
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 07 September 2022
Sec. Coronary Artery Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.929634
A correction has been applied to this article in:
Corrigendum: Higher serum phosphorus and calcium levels provide prognostic value in patients with acute myocardial infarction
 Wei Cao1,2†
Wei Cao1,2† Yilan Li1,3†
Yilan Li1,3† Yao Wen1
Yao Wen1 Shaohong Fang1,3
Shaohong Fang1,3 Bing Zhao1,3
Bing Zhao1,3 Xiaoyuan Zhang1,3
Xiaoyuan Zhang1,3 Yanxiu Zhang1,3
Yanxiu Zhang1,3 Xueyan Lang1,3
Xueyan Lang1,3 Bo Yu1,3*
Bo Yu1,3* Yao Zhang1,3*
Yao Zhang1,3*Background: Although traditional cardiovascular risk factors are closely related to the poor prognosis of acute myocardial infarction (AMI) patients, there are few studies on the relationship of serum phosphorus and calcium with prognosis in AMI patients. The relationship of serum phosphorus and calcium with prognostic biomarkers in AMI remains unclear.
Methods and results: A total of 3,891 AMI patients were enrolled from a prospective cohort study. We investigated the association of serum phosphorus and calcium with prognostic biomarkers. The risk of in-hospital heart failure (HF), post-discharge HF, all-cause mortality and cardiac mortality was estimated across quartiles of serum phosphorus and calcium levels. Serum phosphorus and calcium levels were associated with biomarkers of prognosis. Overall, 969 patients developed in-hospital HF during hospitalization, 549 patients developed post-discharge HF during a median follow-up of 12 months, and 252 patients died, with 170 cardiac deaths since admission. In the fully adjusted model, compared with patients in quartile 2 (Q2), patients with serum phosphorus levels in Q4 were at greater risk of post-discharge HF [sub-distributional hazard ratios (SHR) 1.55; 95% confidence interval (CI), 1.21–1.99], in-hospital HF [odds ratio (OR) 1.84; 95% CI, 1.47–2.31], all-cause mortality (HR 1.59; 95% CI, 1.08–2.32), and cardiac mortality (SHR 1.68; 95% CI, 1.03–2.75). Compared with patients in Q2, patients with corrected calcium levels in Q4 had a higher risk of in-hospital HF (OR 1.62; 95% CI, 1.29–2.04), all-cause mortality (HR 1.99; 95% CI, 1.37–2.88), and cardiac mortality (SHR 1.87; 95% CI, 1.19–2.96; all p-trend < 0.05).
Conclusion: Serum phosphorus and calcium levels were associated with AMI prognostic biomarkers in AMI. Higher serum phosphorus was independently related to the increased risk of in-hospital HF, postdischarge HF, all-cause mortality and cardiac mortality, and higher serum calcium was independently related to the increased risk of in-hospital HF, all-cause mortality and cardiac mortality after AMI.
Patients with acute myocardial infarction (AMI) have a high risk of heart failure (HF) and mortality (1). Although advances in the diagnosis and treatment of AMI have reduced the risk of mortality in AMI patients, the incidence of HF is still on the rise after myocardial infarction (MI), which significantly increases the risk of short-term and long-term adverse events in AMI patients (2). Although some prognostic biomarkers (such as troponin, natriuretic peptide, and C-reactive protein levels) are related to poor prognosis after MI (3–5), there are still great challenges in the early prediction of clinical outcomes.
In the past, more attention has been given to the correlation between poor prognosis and traditional cardiovascular risk factors such as blood glucose, blood lipids, and smoking in AMI patients. However, prior studies have shown that phosphorus and calcium are associated with cardiovascular disease (CVD) as non-traditional risk factors (6–11).
Although most studies found that higher serum phosphate levels were related to post-discharge HF risk or mortality in various populations, including MI (6–9), controversy remained in another study (12), in which there was no relationship between serum phosphorus and cardiovascular events.
In addition, the link between serum calcium levels and poor outcome has been debated in different populations. Lower serum calcium levels were related to the risk of all-cause death in patients with acute coronary syndrome (ACS) (10) or in HF with preserved EF (HFpEF) patients (13), but both high and low serum calcium levels were related to an increased risk of in-hospital mortality in another study (14). Moreover, higher serum calcium levels were associated with HF risk in a community-based cohort study (11).
Prior studies were carried out in a part of the MI population or non-MI population alone, and most were limited by small sample sizes. Few studies have evaluated the relationship between serum phosphorus and serum calcium levels with in-hospital HF. In this study, we systematically evaluated the prognostic value of serum phosphorus and serum calcium levels on in-hospital HF, post-discharge HF, all-cause mortality, and cardiac mortality in a large AMI population.
All patients were from a prospective hospital-based AMI cohort study, which was supported by the National Key Research and Development Program of China. From 1 April 2017 to 31 March 2019, 4,335 patients with AMI, aged ≥18 years, were admitted to the Department of Cardiology of the Second Affiliated Hospital of Harbin Medical University. Patients were excluded if they met any of the following criteria: (1) treatment with glucocorticosteroids, bisphosphonates, calcium or vitamin D supplements, or diuretics within the past 1 month; (2) medical history of HF, active infection, liver disease, uremia [estimated glomerular filtration rate (eGFR) lower than 15 mL/min/1.73 m2, or dialysis or transplantation is needed], or malignancy; and (3) insufficient medical records (Figure 1). A total of 3,891 patients were finally included in this complete data cohort. A total of 3,830 patients who survived at discharge agreed to be followed.
All patients were followed from admission to death or the last follow-up date. The regular follow-up times were 1, 3, 6, 12, 18, and 24 months.
AMI should be diagnosed according to the Fourth Universal Definition of Myocardial Infarction (2018) (15). HF after AMI was defined as typical signs and symptoms of congestion that required treatment with a diuretic or intravenous vasodilator. Participants with N-terminal pro-B-type natriuretic peptide (NT-proBNP) < 125 pg/mL in the non-acute setting or NT-proBNP < 300 pg/mL in the acute setting were excluded from HF (16). Multi-vessel disease was defined as stenosis of at least two main blood vessels (≥2 mm diameter) >70%, measured by quantitative coronary angiography (17).
We collected blood samples on admission and sent them to the laboratory for testing immediately. Serum phosphorus was measured by using methods based on ammonium molybdate, and serum calcium was measured with an approach based on o-cresolphthalein complexone on the Siemens Dimension Clinical Chemistry System. The baseline population characteristics were collected from medical records, prior medication, and self-reports. Echocardiography characteristics were examined at admission and reviewed when necessary.
The serum calcium level was corrected according to the formula: albumin-corrected serum calcium (mmol/L) = measured serum calcium (mmol/L) – 0.025* serum albumin (g/L) +1.0 (mmol/L) (18).
The primary outcome was post-discharge HF after AMI. The secondary outcomes were in-hospital HF and all-cause death, including cardiac death after AMI. Cardiac death was defined as death due to recurrent MI (RMI), HF, severe arrhythmias and sudden death (19).
For baseline characteristics, the median [interquartile range (IQR)] or number (percentage) was used as appropriate. Serum phosphorus and calcium levels were analyzed as continuous variables and as quartiles. Characteristics among different serum phosphorus and corrected serum calcium quartile groups were compare by Kruskal–Wallis test, chi-square test or Fisher's exact test. We used multivariate multiple imputation with chained equations to impute missing values. The correlation of serum phosphorus and calcium with prognostic biomarkers was assessed by Spearman correlation analysis. The non-linear association of serum phosphorus and calcium levels with clinical outcomes was examined by restricted cubic splines. Kaplan–Meier curves were used to perform the proportion of patients free of clinical outcomes according to quartiles of serum phosphorus or corrected serum calcium, which were compared by log-rank. The association of serum phosphorus or calcium with the incidence of in-hospital HF was evaluated by logistic regression analysis. Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% CIs for all-cause mortality. Fine-Gray proportional sub-distribution hazards models were used to evaluate the relationship of serum phosphorus and calcium levels with the incidence of post-discharge HF or cardiac death, and death without HF was regarded as a competitive risk of post-discharge HF and non-cardiac death as a competitive risk of cardiac death. The regression results were assessed according to serum phosphorus or corrected serum calcium quartiles using quartile 2 (Q2) as the reference. Four multivariable models were constructed. The first model was adjusted according to sex, age, and body mass index (BMI). In Model 2, AMI types, smoking status, prior history of hypertension, diabetes, MI, percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) were further adjusted. In Model 3, heart rate, PCI information and multi-vessel disease were further adjusted. In Model 4, cardiac troponin I (cTNI), NT-proBNP, eGFR, total cholesterol (TC), triglyceride (TG) two kinds of serum phosphorus, corrected serum calcium and magnesium were further adjusted. Stratified analysis based on subgroup variables was performed with full adjustments. Interaction terms were used to evaluate whether subgroup variables modified the associations between serum phosphorus and calcium with the relative risk of clinical outcomes. Stata Statistical Software (Version 15.1; Stata Corp, College Station, TX, USA), and IBM SPSS Statistics (Version 25.0; IBM, Armonk, NY, USA) were used for all statistical analyses, and the results were considered statistically significant at P < 0.05.
Among the 3,891 participants, 67.9% were ST-segment elevation MI (STEMI) patients, 69.1% were male, and the median age was 61 years (IQR 53–69). The median serum phosphate level was 1.21 mmol/L (IQR 1.07–1.36), and the median corrected serum calcium level was 2.17 mmol/L (IQR 2.09–2.27) on hospital admission. According to quartiles of serum phosphate and corrected serum calcium, the cohort characteristics are shown in Tables 1, 2.
Patients with higher phosphorus were more likely to be younger, females, and non-smokers and had a higher prevalence of hypertension, diabetes and multi-vessel diseases, lower systolic blood pressure, higher cTNI, higher NT-proBNP, higher hs-CRP, higher corrected serum calcium, higher TC and TG, lower EF, and lower eGFR. Patients with higher serum phosphorus were less likely to be treated with antiplatelet drugs and statins during hospitalization.
What's more, patients with higher corrected calcium were more likely to be older, females, non-smokers, and non-STEMI and had a higher prevalence of diabetes or prior CABG, lower BMI, lower systolic blood pressure, lower heart rate, lower cTNI, higher NT-proBNP, higher hs-CRP, higher serum phosphate, higher left atrial diameter, lower EF, lower eGFR and lower serum albumin. Patients with higher serum phosphorus were less likely to be treated with antiplatelet drugs, β- blockers, angiotensin converting enzyme inhibitors or angiotensin II receptor blockers, PCI, and coronary angiography during hospitalization.
With Spearman correlation analysis, serum phosphorus was positively correlated with NT-proBNP (r = 0.171; p < 0.001), hs-CRP (r = 0.066; p < 0.001), and cTNI (r = 0.058; p < 0.001) and negatively correlated with EF (r = −0.094; p < 0.001) and eGFR (r = −0.044; p = 0.006). Corrected serum calcium was also positively correlated with NT-proBNP (r = 0.230; p < 0.001) and hs-CRP (r = 0.207; p < 0.001) and negatively correlated with cTNI (r = −0.110; p < 0.001), EF (r = −0.095; p < 0.001) and eGFR (r = −0.225; p < 0.001; Supplementary Table S1).
The median follow-up was 12 months (range 12–24 months). During hospitalization, in-hospital HF occurred in 969 (24.9%) patients. During follow-up, 549 (14.3%) patients developed post-discharge HF. A total of 252 (6.6%) patients died, and 170 (4.4%) patients experienced cardiac death after AMI.
With increasing quartiles of phosphorus levels, the proportion of patients free of post-discharge HF decreased at the 12-month median follow-up (Figure 2A). Serum phosphorus levels had a S-shaped relationship with post-discharge HF (in unadjusted analysis, Figure 3B).
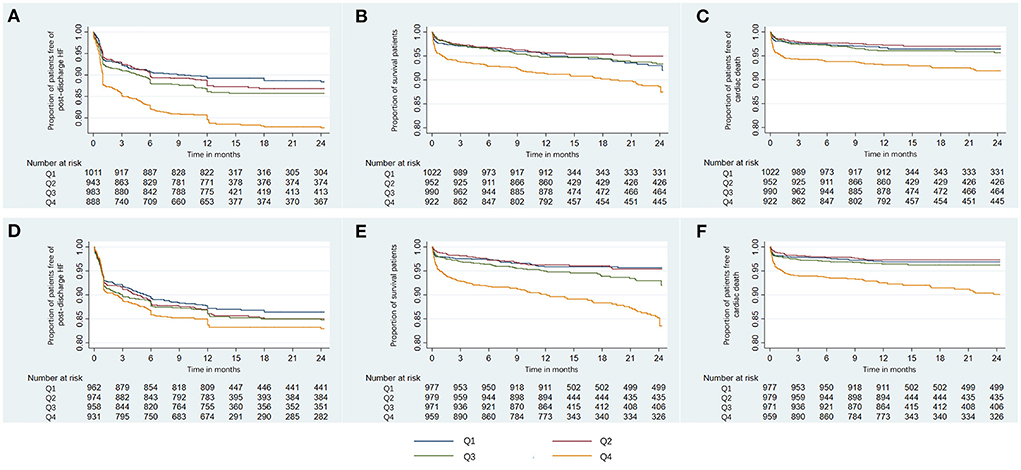
Figure 2. Kaplan-Meier curves of the proportion of AMI patients free of clinical outcomes. Shown are the proportion of patients free of post-discharge HF (A), survival rate (B), and the proportion of patients free of cardiac death (C) for serum phosphorus, and the proportion of patients free of post-discharge HF (D), survival rate (E), and the proportion of patients free of cardiac death (F) for corrected serum calcium. AMI, acute myocardial infarction; HF, heart failure; Q, quartile.
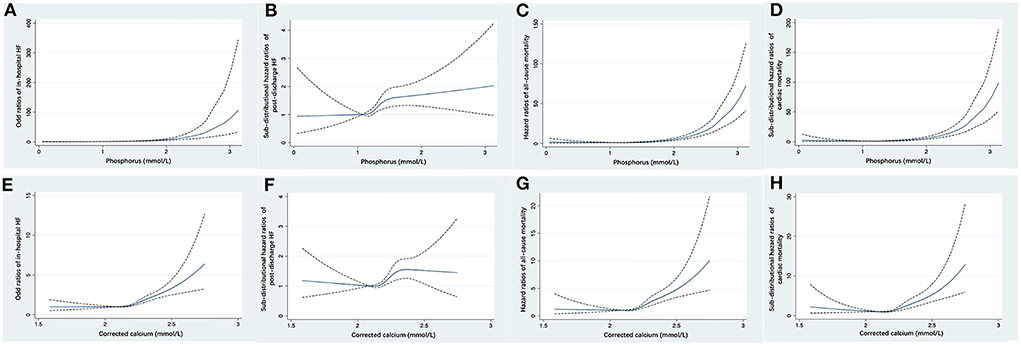
Figure 3. Restricted cubic spline fitting for the association of serum phosphorus with the risk of in-hospital HF (A), post-discharge HF (B), all-cause mortality (C), and cardiac mortality (D), and the association of corrected serum calcium with the risk of in-hospital HF (E), post-discharge HF (F), all-cause mortality (G), and cardiac mortality (H). ORs or HRs were evaluated based on a univariate logistic regression or Cox proportional regression model. The area between dotted lines represents the 95% CI. CI, confidence interval; HF, heart failure; HR, hazard ratio; OR, odds ratio.
In competing risk regression analysis, there was a statistically significant trend of rising post-discharge HF risk across quartiles of phosphorus (all p-trend < 0.001) (Table 3). Patients in Q4 of phosphorus were 1.77 (95% CI: 1.40–2.22) times more likely to develop post-discharge HF than patients in Q2 before adjustment. After full adjustments, the results of the primary analysis remained robust [sub-distributional hazard ratios (SHR): 1.55, 95% CI: 1.21–1.99] (Table 3 and Figure 4A). Furthermore, stratified analysis showed that there was a consistent prognostic effect of serum phosphorus on post-discharge HF across most subgroups (Table 4).
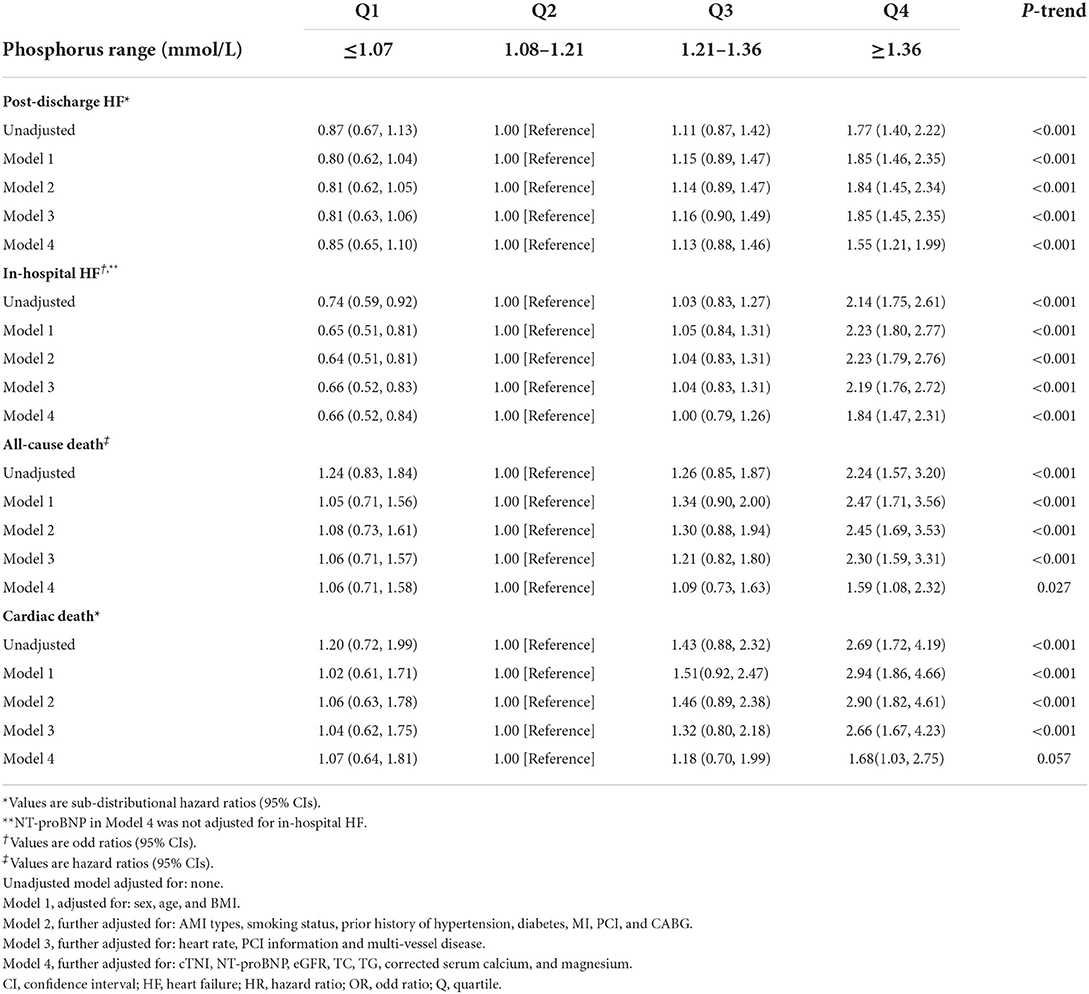
Table 3. Sub-distributional HRs, ORs, or HRs (95% CIs) associated with serum phosphorus for clinical outcomes after acute myocardial infarction.
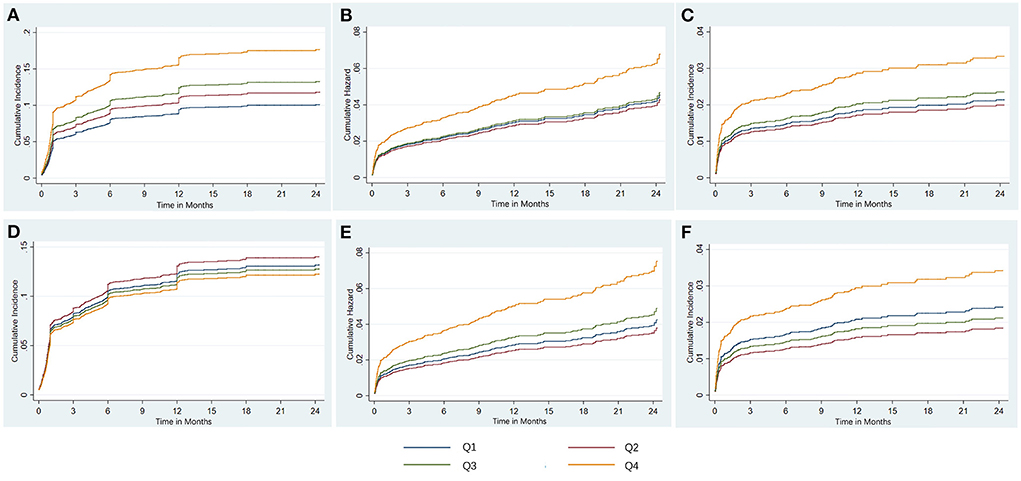
Figure 4. The risk regression analysis of clinical outcomes based on fully adjusted models. Shown are the cumulative incidence of post discharge HF treating death without HF as competing risk (A), the cumulative hazard of all-cause death (B), and the cumulative incidence of cardiac death treating non-cardiac death as competing risk (C) for serum phosphate, and the cumulative incidence of post-discharge HF treating death without HF as competing risk (D), the cumulative hazard of all-cause death (E), and the cumulative incidence of cardiac death treating non-cardiac death as competing risk (F) for corrected serum calcium. HF, heart failure; Q, quartile.
The restricted cubic spline curve showed that serum phosphorus levels had a J-shaped relationship with in-hospital HF (in unadjusted analysis, Figure 3A).
In logistic analysis, compared with patients in Q2, patients in the highest quartile of phosphorus levels had a higher in-hospital HF risk in the unadjusted model (OR: 2.14, 95% CI: 1.75–2.61, p-trend < 0.001), and this association was still stable after full adjustments (OR: 1.84, 95% CI: 1.47–2.31, p-trend < 0.001; Table 3).
Furthermore, stratified analysis showed that there was a consistent prognostic effect of serum phosphorus on in-hospital HF across most subgroups, which was more pronounced among patients with hypertension (p for interaction = 0.001) or eGFR<60 mL/min/1.73 m2 (p for interaction = 0.028; Table 4).
At the 12-month median follow-up, patients in Q4 of phosphorus levels also had the lowest survival rate (Figure 2B) and the lowest proportion of patients free of cardiac death (Figure 2C). Serum phosphorus levels had a J-shaped relationship with all-cause mortality and cardiac mortality (in unadjusted analysis, Figures 3C,D).
Compared with Q2, the highest quartile of phosphorus had more than a 2-fold increase in all-cause death risk (HR: 2.24, 95% CI: 1.57–3.20; p-trend< 0.001) and cardiac death risk (SHR: 2.69, 95% CI: 1.72–4.19; p-trend < 0.001) before adjustment, and this association was still stable in all-cause death risk (HR: 1.59, 95% CI: 1.08–2.32, p-trend = 0.027) (Table 3 and Figure 4B) and cardiac death risk (SHR: 1.68, 95% CI: 1.03–2.57) after full adjustments, but p-trend value was insignificant in cardiac death risk (Table 3 and Figure 4C).
Furthermore, stratified analysis showed that there was a consistent prognostic effect of serum phosphorus on all-cause death or cardiac death across most subgroups, which was more pronounced among non-smokers or patients with eGFR < 60 mL/min/1.73 m2 (all p for interaction < 0.05; Table 5).
With increasing quartiles of corrected serum calcium levels, the proportion of patients free of post-discharge HF decreased at the 12-month median follow-up (Figure 2D). The restricted cubic spline curve showed that corrected serum calcium levels had a S-shaped relationship with post-discharge HF (in unadjusted analysis, Figure 3F).
However, in competing risk regression analysis, the association between corrected serum calcium levels and the risk of post-discharge HF was non-significant before adjustments and after full adjustments (Table 6 and Figure 4D). Stratified analysis showed higher corrected serum calcium was related to a lower risk of post-discharge HF in patients ≥65 years old (p for interaction = 0.004) (Table 7).
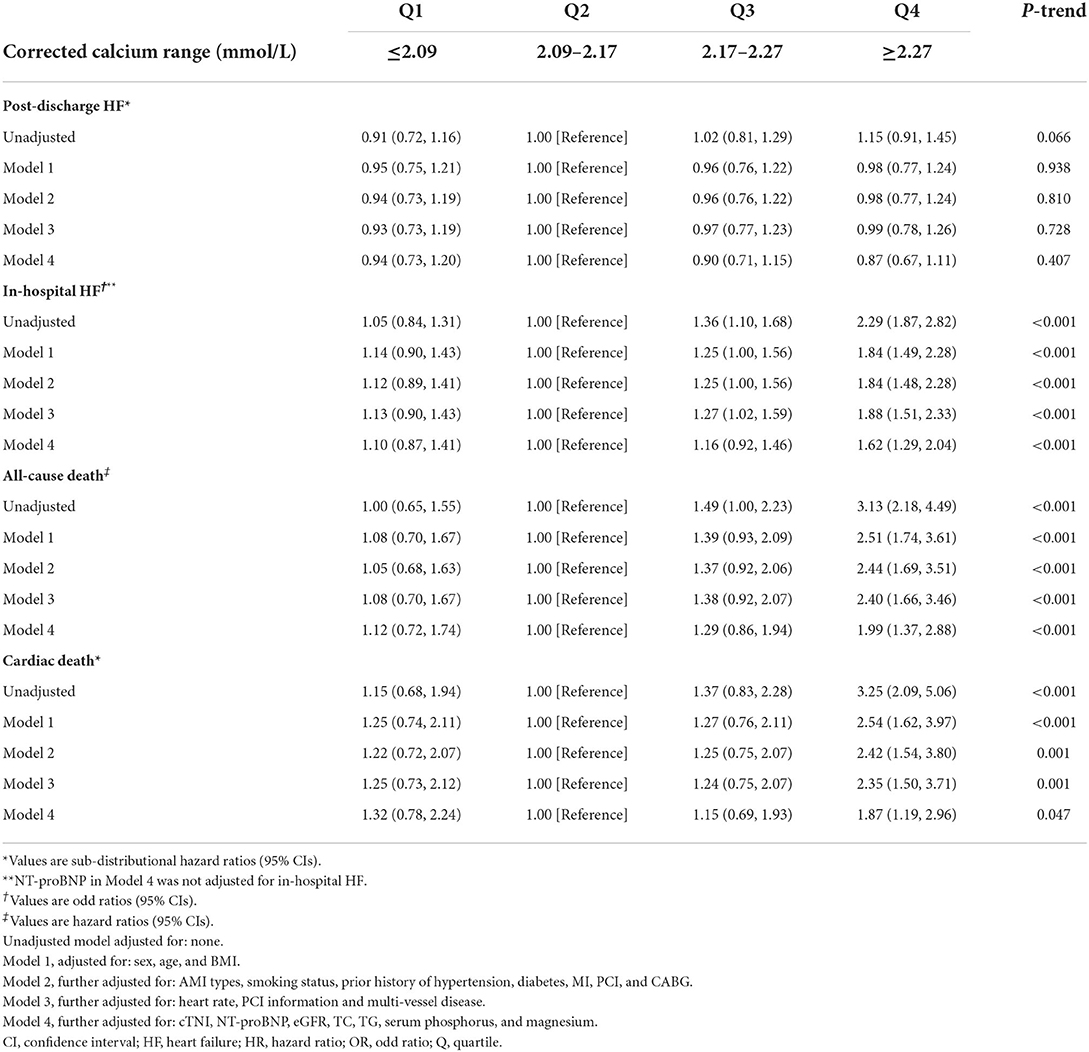
Table 6. Sub-distributional HRs, ORs, or HRs (95% CIs) associated with corrected calcium for clinical outcomes after acute myocardial infarction.
Corrected serum calcium levels had a J-shaped relationship with in-hospital HF (in unadjusted analysis, Figure 3E). In logistic analysis, patients with Q4 of corrected serum calcium had more than a 2-fold increased risk of in-hospital HF than those in Q2 (OR: 2.29, 95% CI: 1.87–2.82; p-trend < 0.001) before adjustment, and this association was still stable after full adjustments (OR: 1.62, 95% CI: 1.29–2.04; p-trend < 0.001; Table 6). Furthermore, stratified analysis showed that there was a consistent prognostic effect of corrected serum calcium on in-hospital HF across all subgroups, which was more pronounced among patients <65 years old (p for interaction = 0.046) (Table 7).
At the 12-month median follow-up, patients in Q4 of corrected serum calcium levels also had the lowest survival rate (Figure 2E) and the lowest proportion of patients free of cardiac death (Figure 2F). Corrected serum calcium levels had a J-shaped relationship with all-cause mortality and cardiac mortality (in unadjusted analysis, Figures 3G,H).
There was a statistically significant trend of rising all-cause death and cardiac death risk across quartiles of corrected serum calcium (all p-trend < 0.05; Table 6). Patients with Q4 of corrected serum calcium had a more than 200% increased risk of all-cause death (HR: 3.13, 95% CI: 2.18–4.49; p-trend < 0.001) or cardiac death (SHR: 3.25, 95% CI: 2.09–5.06; p-trend < 0.001) than those with Q2 before adjustment. This association was still stable in all-cause death risk (HR: 1.99, 95% CI: 1.37–2.88; p-trend < 0.001) or cardiac death risk (SHR: 1.87, 95% CI: 1.19–2.96; p-trend = 0.047) after adjusting for prognostic biomarkers, PCI information, multi-vessel disease and other clinical characteristics at baseline (Table 6 and Figures 4E,F). Moreover, there was a consistent prognostic effect of corrected serum calcium on all-cause mortality or cardiac mortality across most subgroups, which was more pronounced among patients without diabetes (all p for interaction < 0.05; Table 8).
To the best of our knowledge, the current study is the first to explore the prognostic value of serum phosphorus and calcium on admission in the largest AMI population. The key findings are as follows: (1) Serum phosphorus and calcium levels were associated with biomarkers of prognosis in AMI patients; (2) Higher serum phosphorus levels were independently related to the increased risk of in-hospital HF, post-discharge HF, all-cause death and cardiac death; (3) Higher corrected calcium levels were independently related to a greater risk of in-hospital HF, all-cause death and cardiac death. Our findings provide novel insights into the risk factors for incident HF and poor prognosis in AMI patients.
Some studies found an independent relationship of higher serum phosphate levels with incident HF risk in patients with previous MI (6, 7), which was similar to our findings. However, few of them focused on the relationship between serum phosphate levels and in-hospital HF, and few of them investigated the effect of serum phosphorus on post-discharge HF by subgroup analysis. Moreover, these studies only made minimal adjustments, and few of them evaluated the correlation of serum phosphorus and prognostic biomarkers.
In the present study, we first demonstrated that higher serum phosphorus levels were independently related to an increased risk of in-hospital HF. The factors leading to in-hospital HF include myocardial damage caused by myocardial necrosis, myocardial stunning and mechanical complications (20). Reactive oxygen species, the inflammatory response, and comorbidities such as chronic kidney disease (CKD) also result in the development of in-hospital HF (20). The occurrence of post-discharge HF is the result of myocardial cell death and scar formation, which leads to chronic neurohumoral activation and ventricular remodeling (20). In our study, we found that serum phosphorus was positively correlated with NT-proBNP, cTNI and hs-CRP and negatively correlated with EF and eGFR, which indicated that serum phosphorus was related to neurohumoral activation, ventricular remodeling, myocardial injury, inflammation, and impaired kidney function after AMI.
Several mechanisms may explain the relationship between elevated serum phosphorus and the increased risk of HF.
First, serum phosphorus is closely related to calcification of blood vessels and valves (21) and arterial stiffness (22). The decrease in vascular compliance caused by serum phosphorus may lead to left ventricular (LV) overload and remodeling, which is the structural basis of HF. In our study, patients with higher phosphorus levels had a higher prevalence of multi-vessel diseases. It has been reported that the risk of ventricular remodeling in patients with multi-vessel diseases is 1.2 times higher than that in patients with single-vessel disease (23). Importantly, in the current study, the correlation between serum phosphorus and HF risk remained robust after adjustment for the prevalence of multi-vessel diseases, which indicates that non-ischemic mechanisms may also contribute to the correlation. In addition, we found that there was a greater prognostic effect of serum phosphorus on in-hospital HF among patients with hypertension, which may be related to arterial stiffness due to higher phosphorus (22). What's more, higher phosphorus was more closely related to in-hospital HF risk in patients with impaired renal function than those with preserved renal function in our study, which was similar to the previous study.
Second, serum phosphorus may have a direct impact on the heart. In animal and human studies, serum phosphorus is independently related to LV mass (24, 25). Higher serum phosphorus levels are associated with greater LV mass in men with stable CVD (25). We also found that elevated serum phosphorus was more closely related to the risk of in-hospital HF in men than women, but there was no interaction in the subgroup analysis. Phosphorus binder therapy improves aortic stiffness and diastolic dysfunction and protects against LV hypertrophy (LVH) (24). The increase in serum phosphorus was related to the decrease in LV diastolic function and LVH in participants without prior HF (26). However, there were non-significant differences in echocardiographic characteristics except EF among the different serum phosphorus quartile groups. The reason for the inconsistent results may be due to different races and populations.
Third, higher serum phosphorus may indirectly influence the heart through its interactions with vitamin D deficiency, high parathyroid hormone (PTH) and fibroblast growth factor 23 (27). Unfortunately, the mechanisms of the above hormones were not evaluated in our study.
In the present study, higher serum phosphorus was independently related to a greater risk of all-cause mortality or cardiac mortality, which was similar to prior studies. Importantly, prior studies have shown that higher serum phosphorus is related to an increase in all-cause mortality in MI survivors (8), in STEMI populations (6, 9), or in AMI populations with a small sample size (7), and the relation of higher serum phosphorus and cardiac mortality is showed in CKD (28), which are different from our study. The underlying mechanisms may include vascular calcification, endothelial dysfunction, ventricular hypertrophy, and atherosclerosis (6, 9). What's more, the relationship between elevated serum phosphorus and mortality was more pronounced in patients with impaired kidney function, which suggested impaired kidney function was an important factor for the relationship. It was similar to a prior study, in which higher serum phosphorus is independently associated with the risk of adverse events in patients with AMI, and the association is more pronounced in patients with CKD (7).
In our study, we first found that there was an independent association between higher corrected serum calcium levels and the increased risk of in-hospital HF in AMI population. In prior studies, higher serum calcium was independently related to HF risk in a community-based cohort study (11), and elevated serum calcium levels were an independent risk factor for HFpEF in patients with type 2 diabetes mellitus (T2DM) (29). Some potential mechanisms may contribute to the association. First, elevated serum calcium may promote left ventricular diastolic function through the link with blood pressure and various metabolic abnormalities, such as diabetes, obesity, metabolic syndrome (30). Second, prior studies have shown that elevated serum calcium is closely related to left ventricular hypertrophy (31) and vascular stiffness (30). What's more, we found that there were interactions between age and corrected serum calcium for the relationship of corrected serum calcium and HF, which suggested age was an important factor for the relationship.
In the current study, higher serum calcium was independently related to all-cause mortality and -hospital HF and post-discharge HF for serum phosphate. cardiac death after full adjustments in AMI patients without calcium and vitamin D supplements. The possible mechanisms by which elevated serum calcium affects vascular calcification, blood coagulation, altered gene expression induced by effects on arterial wall calcium-sensing receptor (32), and cardiovascular risk factors (30), such as lipid and glucose metabolism. However, Lu et al. (10) found that lower calcium levels are independently related to mortality during hospitalization in STEMI patients. Both the increase and decrease in serum calcium levels are correlated with the increased risk of in-hospital mortality in AMI patients (14). Although our findings were different from these prior studies, our findings were observed in a larger prospective cohort study with a longer follow-up and more confounders were adjusted.
Furthermore, we first investigated the association between serum calcium levels and mortality in different subgroups of traditional cardiovascular risk factors. In the subgroup analysis, there was a greater prognostic effect of increased serum calcium on mortality among patients without diabetes. It is suggested that more attention should be given to the effect of serum calcium levels on the prognosis of AMI patients without diabetes.
There are several limitations of the study. First, this was a single-center study. Most of our research participants come from Northeast China, which limits the universality of our results. Second, at baseline, there were no data about the levels of serum PTH, vitamin D, fibroblast growth factor 23, or intake of calcium and phosphorus in the diet, which were available to clarify the potential mechanism of the observed relationships. Third, we did not repeat the measurement and observe the changes in serum calcium and phosphorus levels, but the baseline phosphorus and calcium levels also provide strong prognostic value in AMI patients. Fourth, confounding may still be caused by residual confounding and unmeasured confounders in these observational data despite the efforts of adjustments.
Our study demonstrated that serum phosphorus and calcium levels were associated with AMI prognostic biomarkers, higher serum phosphorus was an independent predictor of a higher incidence of in-hospital HF, post-discharge HF, all-cause mortality and cardiac mortality, and higher serum calcium was independently related to the increased risk of in-hospital HF, all-cause mortality, and cardiac mortality in AMI patients. These findings will be beneficial to the early risk stratification of incident HF and death after AMI and early standardized treatment of post-AMI HF.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of Harbin Medical University (Reference Number: KY2017-249). The patients/participants provided their written informed consent to participate in this study.
WC designed the analysis and wrote the original draft. YL and XZ conducted the model parameterization and the statistical analyses. YW, BZ, YanZ, and XL collected the data. YaoZ and BY provided resources and supervised the study. YaoZ, BY, and SF revised the manuscript. All authors have read and approved the final manuscript.
This research was supported by the National Key Research and Development Program of China to BY, grant number 2016YFC1301100; the National Natural Science Foundation of China to YaoZ, grant number 81770255; the National Key Research and Development Program of China to YaoZ, grant numbers 2016YFC1301001, 2016YFC1301002, and 2016YFC1301004; and the Key Laboratory of Myocardial Ischemia, Harbin Medical University of Education to YaoZ, grant number KF202103.
We thank the hospital staff who contributed to data collection for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.929634/full#supplementary-material
1. Minicucci MF, Azevedo PS, Polegato BF, Paiva SA, Zornoff LA. Heart failure after myocardial infarction: clinical implications and treatment. Clin Cardiol. (2011) 34:410–4. doi: 10.1002/clc.20922
2. Cleland JG, Torabi A, Khan NK. Epidemiology and management of heart failure and left ventricular systolic dysfunction in the aftermath of a myocardial infarction. Heart. (2005) 91(Suppl.2):ii7–1. doi: 10.1136/hrt.2005.062026
3. Gerber Y, Jaffe AS, Weston SA, Jiang R, Roger VL. Prognostic value of cardiac troponin T after myocardial infarction: a contemporary community experience. Mayo Clin Proc. (2012) 87:247–54. doi: 10.1016/j.mayocp.2011.11.013
4. Al Aseri ZA, Habib SS, Marzouk A. Predictive value of high sensitivity C-reactive protein on progression to heart failure occurring after the first myocardial infarction. Vasc Health Risk Manag. (2019) 15:221–7. doi: 10.2147/VHRM.S198452
5. Scirica BM, Cannon CP, Sabatine MS, Jarolim P, Sloane S, Rifai N, et al. Concentrations of C-reactive protein and B-type natriuretic peptide 30 days after acute coronary syndromes independently predict hospitalization for heart failure and cardiovascular death. Clin Chem. (2009) 55:265–73. doi: 10.1373/clinchem.2008.117192
6. Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G, Cholesterol, et al. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. (2005) 112:2627–33. doi: 10.1161/CIRCULATIONAHA.105.553198
7. Aronson D, Kapeliovich M, Hammerman H, Dragu R. The relation between serum phosphorus levels and clinical outcomes after acute myocardial infarction. PLoS ONE. (2013) 8:e58348. doi: 10.1371/journal.pone.0058348
8. Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the atherosclerosis risk in communities (aric) study. Am Heart J. (2008) 156:556–63. doi: 10.1016/j.ahj.2008.05.016
9. Zhu GH, Sun XP, Liu Z, Fan ZX, Wang YL, Tan J, et al. The relation between serum phosphorus levels and long-term mortality in chinese patients with St-segment elevation myocardial infarction. J Geriatr Cardiol. (2019) 16:775–81. doi: 10.11909/j.issn.1671-5411.2019.10.004
10. Lu X, Wang Y, Meng H, Chen P, Huang Y, Wang Z, et al. Association of admission serum calcium levels and in-hospital mortality in patients with acute st-elevated myocardial infarction: an eight-year, single-center study in China. PLoS ONE. (2014) 9:e99895. doi: 10.1371/journal.pone.0099895
11. Lutsey PL, Alonso A, Michos ED, Loehr LR, Astor BC, Coresh J, et al. Serum magnesium, phosphorus, and calcium are associated with risk of incident heart failure: the atherosclerosis risk in communities (aric) study. Am J Clin Nutr. (2014) 100:756–64. doi: 10.3945/ajcn.114.085167
12. Slinin Y, Blackwell T, Ishani A, Cummings SR, Ensrud KE, Investigators M. Serum calcium, phosphorus and cardiovascular events in post-menopausal women. Int J Cardiol. (2011) 149:335–40. doi: 10.1016/j.ijcard.2010.02.013
13. Liu F, Zhang H, Li Y, Lu X. Hypocalcaemia predicts 12-month re-hospitalization in heart failure. Eur J Clin Invest. (2020) 2020:e13261. doi: 10.1111/eci.13261
14. Shiyovich A, Plakht Y, Gilutz H. Serum calcium levels independently predict in-hospital mortality in patients with acute myocardial infarction. Nutr Metab Cardiovasc Dis. (2018) 28:510–6. doi: 10.1016/j.numecd.2018.01.013
15. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72:2231–64. doi: 10.1016/j.jacc.2018.08.1038
16. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
17. Thiele H, Akin I, Sandri M, de Waha-Thiele S, Meyer-Saraei R, Fuernau G, et al. One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. (2018) 379:1699–710. doi: 10.1056/NEJMoa1808788
18. Payne RB, Carver ME, Morgan DB. Interpretation of serum total calcium: effects of adjustment for albumin concentration on frequency of abnormal values and on detection of change in the individual. J Clin Pathol. (1979) 32:56–60. doi: 10.1136/jcp.32.1.56
19. Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol. (1999) 34:618–20. doi: 10.1016/S0735-1097(99)00250-8
20. Jenca D, Melenovsky V, Stehlik J, Stanek V, Kettner J, Kautzner J, et al. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail. (2021) 8:222–37. doi: 10.1002/ehf2.13144
21. Scialla JJ, Wolf M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol. (2014) 10:268–78. doi: 10.1038/nrneph.2014.49
22. Ix JH, De Boer IH, Peralta CA, Adeney KL, Duprez DA, Jenny NS, et al. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in mesa. Clin J Am Soc Nephrol. (2009) 4:609–15. doi: 10.2215/CJN.04100808
23. Tarantini G, Napodano M, Gasparetto N, Favaretto E, Marra MP, Cacciavillani L, et al. Impact of multivessel coronary artery disease on early ischemic injury, late clinical outcome, and remodeling in patients with acute myocardial infarction treated by primary coronary angioplasty. Coron Artery Dis. (2010) 21:78–86. doi: 10.1097/MCA.0b013e328335a074
24. Maizel J, Six I, Dupont S, Secq E, Dehedin B, Barreto FC, et al. Effects of sevelamer treatment on cardiovascular abnormalities in mice with chronic renal failure. Kidney Int. (2013) 84:491–500. doi: 10.1038/ki.2013.110
25. Saab G, Whooley MA, Schiller NB, Ix JH. Association of serum phosphorus with left ventricular mass in men and women with stable cardiovascular disease: data from the heart and soul study. Am J Kidney Dis. (2010) 56:496–505. doi: 10.1053/j.ajkd.2010.03.030
26. Poudel K, Shah AM, Michos ED, Folsom AR, Konety S, Lutsey PL. Association of serum calcium and phosphorus with measures of left ventricular structure and function: the aric study. Nutr Metab Cardiovasc Dis. (2020) 30:758–67. doi: 10.1016/j.numecd.2020.01.003
27. Lederer E. Regulation of serum phosphate. J Physiol. (2014) 592:3985–95. doi: 10.1113/jphysiol.2014.273979
28. Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum Po(4), Ca X Po(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. (2001) 12:2131–8. doi: 10.1681/ASN.V12102131
29. Li J, Wu N, Dai W, Jiang L, Li Y, Li S, et al. Association of serum calcium and heart failure with preserved ejection fraction in patients with type 2 diabetes. Cardiovasc Diabetol. (2016) 15:140. doi: 10.1186/s12933-016-0458-6
30. Reid IR, Gamble GD, Bolland MJ. Circulating calcium concentrations, vascular disease and mortality: a systematic review. J Intern Med. (2016) 279:524–40. doi: 10.1111/joim.12464
31. Li J, Wu N, Li Y, Ye K, He M, Hu R. Cross-sectional analysis of serum calcium levels for associations with left ventricular hypertrophy in normocalcemia individuals with type 2 diabetes. Cardiovasc Diabetol. (2015) 14:43. doi: 10.1186/s12933-015-0200-9
Keywords: serum phosphate, serum calcium, acute myocardial infarction, prognosis, heart failure, mortality
Citation: Cao W, Li Y, Wen Y, Fang S, Zhao B, Zhang X, Zhang Y, Lang X, Yu B and Zhang Y (2022) Higher serum phosphorus and calcium levels provide prognostic value in patients with acute myocardial infarction. Front. Cardiovasc. Med. 9:929634. doi: 10.3389/fcvm.2022.929634
Received: 27 April 2022; Accepted: 08 August 2022;
Published: 07 September 2022.
Edited by:
Yuli Huang, Southern Medical University, ChinaCopyright © 2022 Cao, Li, Wen, Fang, Zhao, Zhang, Zhang, Lang, Yu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao Zhang, eWFvemhhbmdfZ3JhY2VAMTYzLmNvbQ==; Bo Yu, eXVib2RyQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.