- 1Department of Biomedical Engineering, School of Medicine, University of Alabama at Birmingham, Birmingham, AL, United States
- 2Department of Biomedical Engineering, School of Engineering, University of Alabama at Birmingham, Birmingham, AL, United States
Extracellular vesicles (EVs) are lipid bilayer particles naturally released from most if not all cell types to mediate inter-cellular exchange of bioactive molecules. Mounting evidence suggest their important role in diverse pathophysiological processes in the development, growth, homeostasis, and disease. Thus, sensitive and reliable assessments of functional EV cargo transfer from donor to acceptor cells are extremely important. Here, we summarize the methods EV are labeled and their functional transfer in acceptor cells are evaluated by various reporter systems.
Introduction
Extracellular vesicles (EVs) are lipid-bilayer membrane-enclosed structures released by most if not all cell types in physiological and pathological environments. They are classified into subtypes, including exosomes, ectosomes, and apoptotic bodies, based on the origin of biogenesis, and have different yet overlapping sizes and compositions (1–6). Exosomes (50–150 nm) are formed as intraluminal vesicles of endosomes and released into extracellular space upon endosome and cell membrane fusion, whereas ectosomes (0.1–1 μm) bud out directly from the plasma membrane (6, 7). Apoptotic bodies (50–5000 nm), on the other hand, dislodge from dying and disintegrating cells (8, 9). Loaded with a large spectrum of bioactive agents (i.e., proteins, RNAs, lipids, and metabolites) from donor cells (3, 4), EVs mediate effective transfer of cargos locally to neighboring cells or remotely via blood circulation to cells of other organs, thereby impacting the functional states of acceptor cells, tissue homeostasis, and disease pathophysiology (4, 10–16). Upon interacting with acceptor cells, EVs can affect their function via triggering membrane-mediated intracellular signaling, fusing with cell membrane to release bioactive cargos into the cytoplasm, or being endocytosed into endosomal system to evade lysosomal degradation through largely unknown mechanisms to regulate cellular activities. For example, exosome-derived ectopic mRNA or miRNA can translate into functional proteins or suppress mRNA translation, respectively, in acceptor cells. Given the often low abundance of exosome-delivered molecules in acceptor cells relative to endogenously expressed biomolecules and the complex intracellular feedback networks, accurate measurements of EV-mediated effects is vital to EV research. Here we summarize a number of reported methods EVs are labeled and their functional transfer are evaluated.
Extracellular Vesicle Labeling
Labeling of Isolated Extracellular Vesicles With Chemical Dyes
Labeling of EVs with detectable molecules is widely used for tracking EV biodistribution and uptake into target cells. Various components of EV membrane, particularly lipids and proteins, can be labeled with chemical dyes. Available lipophilic fluorescent dyes include PKH26 (excitation/emission wavelength maxima, λex/λem = 551/567 nm), PKH67 (λex/λem = 490/502 nm), DiO (λex/λem = 484/501 nm), DiI (λex/λem = 549/565 nm), DiR (λex/λem = 750/780 nm), and FM 4–64 (λex/λem = 558/734 nm). While these dyes insert into the membrane lipid bilayer of EVs, generating stable and long-lasting fluorescence signal (17–23), it was reported that at least PKH dyes can trigger EV enlargement as the result of membrane fusion or intercalation (24). Protein fluorescent membrane dyes include carboxyfluorescein succinimidyl diacetate ester fluorescent (25, 26) and maleimide flours (27), which may overcome certain limitations associated with other lipophilic (DiI, PKH67), non-lipophilic (ExoGlow-Vivo), and RNA (SYTO) dyes (27–29).
Labeling of Isolated Extracellular Vesicles With Aptamers
An aptamer is a short single-stranded DNA or RNA molecule with unique structural features that ensure binding to specific molecular target with known or unknown identity (30). Aptamers are usually selected from synthetic libraries using systematic evolution of ligands by exponential enrichment (SELEX). LZH8, an aptamer selected by using whole HepG2 hepatocytes (31), demonstrates impressive binding affinity to HepG2 cell-derived exosomes (32). Wan et al. linked LZH8 to a “trigger” sequence, so that the LZH8-trigger was able to bind to HepG2-exosomes meanwhile extending by base pairing between the “trigger” sequence and fluorescein (FITC)-conjugated oligos to amplify the FITC signal and enlarge the overall structure, allowing direct flow cytometry analysis of these modified exosomes (32). Nevertheless, whether LZH8 aptamer conjugation affects exosome tropisms or uptake by target cells remain to be elucidated.
Labeling of Isolated Extracellular Vesicles With Radioisotope or Magnetic Resonance Imaging Contrast Agents
Recently, evidence suggests that radionuclides or magnetic resonance imaging (MRI) contrast fluid can be loaded into the isolated EVs, enabling imaging of administered EVs in vivo by nuclear and MRI approaches (33–36). This method is particularly beneficial for deep tissue imaging with the potential of clinical application.
Tagging of Extracellular Vesicle Surface Protein via Genetic Engineering
Exosome membrane contains abundant tetraspanins (CD63, CD81, CD9) and lactadherin, which are often used as exosome biomarkers (37). These molecules were genetically engineered to fuse with fluorescent proteins/bioluminescence-generating enzymes, so the exosomes can be visualized under fluorescent microscope or by supplementation with bioluminogenic substrates (38–41). For example, CD63 fused with pHluorin, a pH-sensitive form of green fluorescent protein (GFP), permits tracking of endogenous EVs in the transparent zebrafish with high spatiotemporal accuracy, leading to the finding that the yolk syncytial layer-derived exosomes are endocytosed by macrophages and endothelial cells of the caudal vein plexus (CVP) in a scavenger receptor- and dynamin-dependent manner, thereby providing the trophic support for CVP growth (42).
Among different bioluminescence-generating enzymes, it appears that NanoLuc or ThermoLuc, when tethered to CD63, are preferred for sensitive imaging and tracking of EVs in vivo and in vitro (43). Luo et al. generated a transgenic mouse line that expresses CD63-NanoLuc fusion protein specifically in cardiomyocytes, thus cardiomyocyte-derived EVs, and found NanoLuc signals in thymus, testis, lung, and kidney, supporting the notion that cardiomyocyte-derived EVs mediate molecular exchange between heart and other organs (44). Besides CD63, lactadherin was also engineered, such as by fusion with Gaussia luciferase to visualize and track exosomes in vivo (45, 46). In a sophisticated system, EV surface was tagged with a membrane-bound biotin acceptor peptide linked outward to Gaussia luciferase fusion protein and biotin ligase, thus the EVs can be labeled with supplemented biotin and visualized in vivo with duo modal imaging, bioluminescence (with luciferin) and fluorescence (with streptavidin-fluorophore) (47). Lastly, genetic engineering can also achieve radiolabeling. Takakura et al. engineered lactadherin–streptavidin fusion protein on exosomes and used iodine-125 (125I)-labeled biotin to label exosomes (48, 49).
Genetic engineering of donor cells to molecularly tag EV surface proteins avoids additional labeling processes and has the advantage of achieving cell-specific EV labeling in vivo. Also, it is likely that the engineered proteins, displaying on EV membrane, have significantly lesser untoward effects on the biochemical property of EVs, compared to chemical dyes. However, it should be aware that EVs are diverse in the expression of biomarkers, therefore these methods may be limited by labeling only a certain subgroup of EVs. In addition, although reports were mostly focused on EV membrane proteins, intraluminal soluble proteins, such as exosome-enriched ALIX and TSG101, may also have the potential for tagging, which would indicate the uptake and intracellular trafficking of exosome-derived non-membranous proteins. Lastly, the results obtained from these experiments may still need to be interpreted with care, as the signals are subject to the processing of individual fusion proteins and may not indicate the function of the entire EV proteome.
Bio-Conjugation of Extracellular Vesicle Proteins
Azide–alkyne cycloaddition (click chemistry) is a powerful tool that permits covalent conjugation, thus tagging, of exosomes. Azide or alkyne group can be incorporated into EVs by supplementing azide or alkyne bearing amino acids (e.g., AHA) (50, 51) or glycans/proteoglycans (52) to EV-producing cells, in vitro or in vivo, or by directly adding azide or alkyne bearing chemicals to the isolated EVs (53). The click reaction is catalyzed by Cu, but also can occur in Cu-free physiological fluids permitting in vivo labeling (54, 55). Diverse imaging modalities (fluorescence, luminescence, radioactive imaging, and MRI) can be adapted to the system for in vivo tracking of the labeled EVs. Interestingly, click reactions seem not to affect the size of the exosomes, nor exosome adhesion or internalization in target cells (53).
Notably, David Tirrell group has identified a methionyl-tRNA synthetase L274G mutant (MetRS*), which utilizes the non-canonical azide-bearing amino acid azidonorleucine (ANL) as surrogate of methionine to incorporate into newly synthesized proteins (56). Engineered expression of MetRS* in the neuron enabled ANL-labeling and click-reaction based identification of neuronal specific proteomes in vivo (57). Our group introduced MetRS* into mesenchymal stem cells (MSCs) and administered these cells into the ischemic heart of mice supplemented with ANL, followed by serial isolation of azide-labeled (i.e., MSC-derived) proteins from total cardiac protein lysates. MSCs are believed to exert beneficial effects via paracrine mechanisms, and our study for the first time revealed MSC proteome real-time in situ in the injured cardiac tissue, revealing new insights into MSC mediated cardiac protection and repair (58). We also isolated EVs from ANL-treated MSCs and administered these EVs to mice with surgically induced myocardial infarction. The ANL-labeled (i.e., MSC exosome-derived) proteins were isolated with click-catalyzed alkyne-agarose capture from various organs at different time points and identified with mass spectrometry; the MSC exosomal proteins were also localized histologically via fluorescent non-canonical amino-acid tagging (FUNCAT) in situ. We found that MSC exosomal proteins distributed in different organs are highly diverse, that ischemic injury significantly augments the tissue intake of exosomes, and that in the injured tissue, the exosomal proteins are predominantly associated with cytosol vs. membrane (51).
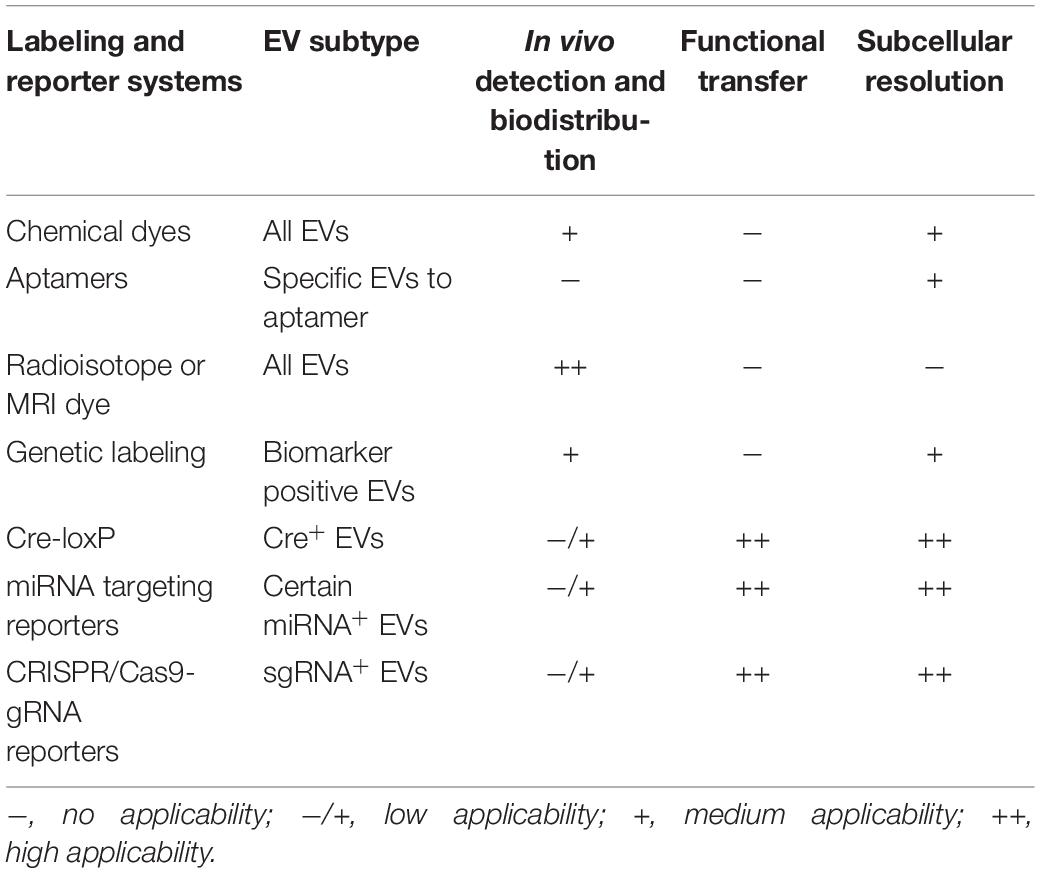
Collectively, labeling of EVs has considerably facilitated assessments of their biodistribution and cellular uptake (Table 1), however, it does not allow discrimination between non-functional uptake (lysosomal degradation) and functional transfer or delivery (protein-mediated signaling, mRNA translation, or miRNA repression of target mRNA).
Reporters of Extracellular Vesicle Transfer in Acceptor Cells
While EVs carry various types of bioactive cargos, their mRNA and miRNA activities in the recipient cells are most frequently used to evaluate EV functional transfer (47, 59). An ideal reporter system entails low basal reporter activity in the absence of EVs but highly induced reporter activity when EVs are present. Frequently used reporters are based on the Cre-loxP system, miRNA recognition size on target mRNA 3′UTR, and CRISPR/Cas9 system (Table 1).
Cre-loxP
The reporter gene is placed downstream of a floxed stop codon in the expression cassette introduced in recipient cells and activated when the cells are transduced with EVs from Cre-expressing donor cells (60). Ridder et al. observed LacZ reporter activation in neurons after taking up EVs carrying functional Cre messenger RNA from immune cells, establishing a unique EV-mediated immune cells to neuron crosstalk (61, 62). In another study, by comparing three Cre reporter mice, Rosa26-LacZ, Rosa26-EGFP, and Rosa26-EYFP, the authors found that Gr1+CD11b+ myeloid-derived suppressor cells (MDSCs) is a major cell population targeted by tumor-released EVs, and that EV transfer augments the immunosuppressive phenotype of these cells (63).
Zomer et al. optimized this Cre-loxP system where the transfer of Cre-bearing EVs induces a switch of DsRed to eGFP expression in the Cre-reporter cells (60). Interestingly, Cre mRNA but not Cre protein was detected in EVs, suggesting that Cre mRNA transfer was primarily responsible for the DsRed-to-eGFP switch (60). Furthermore, the authors applied this Cre-mediated DsRed to eGFP conversion system in vivo and found that the recipient tumor cells taking up EVs from highly malignant tumor cells display an augmented migratory and metastatic activity, suggesting that EVs are able to transfer the malignant property of tumors (64). Interestingly, Sterzenbach et al. engineered the expression of Cre protein fused with a WW tag (WW-Cre), which can be recognized by the evolutionarily conserved late-domain L-domain-containing protein Ndfip1, leading to increased Cre protein ubiquitination, thus packing into exosomes; using this system, the authors demonstrated that administered exosomes via nasal route reached brain cells, leading to Cre-mediated recombination in mT/GFP mice (65). This study, however, indicated functional delivery of Cre protein through exosomes.
Taken together, the Cre-loxP based reporters have proven that exosomes can mediate functional transfer of Cre mRNA and protein. It should be noted that Cre-loxP system has been used extensively in lineage tracing studies in the past and the Cre mediated reporter expression is rarely in no-Cre expressing cells. Given that exosomes pack predominantly small RNAs or mRNA fragments (66), it is likely that the Cre-loxP system, without enhancement of Cre loading into exosomes, would underestimate EV transfer.
miRNA Targeting Reporters
miRNAs are predominantly enriched in EVs (59) and well recognized as important effectors responsible for EV induced biological response (66). miRNA target mRNA at specific sequence primarily located in the 3′UTR, inhibiting mRNA translation and inducing mRNA degradation. Dual-luciferase reporter systems are engineered by placing wild-type 3′UTR or miRNA-targeting-sequence mutated 3′UTR downstream of a firefly luciferase reporter; overexpression of miRNA decreases luciferase expression and activity with wild-type 3′UTR but not with mutated 3′UTR (67). Using this system, Thomou et al. engineered an adenoviral vector containing 3′UTR luciferase reporter for human-specific miRNA hsa_miR-302f and transduced mouse liver. They found that expression of pre-hsa_miR-302f but not control miRNA in the brown adipose tissue (BAT) led to >95% reduction of luciferase activity in the liver, indicating EV transfer of hsa_miR-302f from BAT to liver (68). In the mice with adipose-specific knockout of the miRNA-processing enzyme Dicer (ADicerKO), which has a markedly reduced mature miRNAs, the authors found that serum EVs from wild-type mice significantly decreased the level of luciferase activity with a FGF21 mRNA 3′UTR reporter in the ADicerKO liver, further confirming that BAT-derived EVs transfer miRNA to affect gene regulation of the liver (68).
CRISPR/Cas9 – gRNA Reporters
Recently, de Jong et al. generated an elegant CRISPR/Cas9 based reporter system (69). It is known that Cas9 nuclease mediates non-homologous end joining (NHEJ) repair that creates frameshifts. The reporter construct is driven by CMV promoter; the expression cassette contains genes of mCherry and two alternative reading frames of F2A (a cleavable peptide) fragment and eGFP, which are linked by a “linker-STOP” sequence that contains the specific recognition site of a short single guide RNA (sgRNA) and generates +1 or +2 nt frameshift (eGFP transactivation) at a total frequency of ∼80%. The recipient cells were transduced permanently with the reporter construct, and EV transfer was assessed by co-culture with sgRNA expressing donor cells (close contact or no close contact) or by directly adding donor-derived EVs. The EV-mediated sgRNA transfer permanently activates the expression of eGFP (i.e., mCherry and eGFP double positive). Thus, the system allow visualization of EV-mediated functional sgRNA delivery in single cell level. In a co-culture experiment with cell number ratio of 50:1 (sgRNA donor cells: reporter-transduced recipient cells), an encouraging transfer rate of ∼0.2% was observed (69). Nevertheless, like Cre-loxP based reporter, CRISPR/Cas9 based reporter also causes permanent activation of the reporter gene, the effects are cumulative and not real-time.
Collectively, the reporter systems based on the Cre-loxP system, miRNA and their recognize sites, and the CRISPR/Cas9 system have been used to verify EV mediated intracellular delivery of functional mRNA, protein, and small RNAs in target cells, which are instrumental to our understanding of EV biology.
Outlook
Conceivably, all direct EV labeling methods (for assessment of EV uptake) and reporter systems (for evaluation of functional transfer) have limitations. Direct EV labeling has the potential of affecting the chemical property of EVs’ membrane, which may alter the EV biodistribution or uptake tropism and dynamics. Genetic engineering of donor cells have the advantage of directly studying endogenous EVs in a tissue specific fashion, however, difficult to distinguish between exosomes, microvesicles, and non-EV associated transport, not to mention the effects of transgenic expression on cellular activities. Current reporter systems, on the other hand, primarily indicate the functional transfer of single molecules (mRNA, protein, or small RNA) in recipient cells, thus subject to the unique dynamics of these individual molecules, and the sensitivity may not be at the same scale as the EV-carried biomolecules to be studied. The choices of reporter systems should be based on the biological questions to be addressed in specific studies and corroborated by other functional readouts. For example, the power of these reporter systems would be significantly enhanced if combined with other cutting-edge technologies, such as single cells sequence or spatial transcriptome analysis. For real-time monitoring of functional mRNA, protein, or sgRNA transfer, some sensitive and specific binary systems, such as the TetR-based transactivators (70) or dCas9-VPR transactivators (71), may be used. The sensitivity of the reporter systems may also be enhanced by increasing the loading of bioactive cargos and/or the membrane fusion, back fusion, or lysosomal escape within recipient cells. Ideally, reporter systems should be able to indicate detailed life-cycle events of EVs, their biogenesis and diversity in donor cells, extracellular navigation, systemic distribution, as well as internalization, intracellular trafficking (between subcellular organelles), cargo delivery or escape, and fate in target cells. The advancement in EV reporter systems will likely provide more powerful and invaluable tools for the field of research in EV biology and therapeutics.
Author Contributions
CH: conceptual contributions and writing the draft. GQ: conceptual contributions and editing. Both authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institute of Health (Grants #HL138990, HL131110, HL130052, and HL113541 to GQ); American Diabetes Association (Grant #1-15-BS-148 to GQ); American Heart Association (Grants #19TPA34910227 to GQ and #830472 to CH).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (misev2018): a position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J Extracell Vesicles. (2018) 7:1535750. doi: 10.1080/20013078.2018.1535750
2. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. (2018) 19:213–28. doi: 10.1038/nrm.2017.125
3. Sahoo S, Adamiak M, Mathiyalagan P, Kenneweg F, Kafert-Kasting S, Thum T. Therapeutic and diagnostic translation of extracellular vesicles in cardiovascular diseases: roadmap to the clinic. Circulation. (2021) 143:1426–49. doi: 10.1161/CIRCULATIONAHA.120.049254
4. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. (2020) 367:eaau6977. doi: 10.1126/science.aau6977
5. Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. (2019) 21:9–17. doi: 10.1038/s41556-018-0250-9
6. Han C, Yang J, Sun J, Qin G. Extracellular vesicles in cardiovascular disease: biological functions and therapeutic implications. Pharmacol Ther. (2021) 233:108025. doi: 10.1016/j.pharmthera.2021.108025
7. Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of exosome composition. Cell. (2019) 177:428–445.e18. doi: 10.1016/j.cell.2019.02.029
8. Battistelli M, Falcieri E. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology (Basel). (2020) 9:21. doi: 10.3390/biology9010021
9. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. (1972) 26:239–57. doi: 10.1038/bjc.1972.33
12. Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F. Extracellular vesicles in angiogenesis. Circ Res. (2017) 120:1658–73.
13. de Abreu RC, Fernandes H, da Costa Martins PA, Sahoo S, Emanueli C, Ferreira L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat Rev Cardiol. (2020) 17:685–97. doi: 10.1038/s41569-020-0389-5
14. Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. (2017) 14:259–72.
15. Zhao M, Liu S, Wang C, Wang Y, Wan M, Liu F, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate mitochondrial damage and inflammation by stabilizing mitochondrial DNA. ACS Nano. (2021) 15:1519–38.
16. Jiang F, Chen Q, Wang W, Ling Y, Yan Y, Xia P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microrna-1. J Hepatol. (2020) 72:156–66. doi: 10.1016/j.jhep.2019.09.014
17. Jung KO, Jo H, Yu JH, Gambhir SS, Pratx G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials. (2018) 177:139–48. doi: 10.1016/j.biomaterials.2018.05.048
18. Otero-Ortega L, Gomez de Frutos MC, Laso-Garcia F, Rodriguez-Frutos B, Medina-Gutierrez E, Lopez JA, et al. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J Cereb Blood Flow Metab. (2018) 38:767–79. doi: 10.1177/0271678X17708917
19. Puzar Dominkus P, Stenovec M, Sitar S, Lasic E, Zorec R, Plemenitas A, et al. Pkh26 labeling of extracellular vesicles: characterization and cellular internalization of contaminating pkh26 nanoparticles. Biochim Biophys Acta Biomembr. (2018) 1860:1350–61. doi: 10.1016/j.bbamem.2018.03.013
20. Sagar G, Sah RP, Javeed N, Dutta SK, Smyrk TC, Lau JS, et al. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut. (2016) 65:1165–74. doi: 10.1136/gutjnl-2014-308350
21. Sun W, Li Z, Zhou X, Yang G, Yuan L. Efficient exosome delivery in refractory tissues assisted by ultrasound-targeted microbubble destruction. Drug Deliv. (2019) 26:45–50. doi: 10.1080/10717544.2018.1534898
22. Takov K, Yellon DM, Davidson SM. Confounding factors in vesicle uptake studies using fluorescent lipophilic membrane dyes. J Extracell Vesicles. (2017) 6:1388731. doi: 10.1080/20013078.2017.1388731
23. Wang N, Li X, Zhong Z, Qiu Y, Liu S, Wu H, et al. 3D hESC exosomes enriched with miR-6766-3p ameliorates liver fibrosis by attenuating activated stellate cells through targeting the TGFβRII-SMADS pathway. J Nanobiotechnology. (2021) 19:437. doi: 10.1186/s12951-021-01138-2
24. Shimomura T, Seino R, Umezaki K, Shimoda A, Ezoe T, Ishiyama M, et al. New lipophilic fluorescent dyes for labeling extracellular vesicles: characterization and monitoring of cellular uptake. Bioconjug Chem. (2021) 32:680–4. doi: 10.1021/acs.bioconjchem.1c00068
25. Nocera AL, Mueller SK, Stephan JR, Hing L, Seifert P, Han X, et al. Exosome swarms eliminate airway pathogens and provide passive epithelial immunoprotection through nitric oxide. J Allergy Clin Immunol. (2019) 143:1525–1535.e1. doi: 10.1016/j.jaci.2018.08.046
26. Zhou X, Zhang J, Song Z, Lu S, Yu Y, Tian J, et al. Exotracker: a low-ph-activatable fluorescent probe for labeling exosomes and monitoring endocytosis and trafficking. Chem Commun (Camb). (2020) 56:14869–72. doi: 10.1039/d0cc06208a
27. Roberts-Dalton HD, Cocks A, Falcon-Perez JM, Sayers EJ, Webber JP, Watson P, et al. Fluorescence labelling of extracellular vesicles using a novel thiol-based strategy for quantitative analysis of cellular delivery and intracellular traffic. Nanoscale. (2017) 9:13693–706. doi: 10.1039/c7nr04128d
28. Kumakura A, Shikuma J, Ogihara N, Eiki J, Kanazawa M, Notoya Y, et al. Effects of celiac superior mesenteric ganglionectomy on glucose homeostasis and hormonal changes during oral glucose tolerance testing in rats. Endocr J. (2013) 60:525–31.
29. Subiros-Funosas R, Mendive-Tapia L, Sot J, Pound JD, Barth N, Varela Y, et al. A trp-bodipy cyclic peptide for fluorescence labelling of apoptotic bodies. Chem Commun (Camb). (2017) 53:945–8. doi: 10.1039/c6cc07879f
31. Zhang L, Yang Z, Sefah K, Bradley KM, Hoshika S, Kim MJ, et al. Evolution of functional six-nucleotide DNA. J Am Chem Soc. (2015) 137:6734–7. doi: 10.1021/jacs.5b02251
32. Wan S, Zhang L, Wang S, Liu Y, Wu C, Cui C, et al. Molecular recognition-based DNA nanoassemblies on the surfaces of nanosized exosomes. J Am Chem Soc. (2017) 139:5289–92. doi: 10.1021/jacs.7b00319
33. De La Pena H, Madrigal JA, Rusakiewicz S, Bencsik M, Cave GW, Selman A, et al. Artificial exosomes as tools for basic and clinical immunology. J Immunol Methods. (2009) 344:121–32. doi: 10.1016/j.jim.2009.03.011
34. Hu L, Wickline SA, Hood JL. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn Reson Med. (2015) 74:266–71. doi: 10.1002/mrm.25376
35. Hwang DW, Choi H, Jang SC, Yoo MY, Park JY, Choi NE, et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using (99m)tc-hmpao. Sci Rep. (2015) 5:15636. doi: 10.1038/srep15636
36. Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. (2015) 199:145–55. doi: 10.1016/j.jconrel.2014.12.013
37. Salunkhe S, Dheeraj, Basak M, Chitkara D, Mittal A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: strategies and significance. J Control Release. (2020) 326:599–614.
38. Levy D, Do MA, Brown A, Asano K, Diebold D, Chen H, et al. Genetic labeling of extracellular vesicles for studying biogenesis and uptake in living mammalian cells. Methods Enzymol. (2020) 645:1–14. doi: 10.1016/bs.mie.2020.02.001
39. Men Y, Yelick J, Jin S, Tian Y, Chiang MSR, Higashimori H, et al. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun. (2019) 10:4136. doi: 10.1038/s41467-019-11534-w
40. Ren R, Tan XH, Zhao JH, Zhang QP, Zhang XF, Ma ZJ, et al. Bone marrow mesenchymal stem cell-derived exosome uptake and retrograde transport can occur at peripheral nerve endings. Artif Cells Nanomed Biotechnol. (2019) 47:2918–29. doi: 10.1080/21691401.2019.1640713
41. Stickney Z, Losacco J, McDevitt S, Zhang Z, Lu B. Development of exosome surface display technology in living human cells. Biochem Biophys Res Commun. (2016) 472:53–9. doi: 10.1016/j.bbrc.2016.02.058
42. Verweij FJ, Revenu C, Arras G, Dingli F, Loew D, Pegtel DM, et al. Live tracking of inter-organ communication by endogenous exosomes in vivo. Dev Cell. (2019) 48:573–589.e4. doi: 10.1016/j.devcel.2019.01.004
43. Gupta D, Liang X, Pavlova S, Wiklander OPB, Corso G, Zhao Y, et al. Quantification of extracellular vesicles in vitro and in vivo using sensitive bioluminescence imaging. J Extracell Vesicles. (2020) 9:1800222. doi: 10.1080/20013078.2020.1800222
44. Luo W, Dai Y, Chen Z, Yue X, Andrade-Powell KC, Chang J. Spatial and temporal tracking of cardiac exosomes in mouse using a nano-luciferase-cd63 fusion protein. Commun Biol. (2020) 3:114. doi: 10.1038/s42003-020-0830-7
45. Takahashi Y, Nishikawa M, Takakura Y. In vivo tracking of extracellular vesicles in mice using fusion protein comprising lactadherin and gaussia luciferase. Methods Mol Biol. (2017) 1660:245–54. doi: 10.1007/978-1-4939-7253-1_20
46. Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, et al. Visualization and in vivo tracking of the exosomes of murine melanoma b16-bl6 cells in mice after intravenous injection. J Biotechnol. (2013) 165:77–84. doi: 10.1016/j.jbiotec.2013.03.013
47. Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. (2014) 8:483–94. doi: 10.1021/nn404945r
48. Matsumoto A, Takahashi Y, Nishikawa M, Sano K, Morishita M, Charoenviriyakul C, et al. Accelerated growth of b16bl6 tumor in mice through efficient uptake of their own exosomes by b16bl6 cells. Cancer Sci. (2017) 108:1803–10. doi: 10.1111/cas.13310
49. Morishita M, Takahashi Y, Nishikawa M, Sano K, Kato K, Yamashita T, et al. Quantitative analysis of tissue distribution of the b16bl6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice. J Pharm Sci. (2015) 104:705–13. doi: 10.1002/jps.24251
50. Alvarez-Castelao B, Schanzenbacher CT, Langer JD, Schuman EM. Cell-type-specific metabolic labeling, detection and identification of nascent proteomes in vivo. Nat Protoc. (2019) 14:556–75. doi: 10.1038/s41596-018-0106-6
51. Zhang E, Liu Y, Han C, Fan C, Wang L, Chen W, et al. Visualization and identification of bioorthogonally labeled exosome proteins following systemic administration in mice. Front Cell Dev Biol. (2021) 9:657456. doi: 10.3389/fcell.2021.657456
52. Wang M, Altinoglu S, Takeda YS, Xu Q. Integrating protein engineering and bioorthogonal click conjugation for extracellular vesicle modulation and intracellular delivery. PLoS One. (2015) 10:e0141860. doi: 10.1371/journal.pone.0141860
53. Smyth T, Petrova K, Payton NM, Persaud I, Redzic JS, Graner MW, et al. Surface functionalization of exosomes using click chemistry. Bioconjug Chem. (2014) 25:1777–84. doi: 10.1021/bc500291r
54. Sletten EM, Bertozzi CR. From mechanism to mouse: a tale of two bioorthogonal reactions. Acc Chem Res. (2011) 44:666–76. doi: 10.1021/ar200148z
55. Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, et al. Copper-free click chemistry for dynamic in vivo imaging. Proc Natl Acad Sci USA. (2007) 104:16793–7. doi: 10.1073/pnas.0707090104
56. Stone SE, Glenn WS, Hamblin GD, Tirrell DA. Cell-selective proteomics for biological discovery. Curr Opin Chem Biol. (2017) 36:50–7. doi: 10.1016/j.cbpa.2016.12.026
57. Alvarez-Castelao B, Schanzenbacher CT, Hanus C, Glock C, Tom Dieck S, Dorrbaum AR, et al. Cell-type-specific metabolic labeling of nascent proteomes in vivo. Nat Biotechnol. (2017) 35:1196–201.
58. Han D, Yang J, Zhang E, Liu Y, Boriboun C, Qiao A, et al. Analysis of mesenchymal stem cell proteomes in situ in the ischemic heart. Theranostics. (2020) 10:11324–38. doi: 10.7150/thno.47893
59. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. (2007) 9:654–9. doi: 10.1038/ncb1596
60. Zomer A, Steenbeek SC, Maynard C, van Rheenen J. Studying extracellular vesicle transfer by a cre-loxp method. Nat Protoc. (2016) 11:87–101. doi: 10.1038/nprot.2015.138
61. PLoS Biology Staff. Correction: extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. (2018) 16:e1002623. doi: 10.1371/journal.pbio.1002623
62. Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. (2014) 12:e1001874. doi: 10.1371/journal.pbio.1001874
63. Ridder K, Sevko A, Heide J, Dams M, Rupp AK, Macas J, et al. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology. (2015) 4:e1008371. doi: 10.1080/2162402X.2015.1008371
64. Zomer A, Maynard C, Verweij FJ, Kamermans A, Schafer R, Beerling E, et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. (2015) 161:1046–57. doi: 10.1016/j.cell.2015.04.042
65. Sterzenbach U, Putz U, Low LH, Silke J, Tan SS, Howitt J. Engineered exosomes as vehicles for biologically active proteins. Mol Ther. (2017) 25:1269–78. doi: 10.1016/j.ymthe.2017.03.030
66. O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. (2020) 21:585–606. doi: 10.1038/s41580-020-0251-y
67. Jin Y, Chen Z, Liu X, Zhou X. Evaluating the microRNA targeting sites by luciferase reporter gene assay. Methods Mol Biol. (2013) 936:117–27. doi: 10.1007/978-1-62703-083-0_10
68. Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. (2017) 542:450–5.
69. de Jong OG, Murphy DE, Mager I, Willms E, Garcia-Guerra A, Gitz-Francois JJ, et al. A crispr-cas9-based reporter system for single-cell detection of extracellular vesicle-mediated functional transfer of RNA. Nat Commun. (2020) 11:1113.
70. Roukos V, Burgess RC, Misteli T. Generation of cell-based systems to visualize chromosome damage and translocations in living cells. Nat Protoc. (2014) 9:2476–92. doi: 10.1038/nprot.2014.167
Keywords: exosomes, cell–cell communication, reporter, EVs visualization, recipient cells, extracellular vesicle
Citation: Han C and Qin G (2022) Reporter Systems for Assessments of Extracellular Vesicle Transfer. Front. Cardiovasc. Med. 9:922420. doi: 10.3389/fcvm.2022.922420
Received: 17 April 2022; Accepted: 06 May 2022;
Published: 01 June 2022.
Edited by:
Junjie Xiao, Shanghai University, ChinaReviewed by:
Sudad Saman, Elms College, United StatesCopyright © 2022 Han and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gangjian Qin, Z3FpbkB1YWIuZWR1
 Chaoshan Han
Chaoshan Han Gangjian Qin
Gangjian Qin