
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 30 August 2022
Sec. Cardiovascular Imaging
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.924428
This article is part of the Research Topic Multimodality Imaging in Acute Coronary Syndrome, Volume II View all 5 articles
 Yunling Li1†
Yunling Li1† Guokun Wang1†
Guokun Wang1† Xueying Wang1
Xueying Wang1 Ye Li1
Ye Li1 Yanming Zhao1
Yanming Zhao1 Xia Gu1
Xia Gu1 Bing Xu1
Bing Xu1 Jinjin Cui2
Jinjin Cui2 Xuedong Wang2
Xuedong Wang2 Yong Sun2*
Yong Sun2* Shengliang Liu1*
Shengliang Liu1* Bo Yu2
Bo Yu2Aims: Myocardial salvage index (MSI) is attracting increasing attention for predicting prognosis in acute myocardial infarction (AMI); however, the evaluation of MSI is mainly based on contrast agent-dependent cardiac magnetic resonance (CMR) scanning sequences. This study aims to investigate the prognostic value of MSI in reperfused ST-segment elevation myocardial infarction (STEMI) through the contrast agent-free CMR technique.
Methods and results: Nighty-two patients with acute STEMI, who underwent CMR after primary percutaneous coronary intervention (PPCI), were finally enrolled. Patients were subcategorized into two groups according to median MSI. T1 and T2 mapping were conducted for measuring infarct size (IS) and area at risk (AAR). IS was significantly larger in < median MSI group than ≥ median MSI group (P < 0.001). AAR between the two groups showed no obvious differences (P = 0.108). Left ventricular ejection fraction (LVEF) was lower in < median MSI group than ≥ median MSI group (P = 0.014). There was an obvious inverse correlation between MSI and reperfusion time (R = –0.440, P < 0.001) and a strong inverse correlation between MSI and IS (R = –0.716, P = 0.011). As for the relationship LVEF, MSI showed positive but weak correlation (R = 0.2265, P < 0.001). Over a median follow-up period of 263 (227–238) days, prevalence of MACEs was significantly higher in the < median MSI group [HR: 0.15 (0.04–0.62); Log-rank P = 0.008]. The univariate Cox regression analysis revealed that LVEF, IS, and MSI were significant predictors for major adverse cardiovascular events (MACEs) (all P < 0.05). In the stepwise multivariate Cox regression analysis, LVEF and MSI were identified as independent parameters for predicting MACEs (both P < 0.05). In the receiver-operating characteristic analysis, LVEF, IS, and MSI showed prognostic value in predicting MACEs with AUCs of 0.809, 0.779, and 0.896, respectively, all (P < 0.05). A combination of MSI with LVEF showed the strongest prognostic value of MACEs (AUC: 0.901, sensitivity: 77.78%, specificity: 98.80%, P < 0.001). Delong’s test showed that the combination of LVEF with MSI had an incremental value than LVEF itself in predicting MACEs (P = 0.026).
Conclusion: Contrast agent-free CMR technique provides a reliable evaluation of MSI, which contributes to assessing the efficacy of reperfusion therapy and predicting the occurrence of MACEs.
Myocardial salvage index (MSI) is attracting more and more attention for its superiority in assessing the efficacy of reperfusion therapy in acute myocardial infarction (AMI) (1). Reperfusion following AMI is mainly constituted by pharmacological thrombolysis, primary percutaneous coronary intervention (PPCI), and coronary artery bypass grafting, all of which are responsible for effective myocardial salvage. Infarct-related parameters are associated with adverse clinical outcomes; quantification of myocardial injury is necessary for evaluating treatment effects (2). The amount of salvaged myocardium is closely related to the initial myocardial area at risk (AAR) and irreversible infarct myocardium (3), difference between edema-based AAR and infarct area is used to calculate MSI (4). Absolute myocardial infarct size (IS) has been utilized to predict the prognosis of AMI; however, many patients develop extensive myocardial damage even after receiving revascularization by PPCI (5), which draws attention to reversible myocardial injury; thus, MSI might be an effective indicator in predicting AMI prognosis (6).
Single-photon emission computed tomography (SPECT) has been widely confirmed in detecting MSI (7–9), which is an independent predictor of prognosis in AMI (10). However, this technique is unreasonable in an acute condition, as it requires injection of isotope before reperfusion. In addition, inevitable radiation exposure and low spatial resolution make it limited in clinical practice (9). Thus, developing novel and feasible methods to measure MSI and assess reperfusion efficiency is of utmost importance.
Cardiac magnetic resonance (CMR) is a radiation-free and multi-parametric imaging technique with high sensitivity and resolution. It offers reproducible measurement of MSI with excellent consistency with SPECT (7, 11). A previous study proved that combining T2-weighted imaging and late gadolinium enhancement (LGE) offered stable MSI measurement (4). However, contrast agent-mediated LGE imaging is limited to a certain extent, as many patients with AMI also carry chronic kidney diseases simultaneously, in addition, contrast-induced nephropathy following PPCI cannot be ignored as well. Besides that, a comprehensive CMR can be challenging and time-consuming for patients with AMI, obviating some scanning protocols and shortening scanning duration without compromising data acquisition would be an ideal approach (12). Native T1 mapping can identify myocardial fibrosis and quantify IS without utilizing gadolinium agents (13–15), which gives an estimate of IS for particular patient groups. A high T2 signal reflects an increased myocardial water content; T2 mapping is confirmed effective in assessing myocardial edema, which is considered as AAR in AMI (16). Comparing and quantifying T1 and T2 mapping ideally provide MSI and assess reperfusion efficacy.
The purpose of the study is to investigate the prognostic value of MSI in reperfused (AMI) through the contrast agent-free CMR technique.
This retrospective trial was performed at a single cardiac center between September 2020 and November 2021. Patients were eligible if symptoms lasted less than 12 h, and the ST-segment elevated more than 0.1 mV in at least two extremity leads or more than 0.2 mV in at least two precordial leads (17). Patients with first ST-segment elevation myocardial infarction (STEMI) undergoing PPCI were initially enrolled. The CMR study was carried out within 5 days of PPCI. Patients with previous myocardial infarction or contraindications to CMR, such as implanted pacemakers, defibrillators, claustrophobia, or metallic intracranial implants, were excluded. The study was approved by the local ethics committee and all patients gave written informed consent.
Prior to PPCI, all patients orally received a 300-mg loading dose of aspirin and clopidogrel separately. In addition, they received low molecular heparin xintravenously. All patients received PPCI according to standard clinical practice, additional use of thrombectomy was performed depending on the thrombus burden in an occluded artery. After PPCI, aspirin and clopidogrel were administered at a dose of 100 and 75 mg, respectively, per day. All other medications, including glycoprotein IIb/IIIa-inhibitors, angiotensin-converting enzyme inhibitors, beta-blockers, and statins, were recommended according to contemporary guidelines (18).
CMR imaging was performed in all patients by using a 3.0-Tesla system (Ingenia CX, Philips Healthcare, Netherlands), with a 32-channel phased-array abdomen coil. The scanning protocols mainly included cine steady-state free precession imaging, T2-weighted short tau inversion recovery (T2w-STIR), native T1 mapping, T2 mapping, and LGE.
After performing scout imaging, cine imaging with breath-hold and electrocardiogram trigger were used for cardiac morphologic and functional analyses. The scanning was conducted in short-axis slices, 2-chamber, 3-chamber, and 4-chamber planes, for short-axis imaging; left ventricular (LV) was completely encompassed from base to apex, and the parameters were as follows: repetition time (TR)/echo time (TE) = 2.8/1.42 ms, the field of view (FOV) = 300 × 300 mm2, voxel = 1.8 × 1.6 × 8 mm3, flip angle = 45°, and 8-mm slice thickness.
For T2w-STIR imaging, a T2-weighted imaging triple inversion recovery turbo spin-echo sequence based on breath hold was applied; the scanning parameters were as follows: TR/TE = 1,500/75 ms, FOV = 300 × 300 mm2, voxel = 1.3 × 1.65 × 8 mm3, and flip angel = 90°. The T2w-STIR images were acquired on short-axis planes including the base, mid-ventricular, and apex levels.
Modified Look-Locker inversion recovery sequence was performed for native T1 mapping in short-axis slices (basal, mid, and apical) (15, 19); the detailed scanning parameters were as follows: TR/TE = 2.2/1.02 ms, FOV = 300 × 300 mm2, voxel = 2 × 2 × 8 mm3, flip angle = 20°, and 8-mm slice thickness.
T2 mapping was acquired in a gradient-spin echo sequence (20, 21) at the same short-axis positions corresponding to T1 mapping, which included basal, mid, and apical LV. The sequence parameters were TR/TE = 741/8.8 ms, FOV = 300 × 300 mm2, voxel = 2 × 2 × 8 mm3, flip angel = 90°, and 8-mm slice thickness.
LGE images were acquired 10–15 min after injection of gadolinium-based contrast agent (Bayer Healthcare, Germany) by using a three-dimensional phase-sensitive inversion recovery sequence (22), the scanning parameters of LGE were TR/TE = 6.1/3 ms, FOV = 300 × 300 mm2, voxel = 1.8 × 1.68 × 8 mm3, flip angel = 25°, and 8-mm slice thickness. The inversion time was optimized to null the signal in the normal myocardium.
All CMR studies were post-processed with a dedicated workstation (cvi42, Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada) by two experienced operators who were blinded to baseline and outcome data. LV dimensions, mass, and systolic function were calculated from the SSFP cine. Typical CMR images of patients with acute anterior, anteroseptal myocardial infarction and inferior, and inferoseptal myocardial infarction are shown in Figure 1. Validation of infarct region and edema-based AAR are obtained by comparing T1 mapping, T2 mapping against LGE, and T2w-STIR as the reference standards.
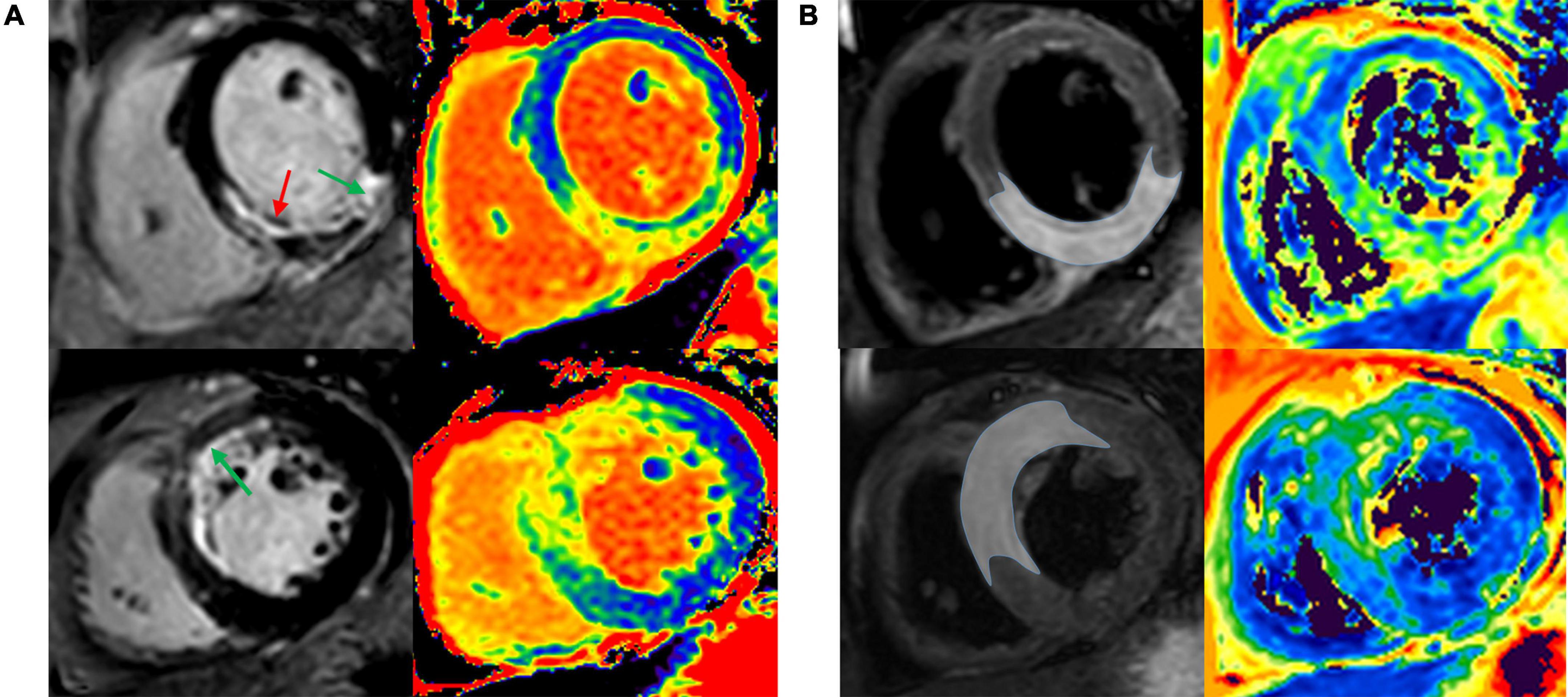
Figure 1. Infarct region and AAR were validated by comparing T1 and T2 mapping against LGE and T2w-STIR. Representative images of patient with acute anterior and anteroseptal STEMI (A), and patient with acute inferior and inferoseptal STEMI (B). AAR, area at risk; LGE, late gadolinium enhancement; T2w-STIR, T2-weighted short tau inversion recovery; STEMI, ST-segment elevation myocardial infarction.
For native T1 mapping, pixel-coded T1 values were provided in a parametric color-encoded anatomical map, followed by analyses that were consistent with current guidelines (23). Epicardial and endocardial contours of LV and the remote myocardium without visible evidence of infarction, edema, or abnormal motion (assessed through LGE, T2w-STIR, and cine images) were delineated. Regions of interest, which are the infarct areas, were defined as myocardium with a signal-intensity threshold of > 5 standard deviations (SDs) above remote myocardium (24). The hypo-intense infarct cores that represented microvascular damage were also included within IS after manual delineation.
T2 mapping sequences provide T2 value per pixel in milliseconds and deliver a good correlation with myocardial water content. AAR was determined when pixel values of myocardium > 2 SDs above remote myocardium (25, 26). In T2 mapping, the epi- and endocardial contours and remote myocardium were correspondingly copied from T1 mapping. Central hypo-intense cores that were deemed to be myocardial hemorrhage were included in the AAR measurement (27). Attention should be paid to excluding high signals caused by slow flow in the blood pool. IS and AAR were normalized as a percentage of LV mass. The following formulas were applied: AAR = volume edema/volume LV mass, IS = volume infarct/volume LV mass, MSI = (AAR–IS)/AAR (4, 24).
The primary endpoint of this study was defined as the emergence of major adverse cardiovascular events (MACEs), which included all-cause death, re-infarction, and new congestive heart failure (1). Re-infarction was diagnosed based on ischemic symptoms, new ST-segment changes, increase in creatine kinase, and troponin I. New congestive heart failure was defined according to New York Heart Association functional class. Clinical follow-up was conducted via a structured questionnaire by telephone and then assessed by two experienced cardiologists. The telephone interview was conducted every 6 months after the CMR test.
Categorical variables were expressed as numbers, with percentages in parentheses, and differences were assessed by the Fisher exact or Chi-square test. Continuous data with normal distribution were compared through Student’s t-test and expressed as mean ± SD, continuous variables with non-normal distribution were compared by the Mann-Whitney U-test and were, therefore, presented as medians with the interquartile range. Pearson’s or Spearman’s correlation coefficients were calculated to assess the correlations between left ventricular ejection fraction (LVEF) and infarct-related parameters. Univariate and multivariate COX regression analyses were performed to calculate hazard ratio (HR) and 95% CI, then characterize predictors of MSI. The Kaplan-Meier method was used for depicting survival curves; differences were assessed by log-rank tests. The area under the curves (AUCs), specificity, sensitivity, and Youden’s index were analyzed by the receiver-operating characteristic curve to define optimal cut-off values for the prediction of clinical endpoints. Intra- and interobserver variabilities were assessed using intraclass correlation coefficients (ICC). Data above were calculated by SPSS 26.0.0 (Inc., Chicago, IL, United States) or by the MedCalc version20 (MedCalc Software, Ostend, Belgium). P < 0.05 was statistically significant.
This retrospective study consisted of 97 consecutive patients with STEMI, 3 patients were excluded because of poor image quality, and 2 patients were excluded because of lacking T2 mapping (Figure 2). Clinical data of the rest of the 92 patients were collected, and images exhibited diagnostic quality, enabling the assessment of myocardial salvage. PPCI was performed in the left anterior descending artery in 47 patients, in the left circumflex artery in 42 patients, and in the right coronary artery in 3 patients. Demographic data and clinical characteristics are presented in Table 1. Patients were separated into two groups according to median MSI, that was a < median MSI group (n = 46) and a ≥ median MSI group (n = 46). The baseline characteristics (age, sex, body mass index) and risk factors (hypertension, diabetes mellitus, and smoking) were similar between the two groups (all P > 0.05). The mean age of the patients with STEMI was 59 ± 10 years old and 75% were male. The ≥ median MSI group tended to show lower level of cholesterol and shorter time from symptom onset to reperfusion in comparison to < median MSI group [cholesterol, 4.6 ± 1 mmol/L vs. 5.1 ± 1.1 mmol/L; reperfusion time, 180 (120, 360) min vs. 330 (180, 480) min; both P < 0.05]. Levels of N-terminal pro-B type natriuretic peptide (NT-proBNP) and peak troponin I of the two groups showed no significant differences [NT-proBNP, 334.5 (97.5, 823.8) pg/ml vs. 405 (100.8, 1,422.5) pg/mL; troponin I, 18.2 (6.7, 85.5) ng/ml vs. 44.9 (11.7, 117.2) ng/mL; both P > 0.05]. There were no statistical differences between the two groups in terms of involved culprit coronary arteries (P = 0.641). Concomitant medications after PPCI between the two groups were similar except for nitrates.
The median time between PPCI and CMR acquisition was 3.9 (3–4.8) days for the whole study group, 3.6 (3–4.7) days for < median MSI group, and 4 (3–4.9) days for ≥ median MSI group. CMR parameters are summarized in Table 2. Regions of increased signal intensity on native T1 and T2 mapping were measured in the territory of corresponding occluded coronary arteries. Mean IS among all the patients was 22.6 ± 12% of LV mass. IS was significantly larger in the < median MSI group relative to ≥ median MSI group (29.2 ± 11.6% vs. 15.9 ± 8.2%, P < 0.001). LGE was more prevalent in the < median MSI group than ≥ median MSI group (28.1 ± 13.5% vs. 16.9 ± 8%, P < 0.001). The mean edema-based AAR of the whole studying cohort was 40.7 ± 14.7% of LV mass. AAR between the two groups showed no obvious differences (38.2 ± 13.7% vs. 43.2 ± 15.3%, P = 0.108). LV mass, end-diastolic volume (EDV), and end-systolic volume (ESV) were similar in the two groups (LV mass, 113.5 ± 26.7 g vs. 107.9 ± 24.9 g; LV EDV, 154.7 ± 35.8 ml vs. 148.2 ± 31.4 ml; LV ESV, 77.8 ± 27.6 ml vs. 73 ± 27.8 ml; all P > 0.05); whereas LVEF was obviously lower in the < median MSI group than that in the ≥ median MSI group (51.5 ± 6.7% vs. 54.5 ± 5%, P = 0.014).
There was an obvious inverse correlation between MSI and reperfusion time (R = –0.440, P < 0.001) and a strong inverse correlation between MSI and IS (R = –0.716, P < 0.001). As for the relationship with LVEF, MSI showed a positive but weak correlation (R = 0.265, P = 0.011) (Figure 3).
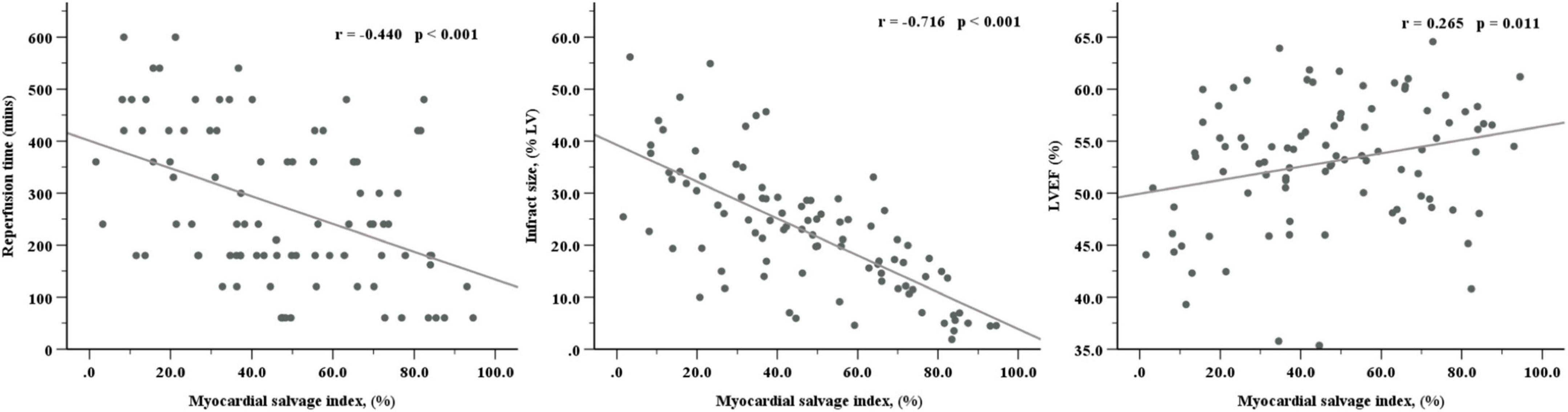
Figure 3. Correlations of myocardial salvage index with reperfusion time, infarct size, and LVEF. LVEF, left ventricular ejection fraction.
Over a median duration follow-up of 263 (227–238) days, the primary endpoint occurred in 9 patients; we observed 9 re-infarctions, among which there were 7 re-infarctions (15.2%) in the < median MSI group and 2 (4.3%) in the ≥ median MSI group. Cardiac death and congestive heart failure were not detected in both groups. Prevalence of MACEs was significantly higher in the < median MSI group (HR: 0.15 [0.04–0.62]; Log-rank P = 0.008) (Figure 4). Several CMR parameters (LVEF, IS, and MSI) were associated with MACEs by univariate COX regression analysis (all P < 0.05), while MSI and LVEF were the independent parameters in predicting clinical endpoint in further multivariate COX regression analysis (both P < 0.05) (Table 3). In the receiver-operating characteristic analysis, LVEF, IS, and MSI showed prognostic value in predicting MACEs with AUCs of 0.809, 0.779, and 0.896, respectively, (all P < 0.05). A combination of MSI with LVEF showed the strongest prognostic value of MACEs (AUC: 0.901, sensitivity: 77.78%, specificity: 98.80%, P < 0.001) (Figure 5). Delong’s test showed that the combination of LVEF with MSI had an incremental value than LVEF itself in predicting MACEs (P = 0.026).
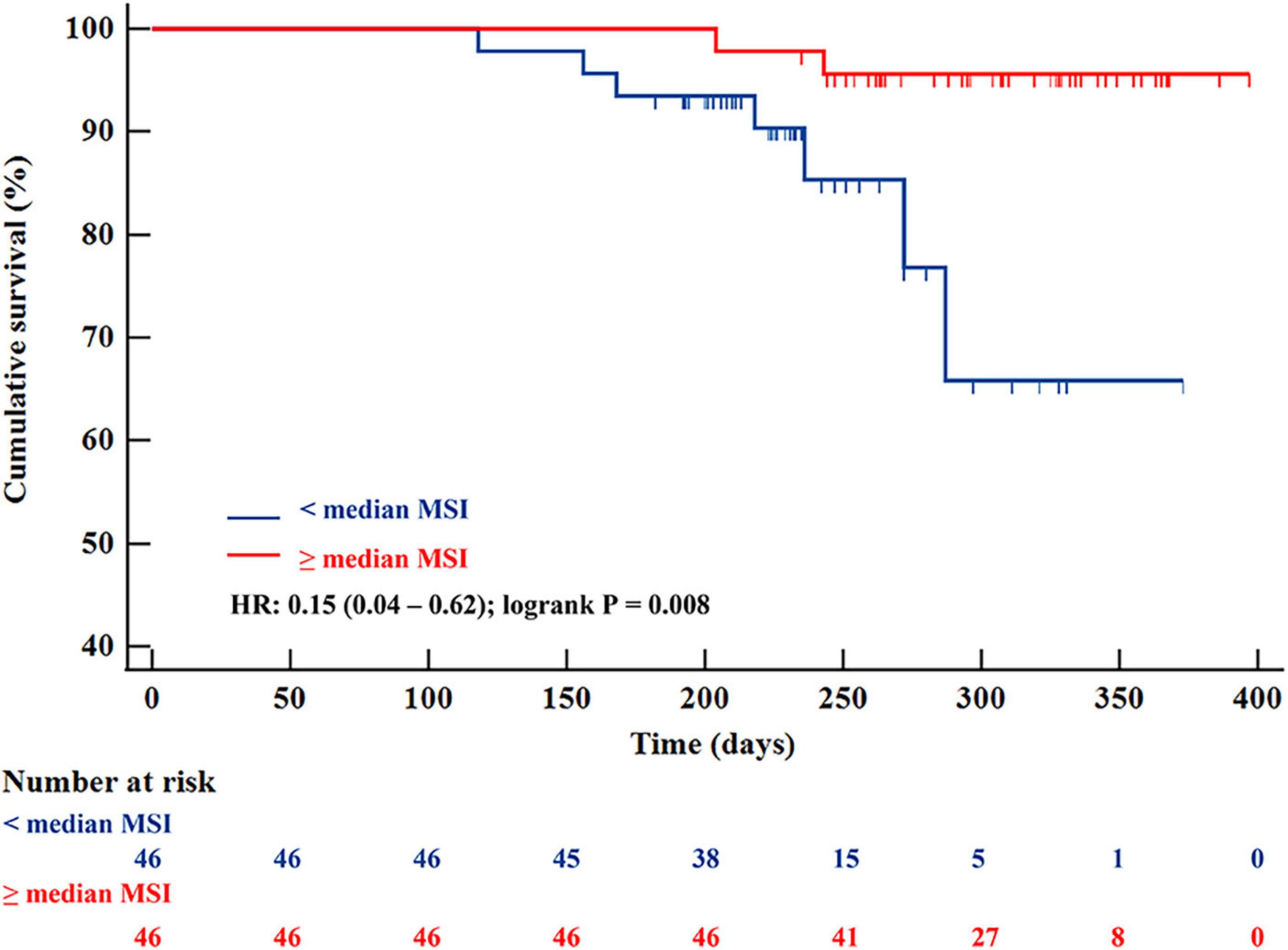
Figure 4. Kaplan-Meier survival curves for 92 patients with STEMI separated into two groups according to median MSI. STEMI, ST-segment elevation myocardial infarction; MSI, myocardial salvage index.
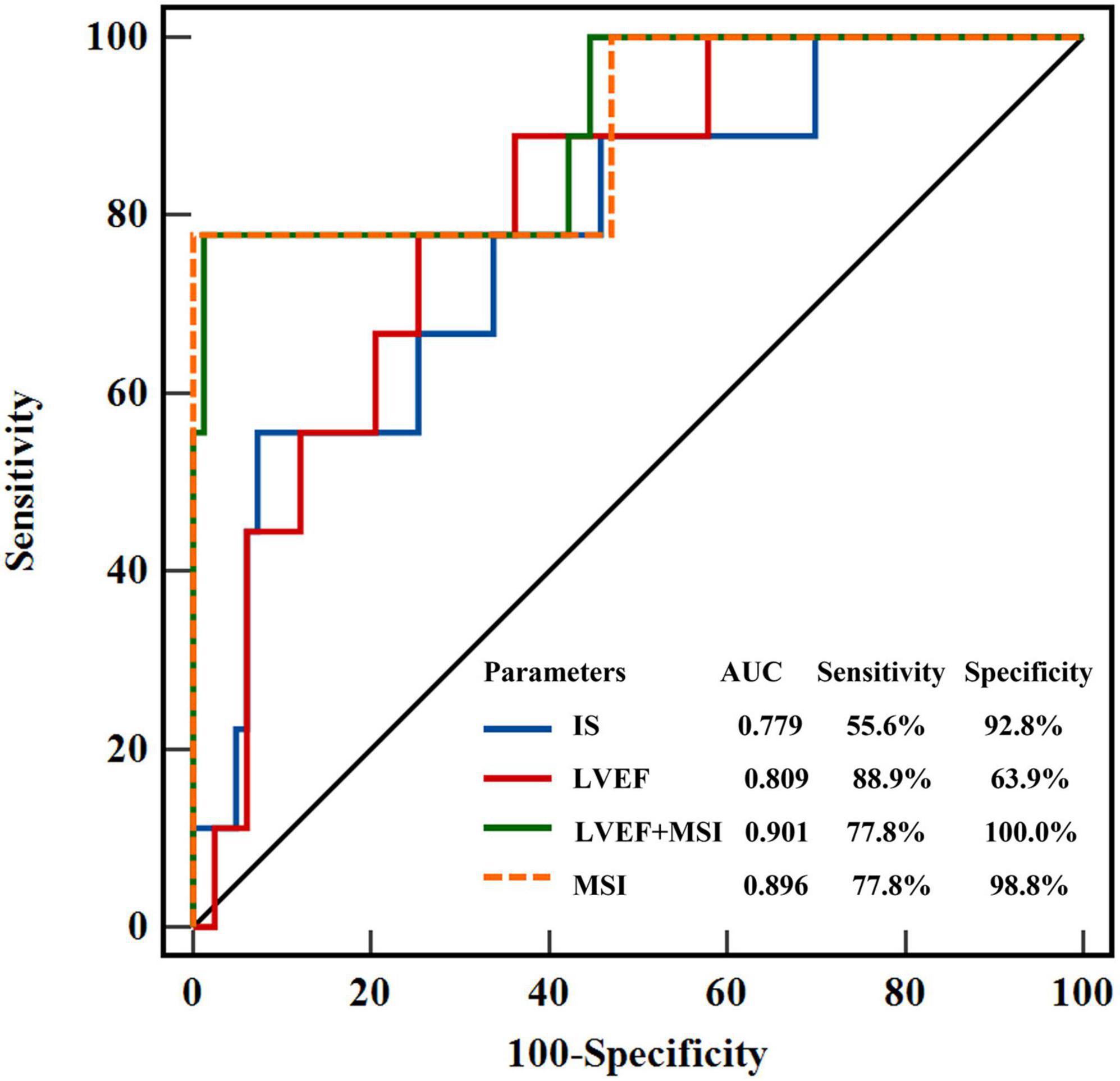
Figure 5. Receiver-operating characteristic curves showed the prognostic value of LVEF, MSI, IS, and the combined model of LVEF + MSI. LVEF, left ventricular ejection fraction; MSI, myocardial salvage index, IS, infarct size.
Quantification of IS and AAR revealed good reproducibility. The intraobserver variability in measuring IS on T1 mapping and LGE were excellent, with ICCs of 0.956 (95% CI: 0.908–0.979), and 0.939 (95% CI: 0.872–0.971), respectively (Figures 6B,C). The interobserver variability in measuring IS on T1 mapping and LGE were good, with ICCs of 0.901 (95% CI: 0.727–0.958) and 0.907 (95% CI: 0.804–0.956), respectively (Figures 6F,G). The ICC for the intraobserver variability ranged between 0.925 (95% CI: 0.798–0.968) for T2 mapping (Figure 6A). The ICC for the interobserver variability ranged between 0.895 (95% CI: 0.770–0.951) for T2 mapping (Figure 6E). Bland-Altman analysis showed good agreement between T1 mapping and LGE for measuring IS (limits of agreement = 0.04 ± 6.13%) (Figure 6D). T1 mapping correlated well with LGE in quantifying IS (y = 3.44 + 0.85x, R2 = 0.765, P < 0.001) (Figure 6H).
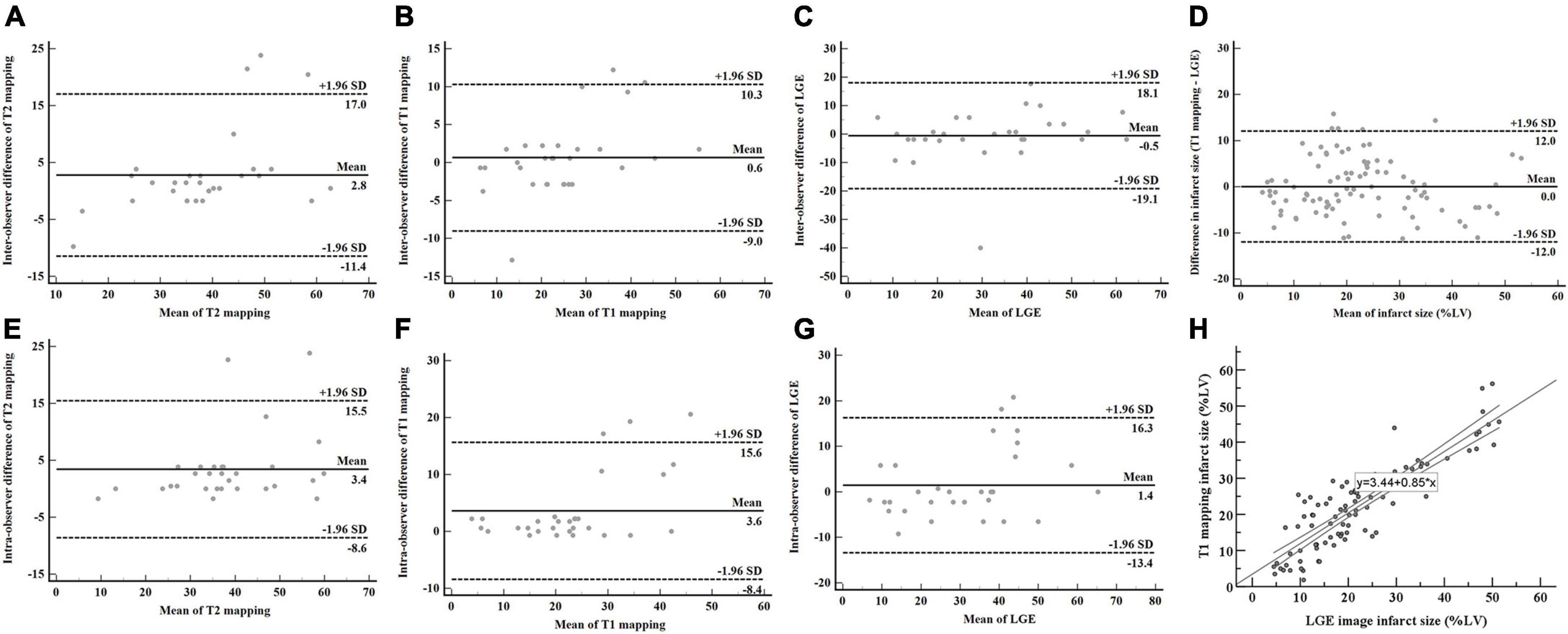
Figure 6. AAR and IS assessed by T2 mapping, T1 mapping, and LGE. The intraobserver and interobserver reproducibility of T2 mapping (A,E), T1 mapping (B,F), and LGE (C,G) were good. Bland-Altman analysis showed good agreement in measuring IS between T1 mapping and LGE (D). T1 mapping correlated well with LGE in quantifying IS (H). AAR, area at risk; IS, infarct size; LGE, late gadolinium enhancement.
Obstruction or relative insufficient supply of blood flow leads to myocardial necrosis and myocytolysis, transmembrane sodium gradients alteration, or inflammatory response after an acute ischemic insult leads to tissue edema and increased intersarcomeric distance (28, 29). The process of reperfusion therapy is a double-edged sword that accounts for salvaged myocardium; paradoxically, it is also a driving factor that contributes to additional myocardial damage (30). In patients with STEMI, myocardial AAR is the proportion of myocardium that is at risk of becoming necrotic, supplied by occluded artery, and it exceeds irreversibly infarct myocardium (13). The amount of salvaged myocardium is derived by subtracting IS from the edematous area (4). CMR is a promising tool that offers robust quantification of reversible and irreversible myocardial injury through analyzing T2w-STIR and LGE; thus, enabling us to determine the amount of salvaged myocardium, and it has been proven to show good consistency with SPECT and histological examinations (11, 31–34).
To the best of our knowledge, this is the first evaluation of MS using CMR mapping sequences. The major findings are as follows: (1) patients with < median MSI have larger IS, lower LVEF, and a significantly higher incidence of MACE at 1-year follow-up; (2) Combining MSI with LVEF obtained a stronger predictive value of MACE rate; and (3) Increased MSI was associated with shorter reperfusion time and decreased IS; on the contrary, MSI correlated positively with LVEF. Reperfusion time, IS, and LVEF have been proven to be associated with adverse patient outcomes (1, 24), and they showed fair correlations with MSI; as a corollary, CMR mapping-derived MSI might serve as a novel and strong predictor of clinical outcomes in patients with STEMI after reperfusion. In our study, a shorter reperfusion time was associated with higher MSI, which predicted a reduced risk of MACE. This confirmed current guidelines for patients with STEMI to accept reperfusion therapy as early as they can (35). LVEF is a typically reliable marker that reflects cardiac function and clinical outcome, combining analysis with MSI increased the prognostic accuracy on the risk of MACE than LVEF itself.
More and more studies demonstrated native T1 mapping-enabled estimation of IS in animal models and patients (15, 36, 37). Messroghli et al. demonstrated that high-resolution T1 mapping enabled the detection of AMI; standard T1 relaxation time thresholds served as a potential tool for the measurement of IS with high sensitivity and specificity (15). Cui et al. proved that the presence and extent of myocardial infarction measured by T1 mapping correlated well with that evaluated by LGE and triphenyl tetrazolium chloride staining in the porcine study (14). Not only in AMI but T1 mapping was also validated as amenable in chronic myocardial infarction (38). Thus, native T1 mapping constitutes a surrogate method for contrast agent-dependent technique. This advantage broadens CMR application by reducing scanning time and especially benefits patients with renal dysfunction.
T2 is sensitive to myocardial water content, edema in infarct tissue prolongs T2 signal decay and depicts a hyperintense region. Aletras et al. showed that increased signal area measuring T2-weighted imaging was comparable with the AAR detected with microspheres in coronary occluded animal models (39). Ugander et al. confirmed that the histologic AAR and T2-defined AAR overlapped to a large extent in the canine infarction model (40). T2 mapping provides estimated T2 values per pixel in milliseconds, and it has been validated to serve as an attractive approach to measuring myocardial edema (33). Traditional T2 sequences introduce several limitations, on the one hand, mistiming the image scanning outside the diastolic R-R interval leads to signal reduction, while on the other hand, remaining blood caused by trabeculae in the ventricular cavity obscures the borders of the myocardium and increases signal intensity. T2 mapping overcomes the aforementioned limitations (41).
T2 values change dynamically over the first few days after STEMI. Bimodal edema pattern has been illustrated in human studies (34). The first peak develops directly after reperfusion; it is caused by cell swelling and reactive hyperemia with capillary leakage, whereas the second peak occurs from 4 to 7 days after PPCI, and results from tissue inflammation and regeneration. Thus, T2 mapping is still under debate for robust evaluation of AAR. Native T1 mapping has been proven to underestimate IS by 10% as validated by histological examination in swine experiments (14). However, histologic findings in animal models cannot directly represent the conditions in the human body. Moreover, microvascular damage, ranging from microvascular obstruction to intramyocardial hemorrhage, always overlaps with edema, thus mitigating T1 prolongations, and leading to underestimation of the resultant T1 value (13). Further clinical trials and multiple parallel compare methods are needed to verify the potential of native T1 mapping in quantifying irreversible infarct areas. This study was conducted in a single center with a small cohort. In addition, only three short-axis slices (basal, mid, and apical) were scanned and analyzed in T1 and T2 mapping, we suppose that more slices covering from ventricular base to apex might increase accuracy in measuring salvaged myocardium.
This work emphasizes the promising prognostic role of contrast agent-free CMR sequences in providing in vivo characterization of myocardial tissue damage in patients with STEMI. MSI contributes to assessing the efficacy of reperfusion therapy and increases the predictive value of the MACE rate in reperfused myocardial infarction.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Committee of The Second Affiliated Hospital of Harbin Medical University (Harbin, China). The patients/participants provided their written informed consent to participate in this study.
YLL and SL designed the study and wrote the manuscript. XYW and YL post-processed the images. XG, JC, and BX collected the data. XDW, GW, and YZ analyzed the data. YS and BY supervised the study. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (grant no. 82072030 to YS).
We appreciate Jianxiu Lian for technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor ZW declared a past collaboration with the author BY.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Eitel I, Desch S, Fuernau G, Hildebrand L, Gutberlet M, Schuler G, et al. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol. (2010) 55:2470–9. doi: 10.1016/j.jacc.2010.01.049
2. Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. (2006) 113:2733–43. doi: 10.1161/CIRCULATIONAHA.105.570648
3. Stork A, Lund G, Muellerleile K, Bansmann P, Nolte-Ernsting C, Kemper J, et al. Characterization of the peri-infarction zone using T2-weighted MRI and delayed-enhancement MRI in patients with acute myocardial infarction. Eur Radiol. (2006) 16:2350–7. doi: 10.1007/s00330-006-0232-3
4. Friedrich M, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. (2008) 51:1581–7. doi: 10.1016/j.jacc.2008.01.019
5. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77.
6. Pennell D. Myocardial salvage: retrospection, resolution, and radio waves. Circulation. (2006) 113:1821–3. doi: 10.1161/CIRCULATIONAHA.105.618942
7. Sorensson P, Heiberg E, Saleh N, Bouvier F, Caidahl K, Tornvall P, et al. Assessment of myocardium at risk with contrast enhanced steady-state free precession cine cardiovascular magnetic resonance compared to single-photon emission computed tomography. J Cardiovasc Magn Reson. (2010) 12:25.
8. Gibbons R, Christian T, Hopfenspirger M, Hodge D, Bailey K. Myocardium at risk and infarct size after thrombolytic therapy for acute myocardial infarction: implications for the design of randomized trials of acute intervention. J Am Coll Cardiol. (1994) 24:616–23. doi: 10.1016/0735-1097(94)90005-1
9. Gibbons R, Miller T, Christian T. Infarct size measured by single photon emission computed tomographic imaging with (99m)Tc-sestamibi: a measure of the efficacy of therapy in acute myocardial infarction. Circulation. (2000) 101:101–8. doi: 10.1161/01.CIR.101.1.101
10. Ndrepepa G, Mehilli J, Schwaiger M, Schuhlen H, Nekolla S, Martinoff S, et al. Prognostic value of myocardial salvage achieved by reperfusion therapy in patients with acute myocardial infarction. J Nucl Med. (2004) 45:725–9.
11. Carlsson M, Ubachs J, Hedström E, Heiberg E, Jovinge S, Arheden H. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc Imaging. (2009) 2:569–76. doi: 10.1016/j.jcmg.2008.11.018
12. Bulluck H, Hammond-Haley M, Fontana M, Knight DS, Sirker A, Herrey AS, et al. Quantification of both the area-at-risk and acute myocardial infarct size in ST-segment elevation myocardial infarction using T1-mapping. J Cardiovasc Magn Reson. (2017) 19:57. doi: 10.1186/s12968-017-0370-6
13. Beijnink CWH, van der Hoeven NW, Konijnenberg LSF, Kim RJ, Bekkers S, Kloner RA, et al. Cardiac MRI to visualize myocardial damage after ST-segment elevation myocardial infarction: a review of its histologic validation. Radiology. (2021) 301:4–18. doi: 10.1148/radiol.2021204265
14. Cui C, Wang S, Lu M, Duan X, Wang H, Jia L, et al. Detection of recent myocardial infarction using native T1 mapping in a swine model: a validation study. Sci Rep. (2018) 8:7391. doi: 10.1038/s41598-018-25693-1
15. Messroghli DR, Walters K, Plein S, Sparrow P, Friedrich MG, Ridgway JP, et al. Myocardial T1 mapping: application to patients with acute and chronic myocardial infarction. Magn Reson Med. (2007) 58:34–40. doi: 10.1002/mrm.21272
16. Gómez-Talavera S, Fernández-Jiménez R, Galán-Arriola C, Agüero J, López-Martín G, González M, et al. Variations in T2-mapping-assessed area at risk after experimental ischemia/reperfusion. J Cardiovasc Transl Res. (2021) 14:1040–2. doi: 10.1007/s12265-021-10120-0
17. Fuernau G, Eitel I, Franke V, Hildebrandt L, Meissner J, de Waha S, et al. Myocardium at risk in ST-segment elevation myocardial infarction comparison of T2-weighted edema imaging with the MR-assessed endocardial surface area and validation against angiographic scoring. JACC Cardiovasc Imaging. (2011) 4:967–76. doi: 10.1016/j.jcmg.2011.02.023
18. Collet J, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt D, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa909
19. Messroghli D, Radjenovic A, Kozerke S, Higgins D, Sivananthan M, Ridgway J. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. (2004) 52:141–6. doi: 10.1002/mrm.20110
20. Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. (2009) 11:56. doi: 10.1186/1532-429X-11-56
21. Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti O, et al. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc Imaging. (2011) 4:269–78. doi: 10.1016/j.jcmg.2010.09.023
22. Kellman P, Arai A, McVeigh E, Aletras A. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. (2002) 47:372–83. doi: 10.1002/mrm.10051
23. Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, et al. Myocardial T1 mapping and extracellular volume quantification: a society for cardiovascular magnetic resonance (SCMR) and CMR working group of the europeansociety of cardiology consensus statement. J Cardiovasc Magn Reson. (2013) 15:92. doi: 10.1186/1532-429X-15-92
24. Francone M, Bucciarelli-Ducci C, Carbone I, Canali E, Scardala R, Calabrese FA, et al. Impact of primary coronary angioplasty delay on myocardial salvage, infarct size, and microvascular damage in patients with ST-segment elevation myocardial infarction: insight from cardiovascular magnetic resonance. J Am Coll Cardiol. (2009) 54:2145–53. doi: 10.1016/j.jacc.2009.08.024
25. Berry C, Kellman P, Mancini C, Chen M, Bandettini W, Lowrey T, et al. Magnetic resonance imaging delineates the ischemic area at risk and myocardial salvage in patients with acute myocardial infarction. Circ Cardiovasc Imaging. (2010) 3:527–35. doi: 10.1161/CIRCIMAGING.109.900761
26. Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor A, Messroghli D, Kumar A, et al. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. (2004) 109:2411–6. doi: 10.1161/01.CIR.0000127428.10985.C6
27. Ganame J, Messalli G, Dymarkowski S, Rademakers FE, Desmet W, Van de Werf F, et al. Impact of myocardial haemorrhage on left ventricular function and emodeling in patients with reperfused acute myocardial infarction. Eur Heart J. (2009) 30:1440–9. doi: 10.1093/eurheartj/ehp093
28. Thygesen K, Alpert J, Jaffe A, Simoons M, Chaitman B, White H. Third universal definition of myocardial infarction. Glob Heart. (2012) 7:275–95. doi: 10.1016/j.gheart.2012.08.001
29. Bragadeesh T, Jayaweera A, Pascotto M, Micari A, Le D, Kramer C, et al. Post-ischaemic myocardial dysfunction (stunning) results from myofibrillar oedema. Heart. (2008) 94:166–71. doi: 10.1136/hrt.2006.102434
30. Li J, Liu S, Yao R, Tian Y, Yao YA. Novel insight into the fate of cardiomyocytes in ischemia-reperfusion injury: from iron metabolism to ferroptosis. Front Cell Dev Biol. (2021) 9:799499. doi: 10.3389/fcell.2021.799499
31. Sörensson P, Heiberg E, Saleh N, Bouvier F, Caidahl K, Tornvall P, et al. Assessment of myocardium at risk with contrast enhanced steady-state free precession cine cardiovascular magnetic resonance compared to single-photon emission computed tomography. J Cardiovasc Magn Reson. (2010) 12:25. doi: 10.1186/1532-429X-12-25
32. Kim R, Fieno D, Parrish T, Harris K, Chen E, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. (1999) 100:1992–2002. doi: 10.1161/01.CIR.100.19.1992
33. Fernández-Jiménez R, Sánchez-González J, Aguero J, Del Trigo M, Galán-Arriola C, Fuster V, et al. Fast T2 gradient-spin-echo (T2-GraSE) mapping for myocardial edema quantification: first in vivo validation in a porcine model of ischemia/reperfusion. J Cardiovasc Magn Reson. (2015) 17:92. doi: 10.1186/s12968-015-0199-9
34. Fernández-Jiménez R, Martin-García A, Barreiro-Pérez M, Sánchez-González J, Fuster V, Sánchez P, et al. Response by fernández-jiménez et al to letters regarding article, “dynamic edematous response of the human heart to myocardial infarction: implications for assessing myocardial area at risk and salvage”. Circulation. (2018) 137:1754–5. doi: 10.1161/CIRCULATIONAHA.117.032882
35. Carrick D, Haig C, Rauhalammi S, Ahmed N, Mordi I, McEntegart M, et al. Prognostic significance of infarct core pathology revealed by quantitative non-contrast in comparison with contrast cardiac magnetic resonance imaging in reperfused ST-elevation myocardial infarction survivors. Eur Heart J. (2016) 37:1044–59. doi: 10.1093/eurheartj/ehv372
36. Kali A, Choi E, Sharif B, Kim Y, Bi X, Spottiswoode B, et al. Native T1 mapping by 3-T CMR imaging for characterization of chronic myocardial infarctions. JACC Cardiovasc Imaging. (2015) 8:1019–30. doi: 10.1016/j.jcmg.2015.04.018
37. Bauner K, Biffar A, Theisen D, Greiser A, Zech C, Nguyen E, et al. Extracellular volume fractions in chronic myocardial infarction. Invest Radiol. (2012) 47:538–45. doi: 10.1097/RLI.0b013e3182631c37
38. Tahir E, Sinn M, Bohnen S, Avanesov M, Säring D, Stehning C, et al. Acute versus chronic myocardial infarction: diagnostic accuracy of quantitative native T1 and T2 mapping versus assessment of edema on standard T2-weighted cardiovascular MR images for differentiation. Radiology. (2017) 285:83–91. doi: 10.1148/radiol.2017162338
39. Aletras A, Tilak G, Natanzon A, Hsu L, Gonzalez F, Hoyt R, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. (2006) 113:1865–70. doi: 10.1161/CIRCULATIONAHA.105.576025
40. Ugander M, Bagi P, Oki A, Chen B, Hsu L, Aletras A, et al. Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging. (2012) 5:596–603. doi: 10.1016/j.jcmg.2012.01.016
Keywords: acute myocardial infarction, cardiac magnetic resonance, myocardial salvage index, infarct size, area at risk
Citation: Li Y, Wang G, Wang X, Li Y, Zhao Y, Gu X, Xu B, Cui J, Wang X, Sun Y, Liu S and Yu B (2022) Prognostic significance of myocardial salvage assessed by cardiac magnetic resonance in reperfused ST-segment elevation myocardial infarction. Front. Cardiovasc. Med. 9:924428. doi: 10.3389/fcvm.2022.924428
Received: 20 April 2022; Accepted: 01 August 2022;
Published: 30 August 2022.
Edited by:
Zhao Wang, University of Electronic Science and Technology of China, ChinaReviewed by:
Maciej Wybraniec, School of Medicine in Katowice, PolandCopyright © 2022 Li, Wang, Wang, Li, Zhao, Gu, Xu, Cui, Wang, Sun, Liu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengliang Liu, bHNoZW5nbGxAMTI2LmNvbQ==; Yong Sun, c3N1bnl5b25nQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.