC 2 bleeding events in the bivalirudin group tended to be lower (10 [2.2%] vs. 12 [3.6%]; HR: 0.629, P = 0.297); however, the difference did not reach statistical significance. Given the low absolute number of BARC 2 bleeding events at 30-day follow-up, our study may not be powered enough to detect significant differences in BARC 2 bleeding events at 30 days.
Our results demonstrated that the anti-ischemic effect of bivalirudin was similar to that of heparin in patients with STEMI who underwent primary PCI. At 30 days, stent thrombosis occurred in 0.2% receiving bivalirudin and 0.3% receiving heparin (P = 0.847). The relatively low rates of stent thrombosis overall compared with previous similar studies (10, 20) and the absence of a significant between-group difference in ischemic event rates might be due to the treatment with ticagrelor and prolonged infusion of bivalirudin after primary PCI. Ticagrelor has shown evidence of a lower incidence of adverse ischemic events and stent thrombosis compared with clopidogrel in several trials (4, 21). A recently published meta-analysis (22) including four trials on bivalirudin therapy in patients undergoing primary PCI has suggested that bivalirudin, with the high-dose delayed application method after PCI, was associated with a lower risk of early definite stent thrombosis compared with treatment with heparin.
This study has some limitations. First, anticoagulant regimen selection was not randomized and was at the discretion of the treating physician, which makes all comparisons subject to potential biases underlying the choice of therapy. Despite multiple adjustments for differences in baseline characteristics between treatment groups, unmeasured confounders influencing outcomes cannot be excluded. Randomized controlled trials are needed to validate the results. Second, the information on the use of other medications during the 12-month follow-up period was not available, which has potential implications for clinical outcomes in the different cohorts. Some evidence suggests that bleeding affects long-term mortality (6). Third, given the low absolute number of events at 30-day follow-up, our study was not powered enough to detect significant differences in stroke, MI, TVR, stent thrombosis, BARC 2 bleeding events, and BARC 3–5 bleeding events.
In patients with STEMI who were undergoing primary PCI and receiving treatment with aspirin and ticagrelor, bivalirudin was associated with decreased rates in NACE and minimal bleeding events without significant differences in the rates of MACCE or stent thrombosis when compared with heparin. Nevertheless, large randomized trials are warranted to confirm these observations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Clinical Research Ethics Committee of The First Affiliated Hospital of University of Science and Technology of China. The patients/participants provided their written informed consent to participate in this study.
X-FY and L-KM conceived and designed the study. H-WC, Q-ZX, JX, X-HZ, B-BL, and B-LX were involved in data collection, interpretation, and analysis. X-FY wrote the manuscript. L-KM was involved in the editing of the manuscript. All authors have read and approved the final manuscript.
This study was supported by the Open Project of Anhui Provincial Cardiovascular Institute (Grant No. KF2018007) and the National Natural Science Foundation of China (Grant No. 82170263).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.932054/full#supplementary-material
1. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. (2018). doi: 10.1093/eurheartj/ehy658
2. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
3. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-Elevation myocardial infarction. J Am Coll Cardiol. (2013) 61:e78–140. doi: 10.1016/j.jacc.2012.11.018
4. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2009) 361:1045–57. doi: 10.1056/NEJMoa0904327
5. Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. (2006) 114:774–82. doi: 10.1161/CIRCULATIONAHA.106.612812
6. Mehran R, Pocock S, Nikolsky E, Dangas GD, Clayton T, Claessen BE, et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. (2011) 4:654–64. doi: 10.1016/j.jcin.2011.02.011
7. Valgimigli M, Costa F, Lokhnygina Y, Clare RM, Wallentin L, Moliterno DJ, et al. Trade-off of myocardial infarction vs. Bleeding types on mortality after acute coronary syndrome: Lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur Heart J. (2017) 38:804–10. doi: 10.1093/eurheartj/ehw525
8. Stone GW. Procedural anticoagulation in myocardial infarction. N Engl J Med. (2017) 377:1198–200. doi: 10.1056/NEJMe1709247
9. Hu Q, Han Y, Zhou T, Wang X, Zhang Q. Efficacy and safety of the reduced bivalirudin in patients undergoing coronary angiography or percutaneous coronary intervention stratified by renal function (REDUCE BOLUS): a Single-Blind, stratified randomized, non-inferiority trial. Front Cardiovasc Med. (2022) 9:864048. doi: 10.3389/fcvm.2022.864048
10. Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. (2008) 358:2218–30. doi: 10.1056/NEJMoa0708191
11. Steg PG. van T Hof A, Hamm CW, Clemmensen P, Lapostolle F, Coste P, et al. Bivalirudin started during emergency transport for primary PCI. N Engl J Med. (2013) 369:2207–17. doi: 10.1056/NEJMoa1311096
12. Han Y, Guo J, Zheng Y, Zang H, Su X, Wang Y, et al. Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction. JAMA. (2015) 313:1336. doi: 10.1001/jama.2015.2323
13. Kołtowski Ł, Legutko J, Filipiak KJ, Dziewierz A, Bartuś S, Buszman P, et al. Bivalirudin use in acute coronary syndrome patients undergoing percutaneous coronary interventions in Poland: clinical update from expert group of the Association on Cardiovascular Interventions of the Polish Cardiac Society. Cardiol J. (2019) 26:1–7. doi: 10.5603/CJ.2019.0029
14. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. (2011) 123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449
15. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. (2007) 115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313
16. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. (2019) 40:237–69. doi: 10.1093/eurheartj/ehy462
17. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. (2006) 17:2937–44. doi: 10.1681/ASN.2006040368
18. Xanthopoulou I, Deftereos S, Sitafidis G, Kanakakis I, Hamilos M, Karayannis G, et al. In-hospital bleeding events in acute coronary syndrome patients undergoing percutaneous coronary intervention in the era of novel P2Y12 inhibitors: Insights from the GReek AntiPlatelet rEgistry-GRAPE. Int J Cardiol. (2014) 174:160–2. doi: 10.1016/j.ijcard.2014.03.161
19. James S, Koul S, Andersson J, Angerås O, Bhiladvala P, Calais F, et al. Bivalirudin versus heparin monotherapy in ST-Segment–elevation myocardial infarction. Circ Cardiovasc Interv. (2021) 14:e008969. doi: 10.1161/CIRCINTERVENTIONS.120.008969
20. Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, et al. Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): Final 3-year results from a multicentre, randomised controlled trial. Lancet. (2011) 377:2193–204. doi: 10.1016/S0140-6736(11)60764-2
21. Sahlen A, Varenhorst C, Lagerqvist B, Renlund H, Omerovic E, Erlinge D, et al. Outcomes in patients treated with ticagrelor or clopidogrel after acute myocardial infarction: Experiences from SWEDEHEART registry. Eur Heart J. (2016) 37:3335–42. doi: 10.1093/eurheartj/ehw284
Keywords: bivalirudin, heparin, ticagrelor, ST-segment elevation myocardial infarction, primary percutaneous coronary intervention
Citation: Yu X-F, Chen H-W, Xu J, Xu Q-Z, Zhang X-H, Li B-B, Xu B-L and Ma L-K (2022) Bivalirudin vs. heparin on a background of ticagrelor and aspirin in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: A multicenter prospective cohort study. Front. Cardiovasc. Med. 9:932054. doi: 10.3389/fcvm.2022.932054
Received: 29 April 2022; Accepted: 05 October 2022;
Published: 28 October 2022.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Guangzhi Cong, General Hospital of Ningxia Medical University, ChinaCopyright © 2022 Yu, Chen, Xu, Xu, Zhang, Li, Xu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Kun Ma, bGttYUB1c3RjLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.