- Department of Cardiology, West China Hospital of Sichuan University, Chengdu, China
The IL-33/ST2 L signaling pathway is involved in the pathophysiological processes of several diseases and mainly exerts anti-inflammatory and antifibrotic effects. Soluble suppression of tumorigenicity 2 (sST2), which serves as a competitive inhibitory molecule of this pathway, is a member of the interleukin (IL)-1 family, a decoy receptor for IL33, thought to play a role in cardiac remodeling and the inflammatory process. However, the association between sST2 and coronary artery disease (CAD), one of the most common causes of heart failure, is still being explored. We therefore reviewed the research on sST2 in the field of CAD, including reflecting the atherosclerosis burden, predicting no-reflow, predicting prognosis, responding to myocardial remodeling, and guiding management, hoping to provide cardiologists with new perspectives.
Introduction
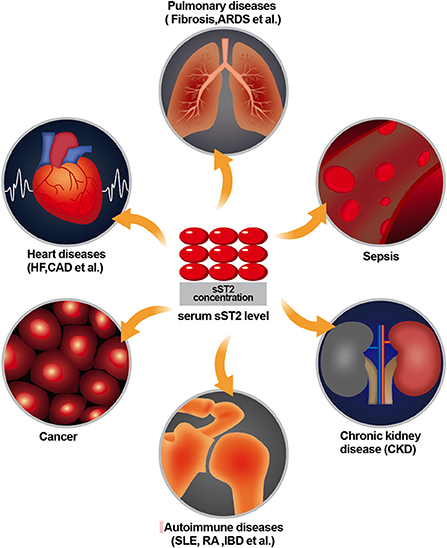
Suppression of tumorigenicity 2 (ST2) is a member of the interleukin 1 (IL-1) receptor family and is formally known as interleukin 1 receptor-like 1 (IL1RL-1). It was first described in 1989 but remained an orphan receptor mainly related to immune and inflammatory diseases for years (1). In 2005, ST2 was reported to be expressed in cardiac cells in response to myocardial stress, and interleukin 33 (IL-33) was reported to be the ligand of ST2 (2). Since then, its role in cardiovascular diseases has been of great concern. The ST2 gene is located on human chromosome 2q12 and encodes two main protein isoforms: transmembrane receptor (ST2 L) and truncated soluble receptor (sST2). The interaction between IL-33 and ST2 L mediates anti-inflammatory and antifibrotic effects (3). For instance, the activation of mitogen-activated protein kinase (MAPK) and nuclear factor (NF-kB) signaling originates from the binding of IL-33 to ST2 L, which produces various downstream effects in target cells in the presence of additional interleukin-1 receptor accessory protein (IL-1RacP) receptor protein molecules. In contrast, when IL-33 binds to sST2, it prevents and blocks these effects (4). While sST2 can be secreted into the circulation and functions as a decoy receptor for IL-33, it is unavailable to ST2 L, which abolishes the cardioprotective effects of IL-33/ST2. Meanwhile, ST2 is established as a selective marker of T helper type 2 (Th2) lymphocytes, which are also expressed on mast cells, epithelial cells, endothelial cells, smooth muscle cells, neonatal cardiac fibroblasts, and cardiac myocytes. More specifically, sST2 can serve as a non-invasive diagnostic and prognostic marker for lung, gastric, breast, pancreatic, colon, and other cancers (5–7); can be present in diseases associated with a predominantly Th2 response, such as asthma, pulmonary fibrosis, and rheumatoid arthritis (8, 9); can be useful for risk stratification and prediction of prognosis in patients with suspected sepsis (10, 11); and can enhance the development of fibrosis, hypertrophy, remodeling of the heart muscle, and progression of heart failure (12, 13) (Figure 1). Although sST2 is involved in the pathophysiology of several diseases, an increasing number of recent studies have focused on heart disease, especially heart failure. Since sST2 is less influenced by age and renal insufficiency than N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity troponin T (hs-TnT) (14, 15), it has been entered into guidelines in heart failure and considered to provide additive prognostic value and as a guide to therapy decision-making of heart failure (16).
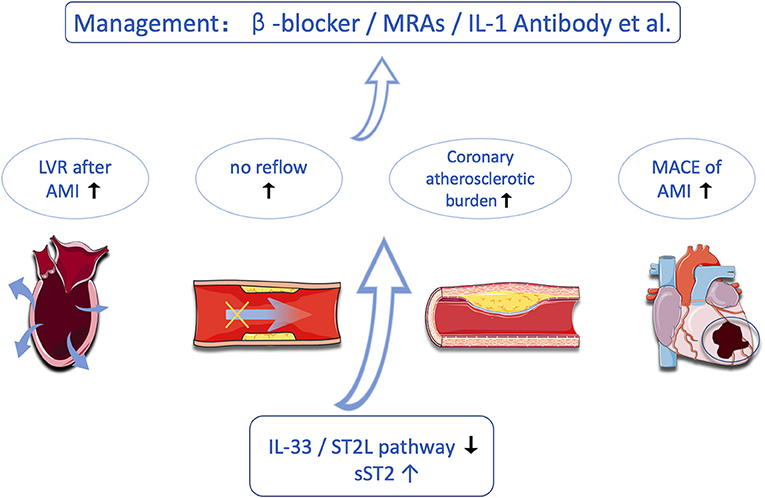
Coronary artery disease (CAD), especially acute coronary syndrome (ACS), is one of the most common causes of mortality throughout the world, despite technological improvements, new drugs, and an increasing level of awareness (17, 18). In fact, timely diagnosis allows physicians to stratify their patients by risk and consequently provide them with the opportunity to select appropriate treatments. Biomarkers that help refine diagnosis, risk stratification, and prognostic assessment are needed. In recent years, the role of ST2 in the pathophysiology of CAD and the clinical value of this biomarker in acute ST-segment elevation myocardial infarction (STEMI) have broadly expanded. In this study, we aimed to reappraise the current knowledge on sST2 in CAD (Figure 2).
Association with coronary atherosclerotic burden
The “inflammatory hypothesis” of atherosclerosis postulates that inflammatory cell signaling drives the formation, growth, and ultimately the instability of atherosclerotic plaques, setting up the substrate for the thrombotic response that causes myocardial damage or infarction (19). In the arterial wall, the interaction between IL-33 and ST2 L directs the immune response toward a T helper 2, macrophage 2 phenotype, limiting plaque inflammation and evolution. In contrast, sST2 blocks the protective effects of IL-33 on atherosclerotic plaques by sequestering IL-33 (20).
Pfetsh et al. observed a strong correlation between elevated sST2 levels and inflammatory markers (hs-CRP and IL-6) that may reflect the presence of chronic inflammation in the pathophysiology of atherosclerosis (21). In addition to being closely related to the progression of atherosclerotic lesions, sST2 has been reported as a biomarker for the stability and complexity of coronary atherosclerosis. Previous studies have shown that an increased sST2 level is a strong marker of increased risk for mortality and adverse cardiac events, such as recurrent MI and stroke, in patients with AMI (22). Zhang et al. revealed that plasma sST2 levels were significantly higher in ACS patients with complex lesions than in those with simple lesions, which indicated that sST2 may be a new marker for assessing the stability and complexity of atherosclerotic plaques (23). However, the above study also showed that there were no correlations between plasma sST2 level and stenosis severity, measured by the number of culprit vessels and Gensini score. Dieplinger et al. came to the same conclusion as above, which elucidated that sST2 was not related to the angiographic severity of CAD (24). The reason for higher sST2 levels in patients with complex lesions than in patients with simple lesions in ACS was explained in the study of Demyanet et al., which showed that sST2 may play a role in the development of vulnerability and plaque rupture, which occurs most predominantly in the ACS population (25).
Based on the above studies, Luo et al. prospectively enrolled 120 patients to assess plaque vulnerability by coronary computed tomography angiography (CCTA) (26). Their study showed that higher serum sST2 levels were associated with higher plaque vulnerability. However, the two prevailing intracoronary imaging techniques in clinical practice, IVUS, which allows macroscopic visualization of the structure of the entire vessel wall, and OCT, which allows microscopic visualization of the subtle structure of the wall, complement each other and can be used as an important tool for identifying vulnerable plaques (27–29). Therefore, studies using IVUS and OCT as outcomes should be conducted to further validate the correlation between sST2 and plaque vulnerability.
Meanwhile, the coronary artery calcium score (CACS) is a marker of atherosclerotic plaque burden and an independent predictor of coronary events. In the study by Oh et al. (30), researchers enrolled 456 subjects to illustrate that, compared with hsCRP, sST2 does not improve net reclassification for predicting a high-risk CACS, defined as CACS ≥300 Agatston units. Overall, sST2 may reflect the atherosclerotic burden in unstable, complex atherosclerotic lesions, but further studies are needed to focus on this issue.
Predict no-reflow phenomenon after percutaneous coronary intervention
The no-reflow phenomenon is defined as insufficiency of myocardial perfusion despite the mechanically responsible lesion being opened. The no-reflow phenomenon rate can reach as high as 50% in ACS patients (31) and restrain the positive effects of percutaneous coronary intervention (PCI). As there is limited treatment of no-reflow, it is more important to prevent it from occurring (32). Clinicians are committed to finding markers or clinical conditions that can predict no-reflow. It was demonstrated that sST2, a biomarker related to inflammatory activity, is one of the independent predictors of the no-reflow phenomenon in STEMI patients undergoing primary PCI. Somuncu et al. (33) included 379 patients who underwent PCI treatment for STEMI to determine the relationship between sST2 and the no-reflow phenomenon. Higher levels of sST2 patients had a significantly higher level of no-reflow compared with lower levels of sST2 (OR: 2.741 CI 95% 1.433–5.244, p = 0.002). Furthermore, after adjustment for potential confounders, it was found that being in a high-sST2 group was one of the independent predictors of no-reflow [area under the curve (AUC), 0.699; 95% confidence interval (CI), 0.65–0.75; P < 0.001].
Since the above study showed that sST2 can predict the no-reflow phenomenon in STEMI patients, experts then turned their attention to non-ST-segment elevation acute coronary syndrome (NSTE-ACS). Zhang et al. (34) revealed similar results, which pointed out that although the predictive ability was low, sST2 had a predictive value for no-reflow [area under the curve (AUC), 0.662; 95% confidence interval (CI), 0.53–0.79; P = 0.015]. It also had independent predictive value after adjusting for confounding factors [odds ratio (OR), 3.802; 95% CI, 1.03–14.11; P = 0.046]. However, both studies were single-center studies, and the number of patients included was relatively small. More importantly, they only detected the sST2 level of patients at the time of admission but did not observe subsequent changes. Thus, more high-quality trials should be carried out to determine the relationship between sST2 and no-reflow.
As a prognostic biomarker of CAD
Shimpo et al. demonstrated that sST2 levels can predict mortality and heart failure in MI patients by extracting sST2 from the serum of 810 AMI patients (35). Then, Sabatine et al. measured ST2 at baseline in 1,239 patients with STEMI from the CLARITY-TIMI 28 trial, which showed that a high baseline ST2 level, irrespective of baseline features and NT-proBNP, is a strong predictor of cardiovascular mortality and heart failure in STEMI, and the combination of ST2 and NT-proBNP greatly enhances risk stratification (36). At the same time, studies have shown that sST2 is also an independent predictor of future death or heart failure in patients with acute chest pain in the emergency department (37). In contrast, sST levels did not predict the occurrence of MACEs in STEMI patients in the study by Kim et al. (38). The reason for this phenomenon may be that sST2 levels begin to rise at 3 h after STEMI and peak at 12 h. The length of time after AMI onset to reperfusion affects myocardial injury, which is associated with an increase in biomechanical strain, leading to higher sST2 levels. The median time from onset to PCI for patients in this study was 2.7–2.8 h, which is shorter than in other studies (38). These patients, on the one hand, had a short period of myocardial ischemia and may not have been severely injured; on the other hand, they may have had an earlier measurement of sST2, resulting in sST2 not yet being elevated to the desired level. As in STEMI patients, the prognostic predictive role of sST2 in patients with chronic coronary artery disease (SCAD) remains controversial. The 13-year follow-up results of the KAROLA study suggest that sST2 levels can be an independent predictor of mortality in SCAD patients but do not predict non-fatal cardiovascular events (21). Similarly, the results from the Ludwigshafen Risk and Cardiovascular Health Study also elucidated that increased sST2 levels were an independent predictor of long-term all-cause mortality in patients with SCAD (24). However, Demyanets et al. came to the opposite conclusion, stating that sST2 was not associated with mortality in SCAD patients, despite a strong relationship with mortality in STEMI patients (25). Hughes et al. also showed that sST2 does not function as a predictor of cardiovascular events in the general population (39). The reason for this discrepancy may be because the latter study included not only patients with unstable coronary plaque but also patients with stable coronary artery disease, which interfered with the results.
Although many studies have confirmed that sST2 has a strong predictive effect on the prognosis of heart failure, the number of studies on sST2 and the prognosis of CAD, both in ACS and chronic coronary syndrome (CCS), is limited, and their findings are controversial. Therefore, more studies should be conducted in the future to further explore the prognostic effect of sST2 on CAD.
Reflect left ventricular remodeling after acute myocardial infarction
Left ventricular remodeling (LVR) refers to changes in the shape and size of the whole left ventricle after acute myocardial infarction (AMI). Important pathological features of postinfarction LV remodeling include infarct expansion, myocardial hypertrophy, cardiac fibrosis, and ventricular dilation, which are mainly due to inflammatory responses and neuroendocrine activation (40, 41). The development of adverse LVR after AMI remains a significant problem despite current achievements in invasive and pharmacological treatment (40).
Meanwhile, ST2 regulates the expression of proinflammatory cytokines from macrophages and prevents uncontrolled inflammatory reactions in the MI region. The sST2 level could be responsible for myocardial fibrosis and LVR, which could affect the prognosis after MI (40, 42). By constructing a mouse model of MI, Ghali et al. illustrated that IL-33 administration was associated with deterioration of cardiac function and ventricular remodeling. This study validated the role of the IL-33/ST2 axis in LVR after MI and laid the theoretical foundation for subsequent clinical studies (42). Thus, several studies using cardiac magnetic resonance (CMR) or echocardiography (ECHO) as a measure of LVR have been performed to demonstrate the relationship between sST2 and LVR after MI. Weir et al. included 100 patients with AMI for whom serum biomarkers were measured and CMR scans were performed and demonstrated a direct relationship between sST2 and LVR (43). It was also shown that sST2 was higher in individuals with microvascular obstruction (MVO), which is related to a more significant LVR and a poorer cardiovascular prognosis following AMI (44, 45). Kercheva et al. reached a similar conclusion from ECHO endpoints that the rise in serum sST2 was strongly associated with LVR after 6 months (46). Another similar study also found that sST2 levels correlated with both early LVR (<3 months) and late LVR (>3 months) (47). Based on these studies, using novel drugs that may antagonize this pathway, e.g., novel interleukin-1 monoclonal antibodies to antagonize the process of LVR, becomes theoretically possible, but this builds on more experiments demonstrating the causal relationship between sST2 and LVR (47).
Currently, mainly instrumental markers, such as the parameters of ECHO and CMR, are used to indicate the development of adverse LVR (48). The quest for a practical and reliable biomarker of adverse LVR, which would allow us to predict this disease in its early stages based on an exact assessment date, appears to be promising (49). Since both hemodynamic stress and an inflammatory nature are involved in the pathophysiological process of LVR, indicators that might reflect this process, such as NT-proBNP and hsCRP, have been of interest to cardiologists (36). Unlike NT-ProBNP, which responds to cardiac mechanical stress, sST2 reflects the degree of necrosis and inflammatory response of cardiomyocytes (50). Meanwhile, the dynamics of serum levels are different, which affects the timing and purpose of their clinical application. The level of sST2 decreased rapidly during the 7 days after MI; however, the level of NT-proBNP decreased effectively after the first 7 days (46). Then, Pecherina et al. performed a correlation analysis of echocardiographic parameters and serum biomarkers in patients with STEMI and preserved left ventricular ejection fraction, which showed that sST2 predicted LVR better than NT-proBNP (AUC 0.8 and 0.7, respectively), and several other biomarkers, such as matrix metalloproteinases (MMPs) and galectin-3, were also included in the study, illustrating that biomarkers combined with imaging findings can better predict the occurrence of LVR (51).
Moreover, several studies have found a link between sST2 and circulating aldosterone, implying that the IL-33/sST2 signaling system and the RAAS are linked (43). This raises the possibility of a direct role for the IL-33/sST2 system in the pathogenesis of postinfarction remodeling. Studies are already underway in this area, and the effects of drugs such as eplerenone, spironolactone, and beta-blockers on the IL-33/sST2 axis are gradually being discovered by cardiologists (52, 53), but more research is still needed in this area to find an optimal strategy to detect and cope with ventricular remodeling in an early stage after MI.
As help for management of myocardial infarction
The IL-33/ST2 signaling pathway is involved in various adverse pathophysiological processes after infarction, such as fibrosis, inflammation, and hypertrophy, which are important targets for a variety of neuroendocrine antagonists currently available to improve the prognosis of MI patients. Xia et al. demonstrated that beta-blockers could inhibit fibrosis, reduce infarct size, and improve cardiac function by enhancing the IL-33/ST2 signaling pathway through the construction of an AMI mouse model and that this effect results in a decrease in serum sST2 levels (54). It is possible that measurement of serum sST2 levels may reflect the efficacy of beta-blockers in patients with AMI. Next, Gaggin et al. performed a post-hoc analysis of the PROTECT study in which the group of patients who received high-dose beta-blockers with low sST2 levels had the best prognosis, suggesting that sST2 levels can assist cardiologists in selecting the best treatment option for patients with MI (53). Like beta-blockers, mineralocorticoid receptor antagonists (MRAs) are also widely used as neuroendocrine antagonists that can inhibit myocardial remodeling and improve prognosis in patients with MI. Other studies have also shown that both eplerenone and spironolactone antagonize aldosterone and are able to enhance the IL-33/ST2 signaling pathway while decreasing serum sST2 levels. Therefore, the detection of serum sST2 levels can also reflect the efficacy of these drugs (52, 55).
Conclusion and clinical perspectives
Soluble ST2 is a promising biomarker in cardiology, not only in heart failure but also in CAD. As a biomarker related to inflammation and fibrosis, sST2 has important clinical value in CAD, which may guide prognosis prediction, treatment plan selection, risk assessment, and long-term management of MI patients. In this article, we reviewed the use of sST2 in coronary artery disease, including reflecting plaque burden, predicting no-reflow events, predicting the prognosis of patients, reflecting LVR, and guiding the management of patients with MI. We hope that, in the near future, new studies will be conducted to better characterize and understand the relationship between sST2 and CAD.
Author contributions
JZ and ZC wrote the manuscript. MM and YH conceived, instructed, reviewed, and revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the Fellowship of China Postdoctoral Science Foundation (No. 2020M683325), the Postdoctoral Research Project, West China Hospital, Sichuan University (No. 2020HXBH048), and the Innovative Scientific Research Project of Medical Youth in Sichuan Province (No. Q20061).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Iwahana H, Yanagisawa K, Ito-Kosaka A, Kuroiwa K, Tago K, Komatsu N, et al. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem. (1999) 264:397–406. doi: 10.1046/j.1432-1327.1999.00615.x
2. Pascual-Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol. (2015) 115:3B−7B. doi: 10.1016/j.amjcard.2015.01.034
3. Homsak E, Gruson D. Soluble ST2: a complex and diverse role in several diseases. Clin Chim Acta. (2020) 507:75–87. doi: 10.1016/j.cca.2020.04.011
4. Lingel A, Weiss TM, Niebuhr M, Pan B, Appleton BA, Wiesmann C, et al. Structure of IL-33 and its interaction with the ST2 and IL-1RAcP receptors–insight into heterotrimeric IL-1 signaling complexes. Structure. (2009) 17:1398–410. doi: 10.1016/j.str.2009.08.009
5. Lu DP, Zhou XY, Yao LT, Liu CG, Ma W, Jin F, et al. Serum soluble ST2 is associated with ER-positive breast cancer. BMC Cancer. (2014) 14:198. doi: 10.1186/1471-2407-14-198
6. Wang C, Chen Z, Bu X, Han Y, Shan S, Ren T, et al. IL-33 signaling fuels outgrowth and metastasis of human lung cancer. Biochem Biophys Res Commun. (2016) 479:461–8. doi: 10.1016/j.bbrc.2016.09.081
7. Zhang Y, Davis C, Shah S, Hughes D, Ryan JC, Altomare D, et al. IL-33 promotes growth and liver metastasis of colorectal cancer in mice by remodeling the tumor microenvironment and inducing angiogenesis. Mol Carcinog. (2017) 56:272–87. doi: 10.1002/mc.22491
8. Boga S, Alkim H, Koksal AR, Ozagari AA, Bayram M, Tekin Neijmann S, et al. Serum ST2 in inflammatory bowel disease: a potential biomarker for disease activity. J Investig Med. (2016) 64:1016–24. doi: 10.1136/jim-2016-000062
9. Pei C, Barbour M, Fairlie-Clarke KJ, Allan D, Mu R, Jiang HR. Emerging role of interleukin-33 in autoimmune diseases. Immunology. (2014) 141:9–17. doi: 10.1111/imm.12174
10. Hur M, Kim H, Kim HJ, Yang HS, Magrini L, Marino R, et al. Soluble ST2 has a prognostic role in patients with suspected sepsis. Ann Lab Med. (2015) 35:570–7. doi: 10.3343/alm.2015.35.6.570
11. Kim H, Hur M, Moon HW, Yun YM, Di Somma S, Network G. Multi-marker approach using procalcitonin, presepsin, galectin-3, and soluble suppression of tumorigenicity 2 for the prediction of mortality in sepsis. Ann Intensive Care. (2017) 7:27. doi: 10.1186/s13613-017-0252-y
12. De la Fuente M, MacDonald TT, Hermoso MA. The IL-33/ST2 axis: role in health and disease. Cytokine Growth Factor Rev. (2015) 26:615–23. doi: 10.1016/j.cytogfr.2015.07.017
13. Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. (2002) 106:2961–6. doi: 10.1161/01.CIR.0000038705.69871.D9
14. Aimo A, Januzzi JL Jr, Vergaro G, Richards AM, Lam CSP, Latini R, et al. Circulating levels and prognostic value of soluble ST2 in heart failure are less influenced by age than N-terminal pro-B-type natriuretic peptide and high-sensitivity troponin T. Eur J Heart Fail. (2020) 22:2078–88. doi: 10.1002/ejhf.1701
15. Kim MS, Jeong TD, Han SB, Min WK, Kim JJ. Role of soluble ST2 as a prognostic marker in patients with acute heart failure and renal insufficiency. J Korean Med Sci. (2015) 30:569–75. doi: 10.3346/jkms.2015.30.5.569
16. Aimo A, Januzzi JL Jr, Bayes-Genis A, Vergaro G, Sciarrone P, Passino C, et al. Clinical and prognostic significance of sst2 in heart failure: JACC review topic of the week. J Am Coll Cardiol. (2019) 74:2193–203. doi: 10.1016/j.jacc.2019.08.1039
17. Madhavan MV, Gersh BJ, Alexander KP, Granger CB, Stone GW. Coronary artery disease in patients >/=80 years of age. J Am Coll Cardiol. (2018) 71:2015–40. doi: 10.1016/j.jacc.2017.12.068
18. Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S, et al. Review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. (2019) 234:16812–23. doi: 10.1002/jcp.28350
19. Kobiyama K, Ley K. Atherosclerosis. Circ Res. (2018) 123:1118–20. doi: 10.1161/CIRCRESAHA.118.313816
20. Aimo A, Migliorini P, Vergaro G, Franzini M, Passino C, Maisel A, et al. The IL-33/ST2 pathway, inflammation and atherosclerosis: trigger and target? Int J Cardiol. (2018) 267:188–92. doi: 10.1016/j.ijcard.2018.05.056
21. Pfetsch V, Sanin V, Jaensch A, Dallmeier D, Mons U, Brenner H, et al. Increased plasma concentrations of soluble ST2 independently predict mortality but not cardiovascular events in stable coronary heart disease patients: 13-year follow-up of the KAROLA study. Cardiovasc Drugs Ther. (2017) 31:167–77. doi: 10.1007/s10557-017-6718-1
22. Dhillon OS, Narayan HK, Khan SQ, Kelly D, Quinn PA, Squire IB, et al. Pre-discharge risk stratification in unselected STEMI: is there a role for ST2 or its natural ligand IL-33 when compared with contemporary risk markers? Int J Cardiol. (2013) 167:2182–8. doi: 10.1016/j.ijcard.2012.05.073
23. Zhang Y, Fan Z, Liu H, Ma J, Zhang M. Correlation of plasma soluble suppression of tumorigenicity-2 level with the severity and stability of coronary atherosclerosis. Coronary Artery Dis. (2020) 2020:851. doi: 10.1097/MCA.0000000000000851
24. Dieplinger B, Egger M, Haltmayer M, Kleber ME, Scharnagl H, Silbernagel G, et al. Increased soluble ST2 predicts long-term mortality in patients with stable coronary artery disease: results from the Ludwigshafen risk and cardiovascular health study. Clin Chem. (2014) 60:530–40. doi: 10.1373/clinchem.2013.209858
25. Demyanets S, Speidl WS, Tentzeris I, Jarai R, Katsaros KM, Farhan S, et al. Soluble ST2 and interleukin-33 levels in coronary artery disease: relation to disease activity and adverse outcome. PLoS ONE. (2014) 9:e95055. doi: 10.1371/journal.pone.0095055
26. Luo G, Qian Y, Sheng X, Sun J, Wu Z, Liao F, et al. Elevated serum levels of soluble ST2 are associated with plaque vulnerability in patients with non-ST-elevation acute coronary syndrome. Front Cardiovasc Med. (2021) 8:688522. doi: 10.3389/fcvm.2021.688522
27. Tian J, Ren X, Vergallo R, Xing L, Yu H, Jia H, et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: a combined optical coherence tomography and intravascular ultrasound study. J Am Coll Cardiol. (2014) 63:2209–16. doi: 10.1016/j.jacc.2014.01.061
28. Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. (2013) 62:1748–58. doi: 10.1016/j.jacc.2013.05.071
29. Jia H, Dai J, Hou J, Xing L, Ma L, Liu H, et al. Effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur Heart J. (2017) 38:792–800. doi: 10.1093/eurheartj/ehw381
30. Oh J, Park S, Yu HT, Chang HJ, Lee SH, Kang SM, et al. Lack of superiority for soluble ST2 over high sensitive C-reactive protein in predicting high risk coronary artery calcium score in a community cohort. Yonsei Medical J. (2016) 57:1347–53. doi: 10.3349/ymj.2016.57.6.1347
31. Durante A, Camici PG. Novel insights into an “old” phenomenon: the no reflow. Int J Cardiol. (2015) 187:273–80. doi: 10.1016/j.ijcard.2015.03.359
32. Rezkalla SH, Stankowski RV, Hanna J, Kloner RA. Management of no-reflow phenomenon in the catheterization laboratory. JACC Cardiovasc Intervent. (2017) 10:215–23. doi: 10.1016/j.jcin.2016.11.059
33. Somuncu MU, Akgun T, Cakir MO, Akgul F, Serbest NG, Karakurt H, et al. The elevated soluble ST2 predicts no-reflow phenomenon in ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Atheroscler Thromb. (2019) 26:970–8. doi: 10.5551/jat.48413
34. Zhang Q, Hu M, Ma S. Association of soluble suppression of tumorigenicity with no-reflow phenomenon and long-term prognosis in patients with non-ST-segment elevation acute coronary syndrome after percutaneous coronary intervention. J Atheroscler Thromb. (2021) 28:1289–97. doi: 10.5551/jat.59832
35. Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. (2004) 109:2186–90. doi: 10.1161/01.CIR.0000127958.21003.5A
36. Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, et al. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation. (2008) 117:1936–44. doi: 10.1161/CIRCULATIONAHA.107.728022
37. Aldous SJ, Richards AM, Troughton R, Than M. ST2 has diagnostic and prognostic utility for all-cause mortality and heart failure in patients presenting to the emergency department with chest pain. J Card Fail. (2012) 18:304–10. doi: 10.1016/j.cardfail.2012.01.008
38. Kim M, Lee DI, Lee JH, Kim SM, Lee SY, Hwang KK, et al. Lack of prognostic significance for major adverse cardiac events of soluble suppression of tumorigenicity 2 levels in patients with ST-segment elevation myocardial infarction. Cardiol J. (2021) 28:244–54. doi: 10.5603/CJ.a2020.0028
39. Hughes MF, Appelbaum S, Havulinna AS, Jagodzinski A, Zeller T, Kee F, et al. ST2 may not be a useful predictor for incident cardiovascular events, heart failure and mortality. Heart. (2014) 100:1715–21. doi: 10.1136/heartjnl-2014-305968
40. Dorn GW. Novel pharmacotherapies to abrogate postinfarction ventricular remodeling. Nat Rev Cardiol. (2009) 6:283–91. doi: 10.1038/nrcardio.2009.12
41. Yousef ZR, Redwood SR, Marber MS. Postinfarction left ventricular remodelling: where are the theories and trials leading us? Heart. (2000) 83:76–80. doi: 10.1136/heart.83.1.76
42. Bayes-Genis A, de Antonio M, Galan A, Sanz H, Urrutia A, Cabanes R, et al. Combined use of high-sensitivity ST2 and NTproBNP to improve the prediction of death in heart failure. Eur J Heart Fail. (2012) 14:32–8. doi: 10.1093/eurjhf/hfr156
43. Weir RA, Miller AM, Murphy GE, Clements S, Steedman T, Connell JM, et al. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol. (2010) 55:243–50. doi: 10.1016/j.jacc.2009.08.047
44. Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, et al. Clinical implications of the 'no reflow' phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. (1996) 93:223–8. doi: 10.1161/01.CIR.93.2.223
45. Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. (1998) 97:765–72. doi: 10.1161/01.CIR.97.8.765
46. Kercheva M, Ryabova T, Gusakova A, Suslova TE, Ryabov V, Karpov RS. Serum soluble ST2 and adverse left ventricular remodeling in patients with ST-segment elevation myocardial infarction. Clin Med Insights Cardiol. (2019) 13:1179546819842804. doi: 10.1177/1179546819842804
47. Biere L, Garcia G, Guillou S, Larcher F, Furber A, Willoteaux S, et al. ST2 as a predictor of late ventricular remodeling after myocardial infarction. Int J Cardiol. (2018) 259:40–2. doi: 10.1016/j.ijcard.2018.02.058
48. Galli A, Lombardi F. Postinfarct left ventricular remodelling: a prevailing cause of heart failure. Cardiol Res Pract. (2016) 2016:2579832. doi: 10.1155/2016/2579832
49. Ciccone MM, Cortese F, Gesualdo M, Riccardi R, Di Nunzio D, Moncelli M, et al. Novel cardiac bio-marker: ST2: a review. Molecules. (2013) 18:15314–28. doi: 10.3390/molecules181215314
50. Yu J, Oh PC, Kim M, Moon J, Park YM, Lee K, et al. Improved early risk stratification of patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention using a combination of serum soluble ST2 and NT-proBNP. PLoS ONE. (2017) 12:e0182829. doi: 10.1371/journal.pone.0182829
51. Pecherina T, Kutikhin A, Kashtalap V, Karetnikova V, Gruzdeva O, Hryachkova O, et al. Serum and echocardiographic markers may synergistically predict adverse cardiac remodeling after ST-segment elevation myocardial infarction in patients with preserved ejection fraction. Diagnostics. (2020) 10:50301. doi: 10.3390/diagnostics10050301
52. Chen B, Geng J, Gao SX, Yue WW, Liu Q. Eplerenone modulates interleukin-33/sST2 signaling and IL-1beta in left ventricular systolic dysfunction after acute myocardial infarction. J Interferon Cytokine Res. (2018) 38:137–44. doi: 10.1089/jir.2017.0067
53. Gaggin HK, Motiwala S, Bhardwaj A, Parks KA, Januzzi JL Jr. Soluble concentrations of the interleukin receptor family member ST2 and beta-blocker therapy in chronic heart failure. Circ Heart Fail. (2013) 6:1206–13. doi: 10.1161/CIRCHEARTFAILURE.113.000457
54. Xia J, Qu Y, Yin C, Xu D. Preliminary study of beta-blocker therapy on modulation of interleukin-33/ST2 signaling during ventricular remodeling after acute myocardial infarction. Cardiol J. (2017) 24:188–94. doi: 10.5603/CJ.a2016.0096
55. Lax A, Sanchez-Mas J, Asensio-Lopez MC, Fernandez-Del Palacio MJ, Caballero L, Garrido IP, et al. Mineralocorticoid receptor antagonists modulate galectin-3 and interleukin-33/ST2 signaling in left ventricular systolic dysfunction after acute myocardial infarction. JACC Heart Fail. (2015) 3:50–8. doi: 10.1016/j.jchf.2014.07.015
Keywords: sST2, coronary artery disease, myocardial infarction, LVR, management
Citation: Zhang J, Chen Z, Ma M and He Y (2022) Soluble ST2 in coronary artery disease: Clinical biomarkers and treatment guidance. Front. Cardiovasc. Med. 9:924461. doi: 10.3389/fcvm.2022.924461
Received: 20 April 2022; Accepted: 22 August 2022;
Published: 26 September 2022.
Edited by:
Dobrin Vassilev, University of Ruse, BulgariaReviewed by:
Udhaya Kumar S., Vellore Institute of Technology, IndiaCopyright © 2022 Zhang, Chen, Ma and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong He, aGV5b25nX2h1YXhpQDE2My5jb20=
†These authors have contributed equally to this work
 Junyan Zhang
Junyan Zhang Zhongxiu Chen†
Zhongxiu Chen† Yong He
Yong He