- 1Department of Gastroenterology, The Third People’s Hospital of Chengdu, The Affiliated Hospital of Southwest Jiaotong University, Chengdu, Sichuan, China
- 2Medical Research Center, The Third People’s Hospital of Chengdu, The Affiliated Hospital of Southwest Jiaotong University, Chengdu, Sichuan, China
- 3Luodian Clinical Drug Research Center, Shanghai Baoshan Luodian Hospital, Shanghai University, Shanghai, China
Background: Although epidemiological studies have shown a positive relationship between inflammatory bowel disease (IBD) and risk of cardiovascular disease (CVD) outcomes, a solid causal relationship has not been established. Thus, a two-sample Mendelian randomization (MR) study was conducted to explore the potential causal effect between IBD and CVD outcomes.
Methods: We performed a two-sample MR analysis to analyze the causal effect of the IBD on CVD outcome by using summary-level genome-wide association studies of European descent. The inverse-variance weighted (IVW) method was used as the main MR analysis, with complementary analyses of MR Egger, maximum likelihood, weighted median, penalized weighted media, simple mode, weighted mode, and MR-PRESSO methods. Multiple sensitivity analyses were used to evaluate the robustness of our results.
Results: All P-values were greater than 0.05 in the IVW method, showing no evidence of a causal association between circulating IBD and CVD. Similar results were observed by using other MR methods. No evidence of heterogeneity, pleiotropy, or outlier single-nucleotide polymorphisms was detected. Sensitivity analyses demonstrated the robustness of the results.
Conclusion: The findings of this study provided no evidence to support that IBD has a large effect on risk of CVD outcomes, which is in contrast to many previous observational reports. Further studies are needed to determine the potential mechanism of association identified in observational studies.
Introduction
Cardiovascular disease (CVD) is a general term for all cardiovascular and brain diseases related to vasculopathy, mainly heart failure, coronary heart disease, arrhythmia, stroke and cerebral infarction (1, 2). Globally, CVDs are the leading cause of death, morbidity and disability worldwide, causing the increase of social and economic burden. Owing to the severe clinical and social consequences of CVD, there is an urgent need for concerted efforts to identify risk factors leading to CVD for early prevention and intervention (3). The occurrence and progression of CVD may be driven by genetic or environmental factors. Besides, some epidemiological studies have shown a strong association between inflammatory disease and CVD outcomes (4).
Inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease, is an immune-mediated chronic inflammatory disease of the gastrointestinal tract, characterized by abdominal pain and diarrhea (5). IBD is often accompanied by systemic inflammation and other extraintestinal manifestations. More than 6.8 million patients are suffering from IBD worldwide (6).
Several studies have proved that IBD patients have higher rates of CVD outcomes. The high levels of C-reactive protein, circulating inflammatory cytokines, immunoglobulins G and M, antineutrophil cytoplasmic antibody, vascular endothelial growth factor, and interleukin-1 in patients with IBD can lead to elevated level of oxidative stress, endothelial dysfunction, prothrombotic state, microvascular, and macrovascular dysfunction, which could further promote the formation of atherosclerosis, hence, resulting in an increased risk of CVD outcomes (7–9). A cohort study of 28,833 IBD patients from Denmark with a 13-year follow-up showed that IBD is associated with the development of coronary atherosclerotic heart disease (10). Findings from previous systematic reviews and meta-analyses also indicated that patients with IBD may possess a higher risk of CVD outcomes (10–12). However, Sridhar et al. found no increased risk of myocardial ischemia, cardiomyopathy, conduction disorders, and embolic strokes in patients with IBD after adjusting for confounding factors such as hypertension, diabetes, and hyperlipidemia (13). Studies evaluating the association between IBD and risk of CVD outcomes have reported inconsistent results, most likely due to sample size limitations. Furthermore, observational epidemiological studies are susceptible to confounding and reverse causation (14). The causal relationship between IBD and CVD outcomes requires further investigations. Although randomized controlled studies (RCTs) can prevent selection bias and blinded analysis is able to improve the reliability of the results, RCTs are difficult or impractical to perform for the sake of high economic cost, intensive labor, a great deal of resource and time, as well as ethical limitations (15).
Mendelian randomization (MR) is a method of studying genetic epidemiology that utilizes one or multiple genetic variants, such as single nucleotide polymorphisms (SNPs), to assess causal effects between exposures and outcomes (16). Compared with traditional observational epidemiological studies, MR follows the principle of allele separation and free recombination of non-alleles and is not affected by confounding factors (17). Because germline genetic variants are fixed at conception, MR studies are unaffected by the disease process and can avoid confounding and reverse causality, further mitigating the influence of confounding factors (18). Besides, the high measurement precision of genetic variation can prevent regression dilution bias caused by measurement error (19). In this study, a two-sample MR study was performed to explore the genetic association between IBD and CVD outcomes using large-scale publicly available genome-wide association study (GWAS) data.
Materials and methods
Data resources
Inflammatory bowel disease GWAS summary statistics were obtained from the International Inflammatory Bowel Disease Genetics Consortium (IIBDGC),1 which included a total sample size of 65,642 participants of predominantly European ancestry (cases/controls for ulcerative colitis: 13,768/33,977 and Crohn’s disease: 17,897/33,977) (20). The large-scale GWAS summary datasets of about 14 CVD outcomes phenotypes were obtained from United Kingdom Biobank, the FinnGen consortium, and large genetic consortia (21–24). Shah et al. (21) conducted a GWAS comprising 47,309 cases and 930,014 controls of European ancestry across 26 studies from the Heart Failure Molecular Epidemiology for Therapeutic Targets Consortium. Malik et al. (24) performed a genome-wide association meta-analysis in 440,328 European individuals (34,217 ischemic stroke patients and 406,111 controls), and discovered 22 new stroke risk loci. These datasets were used for MR analysis. The full data set can be downloaded from https://gwas.mrcieu.ac.uk/ and https://www.finngen.fi/en. The detailed information is provided in Figure 1. MR studies were conducted by using summary statistics from GWAS, which were ethically approved and publicly available on the website. The data are free to download and can be used without restrictions.
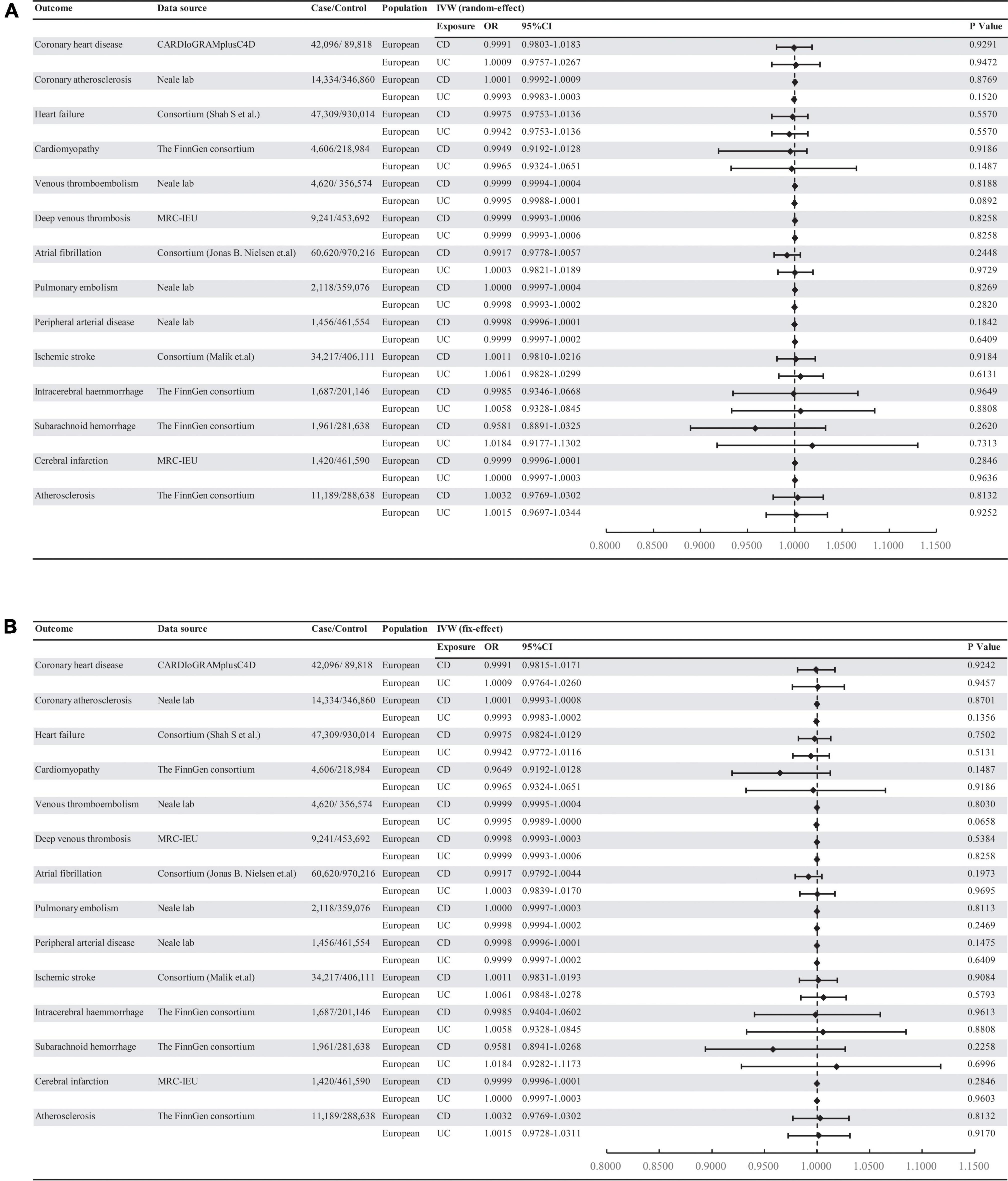
Figure 1. Associations of genetically predicted IBD with risk of 14 CVD outcomes. (A) Random-effect. (B) Fix-effect. UC, ulcerative colitis; CD, Crohn’s disease; MRC-IEU: MRC Integrative Epidemiology Unit.
We selected SNPs that were strongly associated with IBD of genome-wide significance (P < 5 × 10–8) and pruned SNPs in linkage disequilibrium (R2 > 0.001 within a 10,000 kb window) with the clump data function in the TwoSampleMR2 software package in R (25) (Supplementary material 1). In addition, we used the PhenoScanner3 tool to exclude any of the selected SNPs associated with other phenotypes at risk of affecting CVD outcomes. These rigorously selected SNPs were used as the final SNPs for subsequent MR analysis (Figure 2) (26–28). The strength of the genetic instruments was indicated by the F-statistic.
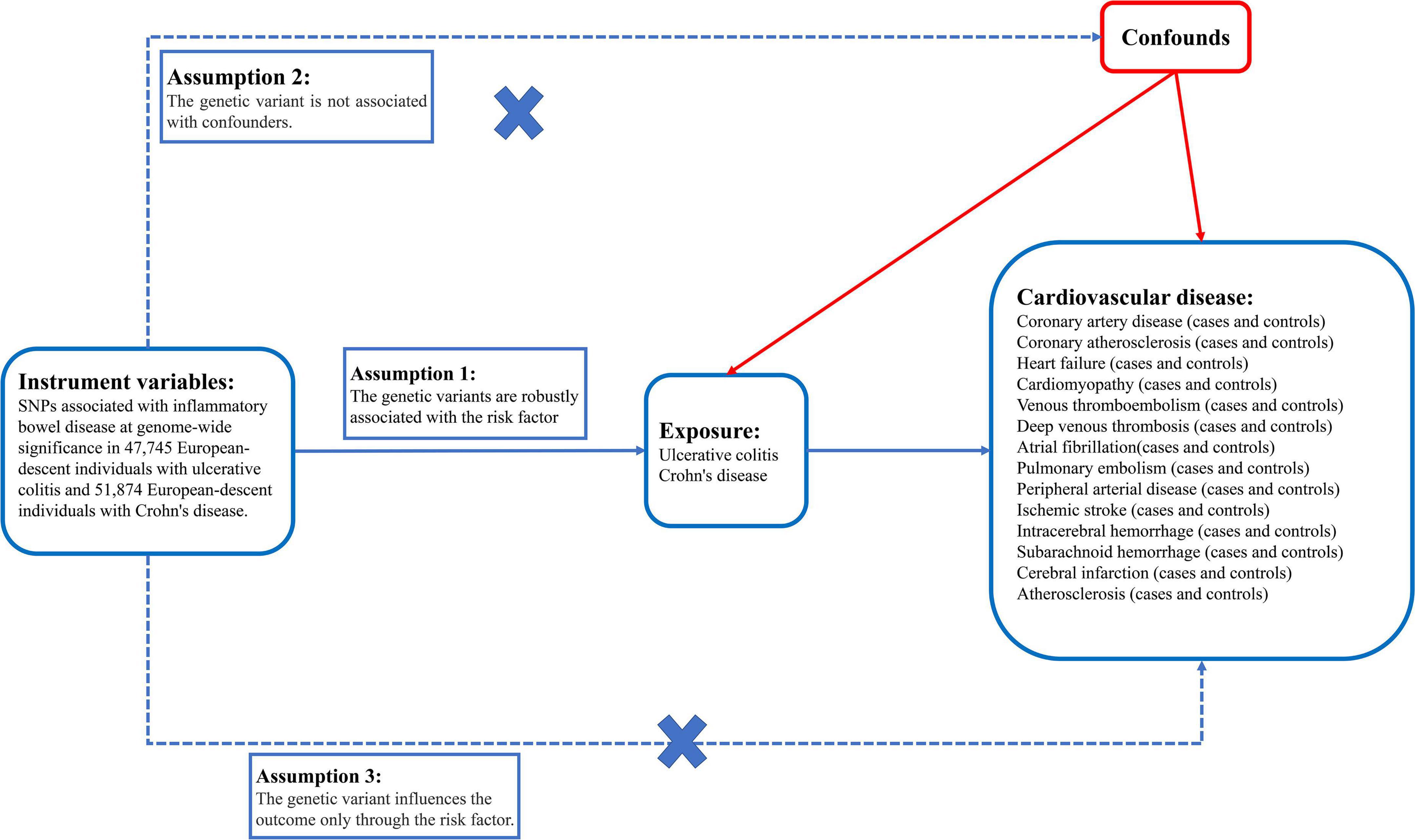
Figure 2. There are three core instrumental variable assumptions for Mendelian randomization analyses.
Mendelian randomization
We used two-sample MR, a powerful statistical method, to infer the causality between the two phenotypes. To obtain MR estimate, an inverse-variance weighted (IVW) meta-analysis of each Wald ratio was performed, which is considered the most reliable when there is no evidence of directional pleiotropy (29). The MR-PRESSO method was used to detect outlier variables in IVW analysis by comparing the actual distance of the genetic variants to the expected distance from the regression, assuming the absence of horizontal pleiotropy, and evaluating the causal estimates after removing outliers (30). We also used alternative analyses, including IVW (fixed effects), MR Egger, maximum likelihood, weighted median, penalized weighted media, simple mode, and weighted mode method. Associations were considered statistically significant at a Bonferroni corrected P-value below 0.0018 (correcting for 2 exposures and 14 outcomes).
Pleiotropy and sensitivity analysis
Inverse-variance weighted and MR-Egger regression were used to detect heterogeneity. Cochran’s Q test was used to quantify heterogeneities, and P < 0.05 was considered statistically significant. Horizontal pleiotropy tests were performed by judging the intercept term in MR-Egger regression. If the intercept term was close to 0 (<0.1) and P > 0.05, it indicated that there was no evidence of horizontal pleiotropy in the analysis, showing that the results of MR analyses were reliable. Asymmetry in a funnel plot is also useful for gauging the reliability of a particular MR analyses. Furthermore, sensitivity analysis was performed for qualified CVDs using the leave-one-out method, where the MR was performed again but leaving out each SNP in turn, to determine whether a single SNP was driving the association.
Mendelian randomization analysis were performed using the “TwoSampleMR” and “MR-PRESSO” R package version 0.4.13.4 The power of MR was calculated by mRnD.5
Results
Single nucleotide polymorphisms selected in Mendelian randomization
We obtained 88 SNPs in ulcerative colitis and 122 SNPs in Crohn’s disease, which met the generally accepted genome-wide significance threshold (P < 5 × 10–8, r2 < 0.001, kb = 10,000) for exposure (Supplementary material 1). To remove confounding factors, 7 SNPs (rs11229555, rs13430791, rs1801274, rs2516440, rs3024493, rs6062496, and rs9271255) in ulcerative colitis and 11 SNPs (rs17622378, rs2641348, rs3024505, rs3129871, rs3184504, rs516246, rs6062496, rs61839660, rs6679677, rs77981966, and rs780094) in Crohn’s disease were eliminated, which were strongly associated with diabetes, total cholesterol, and smoking. Moreover, estimates of the F-statistic indicated no weak instrument bias (all F-statistic > 10).
Association between inflammatory bowel disease and cardiovascular disease risks
Mendelian randomization analysis showed that there was no significant association of IBD with CVD outcomes (all P > 0.05) (Figure 1). The result was consistent with IVW (fixed effects), maximum likelihood, weighted medium, penalized weighted media, and MR-PRESSO (Supplementary material 2). The effect size of each SNP on CVDs is shown in Supplementary materials 3, 4.
Sensitivity analyses
For sensitivity analyses, Cochran’s Q test indicated no evidence of heterogeneity (Supplementary material 5). The horizontal pleiotropy between SNPs and outcome was assessed by MR-Egger regression, and the results showed that there was no evidence of horizontal pleiotropy (Supplementary material 5). The funnel plot displayed symmetric pattern of effect size variation around the point estimate, again indicating no apparent horizontal pleiotropy (Supplementary materials 3, 4). The results of leave-one-out sensitivity analyses demonstrated that there was no potentially influential SNP driving the causal link and the stability of our conclusion (Supplementary materials 3, 4).
Discussion
In this study, a two-sample MR analysis was performed to evaluate the causal effect of IBD on CVD outcomes for the first time. MR analysis did not indicate detrimental effects of genetic increase in IBD on the risk of CVDs. These results were stable in sensitivity analysis by using different Mendelian tools and statistical models, indicating that our analysis is reliable.
Over the past several years, a growing number of observational studies have indicated that IBD is related to an increased risk of CVD outcomes, especially in the active period of IBD, which has been described in studies on large populations (31–33). Meta-analysis showed that there was a positive correlation between IBD and higher incidence of ischemic heart disease and cerebrovascular accidents (11, 34). However, the mechanism of this increased risk remains unclear. Recent fundamental research has suggested that changes in the innate and adaptive immune systems induce an increase in proinflammatory factors and promote the formation of thrombosis and atherosclerosis (35). In addition, changes in gut microbiota composition in patients with IBD may contribute to intestinal epithelial cell apoptosis and barrier dysfunction (36). Both lipopolysaccharide translocation and initiation of an inflammatory cascade result in dysfunction of microvascular and macrovascular endothelial function (37).
Inflammatory bowel disease is less likely to increase the risk of CVD (38). A study that included 31,175 IBD patients (16,779 ulcerative colitis, 10,721 Crohn’s disease, and 3,675 unclassifiable cases) and 154,412 matched controls showed no significant excess of vascular events for IBD patients overall. After adjusting for confounders such as smoking, the risk of stroke, and coronary heart disease was reduced in patients with IBD (39). A meta-analysis also showed that no increased risk of myocardial infarction among patients with ulcerative colitis or Crohn’s disease (39, 40). These results are somewhat inconsistent with previously published observational studies, with a number of possible explanations for this inconsistency. Firstly, the discrepancy between findings from different study designs may be explained by the susceptibility to confound such as socioeconomic, dietary, and lifestyle behavioral factors in traditional observational studies. Hartwig et al. (41) indicated that the risk of coronary artery disease in IBD patients was not increased compared with normal people after excluding traditional risk factors. Thus, we speculated that IBD patients have increased levels of traditional cardiovascular risk factors, resulting in an increased incidence of CVD in observational studies. Recent MR studies showed a lack of genetic causality between IBD and atrial fibrillation (42), which was consistent with our results. Although pleiotropy may cause the bias of the results in MR analysis, there is little possibility in our study (43). Because our MR analysis had more than 80% power to detect the effect of CVDs development (Supplementary material 5). Nonetheless, a very small effect of IBD on CVD outcomes could not be excluded because of tight confidence intervals.
Strengths and limitations
The main strength of this study is that we used a two-sample MR analysis to assess independent effects of the IBD on multiple CVD outcomes, offering the possibility to overcome several limitations in conventional epidemiological studies. As alleles are randomly assorted and fixed at conception, bias caused by reverse causation can be largely avoided (44). An additional strong point is the large scale of the samples size that enabled us to conduct well-powered MR analyses. Finally, our results were unlikely to be impacted by population stratification bias for the sake of GWAS, which comprised individuals who were primarily of European ancestry (45). In addition, we used the STROBE-MR checklist of recommended items to address in reports of MR studies to check our study (Supplementary material 6).
Inevitably, this study had certain limitations. Although we used the GWAS dataset, due to the lack of original data, we could not fully analyze the staging of IBD (such as IBD with periods of disease activation and remission). Therefore, additional MR analysis are still required to estimate the causal relationship between different stages of IBD and CVD outcomes. Though our results suggested that IBD does not increase the risk of CVD outcomes, we could not completely rule out the possibility. A definitive causal relationship requires more in-depth mechanism studies and RCTs in the future.
Conclusion
In this study, we assessed the causal effect of IBD (ulcerative colitis and Crohn’s disease) on the increased risk of CVD outcomes by using a two-sample MR analysis. Our results suggested that genetically predicted IBD has no clear causal effect on CVD outcomes. Therefore, further updated MR analysis should be conducted to confirm our results when more advanced methods and more IBD patients are available to obtain less bias estimation and more accurate accuracy.
Data availability statement
Publicly available datasets were analyzed in this study. The data of ulcerative colitis (ID: ieu-a-970), Crohn’s disease (ID: ieu-a-12), coronary artery disease (ID: ieu-a-7), coronary atherosclerosis (ID: ukb-d-I9_CORATHER), heart failure (ID: ebi-a-GCST009541), venous thromboembolism (ID: ukb-d-I9_VTE), deep venous thrombosis (ID: ukb-b-12040), atrial fibrillation (ID: ebi-a-GCST006414), pulmonary embolism (ID: ukb-d-I26), peripheral arterial disease (ID: ukb-b-4929), ischemic stroke (ID: ebi-a-GCST006908), intracerebral hemorrhage (ID: finn-b-I9_ICH), and cerebral infarction (ID: ukb-b-19350) can be obtained from https://gwas.mrcieu.ac.uk/. The data of cardiomyopathy (ID: Finngen-R7-I9-CARDMYO), subarachnoid hemorrhage (ID: Finngen-R7-I9- SAH), and atherosclerosis (ID: Finngen-R7-I9-ATHSCLE) can be obtained from https://www.finngen.fi/en. The R code to perform the MR analysis is detailed in Supplementary material 7.
Ethics statement
Ethical review and approval was not required for this study in accordance with the local legislation and institutional requirements.
Author contributions
XS designed the study. DX and KW wrote the manuscript. KW and AL contributed to the data analysis and data interpretation. LL and TS contributed to the revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Foundation of Science and Technology, Department of Sichuan Province (2020YJ0485).
Acknowledgments
We thank all of the investigators for sharing summary-level data on GWAS for IBD and CVDs.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.927120/full#supplementary-material
Supplementary material 1 | Instrumental SNPs from ulcerative colitis and Crohn’s disease GWASs.
Supplementary material 2 | Mendelian randomization estimates from each method of the causal effect of inflammatory bowel disease on CVDs.
Supplementary material 3 | Mendelian randomization effect size, leave-one-out analyses, and funnel plot for ulcerative colitis on CVDs.
Supplementary material 4 | Mendelian randomization effect size, leave-one-out analyses, and funnel plot for Crohn’s disease on CVDs.
Supplementary material 5 | Heterogeneity, level pleiotropy test, and the power of Mendelian randomization analyses.
Supplementary material 6 | STROBE-MR checklist of recommended items to address in reports of MR studies.
Supplementary material 7 | The R code was used to perform Mendelian randomization.
Abbreviations
IBD, inflammatory bowel disease; CVD, cardiovascular disease; MR, Mendelian randomization; SNP, single nucleotide polymorphism; OR, odds ratio; CI, confidence interval; HERMES, Heart Failure Molecular Epidemiology for Therapeutic Targets Consortium; MRC-IEU, Medical Research Council-Integrative Epidemiology Unit.
Footnotes
- ^ https://www.ibdgenetics.org/
- ^ https://mrcieu.github.io/TwoSampleMR/reference/clump_data.html
- ^ http://www.phenoscanner.medschl.cam.ac.uk/
- ^ http://github.com/MRCIEU/TwoSampleMR
- ^ https://shiny.cnsgenomics.com/mRnd/
References
1. Roth GA, Mensah GA, Fuster V. The global burden of cardiovascular diseases and risks: A compass for global action. J Am Coll Cardiol. (2020) 76:2980–1. doi: 10.1016/j.jacc.2020.11.021
2. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2019) 140:e596–646. doi: 10.1161/cir.0000000000000678
3. Roger VL, Sidney S, Fairchild AL, Howard VJ, Labarthe DR, Shay CM, et al. Recommendations for cardiovascular health and disease surveillance for 2030 and beyond: A policy statement from the American heart association. Circulation. (2020) 141:e104–19. doi: 10.1161/cir.0000000000000756
4. Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet. (2020) 395:795–808. doi: 10.1016/s0140-6736(19)32008-2
5. Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2021) 18:56–66. doi: 10.1038/s41575-020-00360-x
6. Gbd 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. (2020) 5:17–30. doi: 10.1016/s2468-1253(19)30333-4
7. Chen L, Sun X, Wang Z, Lu Y, Chen M, He Y, et al. The impact of plasma vitamin C levels on the risk of cardiovascular diseases and Alzheimer’s disease: A Mendelian randomization study. Clin Nutr. (2021) 40:5327–34. doi: 10.1016/j.clnu.2021.08.020
8. Bigeh A, Sanchez A, Maestas C, Gulati M. Inflammatory bowel disease and the risk for cardiovascular disease: Does all inflammation lead to heart disease? Trends Cardiovasc Med. (2020) 30:463–9. doi: 10.1016/j.tcm.2019.10.001
9. Łykowska-Szuber L, Rychter AM, Dudek M, Ratajczak AE, Szymczak-Tomczak A, Zawada A, et al. What links an increased cardiovascular risk and inflammatory bowel disease? A narrative review. Nutrients (2021) 13:2661. doi: 10.3390/nu13082661
10. Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: A nationwide Danish cohort study. Gut. (2013) 62:689–94. doi: 10.1136/gutjnl-2012-303285
11. Sun HH, Tian F. Inflammatory bowel disease and cardiovascular disease incidence and mortality: A meta-analysis. European J Prev Cardiol. (2018) 25:1623–31. doi: 10.1177/2047487318792952
12. Filimon AM, Negreanu L, Doca M, Ciobanu A, Preda CM, Vinereanu D. Cardiovascular involvement in inflammatory bowel disease: Dangerous liaisons. World J Gastroenterol. (2015) 21:9688–92. doi: 10.3748/wjg.v21.i33.9688
13. Sridhar AR, Parasa S, Navaneethan U, Crowell MD, Olden K. Comprehensive study of cardiovascular morbidity in hospitalized inflammatory bowel disease patients. J Crohns Colitis. (2011) 5:287–94. doi: 10.1016/j.crohns.2011.01.011
14. Venkatesh SS, Ferreira T, Benonisdottir S, Rahmioglu N, Becker CM, Granne I, et al. Obesity and risk of female reproductive conditions: A Mendelian randomisation study. PLoS Med. (2022) 19:e1003679. doi: 10.1371/journal.pmed.1003679
15. Wu F, Huang Y, Hu J, Shao Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med. (2020) 18:312. doi: 10.1186/s12916-020-01778-5
16. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
17. Zong Y, Li X. Identification of causal genes of COVID-19 using the SMR method. Front Genet. (2021) 12:690349. doi: 10.3389/fgene.2021.690349
18. Yarmolinsky J, Relton CL, Lophatananon A, Muir K, Menon U, Gentry-Maharaj A, et al. Appraising the role of previously reported risk factors in epithelial ovarian cancer risk: A Mendelian randomization analysis. PLoS Med. (2019) 16:e1002893. doi: 10.1371/journal.pmed.1002893
19. Larsson SC, Michaëlsson K, Burgess S. Mendelian randomization in the bone field. Bone. (2019) 126:51–8. doi: 10.1016/j.bone.2018.10.011
20. Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. (2015) 47:979–86. doi: 10.1038/ng.3359
21. Shah S, Henry A, Roselli C, Lin H, Sveinbjörnsson G, Fatemifar G, et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. (2020) 11:163. doi: 10.1038/s41467-019-13690-5
22. Schunkert H, König IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. (2011) 43:333–8. doi: 10.1038/ng.784
23. Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. (2018) 50:1234–9. doi: 10.1038/s41588-018-0171-3
24. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. (2018) 50:524–37. doi: 10.1038/s41588-018-0058-3
25. Howe LJ, Battram T, Morris TT, Hartwig FP, Hemani G, Davies NM, et al. Assortative mating and within-spouse pair comparisons. PLoS Genet. (2021) 17:e1009883. doi: 10.1371/journal.pgen.1009883
26. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: A report from the American heart association. Circulation. (2022) 145:e153–639. doi: 10.1161/cir.0000000000001052
27. Cui Z, Tian Y. Using genetic variants to evaluate the causal effect of serum vitamin D concentration on COVID-19 susceptibility, severity and hospitalization traits: A Mendelian randomization study. J Transl Med. (2021) 19:300. doi: 10.1186/s12967-021-02973-5
28. He Q, Yang Z, Sun Y, Qu Z, Jia X, Li J, et al. The impact of homocysteine on the risk of hormone-related cancers: A Mendelian randomization study. Front Nutr. (2021) 8:645371. doi: 10.3389/fnut.2021.645371
29. Holmes MV, Ala-Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: Challenges in evaluating causality. Nat Rev Cardiol. (2017) 14:577–90. doi: 10.1038/nrcardio.2017.78
30. Choi KW, Chen CY, Stein MB, Klimentidis YC, Wang MJ, Koenen KC, et al. Assessment of bidirectional relationships between physical activity and depression among adults: A 2-sample Mendelian randomization study. JAMA Psychiatry. (2019) 76:399–408. doi: 10.1001/jamapsychiatry.2018.4175
31. Shen X, Wan Q, Zhao R, Wu Y, Wang Y, Cui Y, et al. Inflammatory bowel diseases and the risk of adverse health outcomes: Umbrella review of meta-analyses of observational studies. Dig Liver Dis. (2021) 53:809–16. doi: 10.1016/j.dld.2021.01.018
32. Nevulis MG, Baker C, Lebovics E, Frishman WH. Overview of link between inflammatory bowel disease and cardiovascular disease. Cardiol Rev. (2018) 26:287–93. doi: 10.1097/crd.0000000000000214
33. Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis. (2016) 10:239–54. doi: 10.1093/ecco-jcc/jjv213
34. Feng W, Chen G, Cai D, Zhao S, Cheng J, Shen H. Inflammatory bowel disease and risk of ischemic heart disease: An updated meta-analysis of cohort studies. J Am Heart Assoc. (2017) 6:e005892. doi: 10.1161/jaha.117.005892
35. Kamperidis N, Kamperidis V, Zegkos T, Kostourou I, Nikolaidou O, Arebi N, et al. Atherosclerosis and inflammatory bowel disease-shared pathogenesis and implications for treatment. Angiology. (2021) 72:303–14. doi: 10.1177/0003319720974552
36. Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. (2017) 120:1183–96. doi: 10.1161/circresaha.117.309715
37. McDonnell M, Liang Y, Noronha A, Coukos J, Kasper DL, Farraye FA, et al. Systemic toll-like receptor ligands modify B-cell responses in human inflammatory bowel disease. Inflamm Bowel Dis. (2011) 17:298–307. doi: 10.1002/ibd.21424
38. Gill GS, Fernandez SJ, Malhotra N, Mete M, Garcia-Garcia HM. Major acute cardiovascular events in patients with inflammatory bowel disease. Coron Artery Dis. (2021) 32:73–7. doi: 10.1097/mca.0000000000000899
39. Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: A population-based cohort study. Circulation. (2014) 130:837–44. doi: 10.1161/circulationaha.114.009990
40. Rungoe C, Nyboe Andersen N, Jess T. Inflammatory bowel disease and risk of coronary heart disease. Trends Cardiovasc Med. (2015) 25:699–704. doi: 10.1016/j.tcm.2015.03.010
41. Hartwig FP, Borges MC, Horta BL, Bowden J, Davey Smith G. Inflammatory biomarkers and risk of schizophrenia: A 2-sample Mendelian randomization study. JAMA Psychiatry. (2017) 74:1226–33. doi: 10.1001/jamapsychiatry.2017.3191
42. Chen L, Fu G, Jiang C. Mendelian randomization as an approach to assess causal effects of inflammatory bowel disease on atrial fibrillation. Aging. (2021) 13:12016–30. doi: 10.18632/aging.202906
43. Huang G, Cai J, Li W, Zhong Y, Liao W, Wu P. Causal relationship between educational attainment and the risk of rheumatoid arthritis: A Mendelian randomization study. BMC Rheumatol. (2021) 5:47. doi: 10.1186/s41927-021-00216-0
44. Fu Y, Xu F, Jiang L, Miao Z, Liang X, Yang J, et al. Circulating vitamin C concentration and risk of cancers: A Mendelian randomization study. BMC Med. (2021) 19:171. doi: 10.1186/s12916-021-02041-1
Keywords: cardiovascular diseases, inflammatory bowel disease, Mendelian randomization, genome-wide association study, causation
Citation: Wu K, Li A, Liu L, Shu T, Xia D and Sun X (2022) Inflammatory bowel disease and cardiovascular disease: A two-sample Mendelian randomization analysis. Front. Cardiovasc. Med. 9:927120. doi: 10.3389/fcvm.2022.927120
Received: 23 April 2022; Accepted: 16 August 2022;
Published: 02 September 2022.
Edited by:
Sebhat Erqou, Brown University, United StatesReviewed by:
Long Zhou, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, ChinaChunyu Li, Sichuan University, China
Copyright © 2022 Wu, Li, Liu, Shu, Xia and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Demeng Xia, ZGVtZW5neGlhQDE2My5jb20=; Xiaobin Sun, eGJzdW4xMTk3QDE2My5jb20=
†These authors have contributed equally to this work
 Kaiwen Wu
Kaiwen Wu Aoshuang Li1†
Aoshuang Li1† Lei Liu
Lei Liu Demeng Xia
Demeng Xia Xiaobin Sun
Xiaobin Sun