
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 20 June 2022
Sec. Coronary Artery Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.918167
This article is part of the Research TopicClinical Prospective of SGLT2 Inhibitors in AtherosclerosisView all 9 articles
Background: Contrast-induced acute kidney injury (CI-AKI) is a common complication of patients undergoing percutaneous coronary intervention (PCI). Data regarding the influence of sodium-glucose cotransporter-2 (SGLT2) inhibitor on the CI-AKI incidence and renal outcomes of patients undergoing PCI are limited. This study aimed to examine the real-world risk of CI-AKI in SGLT2 inhibitor users undergoing PCI.
Methods: We used longitudinal data from the medical records of the First Affiliated Hospital of Xi'an Jiaotong University. We selected SGLT inhibitor users and nonusers [patients with type 2 diabetes (T2D) without SGLT2 inhibitor prescription] undergoing PCI. We determined CI-AKI by the ESUR (European Society of Urogenital Radiology, AKIESUR) and KDIGO definition (Kidney Disease: Improving Global Outcomes, AKIKDIGO). We performed 1:1 nearest-neighbor propensity matching and calculated unadjusted odds ratios (ORs) and adjusted ORs (aORs; accounting for covariates poorly balanced) for AKI in primary and sensitivity analyses. We compared the renal function indicators in users and nonusers at 24, 48, and 72 h post-PCI.
Results: We identified 242 SGLT2 inhibitor users and 242 nonusers in the cohort. The unadjusted ORs of CI-AKIESUR were 63% lower in users [OR: 0.37 (95% CI: 0.18–0.68); P = 0.01], which was unchanged [aOR: 0.37 (95% CI: 0.19–0.67); P < 0.01] post adjustment. These estimates did not qualitatively change across several sensitivity analyses. There was no significant difference in urea nitrogen, creatinine, and estimated glomerular filtration rate (eGFR) values between the two groups before PCI, and at 24 h, while the creatinine (48 and 72 h post-PCI) and CyC (24 and 48 h post-PCI) were significantly lower than those in the nonuser group (P < 0.05).
Conclusion: Our findings do not suggest an increased risk of CI-AKI associated with SGLT2 inhibitor use in patients with CAD and T2D undergoing PCI.
Revascularization by the percutaneous coronary intervention (PCI) has achieved great success in reducing mortality for patients with coronary artery disease (CAD) (1). Nevertheless, a proportion of patients with CAD have suffered from an acute renal injury caused by contrast medium (CM) with the incidence ranging from 1.3 to 33.3% (2), which is defined as contrast-induced acute kidney injury (CI-AKI), considered as a new-onset or an exacerbation of renal dysfunction following administration of CM, without other potential causes. CI-AKI is an increasing complication in the general population of patients with CAD and the risk of this serious adverse event is further increased among patients with type 2 diabetes mellitus (T2DM) (3).
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, which specifically inhibit renal tubular reabsorption of glucose, are new medications for the treatment of patients with T2DM. Mechanically, SGLT2 inhibitors (SGLT2i) block the reabsorption of glucose in the kidney, increase glucose excretion, and lower blood glucose levels (4). Several multicenter studies demonstrated lower rates of cardiovascular events and mortality, and a significant reduction in incidence and worsening kidney disease (5–7). However, little is known about the impact of SGLT2i on the incidence of CI-AKI for patients undergoing PCI.
Recently, real-world studies suggest that patients using SGLT2i, including empagliflozin, were at a lower risk of developing AKI and exhibited a smaller eGFR decline than patients using other glucose-lowering drugs (8). This indicates the potential renoprotective effects of SGLT2i against AKI for patients with type 2 diabetes. Few clinical evidence was reported to evaluate the impact of SGLT2i on the incidence of CI-AKI. In our study, we hypothesized that SGLT2i protect the renal function of patients with CAD and reduce CI-AKI incidence among patients undergoing PCI. Our study aims to explore the specific effect of SGLT2i on the incidence of CI-AKI and renal function among patients with CAD undergoing PCI.
This single-center, retrospective, case-control study was approved by the institutional review board of the Medical School of Xi'an Jiaotong University and the need for informed or written consent was waived as part of the study approval. The patients were all admitted to the cardiology department of the First Affiliated Hospital of Xi'an Jiaotong University and underwent coronary angiography (CAG) and PCI between 1 January 2020 and 30 December 2021 (Figure 1). For this study, those patients with a diagnosis of T2DM and CAD and available serum creatinine measurements during hospitalization were included (N = 1,510). The diagnosis of T2DM was confirmed by fasting glucose (>7.0 mmol/L) or by a previous diagnosis when the patient was taking an oral hypoglycemic agent or insulin (9). CAD was defined as the presence of at least 50% luminal diameter narrowing in at least one major coronary artery by two experienced interventional cardiologists (10).
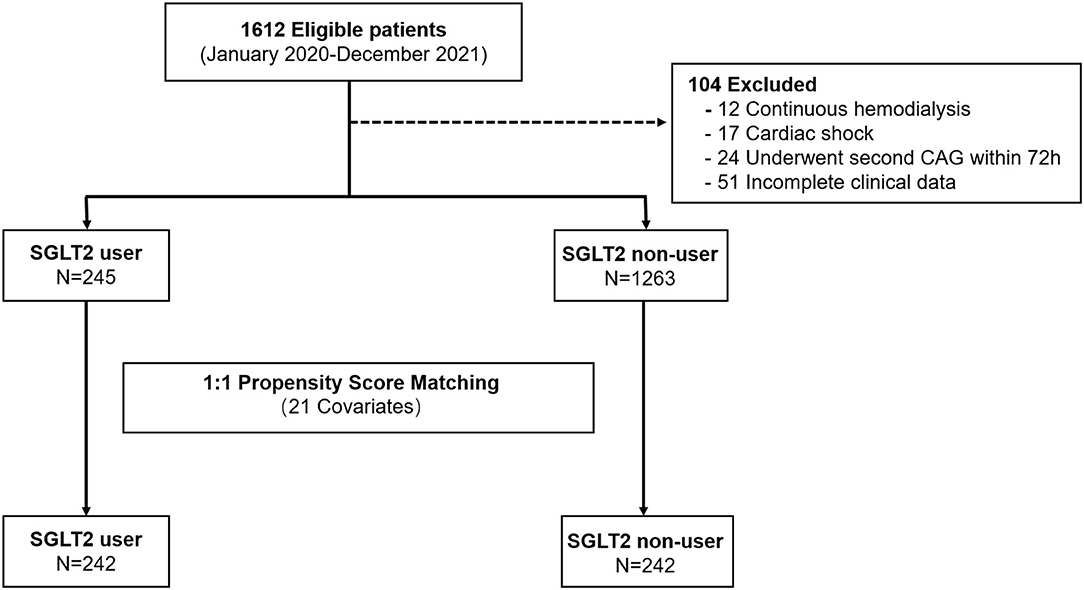
Figure 1. Study flow showing the derivation of unmatched and propensity score–matched patient cohorts in this study.
The study involved no patient or public in the development of the research question, outcome measures, study design, recruitment, conduct, and result dissemination.
The exposure of interest was a prescription of an SGLT2 inhibitor, including canagliflozin, empagliflozin, or dapagliflozin, for at least 6 months till the date of PCI. This was determined by the provider's prescription in the electronic medical record. Six months were used as a cut-off value as SGLT2i may exhibit the beneficial effects after a 6-month period as indicated in several studies (11, 12). For SGLT2 inhibitor users, the index date was defined as the first date on which an SLGT2 prescription was ordered. For nonusers, the index date was defined using the creatinine measurement date from 2020 to 2021.
The primary outcome was the first AKI event during the hospitalization. We identified CI-AKI events using a laboratory-based algorithm, which identifies events based on European Society of Urogenital Radiology (ESUR) serum creatinine criteria (increase in serum creatinine by ≥44.2 μmol/L or 0.5 mg/dL within 72 h or increase in serum creatinine by ≥1.25 times baseline value; hereafter referred to as CI-AKIESUR) (13). As a sensitivity analysis, we also ascertained inpatient episodes of AKI using Kidney Disease: Improving Global Outcomes (KDIGO) serum creatinine criteria (increase in serum creatinine by ≥26.52 μmol/L/0.3 mg/dl within 48 h or increase in serum creatinine by ≥1.5 times baseline value before CAG; hereafter referred to as AKIKDIGO) along with the dates (14). The last laboratory values before undergoing CAG were used as the baseline data for analysis to best reflect each patient's baseline condition. The renal functions (serum creatinine and urea nitrogen) of patients with CAD were collected and measured conducted by automatic biochemical immune analyzer VITROS5600 (JNJ, New Jersey, US) upon admission and at 24, 48, and 72 h after CAG. The eGFR (ml/min/1.73 m2) was calculated via the modification of diet in renal disease equation: 186.3 × (creatinine in mg/dl) – 1.154 × (age in years) – 0.203 × (0.742 if woman) × (1.21 if black).
All new users of SGLT2 inhibitors included in this study were identified from the electronic medical record. After being admitted to the hospital, each patient enrolled in this study by prescription screening was further inquired carefully about their drug history by doctors before PCI. Patients indeed taking SGLT2 inhibitors were then included as described. The exclusion criteria were (1) patients with acute myocardial infarction and requiring emergency PCI; (2) severe heart failure, left ventricular ejection fraction ≤ 40%; (3) complex coronary lesions that require extended operation time, or those with other contrast agents more than 400 ml; (4) severe arrhythmia; (5) severe hepatic (serum alanine transaminase >3 times the upper normal limit) and renal dysfunction (serum creatinine >221 μmol/l) or liver dysfunction; (6) urinary infection; (7) patients with poor glycemic control [HbA1c (%) >8%, fasting blood glucose >8.3 mmol/L or postprandial blood glucose >13.3 mmol/L], for whom the immediate change of glycemic treatment approaches was needed; and (8) patients with obvious hypotension, shock, and other causes of renal insufficiency before or after surgery.
Control subjects were selected among patients who never received a prescription for SLGT2 inhibitors and who had a diagnosis of diabetes since January 1, 2020. The data were collected from the hospital information system and prescription records were accessed centrally. Meanwhile, the prescription history of patients was inquired about by physicians on the first day of admission and double-checked by another doctor before PCI. Propensity scores were calculated as described (15) using logistic regression of SLGT2 inhibitor use on age, sex, creatinine measurement, hypertension status, insulin use, antihypertensive medication use, nonsteroidal anti-inflammatory drug use, eGFR, and HbA1c, with matching on previous pharmacist visit, diabetes duration, previous AKI episode, ACE inhibitor/angiotensin receptor blocker use, and volume of administered contrast, generating a separate score for control subjects. For participants with missing values in any of these covariates, exact matches were required on missing status. Propensity matching was performed with a 1:1 match for case and control subjects in which the nearest neighbor was selected without replacement.
Quantitative variables were summarized using means and SDs while qualitative variables were summarized as absolute and percentage frequencies. All analyses were performed using SAS (version 9.4) software (SAS Institute, Inc.). Baseline characteristics were compared between patients using Student's t-test for near-normal continuous variables, the Mann–Whitney U-test for other continuous variables, and the chi-square test for categorical variables unless more than 20% of cells had expected frequencies <5 in which case Fisher's exact test was used. After propensity score matching, two groups were compared using McNemar's test to evaluate the presence of correlation between CI-AKI events and the use of SGLT2 inhibitor over nonusers. All P values were two-sided and P < 0.050 was considered statistically significant.
We identified a total of 245 SGLT2 inhibitor users and 1,265 patients with T2DM not on SGLT2 inhibitors who underwent PCI before matching. Compared with nonusers, SGLT2 inhibitor users tended to be at a lower age, with significantly lower comorbidities, namely, heart failure and PCI history, compared with SGLT2 inhibitor nonusers. They also had higher eGFR and a higher proportion of metformin use compared with nonusers. These results are shown in Table 1.
After propensity matching, we identified 242 SGLT2 inhibitor users and 242 nonusers. The majority of users were on dapagliflozin (71.1%), followed by empagliflozin (16.9%), and then canagliflozin (12.0%). Users and nonusers were well matched except for PCI history (19.8% vs. 12.0% in users vs. nonusers), HbA1c (6.9% vs. 6.4% in users vs. nonusers), and metformin usage (86.0% vs. 70.2% in users vs. nonusers) in the cohort (Table 2).
The proportion of patients with CI-AKI events was 5.3% (13 out of 245) in the SGLT2 inhibitor user cohort and 121 out of 1,265 (9.6%) in the whole nonuser cohort (Table 1). After propensity matching, the proportion of patients with CI-AKI events in SGLT2 inhibitor users and nonusers was 4.9 and 11.6%, respectively (P < 0.01) (Table 3). Similar results were found using the KDIGO definition of AKI (4.1% in users vs. 9.1% in nonusers, P = 0.04). We then compared the severity of AKI events as defined by changes in creatinine from baseline and peak creatinine measures during an AKI event. Median changes in serum creatinine from baseline for the user and nonuser groups were 54.3 μmol/L [interquartile range (IQR): 32.8–74.5] and 78.7 μmol/L (IQR: 61.3–92.8; P < 0.01), respectively (Table 3). However, the difference in median peak creatinine measures in the user and nonuser groups was not significant (140.8 μmol/L, IQR: 105.5–159.0 vs. 162.5 μmol/L, IQR: 133.3–176.5, P = 0.12) (Table 3). Acute dialysis for CI-AKI occurred in one patient in the user group and two patients in the nonuser group.
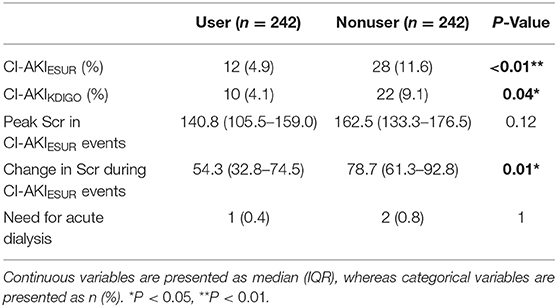
Table 3. CI-AKI outcomes in the SGLT2 inhibitor user and nonuser groups in the propensity-matched cohorts.
We also compared the levels of eGFR, creatinine, and blood urea nitrogen (BUN) at 24, 48, and 72 h post-PCI (Figure 2, Table 4). The levels of BUN were only significantly different at 72 h (5.78 ± 1.97 vs.6.19 ± 2.47, P = 0.044). The eGFR of users at 48 h (93.14 ± 26.51 vs. 87.33 ± 32.12, P = 0.031) and 72 h (91.26 ± 21.38 vs. 84.39 ± 42.76, P = 0.026) after PCI was significantly higher than those in the nonuser group. Accordingly, the levels of creatinine in SGLT2 inhibitor users were lower than that of nonusers at 48 h (63.88 ± 24.55 vs. 69.71 ± 36.81, P = 0.041) and 72 h (66.70 ± 37.47 vs. 74.31 ± 40.50, P = 0.032). The levels of cystatin C (CyC) in users were also lower at 24 h (1.03 ± 0.42 vs. 1.12 ± 0.48, P = 0.029) and 48 h (1.21 ± 0.55 vs. 1.35 ± 0.51, P = 0.004) compared to the nonuser group, but not significantly different at 72 h post-PCI (1.37 ± 0.61 vs. 1.48 ± 0.66, P = 0.058).
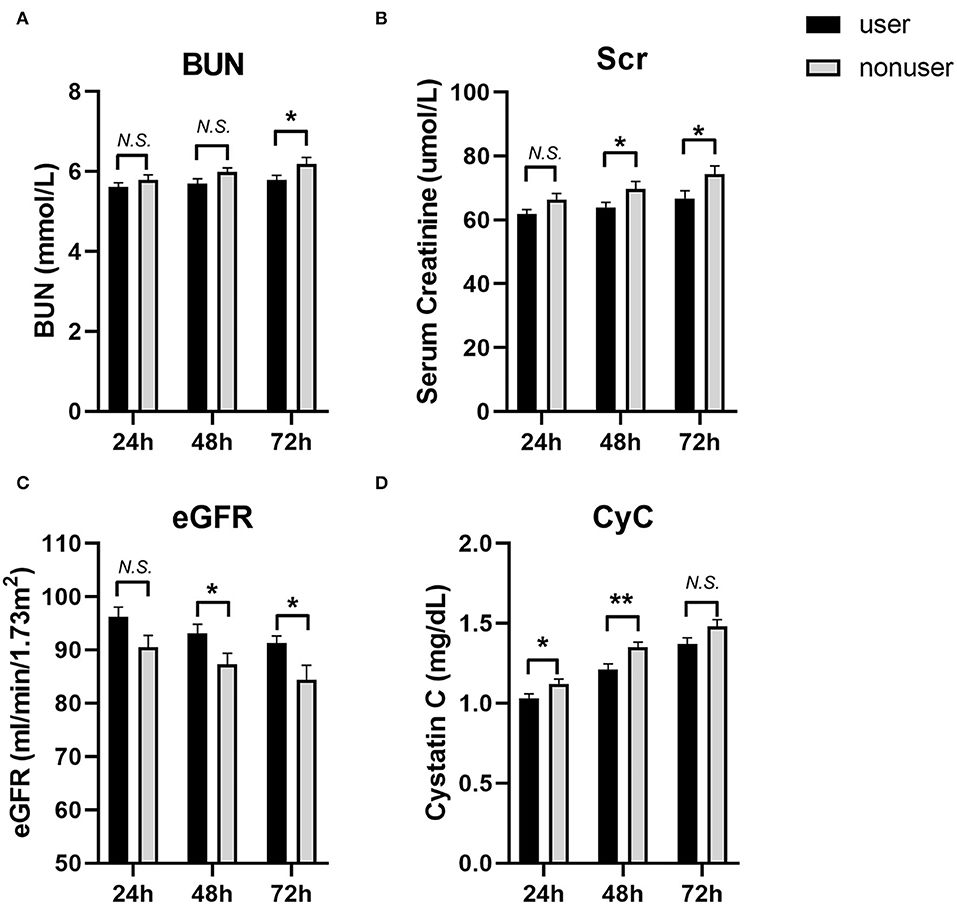
Figure 2. The levels of blood urea nitrogen (BUN), Serum creatinine (Scr), eGFR and Cystatin C (CyC) at 24, 48 and 72 h post-PCI. (A) BUN; (B) Scr; (C) eGFR; (D) CyC. User group: Black bars, N = 242; Nonuser group: Gray bars, N = 242. Data were shown as Mean ± SEM. Student's t-test for variables were used. *P < 0.05; **P < 0.01, N.S, not significant.
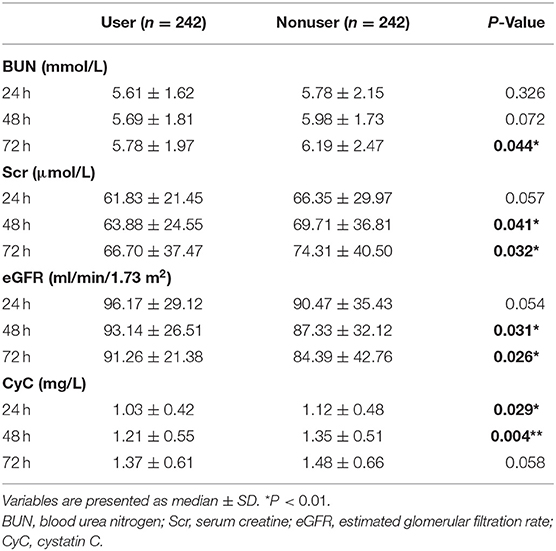
Table 4. Comparison of renal function and incidence of CI-AKI between the two groups at 24, 48, and 72 h after surgery.
The unadjusted ORs of CI-AKIESUR were 63% lower in the SGLT2 inhibitor user group [0.37 (95% CI: 0.16–0.88); P = 0.01] compared with the nonuser group (Figure 3). Sensitivity analysis using KDIGO definition also rendered similar results [0.46 (95% CI: 0.276–0.75); P = 0.02]. After adjusting for age, sex, PCI history, New York Heart Association (NYHA) grade ≥III, HbA1c, and metformin use, the adjusted OR of CI-AKIESUR remained unchanged [0.37 (95% CI: 0.19–0.67); P = 0.02]. We also investigated whether or not there was the differential risk of CI-AKI associated specifically with canagliflozin or dapagliflozin. Results for canagliflozin were not available because of the small sample size and lack of model convergence. The unadjusted and adjusted point estimates were qualitatively similar to the overall results (Figure 3). Furthermore, multivariate logistic regression analysis (Table 5) showed that age (OR: 1.06, 95%CI: 1.02–1.11, P = 0.01), PCI history (OR: 7.84, 95% CI: 3.26–18.84, P = 0.01), and NYHA grade ≥III (OR: 7.92, 95% CI: 1.80–34.91, P < 0.01) are also independent risk factors of CI-AKI for patients undergoing PCI.
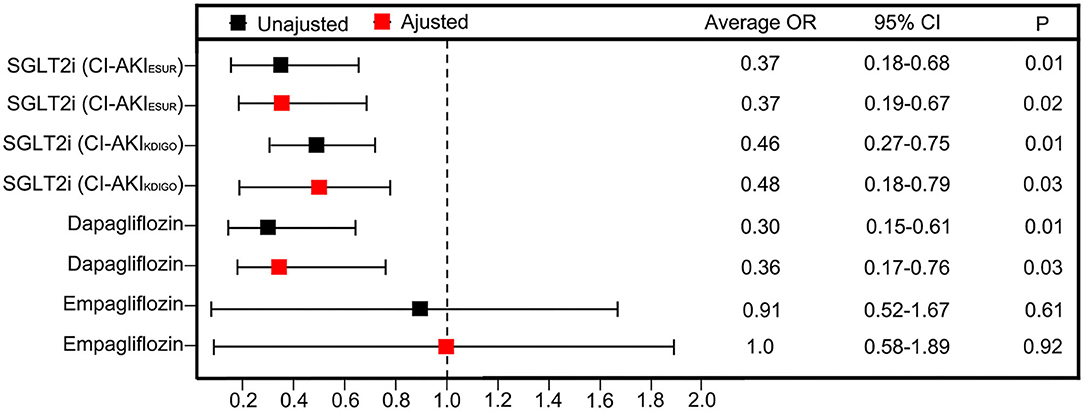
Figure 3. Unadjusted ORs and aORs of CI-AKI with 95% CIs in the propensity-matched cohorts. The ORs are generated after adjustment for covariates including age, sex, PCI histroy, NYHA grade ≥ III, HbA1c and metformin use.
In this large contemporary cohort with different commodity burdens, and differing levels of renal function, we did not observe any increased risk of CI-AKI with SGLT2 inhibitor usage after PCI, consistent with previous study regarding AKI incidence (15). In contrast, we observed trends toward decreased risk of CI-AKI with SGLT2 inhibitor use before and after propensity matching, the estimates of which were qualitatively similar across several sensitivity analyses. The incidence of CI-AKI in patients with CAD and diabetes was lower in the user group, and SGLT2 inhibitor usage was found to be an independent protective factor for the occurrence of CI-AKI for patients after PCI. Moreover, the severity was not worse in the user group when CI-AKI occurred, as estimated by peak serum creatinine or change in creatinine. Finally, there was no increased risk of CI-AKI specifically for dapagliflozin and empagliflozin, for which there is a concern of AKI, and alerts have been issued (6, 15, 16).
Contrast-induced acute kidney injury (CI-AKI) is a common complication of patients undergoing PCI, often caused by diagnostic and therapeutic procedures using iodinated contrast media. Even slight, transient contrast-induced renal damage can have a negative impact on short- and long-term cardiovascular and renal prognoses (13). The common risk factors of CI-AKI are reported to include traditional factors (age, chronic renal failure, heart failure, and diabetes) and factors related to intervention, such as the dosage and types of contrast agents, multiple operations in a short period of time, and the use of intra-aortic balloon counter pulsation (17). Similar to this in our study, results showed such factors as age, NYHA grade, and PCI history risk factors for CI-AKI after PCI. Meanwhile, the point estimates for risk of CI-AKI were qualitatively similar for all the three types of SGLT2 inhibitors, suggesting a class effect. We believe our data complement and build upon the clinical trial data by the implementation of laboratory-based data using both diagnostic criteria of ESUR and KDIGO to ascertain AKI events, before and after propensity matching and additional adjustment, that are free from the selection bias (15).
Besides the overall nephroprotective effect, the risk of AKI with SGLT2 inhibitors is recognized since their clinical use. The Food and Drug Administration reports, and commentaries from various authors, have warned and speculated about AKI associated with SGLT2 inhibitors, namely, dapagliflozin and canagliflozin (16). As SGLT2 inhibitors act through inhibiting tubular glucose reabsorption, which results in a glucose-lowering effect and glucosuria, it is not inconceivable that they might cause acute perturbations in renal function (4). The additional natriuretic effect of SGLT2 inhibition may predispose patients with CAD, particularly those on diuretics and renin-angiotensin-aldosterone system antagonists, to experience abrupt reductions in GFR (16). Recently, several studies have provided evidence that SGLT2 inhibitors do not increase the risk for AKI in patients diagnosed with T2DM or heart failure (15, 18). Some studies also suggest that initiation of an SGLT2 inhibitor was associated with a reduced risk of AKI compared with other glucose-lowering strategies (15, 19, 20). In the Canagliflozin Cardiovascular Assessment Study (5), there were no statistically significant differences in the number of renal-related adverse events vs. placebo (19.7 vs. 17.4/1,000 patient-years). In the DECLARE-TIMI 58 (Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58) study, compared with placebo, dapagliflozin is confirmed to reduce newly increasing or worsening renal disease by 24% (11). In a meta-analysis, including three randomized controlled trials with a total of 1,831 patients, results showed that initiation of SGLT2 inhibitors in patients did not increase the AKI [OR: 0.76; 95% CI: (0.50, 1.16)] (20). These results suggest the renal-protective effects of SGLT2 inhibitors for patients with cardiovascular diseases.
However, in terms of CI-AKI, few studies have provided evidence of the effects of SGLT2 inhibitors on the risk of AKI for CAD patients undergoing PCI. Our study showed that CI-AKI incidence is lower in SGLT2-inhibitor users, suggesting further potential protective effects of SGLT2 inhibitors on the renal function of patients preparing for PCI. The most likely mechanism leading to CI-AKI is a rapid and sustained reduction in renal plasma flow preferentially to the outer medulla (21). This ischemia is due to an imbalance between renal vasodilatory and vasoconstrictive factors. SGLT2 inhibitor caused natriuresis, glycosuria, and following diuretic effect (4). These effects may help fluid administration correct volume depletion and expand IV volume, which increase the clearance of CM, decrease the concentration of CM in the tubule lumen and vasa recta, and counteract the activation of neurohormonal systems that lead to medullary vasoconstriction (22). We also found that levels of serum markers indicating renal function, namely, BUN, creatinine, and CyC, differed in user and nonuser groups. Higher levels of serum creatinine and lower levels of eGFR were observed at 48 and 72 h post PCI in the user group, further suggesting a role of an SGLT2 inhibitor in preserving renal function. On the other hand, CyC levels of patients at 24 h in the user group were lower than that of the control group, as is an earlier marker of AKI, suggesting the protective effects of the SGLT2 inhibitor may exert throughout the peri-PCI period.
The protective effects of SGLT2i have been validated in multiple studies regarding the cardiovascular and renal outcomes in patients with T2DM. In particular, 6 months after randomization in DECLARE-TIMI 58 trial, the mean eGFR in the dapagliflozin group and that in the placebo group started to show differences (11). Besides, according to the (Effects of Empagliflozin on Cardiac Structure in Patients with Type 2 Diabetes) study, SGLT2 inhibition was associated with a significant reduction in left ventricular mass indexed to body surface area after 6 months in people with T2DM and CAD (12). These studies indicate that usage of SGLT2i for more than 6 months may render the beneficial effects of cardioprotection and renoprotection for patients with T2DM. Thus 6-month usage of SGLT2i was used as the determination of inclusion for participants in this study. The impact of a shorter time of SGLT2i usage on the incidence of CI-AKI should be evaluated in future studies.
Our findings need to be interpreted with consideration of some limitations. This is a retrospective study based on a moderately sized cohort emanating from a single-center, thus ascertainment bias because of the retrospective nature of data is a possibility. Plus, the limited number of enrolled patients does not allow the assessment of specific outcome measures and subgroup analysis. Larger cohorts and multicenter studies are necessary to further assess the prognostic impact of SGLT2 inhibitor on CI-AKI risk for patients with T2DM scheduled for cardiac angiography. Meanwhile, as the two groups of patients may differ systematically, the potential effect of SGLT2 on CI-AKI events could be due to considerations for the commencement of SLGT2 inhibitors. In such cases, the comparisons are only suitable for patients who are legitimate candidates for SLGT2 inhibitors.
As American Dental Association recommended (23), injectable glucagon-like peptide 1 (GLP-1) receptor agonists (RAs) are the preferred option for patients with poor glucose control. However, some studies and case reports suggest GLP-1 RAs treatment tended to result in a nonsignificant decrease in risk for AKI (24, 25). Thus, excluding these patients may help to reduce confounding bias generated by GLP-1 RAs therapy in further analysis. Future studies will be needed to address the impact of SGLT2 inhibitors CI-AKI on patients with poor glycemic control.
In conclusion, our findings suggest that the risk of CI-AKI in real-life SGLT2 inhibitor users tended to decrease compared with matched nonusers, for legitimate candidates for SGLT2 inhibitors scheduled for PCI. These data suggest that the usage of SGLT2 inhibitor may benefit patients scheduled for PCI in preventing CI-AKI. Our analyses also demonstrate that advanced pharmacoepidemiologic methods can be leveraged to evaluate the potential risks of new and emerging drug classes. In the meantime, we believe the fear of CI-AKI associated with SGLT2 inhibitor use can be tempered for patients with CAD. Withdraw of SGLT2 inhibitor should not be recommended before PCI, to avoid inappropriate discouragement of the use of this novel class of agents that otherwise appear to afford significant long-term cardiovascular and renal protection in patients with T2DM.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Medical School of Xi'an Jiaotong University (No. 2021-1492). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
ZY and TL designed this study. TL, ND, and HG contributed to the collection and collation of the data. YW contributed to the explanation of the results and the analysis of the data. RH and TL wrote and revised the manuscript. All authors have reviewed the manuscript and the final version was approved by all.
This work was supported by the National Key R&D Program of China Grants (2021YFA1301200 and 2021YFA1301201), the Special Foundation for State Major Basic Research Program of China (No. 92049203), the Youth Project of the National Science Foundation of China (No. 82000474), the Natural Science Foundation of Shaanxi Province of China (No. 2020JM-383), the Innovative Talents Promotion Plan of Shaanxi Province of China (No. 2021KJXX-04), and funding of Xi'an Jiaotong University (No. xzy012019093).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all the participants in the study and the members of the survey teams, and the groups providing financial support.
1. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O'Brien SM, Boden WE, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. (2020) 382:1395–407. doi: 10.1056/NEJMoa1915922
2. Chalikias G, Drosos I, Tziakas DN. Contrast-induced acute kidney injury: an update. Cardiovasc Drugs Ther. (2016) 30:215–28. doi: 10.1007/s10557-015-6635-0
3. Advani A. Acute kidney injury: a bona fide complication of diabetes. Diabetes. (2020) 69:2229–37. doi: 10.2337/db20-0604
4. van Baar MJB, van Ruiten CC, Muskiet MHA, van Bloemendaal L, IJzerman RG, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. (2018) 41:1543–56. doi: 10.2337/dc18-0588
5. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. (2017) 377:644–57. doi: 10.1056/NEJMoa1611925
6. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
7. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. (2016) 375:323–34. doi: 10.1056/NEJMoa1515920
8. Lee YT, Hsu CN, Fu CM, Wang SW, Huang CC, Li LC. Comparison of adverse kidney outcomes with empagliflozin and linagliptin use in patients with type 2 diabetic patients in a real-world setting. Front Pharmacol. (2021) 12:781379. doi: 10.3389/fphar.2021.781379
9. Authors/Task Force M, Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. (2013) 34:3035–87. doi: 10.1093/eurheartj/eht108
10. Hua R, Li Y, Li W, Wei Z, Yuan Z, Zhou J. Apolipoprotein B/A1 ratio is associated with severity of coronary artery stenosis in CAD patients but not in non-CAD patients undergoing percutaneous coronary intervention. Dis Markers. (2021) 2021:8959019. doi: 10.1155/2021/8959019
11. Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol. (2019) 7:606–17. doi: 10.1016/S2213-8587(19)30180-9
12. Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. (2019) 140:1693–702. doi: 10.1161/CIRCULATIONAHA.119.042375
13. James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. (2011) 123:409–16. doi: 10.1161/CIRCULATIONAHA.110.970160
14. Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. (2013) 17:204. doi: 10.1186/cc11454
15. Nadkarni GN, Ferrandino R, Chang A, Surapaneni A, Chauhan K, Poojary P, et al. Acute kidney injury in patients on SGLT2 inhibitors: a propensity-matched analysis. Diabetes Care. (2017) 40:1479–85. doi: 10.2337/dc17-1011
16. Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol. (2016) 12:711–2. doi: 10.1038/nrneph.2016.159
17. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. (2004) 44:1393–9. doi: 10.1016/S0735-1097(04)01445-7
18. Tomasoni D, Fonarow GC, Adamo M, Anker SD, Butler J, Coats AJS, et al. Sodium-glucose co-transporter 2 inhibitors as an early, first-line therapy in patients with heart failure and reduced ejection fraction. Eur J Heart Fail. (2022) 24:431–41. doi: 10.1002/ejhf.2397
19. Zhuo M, Paik JM, Wexler DJ, Bonventre JV, Kim SC, Patorno E. SGLT2 inhibitors and the risk of acute kidney injury in older adults with type 2 diabetes. Am J Kidney Dis. (2021) 79:858–867.e1. doi: 10.1053/j.ajkd.2021.09.015
20. Salah HM, Al'Aref SJ, Khan MS, Al-Hawwas M, Vallurupalli S, Mehta JL, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors initiation in patients with acute heart failure, with and without type 2 diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. (2022) 21:20. doi: 10.1186/s12933-022-01455-2
21. Gassanov N, Nia AM, Caglayan E, Er F. Remote ischemic preconditioning and renoprotection: from myth to a novel therapeutic option? J Am Soc Nephrol. (2014) 25:216–24. doi: 10.1681/ASN.2013070708
22. Maioli M, Toso A, Leoncini M, Musilli N, Grippo G, Ronco C, et al. Bioimpedance-guided hydration for the prevention of contrast-induced kidney injury: the HYDRA Study. J Am Coll Cardiol. (2018) 71:2880–9. doi: 10.1016/j.jacc.2018.04.022
23. American Diabetes A. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2020. Diabetes Care. (2020), 43(Suppl 1):S98–110. doi: 10.2337/dc20-S009
24. Patoulias D, Boulmpou A, Papadopoulos CE, Doumas M. Glucagon-like peptide-1 receptor agonists and the risk of acute kidney injury: alarming, or not? Kidney Med. (2021) 3:674–5. doi: 10.1016/j.xkme.2021.02.014
Keywords: SGLT2 inhibitor, contrast-induced AKI, percutaneous coronary intervention, coronary artery disease, diabetes mellitus
Citation: Hua R, Ding N, Guo H, Wu Y, Yuan Z and Li T (2022) Contrast-Induced Acute Kidney Injury in Patients on SGLT2 Inhibitors Undergoing Percutaneous Coronary Interventions: A Propensity-Matched Analysis. Front. Cardiovasc. Med. 9:918167. doi: 10.3389/fcvm.2022.918167
Received: 12 April 2022; Accepted: 19 May 2022;
Published: 20 June 2022.
Edited by:
Giuseppe Ando', University of Messina, ItalyReviewed by:
Edward Zimbudzi, Monash Health, AustraliaCopyright © 2022 Hua, Ding, Guo, Wu, Yuan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Li, bGl0aW5nMjAwMEBob3RtYWlsLmNvbQ==; Zuyi Yuan, enV5aXl1YW5AbWFpbC54anR1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.