
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 26 July 2022
Sec. Cardiovascular Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.923981
 Min Xu1,2†
Min Xu1,2† Zhiyun Zhao1,2†
Zhiyun Zhao1,2† Feixia Shen3†
Feixia Shen3† Ruying Hu4†
Ruying Hu4† Jieli Lu1,2†
Jieli Lu1,2† Yu Xu1,2†
Yu Xu1,2† Tiange Wang1,2
Tiange Wang1,2 Mian Li1,2
Mian Li1,2 Gang Chen5
Gang Chen5 Li Chen6
Li Chen6 Lulu Chen7
Lulu Chen7 Yuhong Chen1,2
Yuhong Chen1,2 Huacong Deng8
Huacong Deng8 Zhengnan Gao9
Zhengnan Gao9 Yanan Huo10
Yanan Huo10 Qiang Li11
Qiang Li11 Chao Liu12
Chao Liu12 Zuojie Luo13
Zuojie Luo13 Yiming Mu14
Yiming Mu14 Guijun Qin15
Guijun Qin15 Yingfen Qin16
Yingfen Qin16 Lixin Shi17
Lixin Shi17 Qing Su18
Qing Su18 Qin Wan19
Qin Wan19 Guixia Wang20
Guixia Wang20 Shuangyuan Wang1,2
Shuangyuan Wang1,2 Youmin Wang21
Youmin Wang21 Shengli Wu22
Shengli Wu22 Yiping Xu23
Yiping Xu23 Li Yan24
Li Yan24 Tao Yang25
Tao Yang25 Zhen Ye4
Zhen Ye4 Xuefeng Yu26
Xuefeng Yu26 Yinfei Zhang27
Yinfei Zhang27 Jiajun Zhao28
Jiajun Zhao28 Tianshu Zeng7
Tianshu Zeng7 Weiqing Wang1,2*
Weiqing Wang1,2* Yufang Bi1,2*
Yufang Bi1,2* Xulei Tang29*
Xulei Tang29* Guang Ning1,2* on behalf of the 4C Study Group
Guang Ning1,2* on behalf of the 4C Study GroupBackgrounds: Whether longitudinal changes in metabolic status influence the effect of kidney stones on cardiovascular disease (CVD) remains unclarified. We investigated the modification effect of status changes in metabolic syndrome (MetS) in the association of kidney stones with risk of incident CVD events.
Methods: We performed a prospective association and interaction study in a nationwide cohort including 129,172 participants aged ≥ 40 years without CVDs at baseline and followed up for an average of 3.8 years. Kidney stones information was collected by using a questionnaire and validated by medical records. The repeated biochemical measurements were performed to ascertain the metabolic status at both baseline and follow-up.
Results: 4,017 incident total CVDs, 1,413 coronary heart diseases (CHDs) and 2,682 strokes were documented and ascertained during follow-up. Kidney stones presence was significantly associated with 44%, 70% and 31% higher risk of CVDs, CHDs and stroke, respectively. The stratified analysis showed significant associations were found in the incident and sustained MetS patients, while no significant associations were found in the non-MetS at both baseline and follow-up subjects or the MetS remission ones, especially in women. For the change status of each single component of the MetS, though the trends were not always the same, the associations with CVD were consistently significant in those with sustained metabolic disorders, except for the sustained high blood glucose group, while the associations were consistently significant in those with incident metabolic disorders except for the incident blood pressure group. We also found a significant association of kidney stone and CVD or CHD risk in the remain normal glucose or triglycerides groups; while the associations were consistently significant in those with incident metabolic disorders except for the incident blood pressure group. We also found a significant association of kidney stone and CVD or CHD risk in the remain normal glucose or triglycerides groups.
Conclusions: A history of kidney stones in women with newly developed MetS or long-standing MetS associated with increased risk of CVD. The mechanisms link kidney stones and CVD risk in the metabolic and non-metabolic pathways were warranted for further studies.
Compelling data showed that kidney stones independently associated with a higher risk of cardiovascular diseases (CVDs), including coronary heart diseases (CHD) and stroke in large cohort studies and meta-analysis of cohort studies (1–6). Kidney stones, also known as nephrolithiasis, was recognized as a systemic disorder and closely associated with many cardiometabolic diseases such as diabetes, obesity, hypertension, metabolic syndrome (MetS) (7–9) and chronic kidney disease (10). However, whether the dynamic changes in metabolic status possess effect on the association of kidney stones with the risk of CVDs remains unexplored.
An increased risk of myocardial infarction was found in women with kidney stones but not in men (1, 3, 5). While in most studies, the prevalence and risk of forming the kidney stones and CVDs is higher in men than that in women. Whether the sex difference of the risk of CVDs in relation to kidney stone needs to be confirmed. The forming of kidney stones and CVDs shared many lifestyle factors, such as old age and unhealthy diet. It has been suggested that lifestyle modifications, such as weight loss, diet improvement, smoking cessation and exercise, will help prevent the development of both kidney stones and vascular disease. However, less evidence is provided.
Both the prevalence of kidney stones and CVDs increased and varies across countries (11–13). According to National Health and Nutrition Examination Survey, the prevalence of kidney stones is 10.6% in men and 7.1% in women, and the number continues to rise (11, 12). In China, the kidney stone prevalence was from 5.95% to 10.63% from the year 1991 to date (13). Kidney stones can cause significant morbidity including urinary tract infection, flank pain, hydronephrosis, decreased renal function, etc. Due to lack of satisfactory therapeutic options and a high recurrence rate of up to 50% within the subsequent 5–10 years after the first episode, the rate of repeated operative intervention is high. Giving the increasing rate of kidney stones and CVDs and the heavy health burden they bring, identifying the modifiers of associations of kidney stones and risk of CVDs is extremely important for high-risk stratification and precision prevention. In the present study, we aimed to investigate primarily the modification effect of changes in metabolic status on the association between kidney stones and risk of incident CVDs in a large, nation-wide, prospective Chinese cohort; secondarily, the modification effect of sex and lifestyle factors on the associations. To clarify the effect of dynamic changes in metabolic status in the association of kidney stones with the risk of CVDs would be helpful to identify the specific and better management of individuals with kidney stones and at a high risk of CVDs.
The study participants were from an ongoing multicenter, population-based cohort study, the China Cardiometabolic Disease and Cancer Cohort (4C) Study (14, 15). Briefly, during 2011–2012, a total of 20 communities from various geographic regions in China were selected. The eligible men and women aged ≥ 40 years were invited to the study by home visits by the trained community health workers. At baseline, we used a questionnaire to collect the lifestyle factors, disease and medical history, etc. We also performed anthropometry measurements, 2-h oral glucose tolerance tests (OGTTs), blood and urine sampling. During 2014–2015, we conducted the first round of follow-up examinations.
We recruited a total of 193,846 individuals at the baseline and 170,240 were invited and participated in the follow-up. We excluded the baseline CVDs or cancers (n = 2,826) or other kidney diseases (n = 1,666), or those were missing information on kidney stones (n = 13,974), or who failed to be followed up for collecting the information on CVD events (n = 22,602). Thus, a total of 129,172 participants at baseline were finally included in the analysis for risk of CVDs (Supplementary Figure 1) (16).
The incident CVD events were the composite of fatal or non-fatal myocardial infarction or stroke, and hospitalized or treated heart failure. The fatal or non-fatal myocardial infarction was also defined as CHD. Myocardial infarction was defined as changes in troponin T and creatine-kinase-MB isoform levels, or in electrocardiogram results, or with symptoms of myocardial ischemia. Stroke was defined as a fixed neurological deficit at least 24 h because of a presumed vascular cause. Heart failure was defined as a clinical syndrome presenting with multiple signs and symptoms consistent with cardiac decompensation or inadequate cardiac pump function. Deaths and clinical outcomes were collected from local vital registries of the National Disease Surveillance Point System and the National Health Insurance System. The Clinical Outcome Adjudication Committee is from Ruijin Hospital, Shanghai Jiao Tong University School of Medicine and composed of ten unbiased experts in cardiology, oncology, neurology, and endocrine and metabolic diseases. All members of the committee were unaware of baseline risk factors of study participants. Two members of the committee were assigned potential causes of mortality according to a standard clinical outcome adjudication procedure and independently verified each clinical event. Discrepancies were adjudicated by discussions involving other members of the committee.
In the time-to-event analysis, participants were censored at the date of CVD diagnosis, death, or the end of follow-up, whichever occurred first. Person-years was calculated from the enrollment date to the censoring date.
Questions about the history of kidney stones were asked in the baseline questionnaires. History of kidney stones was defined as self-reported presence of kidney stones or nephrolithiasis (ICD-10: N20.000), or receiving a procedure of percutaneous nephrolithotomy (PCNL) (ICD-9-CM-3: 55.0402). Participants reporting a history of kidney stones were asked about the date of occurrence, the diagnosed hospital and the tests or procedures they were advised to perform. The self-reported diagnosis was confirmed by the medical records.
We used questionnaires to collect demographic characteristics, dietary and lifestyle factors, family history, and medical history by face-to-face interviews. Smoking status was categorized into current, former and never smoking. Average alcohol consumption was calculated by multiplying the amount of alcohol consumed per drinking day by frequency (g/day). Education attainment was classified as high school and above or less. International Physical Activity Questionnaire was used to assess physical activity. A food frequency questionnaire was used to collect habitual dietary intake by asking the consumption frequency and portion size of typical food items during the previous 12 months (17). According to the 2020 American Heart Association (AHA) Strategic Impact Goals (18), the healthy diet was defined as the follows, 1) the fruit and vegetable intake of at least 4.5 cups/day, 2) the carbonated beverage <450 Kal per day, 3) the red meat consumption <50 g per day, 4) the bean consumption greater equal 25 g per day, and 5) the fish intake greater or equal 7.0 g per day. We assigned each healthy diet habits as 1, without the healthy habit as 0. We summed the total score of all the 5 healthy diet habit as the dietary score.
Body mass index (BMI) was calculated as body weight (kg) divided by the height (squared meters). Waist circumference was measured at the midway between the lower edge of the costal arch and the upper edge of the iliac crest. Three measurements of systolic and diastolic blood pressure (SBP and DBP) were obtained by using an automated electronic device (OMRON Model HEM-752 FUZZY, Dalian, China) in a seated position after at least a 5-min rest, and the average of the 3 readings were used for analysis.
All participants underwent a 2-h, 75-gram OGTT after an overnight fast of at least 10 h. Fasting and 2-h plasma glucose concentrations were measured locally using a glucose oxidase or hexokinase method. Serum insulin, low- and high-density lipoprotein (LDL and HDL) cholesterol, triglycerides, and serum creatinine were measured at the central laboratory using an auto-analyzer (ARCHITECT ci16200, Abbott Laboratories, Chicago, IL, USA). Insulin resistance was estimated by the homeostasis model assessment of insulin resistance (HOMA-IR) index: fasting insulin (μIU/mL) × fasting glucose (mmol/L) /22.5. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (19).
MetS was defined according to the revised National Cholesterol Education Program/Adult Treatment Panel III (NCEP/ATP III) criteria (2004) (20), when three or more of the following criteria were met: 1) blood pressure ≥ 130/85 mmHg or taking antihypertensive drugs, 2) waist circumference ≥ 90 cm in men and ≥ 80 cm in women, 3) triglycerides ≥ 1.69 mmol/L, 4) HDL cholesterol <1.03 mmol/L in men and <1.29 mmol/L in women, 5) fasting plasma glucose ≥ 5.6 mmol/L or taking hypoglycemic medications. The missing values for each metabolic trait were imputed to the mean values for normal distribution variables and the median values for skewed distribution variables.
The change status of the MetS and its components was grouped into four categories: 1) remain no MetS/or each component at both baseline and follow-up examinations, 2) incident MetS/or each component during follow-up, 3) remission of MetS/or each component from baseline to follow-up, and 4) sustained MetS/or each component from baseline to follow-up.
Baseline characteristics of participants were shown as means with standard deviations (SDs), or medians (interquartile range) for continuous variables and numbers with percentages for categorical variables. Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for CVD events associated with baseline kidney stones in total participants, men and women, and in each category of change status of MetS, with adjustments for traditional CVD risk factors, age (years), baseline BMI (kg/m2), waist circumference (cm), quartiles of physical activity, quartiles of sedentary time, smoking status (current, former and never), alcohol drinking (g/day), and education level (high school and above, or less), SBP and DBP (mmHg), fasting and OGTT 2-h glucose (mmol/L), HOMA-IR, LDL and HDL cholesterol (mmol/L), triglycerides (mmol/L), gall stone (yes or no), diet score and eGFR. If covariate information was missing, we imputed the mean values for continuous variables or used a missing indicator for categorical variables. Multiplicative interaction effects of kidney stones and the interested cardiometabolic risk factors or lifestyles on CVD events were tested by including the product term, e.g., MetS category × kidney stones (yes or no), the MetS category, kidney stones (yes or no), and the covariates in the models simultaneously. The covariates were same as above used in the main analysis. Linear regression models with generalized estimating equations (GEE) were used to examine the association of baseline kidney stones with two time-points (baseline and follow-up) measures of the cardiometabolic traits. Statistical significance was assessed at a two-sided P-value of <0.05 by using the SAS software, version 9.4 (SAS Institute Inc).
The 129,172 adults (44,958 men and 84,214 women) aged ≥ 40 years were followed up for an average of 3.8 years. The presence rate of kidney stones was 4.28% in men and 2.68% in women (Table 1). A total of 4,017 incident CVD events were identified after 579,373 person-years of follow-up.
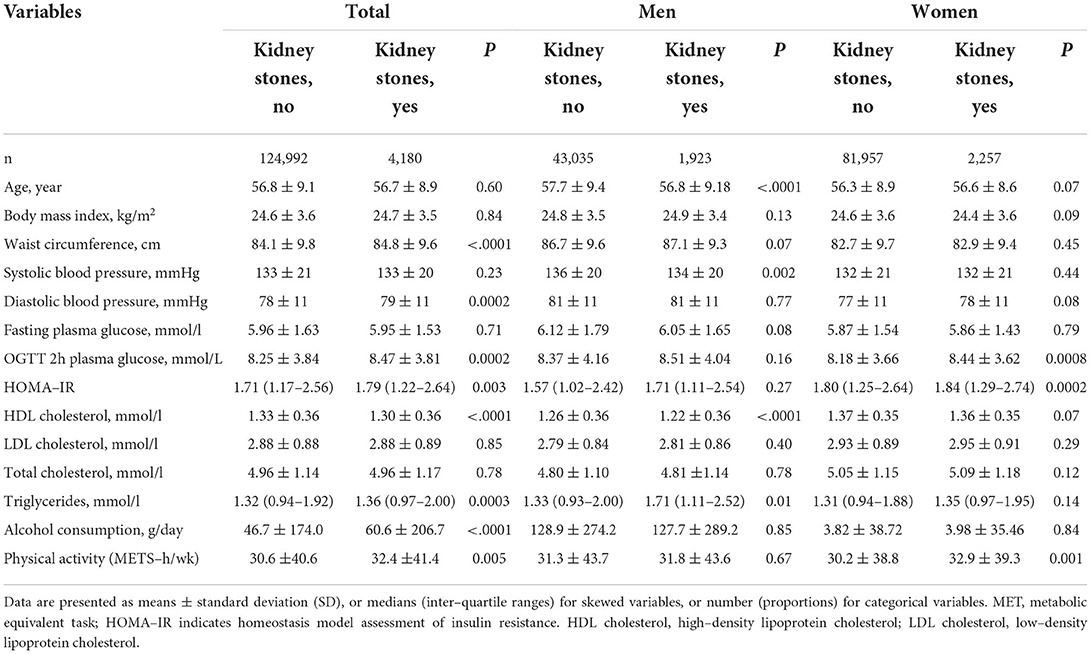
Table 1. Baseline characteristics of study population according to kidney stones presence status by sex.
The presence of kidney stones was associated with a 44% high risk of total CVDs (95% CI, 1.20–1.72; P = 0.0001), 70% of CHDs (95% CI, 1.27–2.28; P = 0.0004) and 31% of stroke (95% CI, 1.04–1.64; P = 0.02). The associations were more prominent in women (the adjusted HR is 1.77 for CVDs [P < 0.0001], 2.50 for CHD [P < 0.0001], and 1.48 for stroke [P = 0.01]); while no significant associations were found in men (all P > 0.35), after adjustments for the conventional risk factors (Table 2). The association was also stronger in participants with MetS (HR 1.66) than that without MetS (HR 1.12) (P for interaction =0.0006) (Supplementary Table 1) (16). We further stratified the participants according to both sex and baseline MetS status, as compared to participants without kidney stones, those with kidney stones was with the most significant higher risk of CVD events in women with MetS (Figure 1). The joint effect analysis showed that in women, as compared to those without MetS or kidney stones, participants with both MetS and kidney stones possessed the highest HRs for incident risk of CVD events (Figure 2A); while in men, the combine effect was not significant either (Figure 2B). The associations were nominally stronger in central obesity, hypertension, dyslipidemia and lower eGFR than those without these disorders (Supplementary Table 1) (16).
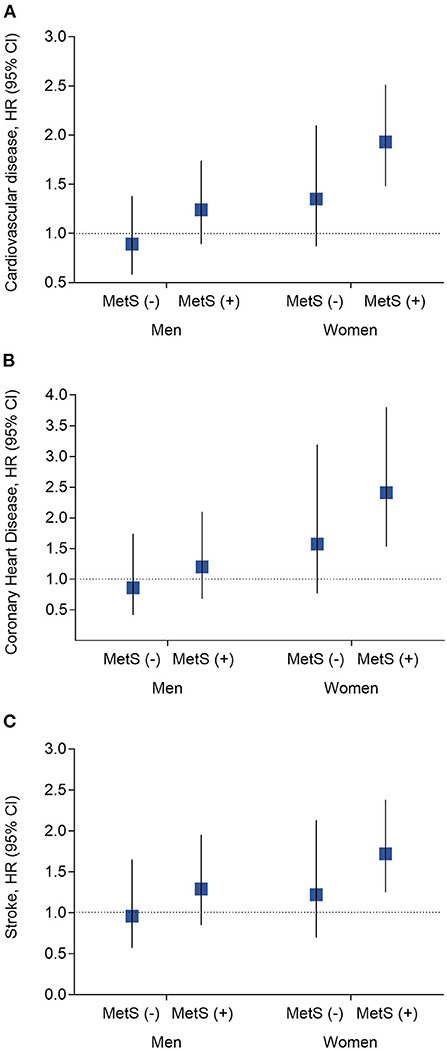
Figure 1. Analysis of the hazard risk of kidney stones with risk of incident cardiovascular events by MetS x sex interaction subgroups. (A) Risk of cardiovascular diseases; (B) Risk of coronary heart disease; (C) Risk of stroke. Data are present as hazard ratio (HR) and 95% confidence interval (CI). P-values were calculated from the multivariable Cox regression models. Adjustments included for age (year), baseline level of body mass index (kg/m2), waist circumference (cm), quartiles of physical activity, quartiles of sedentary time, smoking status (current, former, and never), alcohol drinking (g/l), education level (percentage of high school and above), systolic and diastolic blood pressure (mmHg), fasting plasma glucose (mmol/l), oral glucose tolerance test 2-h glucose (mmol/l), HOMA-IR, low- and high-density lipoprotein cholesterol (mmol/l), and triglycerides (mmol/l), gall stone (yes or no), diet score and eGFR. MetS, metabolic syndrome.
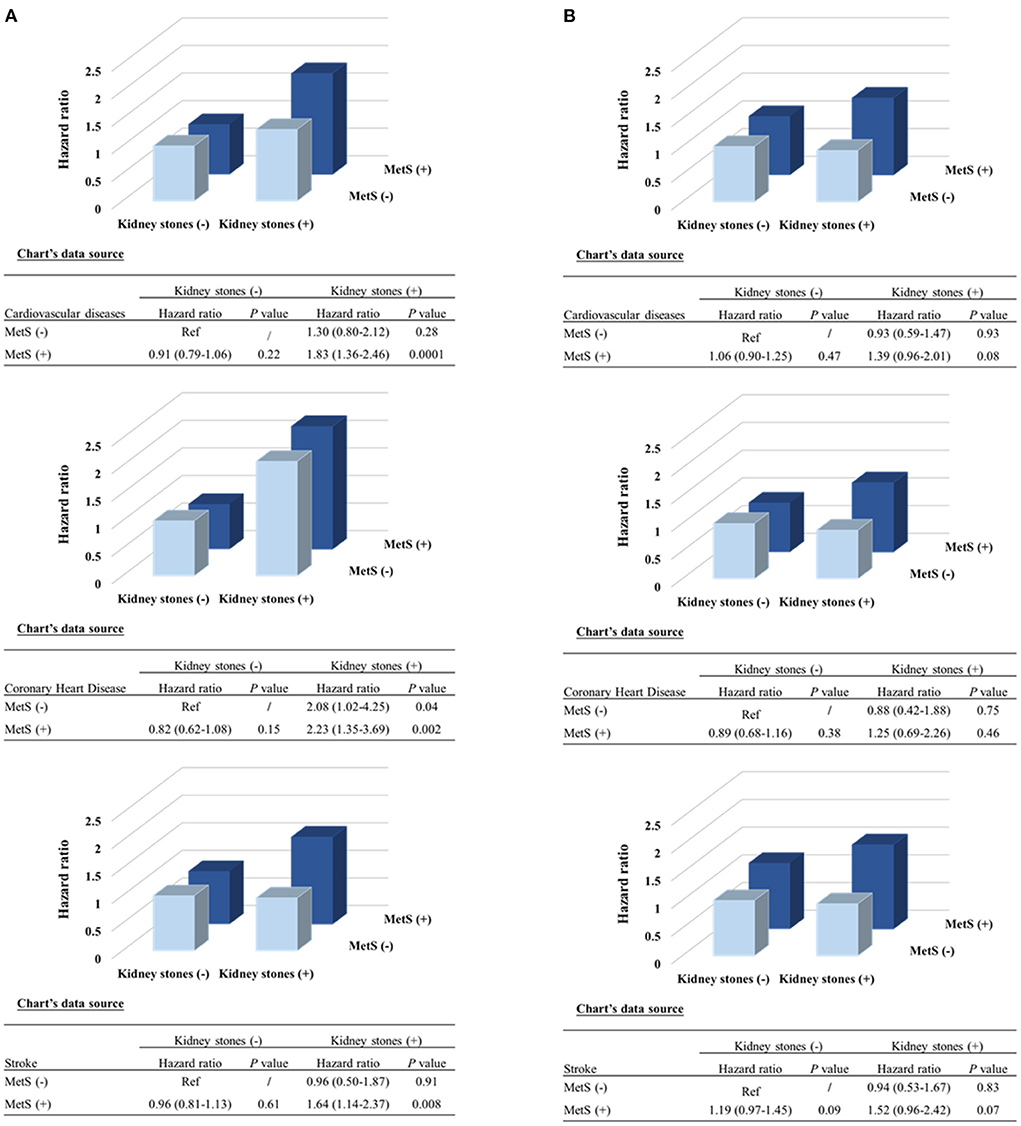
Figure 2. Combined effect of kidney stones and MetS presence on risk of incident cardiovascular events by sex. (A) Women; (B) Men. P-values were calculated from the multivariable Cox regression models, after adjustments for the same covariates as Figure 1. MetS, metabolic syndrome.
The distribution of change status of the MetS and its components were shown in Table 3, Supplementary Figure 2, and Supplementary Table 2 (16). Kidney stones at baseline were significantly associated with a higher risk of incident CVDs and CHDs in the incident MetS group (HR 2.31, 95%CI 1.28–4.18, P = 0.005 for CVD; HR 3.62, 95% CI 1.41–9.27, P =0.007 for CHD) and the sustained MetS group (HR 1.58, 95%CI 1.13–2.22, P= 0.01 for CVD; and HR 2.16, 95% CI 1.22–3.82, P =0.008 for CHD). No significant associations were found in the non-MetS at both baseline and follow-up group (HR 1.33, 95%CI 0.79–2.24 for CVD and 1.67 (0.77–3.64) for CHD, both P ≥ 0.20), or the MetS remission group (HR 1.75, 95%CI 0.91–3.35 for CVD and 1.57 (0.37–6.68) for CHD both P ≥ 0.09). We did not detect a significant association of kidney stones with risk of stroke in each category of the change status of MetS (all P ≥0.12). For the change status of the component of MetS, the trends were not always the same. However, the associations with CVDs were consistently significant in those with sustained metabolic disorders, except for the sustained high blood glucose group; while the associations were consistently significant in those with incident metabolic disorders except for the incident blood pressure group. We also found a significant association of kidney stone and CVD or CHD risk in the remain normal glucose or triglycerides groups. In addition, the association of kidney stone with risk of stroke was significant in the sustained high blood pressure, low HDL_c and high triglycerides group (Table 3). The results were consistently confirmed in women (Supplementary Tables 3, 4) (16).
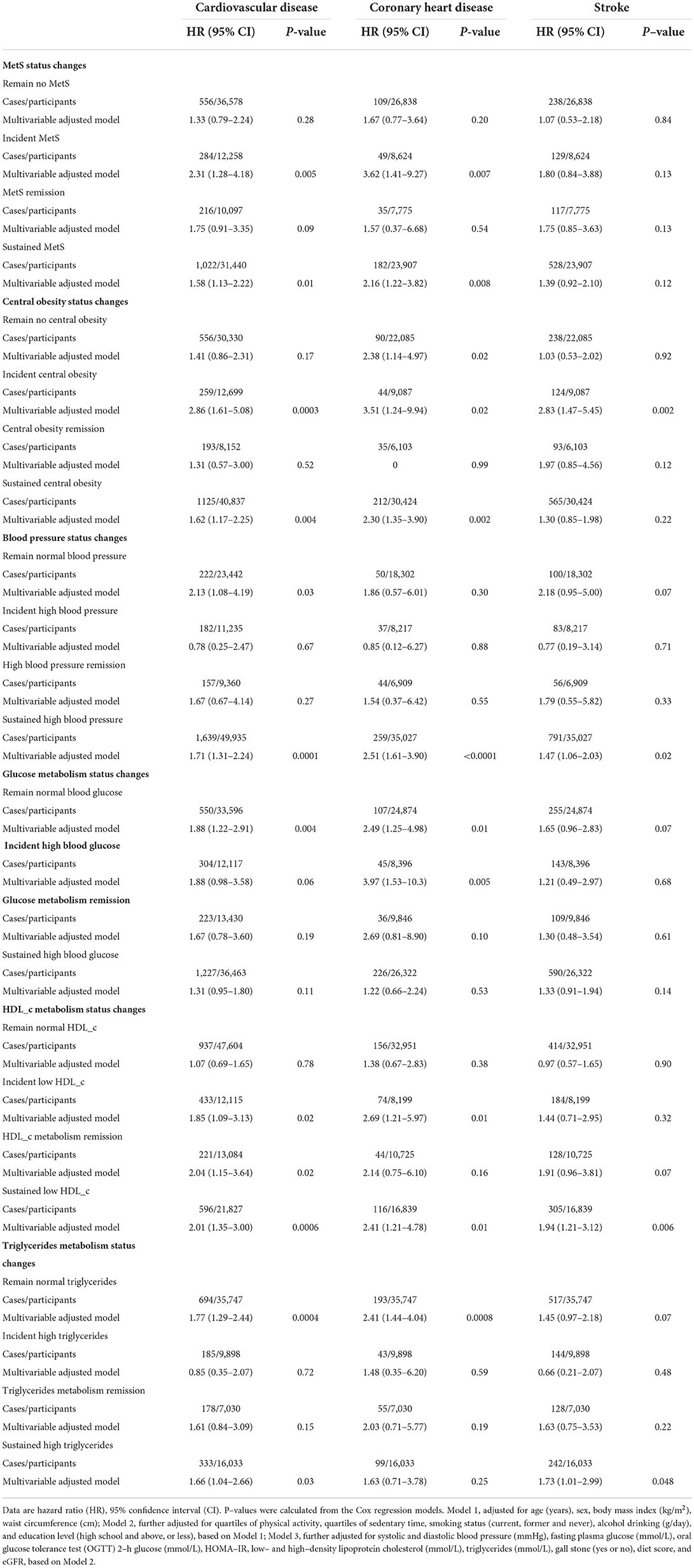
Table 3. Hazard risk of kidney stones with risk of incident cardiovascular events by status changes in metabolic syndrome from baseline to follow–up.
The associations of kidney stones and CVD events were significant in participants who were younger than 65 years, never smoking, non-drinker, with a healthy diet score, and low level of physical activity (Supplementary Figure 3) (16).
We used the GEE models to investigate the presence of baseline kidney stones with the two time points of measurements of cardiovascular profiles (Supplementary Table 5). Baseline kidney stones were significantly associated with a higher level of LDL cholesterol, fasting insulin and HOMA_IR, which are cardio-risk factors, and a lower level of HDL cholesterol. The results were similar in men and women.
From the large nationwide longitudinal study in Chinese, the status changes in MetS and its components significantly modified the associations of kidney stones with the risk of incident CVD events. The presence of kidney stones was associated a higher risk of incident CVDs in the incident and the sustained MetS patients. No significant associations were found in the non-MetS at both baseline and follow-up subjects, or the MetS remission ones. The same trend was found for the risk of incident CHD, while we did not detect a significant association of kidney stones with risk of stroke in each category of the change status of MetS. For the change status of each single component of MetS, the trends were not always the same. However, the associations with CVD were consistently significant in those with sustained metabolic disorders, except for the sustained high blood glucose group; while the associations were consistently significant in those with incident metabolic disorders except for the incident blood pressure group. We also found a significant association of kidney stone and CVD or CHD risk in the remain normal glucose or triglycerides groups. In addition, the association of kidney stone with risk of stroke was significant in the sustained high blood pressure, low HDL_c and high triglycerides group. Our study provides the modifiers of associations of kidney stones with risk of CVDs, and evokes that more attention should be paid to women MetS patients with kidney stones, especially from better prevention and management of metabolic comorbidities and CVD risk point of view.
Kidney stones were found to be associated with a significantly higher risk of CVDs in Caucasians and the western countries. Though there is a considerable ethnicity difference in the prevalence of both kidney stones (12, 13, 21) and CVDs, the estimated risk of kidney stones with CVDs is similar (1, 3, 6). In a meta-analysis including 6 cohort studies that contained 49,597 patients with kidney stones and 3,558,053 controls, with 133,589 cardiovascular events, kidney stones were associated with an increased adjusted risk estimate for CHD (HR, 1.19; 95% CI, 1.05–1.35; P =0.05; n = 6 cohorts) and stroke (HR, 1.40; 95% CI, 1.20–1.64; P = 0.001; n = 3 cohorts). In particular, kidney stones conferred HRs of 1.29 (95% CI, 1.10–1.52; n = 6 cohorts) for myocardial infarction and 1.31 (95% CI, 1.05–1.65; n = 4 cohorts) for coronary revascularization, respectively. In Asians, the urinary calculi were independently associated with a higher risk of developing myocardial infarction (HR, 1.31), stroke (HR, 1.39), and total CVD events (HR, 1.38) from a propensity score-matched cohort study (4). Our results were similar but slightly higher as compared to the reported ones (the HRs for CVDs, CHD and stroke are 1.70, 1.31 and 1.44, respectively), which might mainly be due to different adjustments included. Thus, for the association of kidney stone with the risk of CVDs, our results were largely confirmatory and added evidence based on one large nation-wide Chinese cohorts.
Numerous data showed that the MetS and its components associated with both the forming of kidney stones and the risk of CVDs (22–25). Interestingly, the Alberta Kidney Disease Network reported (3), the associations of kidney stones with acute myocardial infarction events were more significant in subjects without diabetes, hypertension, cancers, or albuminuria. The study provided important stratification strata for the subjects with kidney stones to be cautious of the risk of developing CVDs. However, also there's other studies showed that the association was not significantly different in different age group, history of hypertension, diabetes, or Charlson Comorbidity Index Score (4). In our study, we found a stronger association of kidney stone with risk of CVD (P for interaction = 0.0006), CHD (P for interaction = 0.06) and stroke (P for interaction = 0.002) in those with metabolic syndrome at baseline. The associations were more prominent in those with central obesity, hypertension, dyslipidemia, and eGFR <90, though the interaction tests did not reach significant. The results were mostly consistent with what we found in the modification effect of the change status of the metabolic syndrome and each component of the metabolic syndrome.
Moreover, for the first time, we demonstrated that the longitudinal status changes in MetS and its components modified the associations of kidney stone with the risk of incident CVD events. The kidney stones were significantly associated higher risk of incident CVDs in the incident and the sustained MetS patients. We provided important evidence that those with MetS, especially without any remission of it, would be at much risk for future CVD events, while no significant association was found in those without MetS, even after adjusting for the confounders. We also found that in newly developed and sustained central obesity, newly developed and sustained low HDL cholesterol participants, the associations were stronger than that of without the metabolic disorders.
We did not find a significant modification effect of key lifestyle and dietary factors, such as smoking and drinking habits, physical activities, and healthy dietary habits on the associations of kidney stones and CVD. However, it showed nominally the association between CVD risk and kidney stones was more prominent in younger, never smoker, non-drinker, with healthy diet habit, and a high level of education. In most cases, the one-factor association was more pronounced when other cofounding was not existing. Take the above results in all, we speculated that this might be due to the absence of competing risk factors for CVDs in these relatively healthy subgroups, which is a common phenomenon in the epidemiological studies (3). When it comes to the development of the outcome disease and the change status of the co-existed metabolic disorders, in a more serious disease progression status, the risk of kidney stones, which were considered one of the systematic metabolic dysfunctions, might exert a higher risk for the CVDs risk. It is hard to give an exact explanation about the “contradiction”; however, this modification effect of the lifestyle factors or the other confounding factor at baseline and the longitudinal change in metabolic traits as well needs further investigation, especially to establish the pathophysiological basis of the associations and the modification effect.
We detected a significant sex difference in the association of kidney stone with risk of CVDs and the modification effect of the changes in metabolic status. The presence rate of kidney stones is higher in men than that in women. The incidence rate of CVD, CHD or stroke is higher in men than that in women (3.99% vs. 2.64% CVDs, 1.56% vs. 0.85% CHD, 2.51% vs. 1.85% stroke), too, as well as age, BMI, waist circumference, blood pressure, fasting and 2h-OGTT glucose, HDL-c, TG, and higher level of smoking, alcohol intake and physical activity. The data indicated that it is unlikely because of more metabolic disorders or risk factors in women, in fact, women are in a relatively healthy status.
However, we did find that as compared with men, more women were in sustained metabolic syndrome or incident metabolic syndrome (38.0% vs. 28.5%, and 13.7% and 13.3%, respectively); more sustained or incident central obesity (52.3% vs. 28.9, and 14.1% vs. 13.2%); more sustained or incident HDL-c (29.6% vs. 10.3%, and 14.5% vs. 9.1%); more sustained or incident high blood pressure (61.5% vs. 59.9%, and 12.3% vs. 11.8%) (Supplementary Table 2). It was consistent with that in the above sustained or incident metabolic disorders, the association of kidney stones with risk of CVDs was more prominent. We did not find a higher rate of incident or sustained high blood glucose in women than that in men. Similarly, we did not identify that the presence of kidney stones was associated a higher risk of incident CVDs in the incident and the sustained high blood glucose patients either.
However, we could not exclude the possibility that the difference was due to sex or women may be more likely exposed than men to unknown factors that could increase their cardiovascular and kidney stone risk, which need to be confirmed by further prospective studies. The mechanisms underlying this association also should be directed.
The forming of kidney stones and CVD shared many risk factors, such as obesity, hypertension, insulin resistance, and unhealthy dietary habits (26). The previous study discussed the potential mechanism for the occurs of kidney stones and CVD (27). Both diseases are chronic and characterized by accumulation of oxidized proteins and lipids in the renal tissue and arterial wall, respectively. On one hand, the dyslipidemia, perturbation of gut microbiome, obesity, high-fat diet, genetic factors, and infections produce higher oxalate, uric acid, calcium that leads to supersaturation of salts in urine. Increased reactive oxygen species (ROS) also enhances the crystal nucleation and aggregation it causes the crystal retention and the subsequent stone formation (27). On the other hand, during uric acid formation, ROS is formed from hypoxanthine. ROS causes the oxidation of LDL, and uric acid induces the TG biogenesis, both of which impair the endothelial dysfunction and lead to atherosclerosis (27). However, when we adjusted those shared factors, the kidney stones were still significantly associated with a higher risk of CVD. It may suggest pathway other than the known mechanism in the process of kidney stone formation and CVD events.
Among the several shared risk factors, insulin resistance was the common soil of the MetS, kidney stones and CVD risk, and oxidative stress might play an important role in the process from insulin resistance to endothelial cell dysfunction (28, 29). Dietary factors contributed to the forming kidney stones and increased risk of CVDs, such as high salt and sugar, low fruit and vegetables (26). It has been proposed taking actions in diet changes that are aimed at stone prevention (30) maybe at reduction of CVDs. The ideal cardiovascular health diet (18, 30) or the Mediterranean diet (31) was found not only better for prevention of CVDs, but also for prevention of kidney stone forming.
We have several strengths including the large nationwide sample size, the prospective study design, the repeated blood sampling and biochemical measurements, and the relatively full adjustments for potential confounders, and the well-validated adjudications of the incident CVD events. Limitations should be acknowledged. Firstly, the information on the presence of kidney stones was self-reported. It may underestimate the presence of kidney stones; however, the cases were validated by the medical records by the physician's diagnosis or the undergoing procedures. The recall bias could be minimized to the minimum. For one might not ignore the stone disease because of the obvious symptoms, such as pain, hematuria. Secondly, we could not determine the specific type of kidney stones. The most common types are made from calcium and oxalate, uric acid or cystine. Since uric acid stones are relatively rare in females (32), we speculated that calcium stones may be responsible for the associations. Thirdly, causality could not be determined between kidney stones and CVDs. The large bilateral Mendelian Randomization studies are needed to confirm our findings. Finally, we used the updated NCEP-ATPIII definitions for Asian-Americans. A previous study performed in a rural Chinese population showed that the Joint Interim Statement (JIS), International Diabetes Federation (IDF) and Chinese Diabetes Society (CDS) criteria may not be more suitable than the updated NCEP-ATPIII definitions, for screening high-risk individuals and estimating the risk of CHD and stroke from MetS (33). Thus, it would be acceptable for the present analysis using the updated NCEP-ATPIII definitions. Even though, the association of kidney stones, metabolic status, and CVDs in other age and ethnicity groups need further studies.
In conclusion, the present analysis suggest that a thorough cardiovascular assessment should be considered in patients who develop kidney stones especially in women, and in those with multi-metabolic disorders. Taking measures to prevent metabolic comorbidity may benefit both kidney and CVDs prevention, especially for those with both conditions.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
MX, ZZ, FS, RH, JL, and YuX: conceptualization, formal analysis, and writing–original draft. TW, ML, GC, LiC, LuC, YC, HD, ZG, YH, QL, CL, ZL, YM, GQ, YQ, LS, QS, QW, GW, SWa, YW, SWu, YiX, LY, TY, ZY, XY, YZ, JZ, and TZ: acquisition of data, revision of the manuscript for intellectual content. WW, YB, XT, and GN: supervision, funding acquisition, and revision of the manuscript for intellectual content. MX, WW, YB, XT, and GN: conceptualization, formal analysis, funding acquisition, and critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81941017, 81930021, 81970706, 81970728, 81870560, 82088102, 82022011, and 91857205), the Science and Technology Committee of Shanghai (20Y11905100) and the Clinical Research Plan of SHDC (SHDC2020CR1001A and SHDC2020CR3064B), and the Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant Support (20152508 Round 2). MX, JL, ML, TW, YX, ZZ, YB, WW, and GN are members of innovative research team of high-level local universities in Shanghai.
The authors would like to thank all staff and study participants involved in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.923981/full#supplementary-material
1. Ferraro PM, Taylor EN, Eisner BH, Gambaro G, Rimm EB, Mukamal KJ, et al. History of kidney stones and the risk of coronary heart disease. JAMA. (2013) 310:408–15. doi: 10.1001/jama.2013.8780
2. Rule AD, Roger VL, Melton LJ. 3rd, Bergstralh EJ, Li X, Peyser PA, et al. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol. (2010) 21:1641–4. doi: 10.1681/ASN.2010030253
3. Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Samuel S, Klarenbach SW, et al. Alberta Kidney disease network. Kidney stones and cardiovascular events: a cohort study. Clin J Am Soc Nephrol. (2014) 9:506–12. doi: 10.2215/CJN.04960513
4. Hsu CY, Chen YT, Huang PH, Leu HB, Su YW, Chiang CH, et al. The association between urinary calculi and increased risk of future cardiovascular events: a nationwide population-based study. J Cardiol. (2016) 67:463–70. doi: 10.1016/j.jjcc.2015.07.016
5. Liu Y, Li S, Zeng Z, Wang J, Xie L, Li T, et al. Kidney stones and cardiovascular risk: a meta-analysis of cohort studies. Am J Kidney Dis. (2014) 64:402–10. doi: 10.1053/j.ajkd.2014.03.017
6. Peng JP, Zheng H. Kidney stones may increase the risk of coronary heart disease and stroke: A PRISMA-Compliant meta-analysis. Medicine (Baltimore). (2017) 96:e7898. doi: 10.1097/MD.0000000000007898
7. Devarajan A. Anecdotes of lithogenesis and atherogenesis conversely liable for cardiac dysfunction and kidney stone formation. Urolithiasis. (2015) 43:197. doi: 10.1007/s00240-015-0754-8
9. Sakhaee K, Maalouf NM, Sinnott B. Clinical review. Kidney stones 2012: pathogenesis. diagnosis, and management. J Clin Endocrinol Metab. (2012) 97:1847–60. doi: 10.1210/jc.2011-3492
10. Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Morgan C, Samuel S, et al. Alberta kidney disease network. kidney stones and kidney function loss: a cohort study. BMJ. (2012) 345:e5287. doi: 10.1136/bmj.e5287
11. Scales CD Jr, Smith AC, Hanley JM, Saigal CS. Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. (2012) 62:160–5. doi: 10.1016/j.eururo.2012.03.052
12. Abufaraj M, Xu T, Cao C, Waldhoer T, Seitz C, D'andrea D, et al. Prevalence and trends in kidney stone among adults in the USA: analyses of National Health and Nutrition Examination Survey 2007-2018 Data. Eur Urol Focus. (2021) 7:1468–75. doi: 10.1016/j.euf.2020.08.011
13. Wang W, Fan J, Huang G, Li J, Zhu X, Tian Y, et al. Prevalence of kidney stones in mainland China: a systematic review. Sci Rep. (2017) 7:41630. doi: 10.1038/srep41630
14. Wang T, Lu J, Shi L, Chen G, Xu M, Xu Y, et al. China Cardiometabolic Disease and Cancer Cohort Study Group. Association of insulin resistance and β-cell dysfunction with incident diabetes among adults in China: a nationwide, population-based, prospective cohort study. Lancet Diabetes Endocrinol. (2020) 8:115–24. doi: 10.1016/S2213-8587(19)30425-5
15. Wang T, Lu J, Su Q, Chen Y, Bi Y, Mu Y, et al. 4C Study Group. Ideal cardiovascular health metrics and major cardiovascular events in patients with prediabetes and diabetes. JAMA Cardiol. (2019) 4:874–83. doi: 10.1001/jamacardio.2019.2499
16. Xu M, Zhao Z, Shen F, Hu R, Lu J, Xu Y. Supplementary Data for “Modification Effect of Changes in Cardiometabolic Traits in Association Between Kidney Stones Cardiovascular Events.” (2022). Available online at: https://figshare.com/articles/_resource/Online-Only_Supplementary_Data-Kidneystone_and_CVD_pdf/20297190
17. Bi Y, Jiang Y, He J, Xu Y, Wang L, Xu M, et al. 2010 China Noncommunicable Disease Surveillance Group. Status of cardiovascular health in Chinese adults. J Am Coll Cardiol. (2015) 65:1013–25. doi: 10.1016/j.jacc.2014.12.044
18. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. (2010) 121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703
19. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF. 3rd, Feldman HI, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) a new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
20. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
21. Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. (2010) 12:e86–96.
22. Rendina D, Mossetti G, De Filippo G, Benvenuto D, Vivona CL, Imbroinise A, et al. Association between metabolic syndrome and nephrolithiasis in an inpatient population in southern Italy: role of gender, hypertension and abdominal obesity. Nephrol Dial Transplant. (2009) 24:900–6. doi: 10.1093/ndt/gfn548
23. Gambaro G, Ferraro PM, Capasso G. Calcium nephrolithiasis, metabolic syndrome and the cardiovascular risk. Nephrol Dial Transplant. (2012) 27:3008–10. doi: 10.1093/ndt/gfs139
24. Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. (2005) 293:455–62. doi: 10.1001/jama.293.4.455
25. Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. (2005) 68:1230–5. doi: 10.1111/j.1523-1755.2005.00516.x
26. D'Alessandro C, Ferraro PM, Cianchi C, Barsotti M, Gambaro G, Cupisti A. Which diet for calcium stone patients: a real-world approach to preventive care. Nutrients. (2019) 11:1182. doi: 10.3390/nu11051182
27. Devarajan A. Cross-talk between renal lithogenesis and atherosclerosis: an unveiled link between kidney stone formation and cardiovascular diseases. Clin Sci (Lond). (2018) 132:615–26. doi: 10.1042/CS20171574
28. Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. (2004) 24:816–23. doi: 10.1161/01.ATV.0000122852.22604.78
29. Spatola L, Ferraro PM, Gambaro G, Badalamenti S, Dauriz M. Metabolic syndrome and uric acid nephrolithiasis: insulin resistance in focus. Metabolism. (2018) 83:225–33. doi: 10.1016/j.metabol.2018.02.008
30. Taylor EN, Stampfer MJ, Mount DB, Curhan GC. DASH-style diet and 24-hour urine composition. Clin J Am Soc Nephrol. (2010) 5:2315–22. doi: 10.2215/CJN.04420510
31. Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. (2018) 378:e34. doi: 10.1056/NEJMoa1800389
32. Knoll T, Schubert AB, Fahlenkamp D, Leusmann DB, Wendt-Nordahl G, Schubert G. Urolithiasis through the ages: data on more than 200,000 urinary stone analyses. J Urol. (2011) 185:1304–11. doi: 10.1016/j.juro.2010.11.073
Keywords: kidney stone, CVD, metabolic disorders, modification effect, longitudinal change
Citation: Xu M, Zhao Z, Shen F, Hu R, Lu J, Xu Y, Wang T, Li M, Chen G, Chen L, Chen L, Chen Y, Deng H, Gao Z, Huo Y, Li Q, Liu C, Luo Z, Mu Y, Qin G, Qin Y, Shi L, Su Q, Wan Q, Wang G, Wang S, Wang Y, Wu S, Xu Y, Yan L, Yang T, Ye Z, Yu X, Zhang Y, Zhao J, Zeng T, Wang W, Bi Y, Tang X and Ning G (2022) Modification effect of changes in cardiometabolic traits in association between kidney stones and cardiovascular events. Front. Cardiovasc. Med. 9:923981. doi: 10.3389/fcvm.2022.923981
Received: 20 April 2022; Accepted: 01 July 2022;
Published: 26 July 2022.
Edited by:
Hongliang Li, Wuhan University, ChinaReviewed by:
Yoshihiro Miyamoto, National Cerebral and Cardiovascular Center, JapanCopyright © 2022 Xu, Zhao, Shen, Hu, Lu, Xu, Wang, Li, Chen, Chen, Chen, Chen, Deng, Gao, Huo, Li, Liu, Luo, Mu, Qin, Qin, Shi, Su, Wan, Wang, Wang, Wang, Wu, Xu, Yan, Yang, Ye, Yu, Zhang, Zhao, Zeng, Wang, Bi, Tang and Ning. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiqing Wang, d3Fpbmd3NjFAMTYzLmNvbQ==; Yufang Bi, YnlmMTA3ODRAcmpoLmNvbS5jbg==; Xulei Tang, eHVsZWlfdGFuZ0AxMjYuY29t; Guang Ning, Z25pbmdAc2licy5hYy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.