
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 15 July 2022
Sec. Cardiac Rhythmology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.931845
This article is part of the Research Topic Novel Strategies for Persistent Atrial Fibrillation (AF) Ablation and AF Driver Mapping View all 8 articles
Objective: The objective of this study was to observe the safety and efficacy of electrophysiological mapping following the Cox-Maze IV procedure and to investigate whether a correlation exists between recurrence of atrial fibrillation (AF) with the completeness of bidirectional electrical isolation and the inducibility of AF immediately after the Cox-Maze IV procedure.
Methods: Totally, 80 consecutive patients who suffered from aortic valve or mitral valve disease and persistent AF were randomly enrolled into the control group and electrophysiological mapping following the Cox-Maze IV group (Electrophysio-Maze group). In the Electrophysio-Maze group, patients underwent concomitant Cox-Maze procedure and following electrophysiological mapping of ablation lines in mitral isthmus, left atrial “box,” and tricuspid annulus. If the bidirectional electrical isolation of tricuspid annulus ablation line is incomplete, whether to implement supplementary ablation will be independently decided by the operator. Before and after the Cox-Maze IV procedure, AF induction was performed. All patients in both groups were continuously followed-up and underwent electrocardiogram Holter monitoring after 6 months.
Results: In total, 42 Electrophysio-Maze patients and 38 controls were enrolled. Compared with patients in the control group, there were shorter hospital stay, better cardiac remodeling changes, and higher relief from AF during the follow-up period of 6 months in the Electrophysio-Maze group. Within the Electrophysio-Maze group, the rate of incomplete the bidirectional electrical isolation of “box” ablation lines was zero, and the rate of incomplete bidirectional electrical isolation of mitral isthmus ablation line or tricuspid annulus ablation line was 23.8%. After two cases of successful complementary ablation on the tricuspid annulus ablation line, the final incomplete bidirectional electrical isolation of annulus ablation lines was 19.0%. There were correlations between late AF recurrence after 6 months with incomplete bidirectional electrical isolation of annulus ablation lines and AF induction immediately after the Cox-Maze IV procedure.
Conclusion: Electrophysiological mapping following the Cox-Maze procedure is safe and effective. Electrophysiological mapping in the Cox-Maze procedure can find out the non-transmural annulus ablation lines by assessing the completeness of bidirectional electrical isolation of ablation lines, guide supplementary ablation, and predict AF recurrence after 6 months.
Atrial fibrillation (AF) is the most common progressive arrhythmia in the world, which is the main cause of increased risk of stroke, heart failure, peripheral vascular embolism, and other diseases worldwide (1–4). Especially, in patients undergoing mitral valve surgery, the incidence of AF can be as high as 50–80% (5, 6). For this kind of drug-refractory AF, surgery or percutaneous ablation is recommended (7). The first attempt at surgical ablation for AF can be traced back to the 1980s, including left atrial isolation and corridor surgery. However, the success of surgical ablation was not reliably achieved until the development of the Cox-maze surgery in 1987, which then became the gold standard for surgical treatment of AF (8). Genev et al. (9) conducted a comparative study of catheter ablation, hybrid AF ablation, and open maze surgery in 140 patients and found that the sinus cardioversion rates of the three groups were 20.3, 57.9, and 72.7%, respectively, indicating that open maze surgery is significantly better than catheter ablation and hybrid AF ablation. As far as the effect in sinus cardioversion and stroke reduction is concerned, the maze procedure is definitely superior to drug therapy, especially in patients with persistent or long-standing persistent AF (10). However, maze surgery involves complex incisions, which can cause great trauma to the patient’s heart (11). Due to the complexity of the operation, prolonged surgery time, significant trauma, and obvious impact on cardiac function, the classic Cox-maze III surgery is not currently widely used (12). As the maze-III procedure increases risk during surgery, its use is limited (13). To reduce the trauma associated with maze-III, the Cox-Maze IV procedure was introduced in 2002, which is no less effective than maze-III in reducing stroke. Previous studies have confirmed the excellent effect in lowering postoperative stroke of the maze-III procedure (14), yet Murashita and his colleagues found that the incidence of stroke after Cox-Maze IV surgery was no more than that of maze-III procedure (15). However, the Cox-Maze IV procedure has a higher rate of late AF recurrence than the maze-III procedure (16, 17), which remains the main limitation of the Cox-Maze IV procedure. The recurrence of AF after Cox-Maze IV includes early recurrence and late recurrence, and early recurrence is a powerful independent predictor of late recurrence (18). To lower the incidence of late AF recurrence, it is crucial to identify accurate methods to predict the possibility of late recurrence immediately after the Cox-Maze IV procedure.
During the Cox-Maze IV procedure, bipolar ablation forceps and pens are often used to predict the transmural damage of tissue cells by measuring the tissue impedance between both electrodes. However, impedance of the whole tissue measured with bipolar ablation forceps or pens cannot eliminate the possibility of residual connection of focal survival atrial muscle, which can lead to leakage of the lesion line. Some experts suggest that the bacteriological mapping of bidirectional block of key ablation lines after surgical maze ablation of AF should be beneficial (19), which is expected to be the latent end point of surgical maze ablation. In the domain of traditional interventional AF ablation, electrophysiological mapping can not only evaluate the completeness of bidirectional electrical isolation of ablation lines but also induce AF before and after ablation. Hwang ES et al. evaluated the induction of AF by pacing in 89 patients with AF before and after radio-frequency ablation and found that the induction rate of AF decreased from 95.4% before ablation to 56.3% after ablation (20). Theoretically, a relevant mapping technique in interventional cardiology can also be used in the field of surgical maze ablation. Unfortunately, atrial electrophysiological mapping was seldom used in previous surgical maze procedures. Lanters EAH et al performed AF induction in 44 patients with paroxysmal AF who had recovered from sinus rhythm before surgery, yet found no relation between the inducibility of AF before surgery and early postoperative AF (21). Till present, less is known about the relationship between the inducibility of AF after Cox-Maze surgery and late AF recurrence. Therefore, we performed a prospective randomized controlled clinical trial to observe the safety and efficacy of electrophysiological mapping following the Cox-Maze IV procedure (Electrophysio-Maze) and investigate whether a correlation exists between late AF recurrence 6 months after the Cox-Maze procedure with the completeness of electrical isolation of ablation lines in mitral isthmus, left atrial “box,” and tricuspid annulus and with the inducibility of AF immediately after the Cox-Maze IV procedure.
This clinical study has been registered on the Chinese clinical trial website (ChiCTR1900023775). This research was funded by the National Key Research and Development Program (2018YFC1311204) and was approved by the ethics committee of the Second Xiangya Hospital of Central South University [2019 ethical review (Scientific Research) No. 054]. Written consent was obtained from all patients before surgery.
Between April 2020 and June 2021, 80 consecutive patients who suffered from aortic valve or mitral valve disease accompanied by persistent AF were randomly enrolled into control group and electrophysiological mapping following the Cox-Maze IV group (Electrophysio-Maze group). The inclusion criteria were as follows: (1) patients of either sex aged between 35 and 70 years; (2) history of valvular disease >3 years; (3) persistent atrial fibrillation (the onset of atrial fibrillation lasts for more than 7 days or requires the use of drugs or electrical cardioversion to convert the heart rhythm); (4) ejection faction >40%; and (5) a clinical diagnosis based on at least one of the following factors: rheumatic mitral stenosis or regurgitation with or without tricuspid regurgitation, rheumatic aortic valve stenosis or regurgitation with or without tricuspid regurgitation, rheumatic both mitral and aortic valve stenosis or regurgitation with or without tricuspid insufficiency, and non-rheumatic mitral, and/or aortic valve stenosis or insufficiency with or without tricuspid insufficiency. The diagnostic criteria for persistent atrial fibrillation were: twice electrocardiograph (ECG) Holter monitoring performed both at the first time more than 7 days ago and at the second time exactly before the surgery confirmed that rhythm all the time is atrial fibrillation without sinus rhythm in any time. While calculating duration of AF, the date of AF onset was defined by the earliest ECG record, which confirmed atrial fibrillation. The exclusion criteria were as follows: (1) previous open-heart surgery and (2) schemed concomitant coronary artery bypass grafting. Previous percutaneous balloon mitral valvuloplasty was not considered an exclusion criterion.
Patients in both groups will undergo mitral or aortic valve replacement or angioplasty, and in the Electrophysio-Maze group, patients will undergo additional concomitant Electrophysiological mapping following the Cox-Maze procedure, where electrophysiology mapping of the completeness of electrical isolation of ablation lines in mitral isthmus, left atrial “box,” and tricuspid annulus will be implemented after Cox-Maze IV. The standard Cox-Maze IV procedure was performed as described previously by Damiano et al. with a combination of bipolar radio-frequency ablation clamp (Medtronic, United States) and bipolar radio-frequency ablation pen MAX3 (AtriCure, United States; 22). Briefly, the patient was in the supine position for the procedure, and then a median thoracotomy was performed, followed by heparinization and establishment of cardiopulmonary bypass. Two incisions were made, one of which is on the right atrium and is parallel to the right atrioventricular sulcus, the other of which is on the left atrium and is longitudinally along the interatrial sulcus. The clamp ablation of the mitral isthmus by bipolar ablation forceps could not reach the mitral annulus, so a “spot ablation” by bipolar radio-frequency pen MAX3 was added to fix the ablation at the corresponding point of the coronary sinus on both epicardial and endocardial surface, and a “sliding ablation” by the bipolar radio-frequency pen was performed on the connecting line between the coronary sinus and mitral annulus on both epicardial and endocardial surface. Each ablation procedure was performed twice. If a thrombus was detected in the left atrium, it was cleared before completing the ablation procedure of the left atrium. After all the ablation routes of the left atrial maze surgery, valvuloplasty, replacement, or intracardiac repair was performed. After closing the left atrium, restoring of sinus rhythm, and opening the ascending aorta, the ablation route of the right atrium was performed.
Mapping of mitral isthmus ablation line: A decapolar electrophysiological catheter was inserted into the coronary sinus, and the proximal end polar (the 10th polar) was just located at the ostium of coronary sinus, with every two adjacent electrodes (e.g., polar 1–2 and polar 3–4) forming a pair of recording electrodes. After sinus rhythm recovered, the proximal electrode pair was paced at a cycle of 400–500 ms (>50 ms shorter than the cycle of the patient’s own heart rate), and the delay from the proximal electrode pair to the distal electrode pair was recorded by a 64-channel physiological function recorder (Jinjiang Electronics, China). If the delay from the proximal electrode pair to the distal electrode pair was >120 ms, the conduction block of ablation line from the proximal atrium to distal atrium was identified as complete; otherwise, it was identified as incomplete. Then, the distal electrode pair was paced at the same cycle, and the delay from the distal electrode pair to the proximal electrode pair was also recorded. If the delay from the distal electrode pair to the proximal electrode pair was >120 ms, the conduction block of ablation line from the distal atrium to proximal atrium was identified as complete; otherwise, it was identified as incomplete.
Mapping of left atrial box ablation lines: the distal electrode pairs of the decapolar electrophysiological catheter were placed in the box, and the proximal polar pairs were placed at the left atrium outside of the box. If there was no atrial potential captured or disturbed by the external sinus rhythm at the electrode pairs in box, the conduction block in the direction from the external left atrium to the box was identified as complete; otherwise, it was identified as incomplete. Then, the distal polar pair in the box was paced in a 400–500 ms cycle (>50 ms shorter than the cycle of the patient’s own sinus rate). If the left atria outside of the box was not captured or disturbed by pacing, the conduction block in the direction from the box to the external left atrium was identified as complete; otherwise, it was identified as incomplete.
Mapping of the tricuspid annulus ablation line: A decapolar electrophysiological catheter vertically crossed the tricuspid annulus ablation line, and the overall electrode array was distributed on both sides of the ablation line as symmetrically as possible, with the tip pointing to the free side wall of the right atrium (pointing to the right side of the patient’s body) and the most proximal electrode pair close to the anterior atrial sulcus. A proximal electrode pair was paced at a cycle of 400–500 ms (>50 ms shorter than the cycle of the patient’s own heart rate), and the delay from the proximal electrode pair to the distal electrode pair was recorded. If the delay was more than 120 ms, the conduction block of ablation line from the proximal atrium to distal atrium was identified as complete; otherwise, it was identified as incomplete. Then, a distal electrode pair was paced at a similar cycle, and if the delay from the distal electrode pair to the proximal electrode pair was >120 ms, the conduction block of ablation line from the distal atrium to proximal atrium was identified as complete; otherwise, it was identified as incomplete.
If the electrophysiological mapping immediately after maze operation indicates that the ablation line of tricuspid annulus in incomplete, whether to conduct complementary ablation will be independently decided by operator according to the stability of the patient’s clinical condition. The supplementary ablation method of tricuspid annulus is to use a bipolar radio-frequency pen to perform “sliding ablation” twice on the previous tricuspid annulus ablation line on the epicardial surface. Then, the mapping of tricuspid annulus ablation line was performed again. If electrophysiological mapping indicates that the bidirectional isolation of mitral isthmus or box ablation lines is incomplete, so as to avoid additional risks of supplementary ablation, which needs to flip the heart, complementary ablation was forbidden.
Atrial fibrillation induction before Cox-Maze IV was performed immediately after cannulization and before cardiopulmonary bypass. The tail line of bipolar radio-frequency ablation pen MAX3 was connected to the 64-channel physiological function recorder, with two tip electrodes placed in the high right atrium. The tip electrode pair was stimulated successively at a rate of 400, 300, 250, 230, 210, 190, 170, and 150 ms cycles, for 10 s in each cycle. If AF was induced and sustained for more 30 s, atrial fibrillation induction was identified as positive, and then electrical cardioversion was performed.
After the cardiopulmonary bypass was terminated and before the cannulization was withdrawn, AF induction procedures after Cox-Maze IV were performed again. The induction procedure was the same as that carried out before Cox-Maze IV.
All patients in two groups received amiodarone to prevent early postoperative recurrence of AF for 3 months. Electrical cardioversion was not a choice regardless of possible in-hospital AF recurrence if the hemodynamics was stable. If there was degree 3 atrioventricular conduction block that did not recover 7–10 days after surgery, a rate-responsive atrioventricular pacemaker will be implanted.
All patients in two groups were continuously followed-up and underwent a 24 h ECG Holter monitoring after 6 months, where the late AF reccurrence is defined as AF or atrial flatter lasting for more than 30 s. AF onset within 3 months after surgery, which is known as the postsurgical blanking period, will not be classified into late AF reccurrence. Beside the schemed ECG Holter monitoring after 6 months, if a patient reported persistent symptoms such as palpitation between 3 and 6 months, he/she will be recommended with an immediate ECG examination at the nearest hospital. Under this condition, if any AF onset lasting for more than 30 s was confirmed by ECG, the diagnostic of late AF recurrence is also established. Both follow-up and medical treatment were performed by doctors independent of main researchers.
All statistical analyses were performed using the SPSS software (version 21.0, IBM Corporation, United States). For quantitative variables, normality was assessed using the Shapiro–Wilk test, and mean ± SD was used to summarize normally distributed data. Normal distributions were achieved by logarithmic transformation, if necessary. While comparing continuous variables between two groups, analysis of variance was used to test the homogeneity of the variances of the two groups. If the variances are consistent, Student’s t-test was adopted; otherwise, Dunnett’s t-test should be an alternative selection. For the correlation between qualitative variables, the chi-square test was used, and the corresponding odds ratio was analyzed. All tests were two-tailed, and the significance level was set as 0.05, taking 95% confidence intervals.
Totally, 42 patients were enrolled into the Electrophysio-Maze group, and 38 patients were enrolled into control group. Between two groups, there were no difference in sex, age, duration of AF, long-standing AF percent, Euroscore II score, hypertension, coronary heart disease, preoperative cardiovascular accident, preoperative TIA, left atrial diameter, right atrial diameter, left ventricular diastolic diameter, right ventricular diastolic diameter, ejection fraction, and the use of antiarrhythmic drugs (Table 1).
There were no statistical difference in type of valve operation (percent of mitral valve and aorta valve), left atrial appendage thrombosis and temporary pacing, intraoperative blood loss, and postoperative total drainage volume between two groups. No patient was implanted with a permanent pacemaker, and no patient died or underwent stroke during hospitalization in both groups. Compared with control group, patients in the Electrophysio-Maze group underwent longer bypass time and cross-clamp time. In the Electrophysio-Maze group, a patient underwent rehospitalization due to chest wound infection and recovered after debridement, but there are no statistical differences in rehospitalization between two groups. Interestingly, Electrophysio-Maze can significantly shorten the length of hospitalization stay (Table 2).
In the Electrophysio-Maze group, all patients underwent AF induction before and after the Cox-Maze IV procedure (Figure 1). The induction was performed with cannulization but without cardiopulmonary bypass, making the conditions consistent as much as possible. AF was induced in 88.1% (37/42) of all patients before Cox-Maze IV and was induced in 14.3% (6/42) of all patients after the Cox-Maze IV procedure. The bidirectional electrical isolation of the mitral annular isthmus ablation line (Figure 2), “box” ablation lines (Figure 3), and tricuspid annular ablation line (Figure 4) was successfully mapped in each patient. The rate of incomplete bidirectional electrical isolation of “box” ablation lines immediately after Cox-Maze IV was zero (the rate of complete bidirectional conduction block of “box” ablation lines immediately after Cox-Maze IV was 100%), and the rate of incomplete bidirectional electrical isolation of mitral isthmus ablation lines or tricuspid annulus ablation lines was 23.8% (10/42), wherein the rate of incomplete bidirectional electrical isolation of both mitral isthmus and tricuspid annulus ablation line was 7.14% (3/42), the rate of incomplete bi-directional electrical isolation of single mitral isthmus ablation line was 7.14% (3/42), and the rate of incomplete bidirectional electrical isolation of single tricuspid annulus ablation line was 9.52% (4/42). In two cases of incomplete bidirectional electrical isolation in single tricuspid annulus ablation line, complementary ablation was performed, and then electrophysiological mapping was performed again to ensure that the subsequent electrophysiological mapping confirmed the final complete bidirectional electrical isolation. The final incomplete bidirectional electrical isolation rate of mitral isthmus or tricuspid annulus ablation line was 19.0% (8/42). The incomplete bidirectional electrical isolation rate of ablation lines was correlated with AF inducibility immediately after Cox-Maze IV procedure but not with AF inducibility before Cox-Maze IV (Table 3).
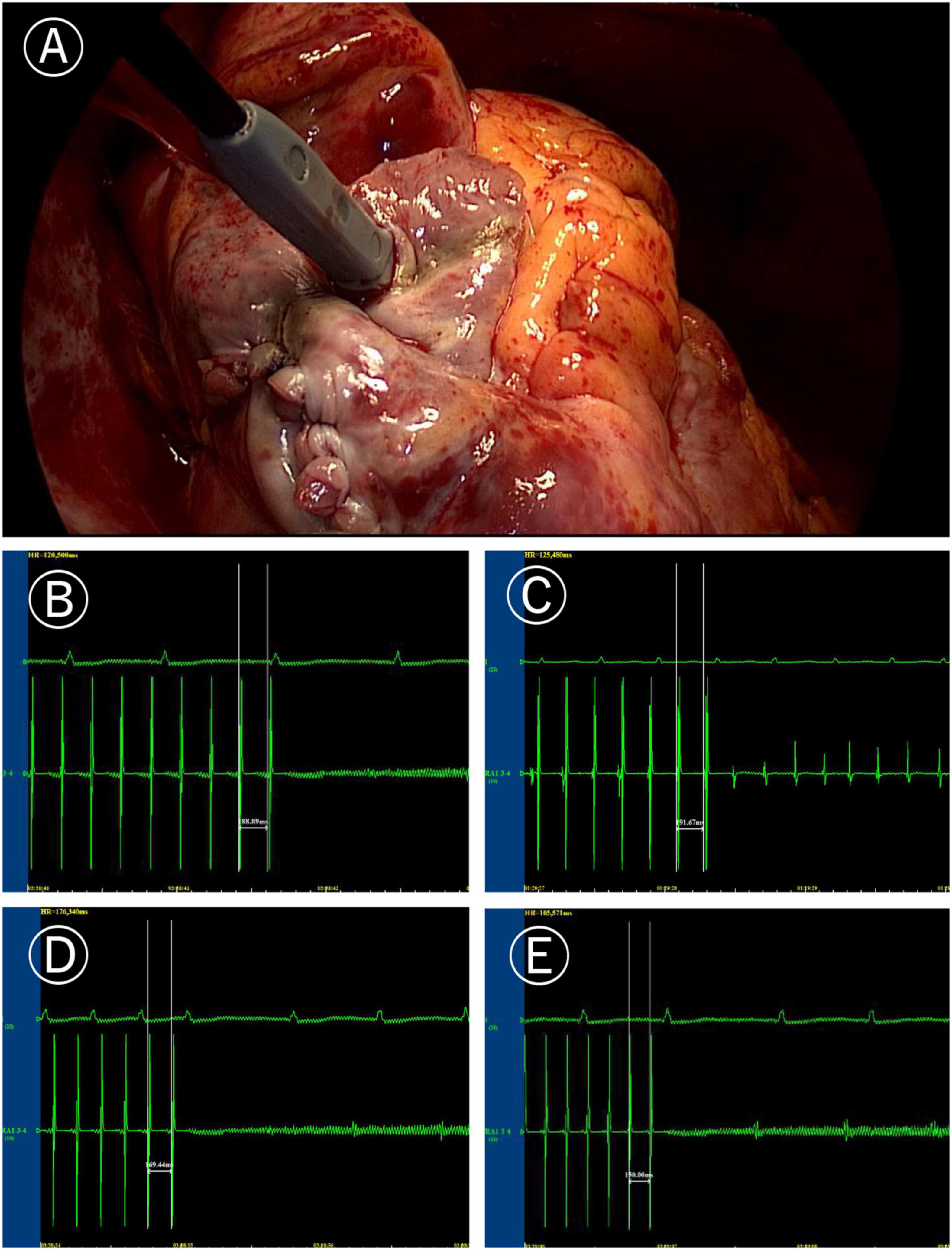
Figure 1. Induction of atrial fibrillation (AF). (A) Image of programmed stimulation at high right atrial for induction of atrial fibrillation (post surgery). (B) AF induced by the 190 ms cycle stimulation before Cox-Maze IV. (C) After Cox-Maze IV, the 190 ms cycle stimulation failed to induce AF again. (D) After Cox-Maze IV, the 170 ms cycle stimulation could not induce AF; and (E) After Cox-Maze IV, the 150 ms cycle stimulation could not induce AF.
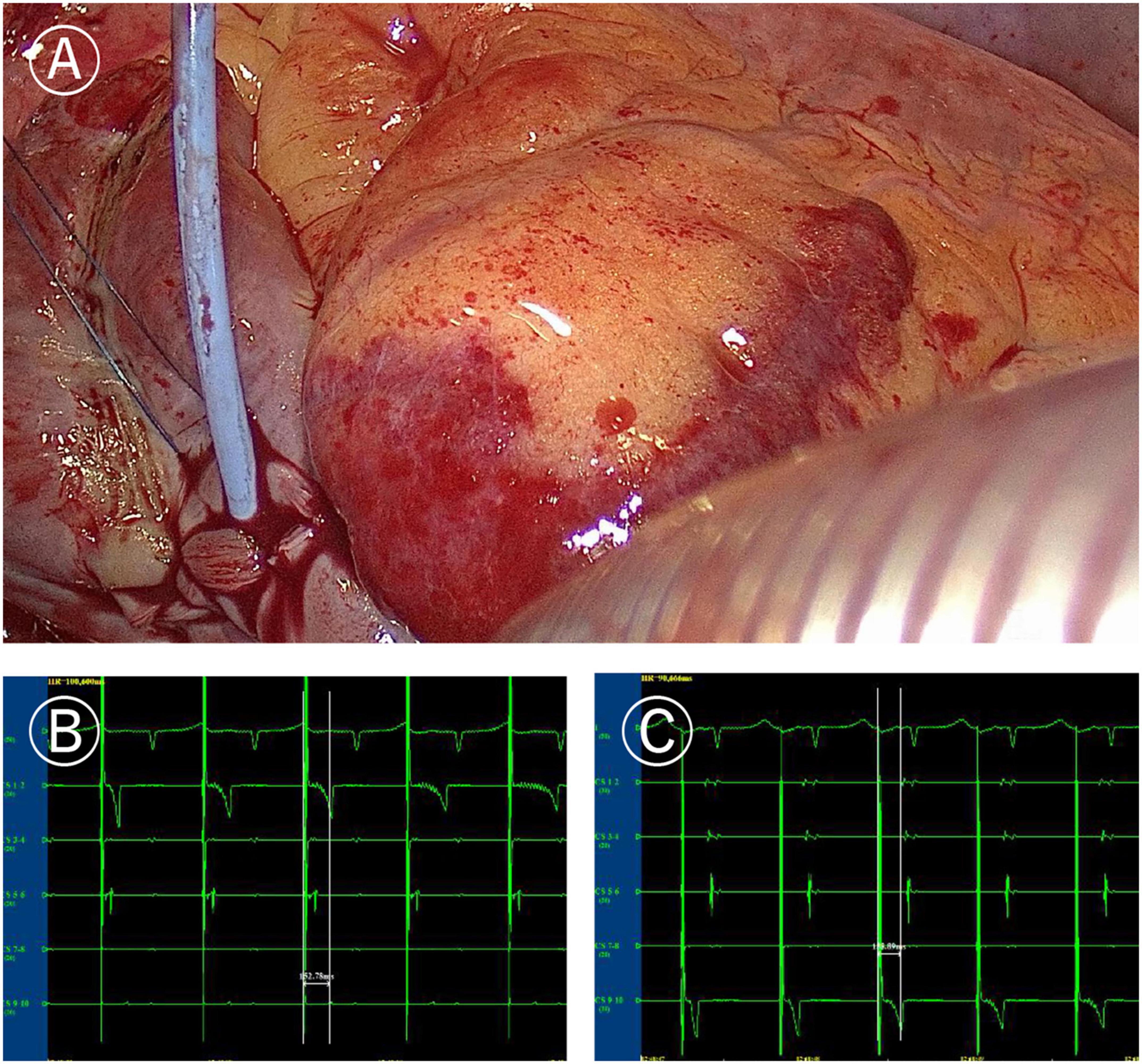
Figure 2. Ablation line mapping of mitral isthmus. (A) Coronary sinus catheter mapping of mitral isthmus ablation line. (B) Distal (CS1-2) delay during proximal (CS9-10) pacing of coronary sinus catheter (more than 120 ms). (C) Proximal delay during distal pacing of coronary sinus catheter (more than 120 ms).
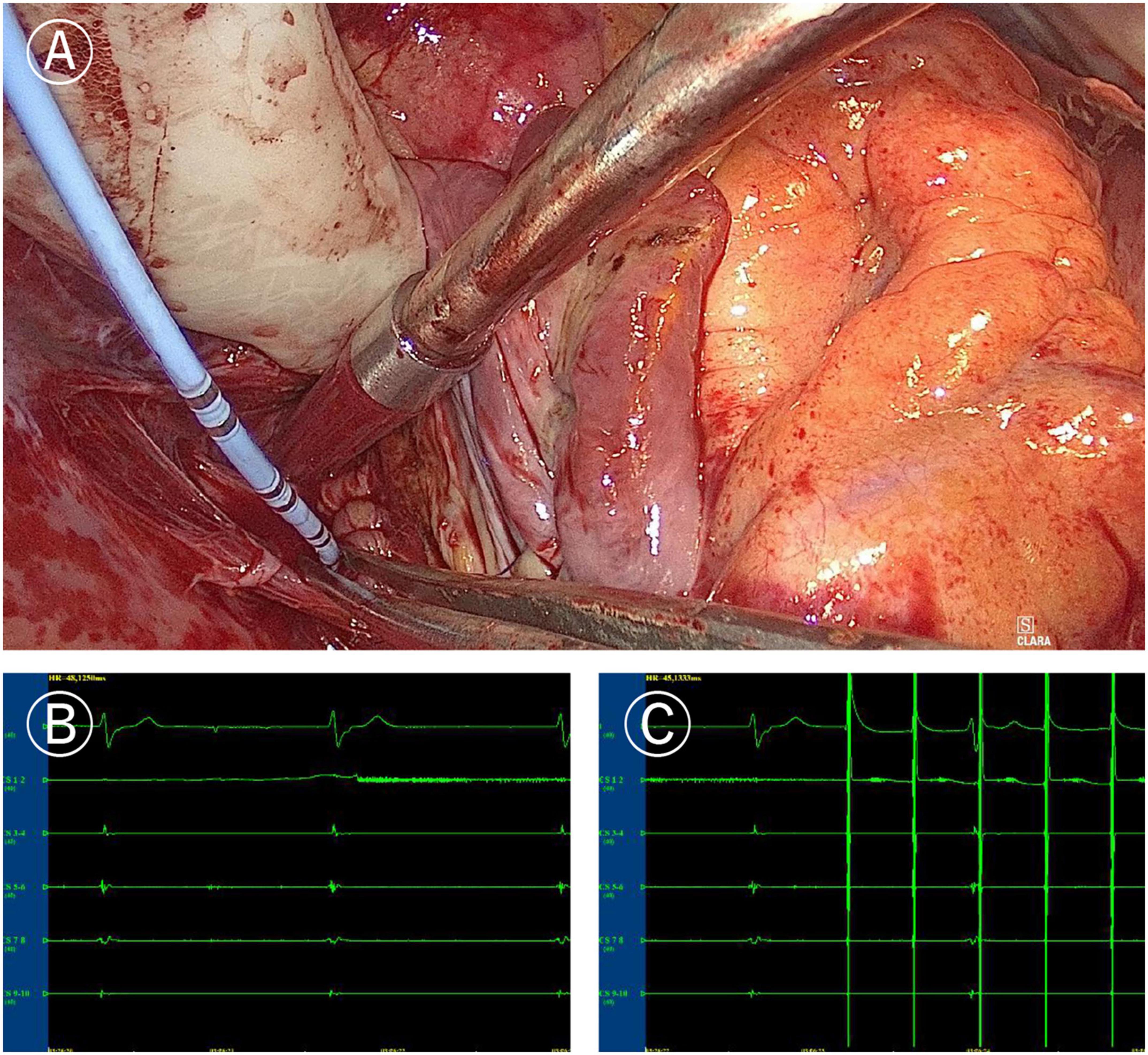
Figure 3. Mapping of left atrial “box” lesion. (A) Coronary sinus catheter mapping of left atrial “box” lesion. (B) Inner-box polar could not sense the outer potential under sinus rhythm. (C) Pacing of inner-box polar could not capture or disturb the sinus rhythm of the outer atrium.
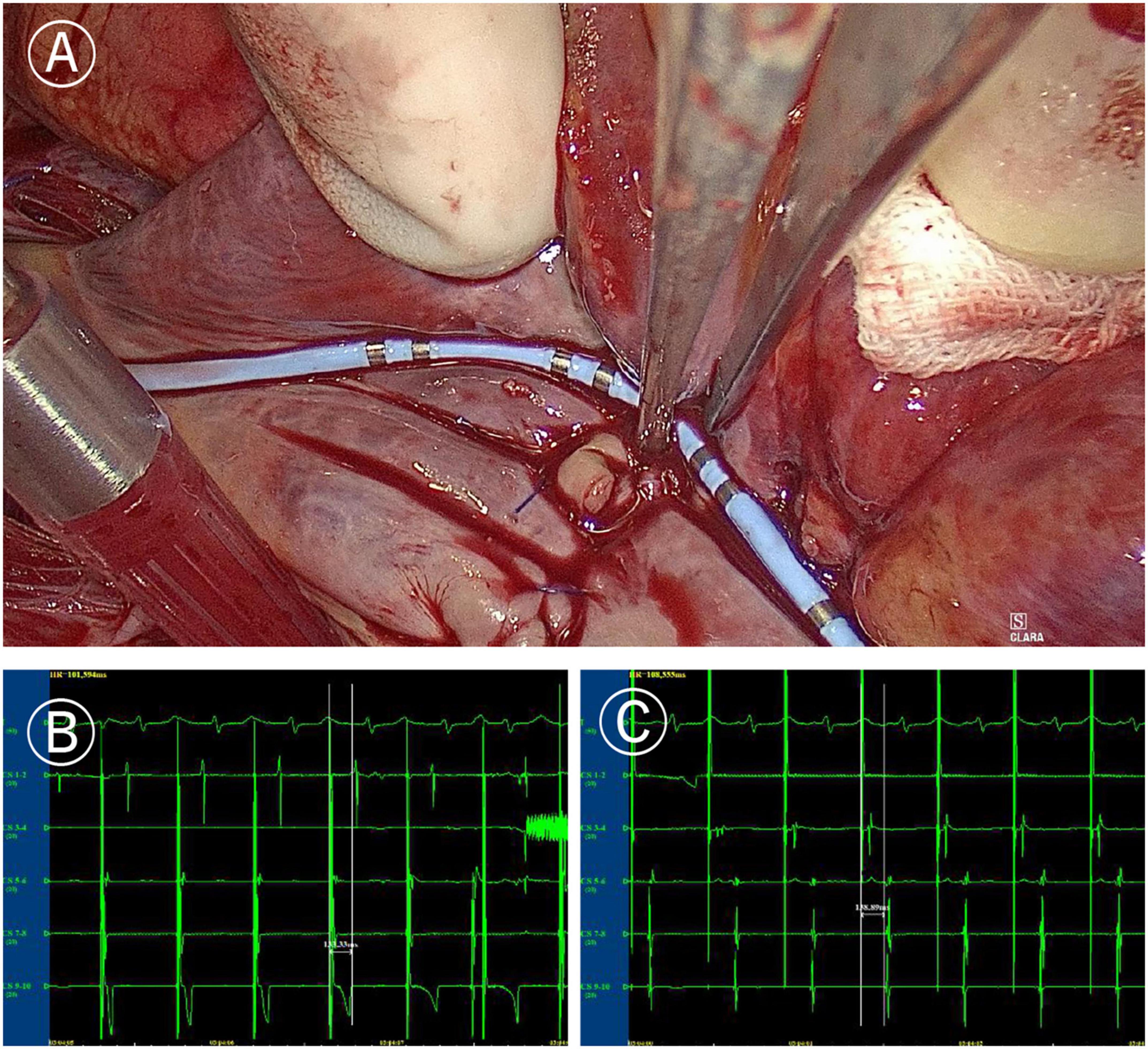
Figure 4. Mapping of tricuspid valve annular ablation line. (A) Coronary sinus catheter mapping of tricuspid valve annular ablation line. (B) Distal polar in free side wall of the right atrium (CS1-2) delay during proximal polar near the anterior atrial sulcus (CS9-10) pacing of coronary sinus catheter (more than 120 ms). (C) Proximal delay during distal pacing of coronary sinus catheter (more than 120 ms).
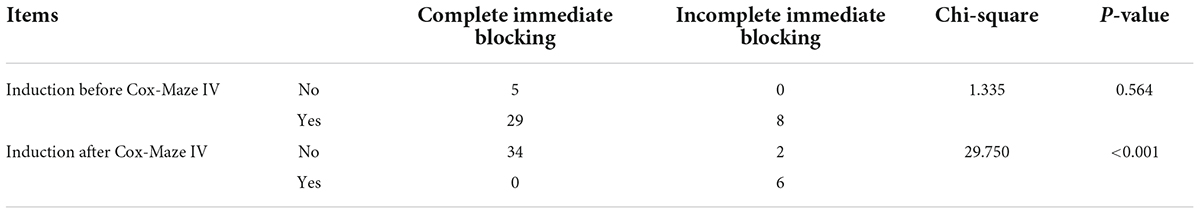
Table 3. Correlation between incomplete conduction blocking of ablation lines with atrial fibrillation (AF) inducibility before and after Cox-Maze IV in the Electrophysio-Maze group.
No patient died or suffered stroke during follow-up in both groups. All patients were prescribed with amiodarone for 3 months and underwent 24 h ECG Holter after 6 months. Compared with patients in the control group, patients in the Electrophysio-Maze group experienced more obvious reduction of the inner diameter of left atrium, right atrium, left ventricle, and right ventricle, a greater increase of left ventricular ejection fraction, and a higher relief from AF at 6 months (88.1 vs. 13.2%, P < 0.001, refer to Table 4).
There was a significant correlation between AF recurrence at 6 months both with incomplete block of ablation lines and with AF inducibility immediately after Cox-Maze IV during the operation. Additionally, AF recurrence at 6 months was correlated with a large left atrium (Table 5).
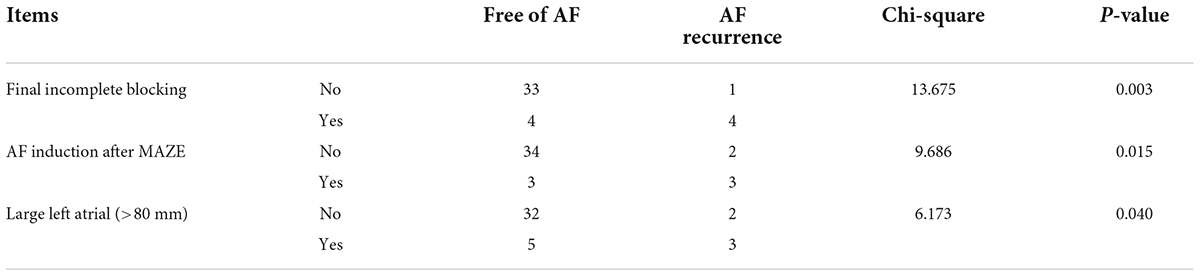
Table 5. Correlation between AF recurrence with final incomplete blocking, AF inducibility, and large left atrial in the Electrophysio-Maze group.
In this study, we reported outcomes of a randomized controlled clinical trial of electrophysiological mapping following the Cox-Maze IV procedure and found that compared with patients in control group, those in the Electrophysio-Maze group experienced shorter hospital stay, better cardiac remodeling changes, and higher relief from AF during follow-up period of 6 months, thus showing similar advantages of Cox-Maze IV as reported in previous studies (23, 24). Most importantly, we found that the rate of bidirectional electrical isolation of “box” ablation lines immediately after Cox-Maze IV were 100%. As the “box” area is the necessary “path” for the four pulmonary vein to connect the main body of left atria, theoretically, all the latent intracavitary pulmonary vein triggers were abolished, which substitutes the basis of the prominent efficacy of maze surgery. However, there was incomplete bidirectional electrical isolation in the mitral isthmus and tricuspid annulus ablation lines in part patients. Interestingly, in two cases of incomplete bidirectional electrical isolation of single tricuspid annulus ablation line, complementary ablation was successful. Both incomplete bidirectional electrical isolation of ablation lines and AF induction immediately after the maze procedure were found to be significantly correlated with late AF recurrence after 6 months.
Incomplete bidirectional isolation of the ablation line has been reported to form the anatomical basis of late AF recurrence in catheter-based AF ablation (25), yet there was no similar concomitant electrophysiological mapping study in domain of open surgery for Cox-Maze IV. Some hybrid ablation studies also reported the result of electrophysiological mapping in pulmonary vein isolation (PVI). Two studies on thoracoscopic encircling catheter-based ablation (26, 27) and a thoracoscopic bipolar radiofrequency clamp ablation (28) all reported 100% leakage in “box” ablation line immediately after ablation, which partly explains why bidirectional electrical isolation test of left atrial “box” ablation lines is not routinely carried out in catheter or thoracoscopic ablation in the real world. In a hybrid maze (minimally invasive surgical ablation combining second-staged catheter mapping and provisional ablation) research on ablation of isolated AF, there was a gap in 23% (5/22) boxes ablation lines and 100% (3/3) mitral isthmus lines (29). In another report on surgical thoracoscopic left atrial box radiofrequency ablation by bipolar clamp combining bipolar linear radiofrequency pen in persistent and long-standing persistent AF patients, gaps in PVI lesions existed in 87% right PV, 77% left PV, 67% roof lines, and 40% inferior lines (30). In this study, the completeness of the surgical Cox-Maze IV procedure in left atrial box ablation lines, mitral isthmus ablation line, and tricuspid valvular ablation line seems much better than interventional catheter ablation and thoracoscopic clamp ablation studies mentioned above. We postulated that the high rate of complete bidirectional electrical isolation in pulmonary vein vestibules insulation and box ablation might result not only from the standard protocol of the Cox-Maze IV procedure in this study but also from the operational stability of a fixed operator who completed the largest number of maze procedures in China.
The induction of AF can be divided into drug-based and electrophysiological-stimulation (pacing) methods, and the latter is used more commonly. In a hybrid AF research, 15 patients with persistent or long-standing persistent AF who failed at least one catheter ablation and one antiarrhythmic drug intervention underwent surgical ablation, followed by AF induction after several days; the AF could be induced by atrial rapid pacing in nearly half of all enrolled patients after surgical ablation (31). Hwang ES evaluated the AF induction rate by pacing before and after catheter radiofrequency ablation in 89 patients with AF and found that ablation of AF reduced the induction rate of AF from 95.4% before operation to 56.3% after ablation (20, 32). Santangeli et al. proposed that the standardized induction scheme of AF should include electrical stimulation-induced AF and infusion of high-dose isoproterenol (33). Theoretically, the combination of drugs and electrical stimulation can improve the induction rate of AF, but it will inevitably increase the false positive rate. Hence, in this study, we only adopted the pacing method in AF induction. Peter Leong-Sit et al conducted a prospective prognostic research of AF induction rate after PVI AF ablation in 144 patients (34), and found that the inducible AF after AF ablation occurred in 52 cases (36.1%). The inducibility of AF after ablation in the above-mentioned studies was much higher than in this study (about 14%). The prognostic value of AF inducibility on the long-term prognosis has been researched in catheter-based ablation by Kosiuk et al. (35), wherein the AF inducibility had no effect on early recurrence but could affect late recurrence, which is consistent with the results of this study.
Generally speaking, almost all previous research in the open Cox-Maze surgery domain (except staged hybrid surgery of surgical and subsequent electrophysiological mapping and complementary catheter ablation) focused solely on ablation strategy based on anatomy, and the importance of electrophysiological mapping was ignored more or less. So, the feasibility and effectiveness of electrophysiological assistance during open Cox-MAZE IV are unclear. This is the first study to demonstrate that electrophysiological mapping can guide supplementary ablation and increase ablation success rate, and that AF induction after the open Cox-MAZE IV procedure can predict the recurrence of atrial fibrillation 6 months after the standard Cox-MAZE IV procedure. Findings in this study bring new strategies not only in understanding the late AF recurrence after Cox-Maze IV but also in screening high-risk populations of late AF recurrence and developing possible preventative measures to lower the incidence of late AF recurrence after Cox-Maze IV. To reduce the risk of late AF recurrence, future studies should focus on lowering the leakage rate in valvular annulus ablation lines and the AF inducibility immediately after ablation. Furthermore, this study can provide a latent protocol of electrophysiological mapping, AF induction, and supplementary ablation for future open Cox-Maze IV studies. It should be pointed out, concerning the complexity of completing electrophysiological mapping, Cox Cox-Maze IV procedure and valvular surgery in an operation, a fixed skilled operator who has passed the learning curve is needed to improve the success rate of AF ablation and avoid unexpected complications associated with concomitant valve surgery. For example, according to the results of a recent randomized trial, the frequency of permanent pacemaker implantation was 7.7% in patients undergoing mitral valve surgery alone, 16.1% in patients undergoing mitral valve surgery plus PVI, and 25% in patients undergoing mitral valve surgery plus biatrial ablation (36), indicating that maze procedure is associated with an increased risk of sinus node dysfunction and permanent pacemaker implantation (37). However, there was no complication of high atrioventricular conduction block requiring permanent pacemaker implantation in this study, which once again proved the importance of sophisticated surgical skills while carrying out the Cox-Maze IV procedure concomitant with valvular surgery.
This study has some limitations. First, this study only mainly focused on the bidirectional electrical isolation of mitral annular isthmus ablation line, left atrial “box” lines, and tricuspid annulus line, without involving other lines such as superior vena cava ablation line, inferior vena cava ablation line, and left atrial appendage ablation line. One of the main reasons is that there is no normal reference value to judge the complete conduction block in such ablation lines at present. Second, although testing for inducibility of AF was performed under some conditions (e.g., both are off pump) before and after ablation, there are residual metabolic changes after extracorporeal circulation that can affect inducibility. Yet, these metabolic changes often increase the risks of atrial fibrillation called postoperative atrial fibrillation (38), which might partially reduce the efficacy of maze surgery, so it would not hinder the rationality of the conclusion that Cox-Maze IV is effective in reducing the rate of atrial fibrillation inducibility. Third, before the surgical procedure, a mitral valvular disease could be related to atrium enlargement and early onset of asymptomatic AF, where the duration of AF in some patients might be underestimated. Fortunately, this impact was exerted on both group, so it was a random error rather than a systemic error, whose influence was controllable in the controlled study. Fourth, secondary causes of AF should be considered and were not investigated. These forms could not respond to ablation approach including an early onset of unknown atrial fibrillation in channelopathies like Brugada (39) and long QT sindrome (40), hypertension, or hyperthyroidism (41), and also lifestyle factors such a regular practice of endurance sport (42). Fifth, although the rate of complete bi-directional electrical isolation of “box” ablation lines was found to be 100% immediately after Cox-Maze IV, yet whether there is pulmonary vein reconnection after 6 months is unknown. As an important trigger of AF recurrence, the latent impact of pulmonary vein reconnection should be considered while analyzing the reason for AF recurrence after 6 months. Due to the difficulty in persuading patients to undergo a postoperative electrophysiological examination, this study did not perform intracardiac electrophysiological examination for all patients suffering late AF recurrent.
Electrophysiological mapping following the Cox-Maze procedure is safety and effective in shortening hospital stay, leading to better cardiac remodeling changes and improving relief from atrial fibrillation during follow-up period of 6 months. Electrophysiological mapping in the Cox-Maze procedure can find out the non-transmural annulus ablation line by assessing the completeness of bidirection electrical isolation of ablation lines, guide supplementary ablation, and predict atrial fibrillation recurrence after 6 months.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. The patients/participants provided their written informed consent to participate in this study.
LL: conceptualization and project administration. ZS, HT, and YS: data curation. ZS: formal analysis and writing–original draft. CF, LS, and LL: investigation. HZ: methodology. ZJ: resources. CF and LL: supervision and writing–review and editing. All authors contributed to the article and approved the submitted version.
This study was supported by the National Key Research and Development Program (No. 2018YFC1311204 to LL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Patel NJ, Atti V, Mitrani RD, Viles-Gonzalez JF, Goldberger JJ. Global rising trends of atrial fibrillation: a major public health concern. Heart. (2018) 104:1989–90. doi: 10.1136/heartjnl-2018-313350
2. Lip GYH, Collet JP, Caterina R, Fauchier L, Lane DA, Larsen TB, et al. Antithrombotic therapy in atrial fibrillation associated with valvular heart disease: a joint consensus document from the European heart rhythm association (EHRA) and European society of cardiology working group on thrombosis, endorsed by the ESC working group on valvular heart disease, cardiac arrhythmia society of Southern Africa (CASSA), heart rhythm society (HRS), Asia Pacific heart rhythm society (APHRS), South African heart (SA Heart) association and sociedad latinoamericana de estimulacion cardiaca y electrofisiologia (SOLEACE). Europace. (2017) 19:1757–8. doi: 10.1093/europace/eux240
3. Zimetbaum P. Atrial fibrillation. Ann Intern Med. (2017) 166:ITC33–48. doi: 10.7326/AITC201703070
4. Garcia-Villarreal OA. Valvular atrial fibrillation: rheumatic mitral valve disease. Europace. (2016) 18:629. doi: 10.1093/europace/euv065
5. Gillinov M, Soltesz EG. Atrial fibrillation in the patient undergoing mitral valve surgery: a once-in-a-lifetime opportunity. J Thorac Cardiovasc Surg. (2018) 155:995–6. doi: 10.1016/j.jtcvs.2017.09.125
6. Lombard FW, Liang Y. Risk factors for mitral valve surgery: atrial fibrillation and pulmonary hypertension. Semin Cardiothorac Vasc Anesth. (2019) 23:57–69. doi: 10.1177/1089253218821694
7. Macle L, Frame D, Gache LM, Monir G, Pollak SJ, Boo LM. Atrial fibrillation ablation with a spring sensor-irrigated contact force-sensing catheter compared with other ablation catheters: systematic literature review and meta-analysis. BMJ Open. (2019) 9:e023775. doi: 10.1136/bmjopen-2018-023775
8. Ruaengsri C, Schill MR, Khiabani AJ, Schuessler RB, Melby SJ, Damiano RJ Jr The cox-maze IV procedure in its second decade: still the gold standard? Eur J Cardiothorac Surg. (2018) 53(Suppl. 1):i19–25. doi: 10.1093/ejcts/ezx326
9. Genev IK, Tompkins LA, Khare MM, Farokhi F. Comparison of the efficancy and complication rates of the hybrid maze, complete cox-maze and catheter ablation in the treatment of atrial fibrillation. J Atr Fibrillation. (2017) 9:1543. doi: 10.4022/jafib.1543
10. Kim HJ, Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Surgical ablation of atrial fibrillation in patients with a giant left atrium undergoing mitral valve surgery. Heart. (2016) 102:1206–14. doi: 10.1136/heartjnl-2015-308482
11. Wu CC, Chang JP, Chen MC, Cheng CI, Chung WJ. Long-term results of radiofrequency maze procedure for persistent atrial fibrillation with concomitant mitral surgery. J Thorac Dis. (2017) 9:5176–83. doi: 10.21037/jtd.2017.11.112
12. Takahashi S, Sueda T. Development of the maze procedure and the contribution of Japanese surgeons. Gen Thorac Cardiovasc Surg. (2017) 65:144–52. doi: 10.1007/s11748-016-0728-y
13. Cao H, Xue Y, Zhou Q, Yu M, Tang C, Wang D. Late outcome of surgical radiofrequency ablation for persistent valvular atrial fibrillation in China: a single-center study. J Cardiothorac Surg. (2017) 12:63. doi: 10.1186/s13019-017-0627-z
14. Gomes GG, Gali WL, Sarabanda AVL, Cunha CRD, Kessler IM, Atik FA. Late results of cox maze III procedure in patients with atrial fibrillation associated with structural heart disease. Arq Bras Cardiol. (2017) 109:14–22. doi: 10.5935/abc.20170082
15. Murashita T, Rankin JS, Wei LM, Roberts HG, Alkhouli MA, Badhwar V. Oral anticoagulation may not be necessary for patients discharged in sinus rhythm after the cox maze IV procedure. J Thorac Cardiovasc Surg. (2018) 155:997–1006. doi: 10.1016/j.jtcvs.2017.10.142
16. Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Chiou YY, et al. Surgical resection versus radiofrequency ablation for single hepatocellular carcinoma </= 2 cm in a propensity score model. Ann Surg. (2016) 263:538–45. doi: 10.1097/SLA.0000000000001178
17. Forkmann M, Schwab C, Edler D, Vevecka A, Butz S, Haller B, et al. Characteristics of early recurrences detected by continuous cardiac monitoring influencing the long-term outcome after atrial fibrillation ablation. J Cardiovasc Electrophysiol. (2019) 30:1886–93. doi: 10.1111/jce.14109
18. Tokuda M, Yamashita S, Matsuo S, Kato M, Sato H, Oseto H, et al. Clinical significance of early recurrence of atrial fibrillation after cryoballoon vs. radiofrequency ablation-A propensity score matched analysis. PLoS One. (2019) 14:e0219269. doi: 10.1371/journal.pone.0219269
19. Nitta T, Ishii Y, Sakamoto S. Surgery for atrial fibrillation: recent progress and future perspective. Gen Thorac Cardiovasc Surg. (2012) 60:13–20. doi: 10.1007/s11748-011-0849-2
20. Hwang ES, Nam GB, Joung B, Park J, Lee JS, Shim J, et al. Significant reduction of atrial defibrillation threshold and inducibility by catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. (2012) 35:1428–35. doi: 10.1111/j.1540-8159.2012.03517.x
21. Lanters EAH, Teuwen CP, Yaksh A, Kik C, van der Does L, Mouws E, et al. Intraoperative inducibility of atrial fibrillation does not predict early postoperative atrial fibrillation. J Am Heart Assoc. (2018) 7:e007879. doi: 10.1161/JAHA.117.007879
22. Ballaux PK, Cathenis KK, Brondeel R, Provenier FP, Francois BA, Goossens DJ, et al. Mid-term follow-up after maze IV procedures for concomitant atrial fibrillation. Acta Chir Belg. (2014) 114:99–104. doi: 10.1080/00015458.2014.11680989
23. Kim WK, Kim HJ, Kim JB, Jung SH, Choo SJ, Chung CH, et al. Concomitant ablation of atrial fibrillation in rheumatic mitral valve surgery. J Thorac Cardiovasc Surg. (2019) 157:1519–28.e5. doi: 10.1016/j.jtcvs.2018.09.023
24. Oi M, Nomura S, Miho M, Kobayashi T, Okabayashi M, Higami H, et al. Rate-dependent and unidirectional conduction block between the left pulmonary vein and left atrium after catheter ablation for atrial fibrillation. J Arrhythm. (2020) 36:1096–9. doi: 10.1002/joa3.12425
25. Gerstenfeld EP, Dixit S, Callans D, Rho R, Rajawat Y, Zado E, et al. Utility of exit block for identifying electrical isolation of the pulmonary veins. J Cardiovasc Electrophysiol. (2002) 13:971–9. doi: 10.1046/j.1540-8167.2002.00971.x
26. Vijayaraman P, Dandamudi G, Naperkowski A, Oren J, Storm R, Ellenbogen KA. Assessment of exit block following pulmonary vein isolation: far-field capture masquerading as entrance without exit block. Heart Rhythm. (2012) 9:1653–9. doi: 10.1016/j.hrthm.2012.06.004
27. Spector P. Principles of cardiac electric propagation and their implications for re-entrant arrhythmias. Circ Arrhythm Electrophysiol. (2013) 6:655–61. doi: 10.1161/CIRCEP.113.000311
28. Chen S, Meng W, Sheng He D, Chen G, Zhang F, Yan Y, et al. Blocking the pulmonary vein to left atrium conduction in addition to the entrance block enhances clinical efficacy in atrial fibrillation ablation. Pacing Clin Electrophysiol. (2012) 35:524–31. doi: 10.1111/j.1540-8159.2012.03343.x
29. Kim JY, Kim SH, Song IG, Kim YR, Kim TS, Kim JH, et al. Achievement of successful pulmonary vein isolation: methods of adenosine testing and incremental benefit of exit block. J Interv Card Electrophysiol. (2016) 46:315–24. doi: 10.1007/s10840-016-0122-9
30. La Meir M, Gelsomino S, Lorusso R, Luca F, Pison L, Parise O, et al. The hybrid approach for the surgical treatment of lone atrial fibrillation: one-year results employing a monopolar radiofrequency source. J Cardiothorac Surg. (2012) 7:71. doi: 10.1186/1749-8090-7-71
31. Bisleri G, Rosati F, Bontempi L, Curnis A, Muneretto C. Hybrid approach for the treatment of long-standing persistent atrial fibrillation: electrophysiological findings and clinical results. Eur J Cardiothorac Surg. (2013) 44:919–23. doi: 10.1093/ejcts/ezt115
32. Lee R, McCarthy PM, Passman RS, Kruse J, Malaisrie SC, McGee EC, et al. Surgical treatment for isolated atrial fibrillation: minimally invasive vs. classic cut and sew maze. Innovations (Phila). (2011) 6:373–7. doi: 10.1097/IMI.0b013e318248f3f4
33. Santangeli P, Marchlinski FE. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart Rhythm. (2017) 14:1087–96. doi: 10.1016/j.hrthm.2017.02.030
34. Leong-Sit P, Robinson M, Zado ES, Callans DJ, Garcia F, Lin D, et al. Inducibility of atrial fibrillation and flutter following pulmonary vein ablation. J Cardiovasc Electrophysiol. (2013) 24:617–23. doi: 10.1111/jce.12088
35. Kosiuk J, Grundig S, Dinov B, Mussigbrodt A, Richter S, Sommer P, et al. Significance of inducibility of atrial fibrillation after pulmonary vein isolation in patients with healthy left atrium substrate. J Cardiovasc Electrophysiol. (2019) 30:2767–72. doi: 10.1111/jce.14234
36. DeRose JJ Jr, Mancini DM, Chang HL, Argenziano M, Dagenais F, Ailawadi G, et al. Pacemaker implantation after mitral valve surgery with atrial fibrillation ablation. J Am Coll Cardiol. (2019) 73:2427–35.
37. Li H, Lin X, Ma X, Tao J, Zou R, Yang S, et al. Biatrial versus isolated left atrial ablation in atrial fibrillation: a systematic review and meta-analysis. Biomed Res Int. (2018) 2018:3651212. doi: 10.1155/2018/3651212
38. Ellam S, Hartikainen J, Korvenoja P, Pitkanen O, Tyrvainen E, Valtola A, et al. Impact of minimal invasive extracorporeal circulation on atrial fibrillation after coronary artery bypass surgery. Artif Organs. (2020) 44:1176–83. doi: 10.1111/aor.13756
39. Mascia G, Della Bona R, Ameri P, Canepa M, Porto I, Brignole M. Brugada syndrome and syncope: a systematic review. J Cardiovasc Electrophysiol. (2020) 31:3334–8. doi: 10.1111/jce.14787
40. Platonov PG, McNitt S, Polonsky B, Rosero SZ, Zareba W. Atrial fibrillation in long QT syndrome by genotype. Circ Arrhythm Electrophysiol. (2019) 12:e007213. doi: 10.1161/CIRCEP.119.007213
41. Frost L, Vestergaard P, Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med. (2004) 164:1675–8. doi: 10.1001/archinte.164.15.1675
Keywords: Cox-Maze IV, ablation lines, surgery, electrical isolation, mapping, recurrence
Citation: Sun Z, Fan C, Song L, Zhang H, Jiang Z, Tan H, Sun Y and Liu L (2022) Effect of electrophysiological mapping on non-transmural annulus ablation and atrial fibrillation recurrence prediction after 6 months of Cox-Maze IV procedure. Front. Cardiovasc. Med. 9:931845. doi: 10.3389/fcvm.2022.931845
Received: 29 April 2022; Accepted: 24 June 2022;
Published: 15 July 2022.
Edited by:
Shuanglun Xie, Sun Yat-sen University, ChinaReviewed by:
Giuseppe Mascia, San Martino Hospital (IRCCS), ItalyCopyright © 2022 Sun, Fan, Song, Zhang, Jiang, Tan, Sun and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liming Liu, liulimingjia@csu.edu.cn
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.