
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 22 July 2022
Sec. Cardiovascular Therapeutics
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.931590
This article is part of the Research Topic Case Reports in Cardiovascular Therapeutics: 2022 View all 11 articles
Pulmonary sequestration with congenital heart disease is a rare congenital malformation. Herein, we report a 19-month-old toddler diagnosed with right lower pulmonary sequestration, right pulmonary artery dysplasia, right lower pulmonary venous ectopic drainage, and a right-sided heart with an atrial septal defect. The pulmonary sequestration had a rare blood supply, such as confluent arteries with the renal vessels draining into the hepatic veins. Arterial embolization and atrial defect closure were used to treat the rare congenital malformation with satisfactory results.
Pulmonary sequestration, categorized as intralobar or extralobar isolation, is a rare congenital anomaly thought to arise from the accessory lung buds (1). The intralobar lesion’s arterial supply typically arises from the thoracic aorta, while it’s venous drainage via the pulmonary veins. On the other hand, the extralobar lesions drain (venous) via the systemic circulation, including the azygos, hemiazygos, or vena cava (2). Notably, extralobar sequestration, less common than intralobar sequestration, presents early in life with respiratory distress or feeding difficulties, is often associated with other congenital malformations, and accounts for 25% of reported cases.
In pulmonary sequestration, confluent arteries with the renal vessels draining into the hepatic veins are extremely rare. Traditional treatments include surgical resection and supplying artery ligation (3). Notwithstanding, transarterial embolization has since been proposed (4). Some reports using supplying artery embolization to treat pulmonary isolation have reported that the embolic lesions eventually become muscled or even disappeared due to ischemia (5–7).
A 19-month-old toddler was admitted to the hospital following a heart murmur discovery. During the fetal period, a routine prenatal color Doppler ultrasound examination at a local hospital detected a congenital cardiac anomaly. However, the examination results were lost by the patient’s family. The patient had recurrent pneumonia after birth. After admission, auscultation revealed a heart murmur and weak breath sounds in the right lung. There was no other notable clinical findings during physical examination and no medical, family, and psychosocial history including genetic information about cardiovascular disease. Cardiac ultrasound examination showed: (i) a dextrorotatory heart; (ii) atrial septal defect (iii) right inferior pulmonary venous drainage; (iv) right pulmonary artery dysplasia; and (v) moderate pulmonary hypertension. The patient was delivered at term with a birth weight of 3.5 kg.
Chest X-ray showed a large area of high density in the lower right lung. Chest CT showed a 9 mm defect in the atrial septum (Figure 1A), a significantly small right pulmonary artery (∼5 mm) and a significantly small right superior pulmonary vein (Figures 1B–D), a small and sparsely branched right lung, and the venous of sequestration lung return to the hepatic vein (Figure 2). Considering the patient’s young age and inability to tolerate cardiac and pulmonary surgery, isolated pulmonary embolism and atrial septal occlusion were performed under general anesthesia. Digital subtraction angiography (DSA) and CT 3D reconstruction (Supplementary Video) showed an abnormal blood vessel branching from the right renal artery opening (∼4 mm; Figures 3A–C), and arching toward the right lower lung for blood supply. During the operation, nine coils of equal size were applied to completely block the abnormal blood supply to the right lower lung. Under transthoracic ultrasound guidance, a 14 mm atrial septal occluder was placed through the delivery sheath and ascertained to be adequately fixed, with no residual shunt and intact valvular apparatus (Figure 3D).
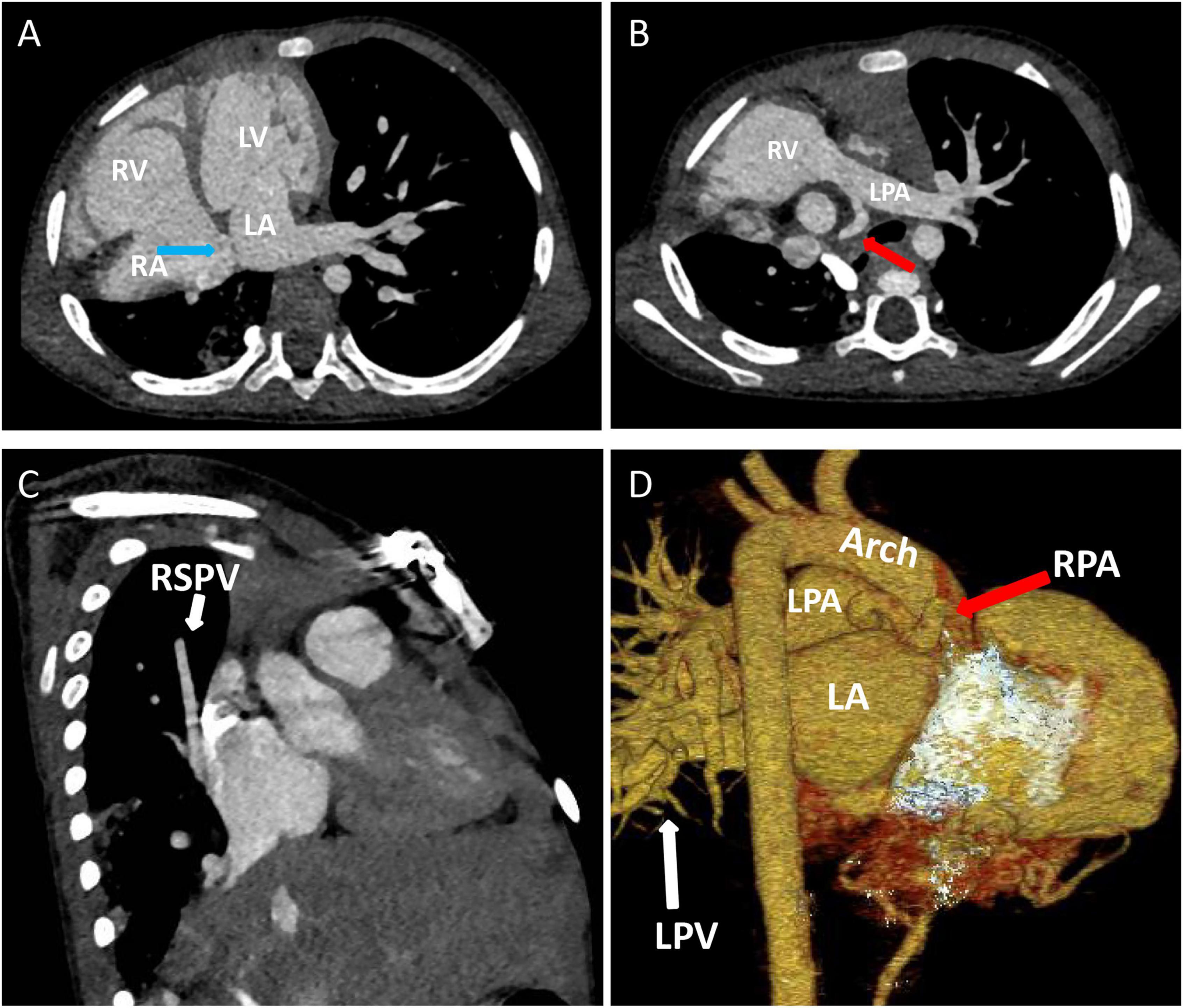
Figure 1. Contrast-enhanced CT of the chest and three-dimensional cardiac computed tomography angiography: (A) Atrial septal defect location, ∼9 mm in size (blue arrow). (B–D) The right pulmonary artery (red arrow) and right superior pulmonary vein are hypoplastic (white arrow). LA, left atrium; LPA, left pulmonary artery; LPV, left pulmonary vein; LV, left ventricle; RA, right atrium; RPA, right pulmonary artery; RSPV, right superior pulmonary vein; RV, right ventricle.
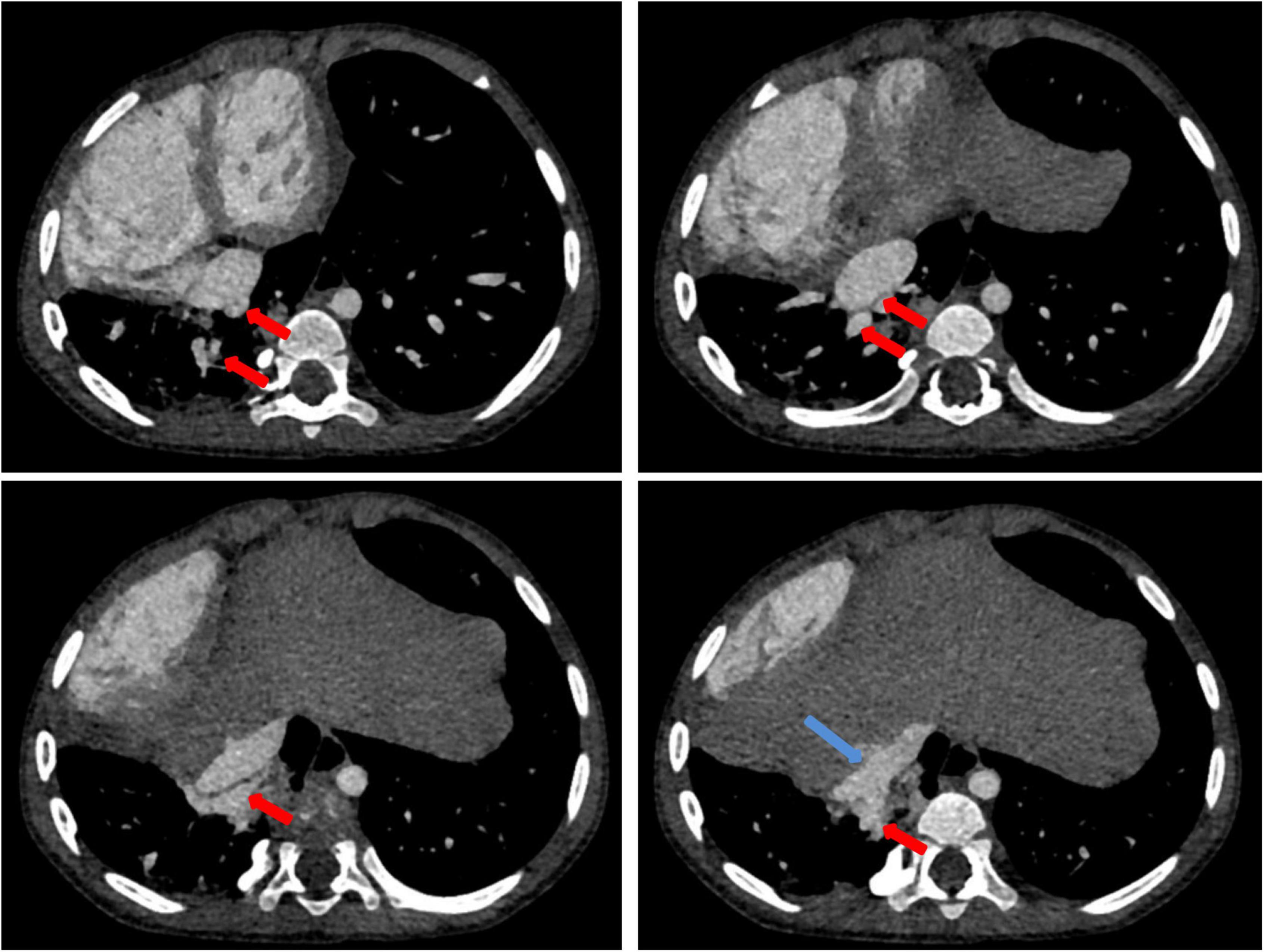
Figure 2. Contrast-enhanced CT of the chest: contrast-enhanced CT shows the isolated pulmonary venous draining (red arrow) into the hepatic vein (blue arrow).
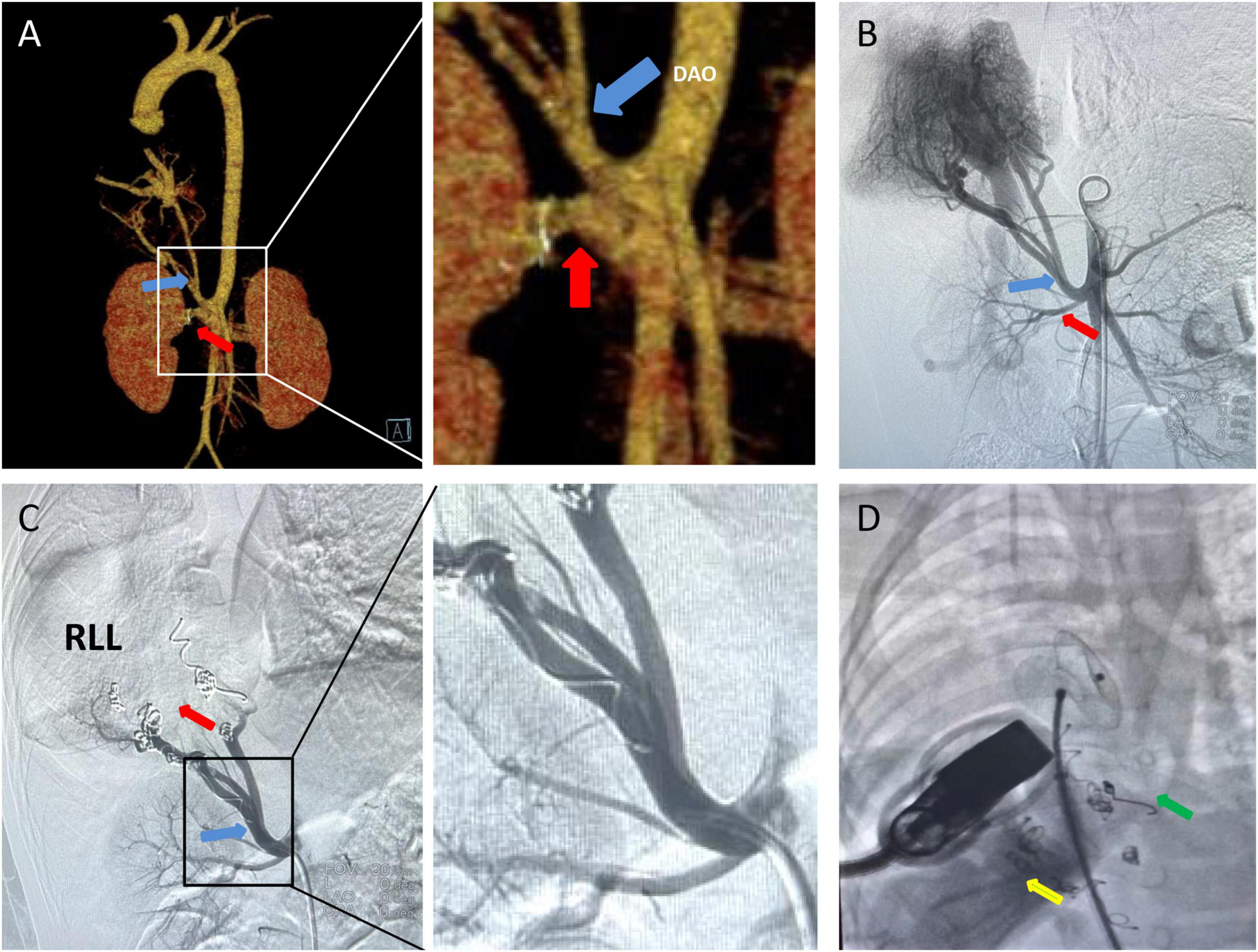
Figure 3. CT 3D reconstruction and Aortic DSA: (A,B) A isolates the pulmonary supplying artery (blue arrow) and the renal artery (red arrow) to the abdominal aorta, and isolates the pulmonary supplying artery by approximately 4 mm. (C) Nine platinum coils were placed in the isolated pulmonary supplying artery (blue arrow), and no contrast agent entered the distal end of the infarct (red arrow). (D) Placement of atrial septal defect occluder (green arrow) under co-guided echocardiography (yellow arrow) and DSA. DAO, decending aorta; RLL, right lower lobe.
The patient recovered well after the operation. Pulmonary infection was controlled with no obvious abnormality following blood examination. Three days postoperatively, echocardiography showed that the occluder was well fixed and there was no residual shunt. The patient had no special discomfort and was discharged 4 days later. No adverse and unanticipated events were indicated during the one-month follow-up period, the patient was strongly advised to come back to the hospital for echocardiography and chest CT reexamination 6 months and 1 year after that.
Pulmonary sequestration refers to the abnormal connection of the lung tissue (part) to the trachea and bronchial tree, with an abnormal vascular supply. Some studies have also suggested that the sequestered lung is a congenital disorder that results from the growth of parapulmonary buds during development. This lung bud is pinched from the caudal foregut, develops its blood supply, and remains independent of the normal tracheobronchial tree (8). However, other authors have suggested that intralobar sequestration can be acquired via bronchial obstruction causing distal infection. It thus stimulates angiogenesis, which utilizes the existing small systemic arteries from the pulmonary ligaments to form a new arterial supply (9).
The presented patient was diagnosed with right lower pulmonary sequestration, right pulmonary artery dysplasia, right lower pulmonary venous ectopic drainage, and a right-sided heart with an atrial septal defect. Pulmonary sequestration occurs in 0.15–6.6% of all pulmonary malformations. Two types can be distinguished: intra-leaf (ILS) and extra-leaf (ELS) isolation. With ILS, the abnormal lung tissue is located within the normal lung and visceral pleura, with venous drainage to the pulmonary veins. Its arterial supply is 73% from the thoracic aorta, 20% from the abdominal aorta, and 3.7% from the intercostal arteries. With ELS; however, the mass is located outside the normal lung and within its visceral pleura, with venous drainage to the systemic venous system. Approximately 80% of its arterial supply comes from the thoracic or abdominal aorta, while 15% arises from the subclavian artery, brachiocephalic, spleen, stomach, and intercostal arteries, and 5% from the pulmonary artery (10). In the present case, the arterial supply arose from a confluent right renal artery (Figure 4A), while the venous drainage was to the hepatic vein (Figure 4B), a form of blood supply and drainage that has never been reported.

Figure 4. Schematic figure shows the presented case of pulmonary sequestration: (A) The arterial supply of the sequestration lung arose from a confluent right renal artery. (B) The venous of sequestration lung return to the inferior vena cava via the hepatic vein. AAO, ascending aorta; DAO, decending aorta; HV, hepatic vein; IVC, inferior vena cava; LK, left kidney; RA, right atrium; RK, right kidney; RLL, right lower lobe.
Extra-leaf (ELS) patients are often accompanied by acute respiratory distress or feeding difficulties after birth. Approximately 60% of children with ELS have congenital malformations, including diaphragmatic hernia, congenital heart disease, elbow syndrome, congenital cyst-adenomatoid malformation (CCAM), or pulmonary dysplasia (11). In the case herein, the patient was born with recurrent episodes of pneumonia and concomitant atrial septal defect.
Traditional treatment is with surgical excision (3). Currently, less invasive endovascular treatments have been reported to prevent complications (12). Platinum coils and polyvinyl alcohol granules are the most frequently used in transcatheter vascular embolization (13). Also, hybrid surgery has been performed for patients with large lung disease and multiple or large feeding arteries or aneurysmal abnormal arteries (14–17). Patients with larger feeding arteries may be at high risk for incomplete embolism, which may require reoperation due to recanalization and symptom recurrence as described in our previous work (18–21). In the present case, the patients feeding artery opening was 4 mm, and the possibility of incomplete embolization was low. Nine platinum coils were placed.
Notably, patients reported having received endovascular therapy recovered better, faster, and had lower complication rates. Given that the abnormal lung parenchyma has not been resected, recurrent hemoptysis and secondary pulmonary infection are the primary complications. Other complications include chest pain and low-grade fever, which might be due to pulmonary infarction after embolization. The patient experienced no postoperative complications, spontaneously recovered after surgery, and was discharged from the hospital 4 days later.
Take-home points: 1. Pulmonary sequestration occurs in 0.15–6.6% of all pulmonary malformations; 2. Pulmonary sequestration can be congenital or acquired via bronchial obstruction causing distal infection; 3. Approximately 80% of its arterial supply comes from the thoracic or abdominal aorta; 4. Transcatheter vascular embolization and hybrid surgery are the most frequently used strategies for the treatment.
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
MT, XW, CF, and JY contributed to the conception and design of the study. JY, XW, MT, CI, and CF wrote sections of the manuscript. DY draw the schematic figure. All authors contributed to the manuscript revision, read, and approved the submitted version.
This study was supported by the Natural Science Foundation of Changsha City (kq2014231).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.931590/full#supplementary-material
Supplementary video | Three-dimensional great artery computed tomography angiography and the kidneys.
1. Savic B, Birtel FJ, Tholen W, Funke HD, Knoche R. Lung sequestration: report of seven cases and review of 540 published cases. Thorax. (1979) 34:96–101.
2. Gamillscheg A, Beitzke A, Smolle-Jüttner FM, Zach M, Stein JI, Steinbrugger B, et al. Extralobar sequestration with unusual arterial supply and venous drainage. Pediatr Cardiol. (1996) 17:57–9. doi: 10.1007/bf02505815
3. Yucel O, Gurkok S, Gozubuyuk A, Caylak H, Sapmaz E, Kavakli K, et al. Diagnosis and surgical treatment of pulmonary sequestration. Thorac Cardiovasc Surg. (2008) 56:154–7. doi: 10.1055/s-2007-965572
4. Zhou H, Tang S, Fu Q, Yu L, Liu L. Hybrid surgery in treatment of pulmonary sequestration with abdominal aorta feeding vessel: a case report. J Cardiothorac Surg. (2018) 13:44. doi: 10.1186/s13019-018-0733-6
5. Borzelli A, Paladini A, Giurazza F, Tecame S, Giordano F, Cavaglià E, et al. Successful endovascular embolization of an intralobar pulmonary sequestration. Radiol Case Rep. (2018) 13:125–9. doi: 10.1016/j.radcr.2017.10.003
6. Qian X, Sun Y, Liu D, Wu X, Wang Z, Tang Y. Pulmonary sequestration: a case report and literature review. Int J Clin Exp Med. (2015) 8:21822–5.
7. Szmygin M, Pyra K, Sojka M, Jargiełło T. Successful endovascular treatment of intralobar pulmonary sequestration – an effective alternative to surgery. Polish J Radiol. (2021) 86:e112–4. doi: 10.5114/pjr.2021.103975
8. Gezer S, Tas̨tepe I, Sirmali M, Findik G, Türüt H, Kaya S, et al. Pulmonary sequestration: a single-institutional series composed of 27 cases. J Thorac Cardiovasc Surg. (2007) 133:955–9. doi: 10.1016/j.jtcvs.2006.11.003
9. Bolca N, Topal U, Bayram S. Bronchopulmonary sequestration: radiologic findings. Eur J Radiol. (2004) 52:185–91. doi: 10.1016/j.ejrad.2004.03.005
10. Corbett HJ, Humphrey GM. Pulmonary sequestration. Paediatr Respir Rev. (2004) 5:59–68. doi: 10.1016/j.prrv.2003.09.009
11. Pikwer A, Gyllstedt E, Lillo-Gil R, Jönsson P, Gudbjartsson T. Pulmonary sequestration – a review of 8 cases treated with lobectomy. Scand J Surg. (2006) 95:190–4. doi: 10.1177/145749690609500312
12. Park ST, Yoon CH, Sung KB, Yoon HK, Goo DE, Kim KS, et al. Pulmonary sequestration in a newborn infant: treatment with arterial embolization. J Vasc Int Radiol. (1998) 9:648–50. doi: 10.1016/s1051-0443(98)70337-9
13. Leoncini G, Rossi UG, Ferro C, Chessa L. Endovascular treatment of pulmonary sequestration in adults using Amplatzer§vascular plugs. Interact Cardiovasc Thorac Surg. (2011) 12:98–100. doi: 10.1510/icvts.2010.246546
14. Hewett L, Kwon J, Adams JD, Denlinger CE, Klapper JA. Intralobar pulmonary sequestration with aneurysmal feeding vessel: use of hybrid surgical management. Ann Thorac Surg. (2016) 102:e533–5.
15. Sridhar P, Hardouin S, Cheng T, Farber A, Suzuki K, Jones DW. Endovascular exclusion and open resection of aberrant pulmonary artery aneurysm associated with intralobar pulmonary sequestration. J Vasc Surg. (2019) 70:1328–9. doi: 10.1016/j.jvs.2019.06.182
16. Sakai M, Ozawa Y, Konishi T, Watanabe A, Shiigai M. Endostapling the aberrant artery filled with embolized coils for intralobar pulmonary sequestration: a report of two cases. J Thorac Dis. (2018) 10:E304–8. doi: 10.21037/jtd.2018.04.21
17. Petty L, Joseph A, Sanchez J. Case report: pulmonary sequestration in an adult. Radiol Case Rep. (2018) 13:21–3. doi: 10.1016/j.radcr.2017.09.029
18. Guan Q, Li J, Deng K, Wu X, Tang S, Fan C, et al. Clinical study to individual treatment for major aortopulmonary collaterals of tetralogy of fallot. Biomed Res Int. (2019) 2019:1603712. doi: 10.1155/2019/1603712
19. Fan C, Cheng J, Wu S, Tang S, Chen W, Yang J. Pulmonary arteriovenous malformation detected by three-dimensional computed tomographic angiography. Heart Lung Circ. (2017) 26:e59–61. doi: 10.1016/j.hlc.2017.02.016
20. Cheng J, Fan C, Tang M, Shu Y, Yang J. Initial research on postoperative management of tetralogy of fallot with major aortopulmonary collaterals. Cardiology. (2016) 134:406–10. doi: 10.1159/000445046
Keywords: pulmonary sequestration, atrial septal defect, atrial septal occlusion, interventional therapy, pulmonary vein drainage
Citation: Tang M, Wu X, Hu S, Wu Q, Yang D, Iroegbu CD, Fan C and Yang J (2022) A case of rare pulmonary sequestration complicated with congenital heart disease treated by arterial embolization and atrial defect closure: A case report and review of literature. Front. Cardiovasc. Med. 9:931590. doi: 10.3389/fcvm.2022.931590
Received: 29 April 2022; Accepted: 28 June 2022;
Published: 22 July 2022.
Edited by:
Jun Yu, Temple University, United StatesReviewed by:
Fang Liu, Children’s Hospital, Fudan University, ChinaCopyright © 2022 Tang, Wu, Hu, Wu, Yang, Iroegbu, Fan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengming Fan, ZmFuY2hlbmdtaW5nQGNzdS5lZHUuY24=; Jinfu Yang, eWpmMTk2ODIwMDVAY3N1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.