
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Cardiovasc. Med., 15 September 2022
Sec. Cardiovascular Therapeutics
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.928695
This article is part of the Research TopicNew Mechanisms and Drugs for the Treatment of Cardiovascular Disease with DiabetesView all 17 articles
 Mingzhi Shen1,2†
Mingzhi Shen1,2† Jihang Wang1†
Jihang Wang1† Dongyun Li3†
Dongyun Li3† Xinger Zhou1,2
Xinger Zhou1,2 Yuting Guo1,2
Yuting Guo1,2 Wei Zhang4
Wei Zhang4 Yi Guo1
Yi Guo1 Jian Wang1
Jian Wang1 Jie Liu5
Jie Liu5 Guang Zhao6
Guang Zhao6 Shihao Zhao1*
Shihao Zhao1* Jinwen Tian1,2*
Jinwen Tian1,2*Background: Type 2 diabetes (T2DM) is a major risk factor for myocardial infarction. Thrombus aspiration was considered a good way to deal with coronary thrombus in the treatment of acute myocardial infarction. However, recent studies have found that routine thrombus aspiration is not beneficial. This study is designed to investigate whether intracoronary artery retrograde thrombolysis (ICART) is more effective than thrombus aspiration or percutaneous transluminal coronary angioplasty (PTCA) in improving myocardial perfusion in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PPCI).
Methods/Design: IntraCoronary Artery Retrograde Thrombolysis (ICART) vs. thrombus aspiration or PTCA in STEMI trial is a single-center, prospective, randomized open-label trial with blinded evaluation of endpoints. A total of 286 patients with STEMI undergoing PPCI are randomly assigned to two groups: ICART and thrombus aspiration or PTCA. The primary endpoint is the incidence of >70% ST-segment elevation resolution. Secondary outcomes include distal embolization, myocardial blush grade, thrombolysis in myocardial infarction (TIMI) flow grade, and in-hospital bleeding.
Discussion: The ICART trial is the first randomized clinical trial (RCT) to date to verify the effect of ICART vs. thrombus aspiration or PTCA on myocardial perfusion in patients with STEMI undergoing PPCI.
Clinical Trial Registration: [https://www.chictr.org.cn/], identifier [ChiCTR1900023849].
Patients with type 2 diabetes (T2DM) are at high risk and have a poor prognosis of myocardial infarction, especially after ST-segment elevation myocardial infarction (STEMI). STEMI is due to plaque rupture leading to intracoronary thrombosis, thus blocking the coronary artery (1, 2). The strategy to treat myocardial infarction is to open the infarct-related coronary artery to achieve reperfusion as soon as possible (3). However, in the process of opening the occluded vessels, it leads to myocardial damage. This phenomenon is called reperfusion injury (4). Myocardial death caused by reperfusion accounts for 25% of the total death area. Animal experiments show that the proportion is even as high as 50% (5). Reperfusion injury can cause reperfusion arrhythmia, myocardial stunning, microvascular occlusion, intramyocardial hemorrhage, and lethal myocardial reperfusion injury (6).
At present, methods of opening occluded vessels include intravenous thrombolysis, primary percutaneous coronary intervention (PPCI), and emergency coronary artery bypass grafting (CABG) (7, 8). The advantage of intravenous thrombolysis is that it can be implemented in general hospitals and even primary hospitals, and can be carried out quickly before hospitalization or even in ambulances (9–11). However, the success rate of intravenous thrombolysis is relatively low (12, 13). Even after intravenous thrombolysis success, emergency percutaneous coronary intervention (PCI) should be performed within 24 h, which increases the risk of hemorrhagic events or even fatal hemorrhage. Therefore, intravenous thrombolysis was gradually reduced or even stopped in the hospitals that could perform primary PCI in time or quickly transfer to primary PCI hospitals (7). Because of the complexity of CABG and the high requirement of patients’ own conditions, it is relatively difficult to carry out emergency CABG (8).
Primary percutaneous coronary intervention is still the best way to treat acute myocardial infarction because of its simple operation and exact effect (14). Thrombus load is an independent risk factor for PPCI. Slow-flow and no-reflow after stent implantation affect the prognosis (15). There are two ways to deal with thrombus before PPCI. The first is thrombus aspiration (16). However, routine thrombus aspiration before PPCI does not reduce the rate of all-cause mortality, rehospitalization of myocardial infarction, or stent thrombosis, and even increase the risk of stroke (17). Even in patients with high thrombus burden, routine thrombus aspiration does not improve outcomes (18). The second is to give antithrombotic or thrombolytic agents through the transcatheter, such as glycoprotein (GP) IIb/IIIa inhibitors, alteplase, et al. (19). However, due to the blood flow erosion, antithrombotic agents, and thrombolytic agents cannot stay for a long time at the thrombus site, affecting the drug effect; if the dosage is increased, the hemorrhagic risk will increase at the same time (20). It has also been studied that thrombolytic drugs are given after thrombus aspiration (21), the efficacy is similarly affected for the above reasons. There is still a long way to go to solve the problem of intracoronary thrombus, especially in patients with large thrombus burdens.
The sequence of continuous thrombosis is white thrombus, mixed thrombus, and red thrombus. The white thrombus in the head is mainly composed of platelets. The red thrombus at the tail end is mainly composed of red cells within the fibrin network (22). According to the characteristics of thrombus, we put forward the method of ICART, applied it to a small sample population, and achieved a certain effect (23). Only 10% of intravenous thrombolysis is needed for the ICART procedure. Before the newly formed thrombus is destroyed, a very high concentration of thrombolytic agent is produced in the red thrombus of the occluded vessel, which could stay for a long time, resulting in a very high thrombolytic efficiency, and reducing the incidence of no-reflow and slow-flow. At the same time, due to the slow opening of occluded vessels, there is a reperfusion preadaptation, which can reduce the reperfusion injury in theory. At the same time, low-dose thrombolysis does not increase the risk of hemorrhage and stroke. ICART may provide a new plan to solve thrombus burden in STEMI. However, there were only eight cases in the previous study (23). Therefore, we intend to verify the effect of ICART vs. thrombus aspiration or PTCA on myocardial perfusion in patients with STEMI undergoing PPCI.
The ICART trial is a single-center, prospective, randomized controlled trial with a blinded evaluation of endpoints (Figure 1). A total of 286 patients with STEMI undergoing PPCI were randomly divided into ICART or thrombus aspiration or PTCA groups. Randomization is performed by the Empower network random system when deciding to perform PCI. The study was approved by the institutional committee on human research of the Chinese People’s Liberation Army General Hospital and complies with the declaration of Helsinki. The protocol of this trial has been registered at chictr.org.cn (ChiCTR1900023849).
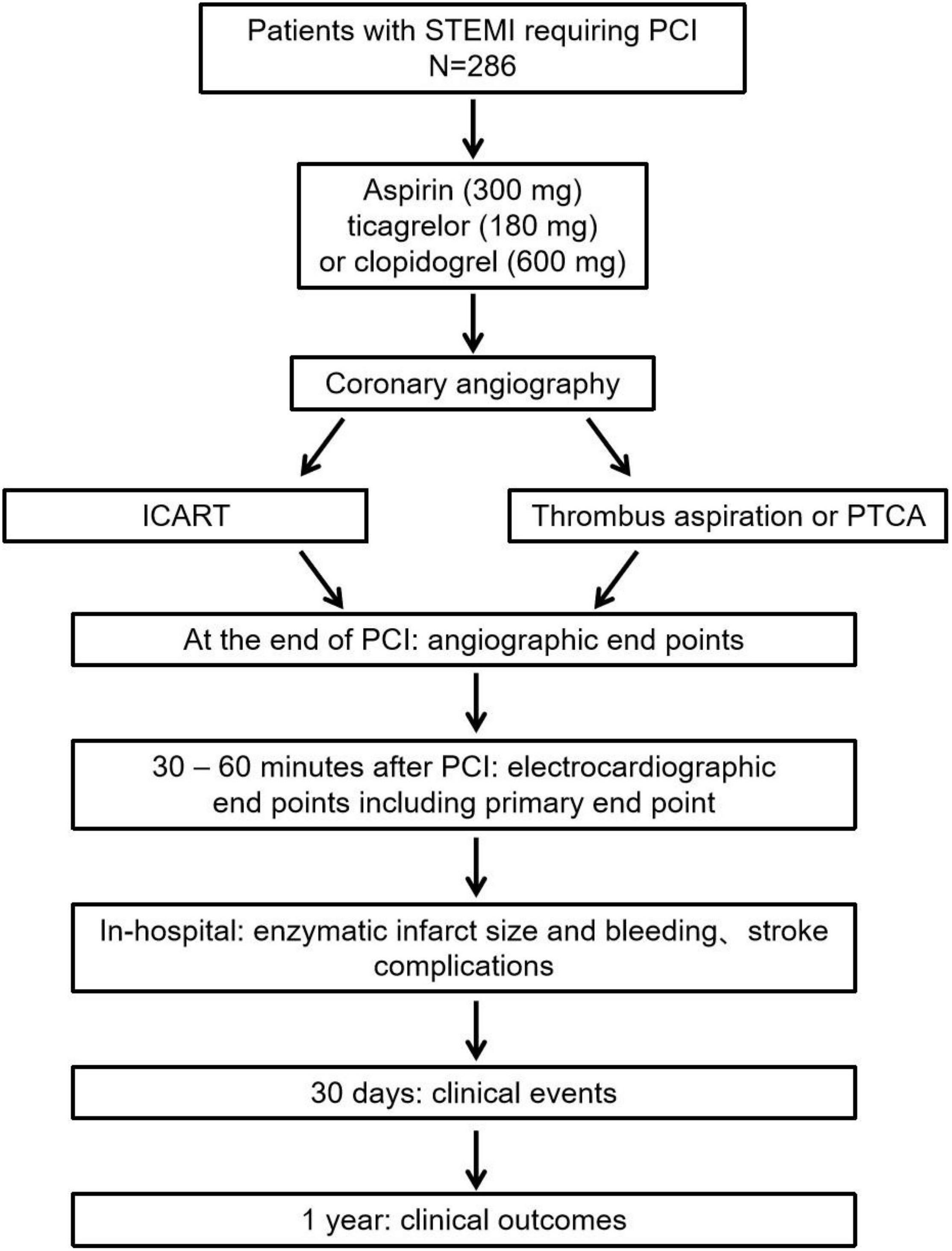
Figure 1. The ICART trial flow chart. ICART, intracoronary artery retrograde thrombolysis; STEMI, ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty.
All consecutive STEMI patients who are ready to receive primary PCI are suitable for inclusion. The inclusion criterion includes: (1) STEMI with chest pain lasting 30 min to 12 h; (2) older than 18 years old; (3) volunteered and signed informed consent. The exclusion criteria includes: (1) active peptic ulcer; (2) severe hepatic or renal insufficiency; (3) pregnancy; (4) uncontrolled hypertension; (5) rescue PCI after thrombolytic therapy; (6) unable to sign informed consent; (7) less than 18 years old; (8) left main coronary artery disease; (9) cardiogenic shock; (10) mechanical complications after myocardial infarction (e.g., interventricular septum perforation, papillary muscle rupture); (11) recent history of major surgery, trauma, hemorrhagic disease, cerebrovascular accident, or thrombocytopenia; (12) previous history of CABG; (13) other obvious abnormal signs, laboratory tests, and clinical diseases. According to the judgment of the clinician, the patients are not suitable for the study.
As we previously described (23), visualized thrombolytic agents are made by dissolving 100,000 units urokinase or 5 mg prourokinase with 15 ml physiological saline and 5 ml iopromide.
The flow diagram for the ICART system is shown in Figure 2. Coronary angiography identifies the occluded coronary artery. First, the guidewire is sent to the distal end of the culprit’s vessel. A microcatheter or a cut balloon is sent to the occluded section through the guidewire. One milliliter of thrombolytic cocktail is bolus-injected through the microcatheter, which is repeated every 30 s. The effect of thrombolysis is observed by X-ray.

Figure 2. Pattern diagram of intracoronary artery retrograde thrombolysis system. After the passing of the wire, a microcatheter or a cut balloon is sent to the occluded section through the guidewire. One milliliter of thrombolytic cocktail is bolus-injected through the microcatheter to the distal lumen of the vessel, which is repeated every 30 s.
Before PCI, ICART or thrombus aspiration is decided according to the grouping. Thrombus is aspirated by the Export aspiration catheter (Medtronic Inc., Santa Rosa, CA, United States) as previously described (24). Finally, a stent is implanted. In certain patients, pre- or postdilatation with a balloon may be necessary.
Patients are given a loading dose of aspirin (300 mg) and ticagrelor (180 mg) or clopidogrel (600 mg), and are diagnosed STEMI by electrocardiography. It’s up to the operator to decide whether to use heparin or bevaludine for anticoagulation. In the setting of STEMI, radial access is preferred. In patients with radial artery pathways, sheaths are pulled out immediately following the PCI procedure. The femoral approach is reserved for patients without the radial approach. Sheaths are exchanged immediately at the end of the PCI procedure by the Angio-Seal device (St. Jude Medical, Inc., St. Paul, MN, United States). After PCI, tirofiban is used for 36 h. Then low molecular weight heparin is given for 1–3 days after tirofiban. According to the international guidelines, standard post PCI medication includes aspirin (100 mg), ticagrelor (90 mg bid), or clopidogrel (75 mg), beta-blockers, lipid-lowering drugs, and angiotensin converting enzyme inhibitors or angiotensin II receptor blockers (8).
A standard 18-lead ECG is acquired at first medical contact. A standard 12 lead ECG is performed 30–60 min after PPCI. Times of symptom manifestation, arrival at the hospital, guidewire passing, thrombolysis, balloon dilatation, end of PCI, and ECG are recorded. The magnitude of ST-segment deviation is calculated 60 ms from the J-point. The ST segment of post-intervention ECG at 30–60 min is compared with that of the ECG at presentation. ST-segment elevation resolution is defined as complete (>70%), partial (30–70%), or absent (<30%) (Trials 2009, 10:90). Residual ST-segment deviation after PCI is counted as the sum of residual ST-segment depression and elevation in all leads (25). All ECG data are analyzed by a physician who is blinded to the clinical grouping and data.
The baseline, peri-, and post-procedural angiographic features will be recorded: the presence of thrombus, treatment of a non-culprit vessel during the same procedure, myocardial blush grade (MBG), thrombolysis in myocardial infarction (TIMI) flow grades, side branch occlusion, the presence of angiographically visible distal embolization, minimum lumen diameter, stent diameter, stent length, no-reflow, and slow flow. TIMI flow grades are evaluated according to the previously described method (26). The evaluation of thrombus adopts the previous evaluation standard (27). The thrombus is assessed according to TIMI thrombus classification: 0 = none, 1 = suspected thrombus, 2 = thrombus, linear size ≤ 1/2 vessel diameter, 3 = thrombus, 1/2 ≤ linear size < two times of vessel diameter, 4 = thrombus, linear size ≥ two times of vessel diameter, 5 = complete occlusion due to thrombosis. MBG’s evaluation is based on the previous description (28): 0 = no myocardial staining, 1 = slight myocardial staining, or contrast density, 2 = moderate myocardial staining or contrast density, but less than that of a contra- or ipsilateral non-infarct-related area, and 3 = normal myocardial staining or contrast density, comparable with that of a contra- or ipsilateral non-infarct-related area. Continuous myocardial staining indicates that the contrast agent leaks into the extravascular space, which is defined as grade 0. Distal embolization is defined as filling defects and/or abrupt cutoff of distal vessels in the target lesion (29). The coronary angiograms are analyzed by a physician who does not know the treatment allocation and clinical data.
The infarct size is estimated by serial monitoring of cardiac markers such as creatine kinase (CK), creatine kinase-MB (CK-MB), and troponin T. Blood samples are taken at baseline and 3, 6, 9, 12, 18, 24, and 48 h after PCI. Peak value, time to peak release as well as area under the curve are determined.
The primary endpoint is to observe the probability of ST-segment resolution > 70% acquired before and 30–60 min after PPCI.
Secondary endpoints include:
1. Angiographic endpoints: TIMI flow grade, distal embolization, no reflow, slow flow, side branch occlusion, myocardial blush grade post-PPCI.
2. Electrocardiographic end points: residual ST-segment deviation 30–60 min after primary PCI.
3. Enzymatic infarct size.
4. Mortality and Major Adverse Cardiac Events: All-cause death, cardiogenic death, cardiogenic shock, reinfarction, revascularization of target vessels, malignant arrhythmia, NYHA class IV heart failure, stent thrombosis, and myocardial infarction rehospitalization are to be registered at 30 days and 1 year.
Safety endpoints consist of in-hospital hemorrhagic and stroke complications.
Furthermore, the primary and secondary endpoints will be analyzed in a preset subgroup, which is defined as:
1. Age (<65 vs. >65 years)
2. Gender
3. Smoker
4. Diabetes mellitus
5. ST-segment resolution
6. Number of diseased vessels (multi-vessel vs. single vessel)
7. Proximal lesions
8. Infarct-related artery [left anterior descending artery (LAD) vs. non-LAD]
9. Bivalirudin
10. Ischemic time (<3 vs. >3 h)
11. Initial TIMI thrombus grade
12. Angiographic presence of thrombus
13. Intra-aortic artery balloon pump
14. Left ventricular ejection fraction
15. Type of P2Y12 inhibitor
16. Killip class
17. Symptom onset
18. Pre-procedural TIMI flow
19. Post-procedural TIMI flow
20. Post-PCI myocardial blush grade
21. Pacing use.
All-cause death, cardiogenic death, cardiogenic shock, reinfarction, revascularization of target vessels, stroke, malignant arrhythmia, NYHA class IV heart failure, bleeding complications, stent thrombosis, and myocardial infarction rehospitalization are to be registered at 30 days and 1 year. The follow-up information will be obtained from hospital records as well as by telephone follow-up with the patients and/or their relatives.
Data and instrumental measurements will be collected from all subjects using an electronic data capture system (EmpowerEDC, Shanghai, China). Data entry and management will be completed by an independent data administrator to guarantee data accuracy. Only the principal investigator could access the data. The final dataset can be acquired only by the principal investigator and the independent statistician. All procedures will comply with the confidentiality standards for medical data. All documents related to clinical trial implementation will be retained by the principal investigator. Important scheme modifications during this project will be communicated to the institutional review board, trial registry, investigators, trial participants, and the journal of publication. Management and analysis of data will be carried out by an independent expert statistician.
It has been reported that the incidence of ST-segment elevation resolution >70% has been reported to be 56.6% in STEMI patients treated with thrombus aspiration (24). We hypothesize that ICART administration during PCI increases the incidence of ST-segment elevation resolution >70–75%. To detect this difference between ICART and thrombus aspiration or PTCA groups, 286 patients are required to reach a 5% significance level (two-sided) with 90% power, which allows 5% of cases to fall off.
Statistical analysis will be carried out using the SPSS statistical software package (version 18.0; SPSS, Inc., Chicago, IL, United States), and the level of significant difference will be established at α = 0.05. The data analysis will be performed by an independent professional statistician who is blinded to allocation. Analysis of efficacy will include all participants who complete the whole study.
Descriptive statistics will be used to compare the baseline characters between ICART and thrombus aspiration or PTCA group. If the normality test is satisfied, the independent t-test will be used; otherwise, the Mann-Whitney U test will be carried out. Regarding the primary and secondary endpoint measures, multiple logistic regression will be used to compare the differences. The mean and standard deviation values of these parameters will be reported.
The ICART project is a single-center, prospective, randomized controlled trial to make clear whether ICART procedure is more effective than thrombus aspiration or PTCA in improving myocardial perfusion and reducing reperfusion injury in STEMI patients undergoing primary PCI. This is the first RCT trial to clarify the effect of ICART vs. thrombus aspiration or PTCA on myocardial perfusion in STEMI patients undergoing PPCI.
The trial is currently in the recruitment phase. The current protocol version number is V1.0 from 14 June 2019. The start recruitment date is January 2022. The expected end date is December 2023.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
This study has been approved by the Ethics Committee Board of the Chinese PLA General Hospital, Beijing, China on 25 March 2014. Written, informed consent to participate will be obtained from all participants.
MS designed this project in collaboration with JT, SZ, JW, and DL. MS also drafted this manuscript. DL, XZ, YG, WZ, YG, JW, JL, and GZ revised the manuscript critically. DL was also responsible for the study of some cases in another center. All authors read and approved the final manuscript.
This study was supported by the Hainan Science and Technology project (ZDYF2020123, LCYX202106, ZDYF2017096, ZDYF2020027, and ZDKJ2019012), Hainan Province Clinical Medical Center, National Key R&D plan (2020YFC2004706), and National Natural Science Foundation of China (fund numbers 81500202). Open subject of National Clinical Research Center of Geriatrics Disease NCRCG-PLAGH-2018014 (MS). All funders have no influence in the study design, data collection and analysis, or publishment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ndrepepa G, Tiroch K, Fusaro M, Keta D, Seyfarth M, Byrne RA, et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. (2010) 55:2383–9. doi: 10.1016/j.jacc.2009.12.054
2. Napodano M, Dariol G, Al Mamary AH, Marra MP, Tarantini G, D’Amico G, et al. Thrombus burden and myocardial damage during primary percutaneous coronary intervention. Am J Cardiol. (2014) 113:1449–56. doi: 10.1016/j.amjcard.2014.01.423
3. Mohammed MAH, Isa WYHW, Yusof Z. Percutaneous coronary intervention during index admission versus pharmaco-invasive strategy for patients with acute ST-elevation myocardial infarction. Int J Cardiol. (2019) 297:24
4. Jenning RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis Induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. (1960) 70:68–78.
6. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia / reperfusion. Compr Physiol. (2016) 7:113–70.
7. Widimsky P, Wijns W, Fajadet J, de Belder M, Knot J, Aaberge L, et al. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur Heart J. (2010) 31:943–57. doi: 10.1093/eurheartj/ehp492
8. O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr., Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myo-cardial infarction: executive summary: a report of the Ameri-can College of Cardiology Foundation/American Heart Asso-ciation Task Force on Practice Guidelines. J Am Coll Cardiol. (2013) 61:485–510.
9. Lincoff AM, Topol EJ. Illusion of reperfusion. Does anyone achieve optimal reperfusion during acute myocardial infarc-tion? Circulation. (1993) 88:1361–74. doi: 10.1161/01.cir.88.3.1361
10. Boersma E, Maas AC, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet. (1996) 348:771–5. doi: 10.1016/S0140-6736(96)02514-7
11. Fibrinolytic Therapy Trialists’ Collaborative Group. Indica-tions for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and ma-jor morbidity results from all randomized trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet. (1994) 343:311–22.
12. Tiefenbrunn AJ, Sobel BE. Timing of coronary recanalization:paradigms, paradoxes, and pertinence. Circulation. (1992) 85:2311–5. doi: 10.1161/01.cir.85.6.2311
13. Holmes DR Jr., Califf RM, Topol EJ. Lessons we have learned from the GUSTO trial. Global utilization of streptokinase and tissue plasminogen activator for occluded arteries. J Am Coll Cardiol. (1995) 25:10S–7S. doi: 10.1016/0735-1097(95)00188-a
14. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. (2003) 361:13–20. doi: 10.1016/S0140-6736(03)12113-7
15. Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. (2009) 54:281–92. doi: 10.1016/j.jacc.2009.03.054
16. Vlaar PJ, Svilaas T, van der Horst IC, Diercks GFH, Fokkema ML, de Smet BJGL, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial in-farction Study (TAPAS): a 1-year follow-up study. Lancet. (2008) 371:1915–20. doi: 10.1016/S0140-6736(08)60833-8
17. Frobert O, Lagerqvist B, Gudnason T, Thuesen L, Svensson R, Olivecrona GK, et al. Thrombus aspiration in ST-elevation myocardial infarction in Scandinavia (TASTE trial). A multicenter, prospective, randomized, controlled clinical registry trial based on the Swedish angiography and angioplasty registry (SCAAR) platform. Study design and rationale. Am Heart J. (2010) 160:1042–8. doi: 10.1016/j.ahj.2010.08.040
18. Jolly SS, Cairns JA, Lavi S, Cantor WJ, Bernat I, Cheema AN, et al. Thrombus aspiration in patients with high thrombus burden in the TOTAL trial. J Am Coll Cardiol. (2018) 72:1589–96. doi: 10.1016/j.jacc.2018.07.047
19. Eitel I, Wöhrle J, Suenkel H, Meissner J, Kerber S, Lauer B, et al. Intracoronary compared with intravenous bolus Abciximab application during primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: cardiac magnetic resonance substudy of the AIDA STEMI trial. J Am College Cardiol. (2013) 61:1447–54. doi: 10.1016/j.jacc.2013.01.048
20. Thiele H, Schindler K, Friedenberger J, Eitel I, Fürnau G, Grebe E, et al. Intracoronary compared with intravenous bolus abciximab application in pa-tients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: the randomized Leipzig immediate percutaneous coronary intervention ab-ciximab IV versus IC in ST elevation myocardial infarction trial. Circulation. (2008) 118:49–57. doi: 10.1161/CIRCULATIONAHA.107.747642
21. Fu Y, Gu X-S, Hao G-Z, Jiang YF, Fan WZ, Fan YM, et al. Comparison of myocardial microcirculatory perfusion after catheter-administered intracoronary thrombolysis with anisodamine versus standard thrombus aspiration in patients with ST-elevation myocardial infarction. Catheter Cardiovasc Interv. (2019) 93:839–45. doi: 10.1002/ccd.28112
22. Yan H, Naadiya C, Yiming W, Reid CG, Alexandra M, Heyu N, et al. Platelets in hemostasis and thrombosis: novel mechanisms of fibrinogen-independent platelet aggregation and fibronectin-mediated protein wave of hemostasis. J Biomed Res. (2015) 29:437–44. doi: 10.7555/JBR.29.20150121
23. Tian JW, Zhu M, Wang FQ, Li K, Zhou CF, Li B, et al. Intracoronary arterial retrograde thrombolysis with percutaneous coronary intervention: a novel use of thrombolytic to treat acute ST-segment elevation myocardial infarction. J Geriatric Cardiol. (2019) 16:458–67. doi: 10.11909/j.issn.1671-5411.2019.06.004
24. Svilaas T, Vlaar PJ, van der Horst IC, Diercks GFH, de Smet BJGL, van den Heuvel AFM, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. (2008) 358:557–67.
25. De Luca G, Maas AC, Suryapranata H, Ottervanger JP, Hoorntje JCA, Gosselink ATM, et al. Prognostic significance of residual cumulative ST-segment deviation after mechanical reperfusion in patients with ST-segment elevation myocardial infarction. Am Heart J. (2005) 150:1248–54. doi: 10.1016/j.ahj.2005.01.056
26. Timi Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med. (1985) 312:932–6.
27. Mabin TA, Holmes DR Jr., Smith HC. Intracoronary thrombus: role in coronary occlusion complicating percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. (1985) 5(Pt 1):198–202. doi: 10.1016/s0735-1097(85)80037-1
28. van ’t Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F, et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. (1998) 97:2302–6. doi: 10.1161/01.cir.97.23.2302
Keywords: ST-segment elevation myocardial infarction, intracoronary artery retrograde thrombolysis, thrombus aspiration, reperfusion preconditioning, percutaneous coronary intervention (PCI)
Citation: Shen M, Wang J, Li D, Zhou X, Guo Y, Zhang W, Guo Y, Wang J, Liu J, Zhao G, Zhao S and Tian J (2022) IntraCoronary Artery Retrograde Thrombolysis vs. Thrombus Aspiration in ST-Segment Elevation Myocardial Infarction: Study Protocol for a Randomized Controlled Trial. Front. Cardiovasc. Med. 9:928695. doi: 10.3389/fcvm.2022.928695
Received: 26 April 2022; Accepted: 18 May 2022;
Published: 15 September 2022.
Edited by:
Yuli Huang, Southern Medical University, ChinaReviewed by:
Li Na, Shanxi Medical University, ChinaCopyright © 2022 Shen, Wang, Li, Zhou, Guo, Zhang, Guo, Wang, Liu, Zhao, Zhao and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinwen Tian, dGp3c3FyLjIwMDBAMTYzLmNvbQ==; Shihao Zhao, NzY1NTk1NzIwQHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.