- 1Department of Cardiology, The First College of Clinical Medical Science, China Three Gorges University and Yichang Central People's Hospital, Yichang, China
- 2Hubei Key Laboratory of Cardiology, Department of Cardiology, Cardiovascular Research Institute, Renmin Hospital, Wuhan University, Wuhan, China
Objective: Studies on extracorporeal membrane oxygenation (ECMO) with and without an intra-aortic balloon pump (IABP) for cardiogenic shock (CS) have been published, but there have been no meta-analyses that compare the efficacy of these two cardiac support methods. This meta-analysis evaluated the outcomes of these two different treatment measures.
Methods: The PubMed, Embase, Cochrane Library, Web of Science, and Clinical Trials databases were searched until March 2022. Studies that were related to ECMO with or without IABP in patients with CS were screened. Quality assessments were evaluated with the methodological index for nonrandomized studies (MINORS). The primary outcome was in-hospital survival, while the secondary outcomes included duration of ECMO, duration of ICU stay, infection/sepsis, and bleeding. Revman 5.3 and STATA software were used for this meta-analysis.
Results: In total, nine manuscripts with 2,573 patients were included in the systematic review. CS patients who received ECMO in combination with IABP had significantly improved in-hospital survival compared with ECMO alone (OR = 1.58, 95% CI = 1.26–1.98, P < 0.0001). However, there were no significant differences in the duration of ECMO (MD = 0.36, 95% CI = −0.12–0.84, P = 0.14), duration of ICU stay (MD = −1.95, 95% CI = −4.05–0.15, P = 0.07), incidence of infection/sepsis (OR = 1.0, 95% CI = 0.58–1.72, P = 1.0), or bleeding (OR = 1.28, 95% CI = 0.48–3.45, P = 0.62) between the two groups of patients with CS.
Conclusion: ECMO combined with IABP can improve in-hospital survival more effectively than ECMO alone in patients with CS.
Introduction
Low cardiac output and hypoperfusion are highly associated with multiorgan damage and are typical manifestations of cardiogenic shock (CS). Moreover, treating CS is challenging for the attending doctors in the intensive care units (ICUs) (1). Cardiogenic shock is a life-threatening condition and patients with CS tend to have high rates of mortality (2). Mechanical heart assist devices are often needed to save patients' lives because drug therapy may be ineffective. To maintain the circulation state of CS patients and ensure the perfusion of important organs, extracorporeal membrane oxygenation (ECMO) and intra-aortic balloon pumps (IABPs) are frequently used as rescue options.
ECMO has been frequently used to treat CS in patients with myocardial infarction, explosive myocarditis, and sudden cardiac arrest (3–5). During the procedure, the venous blood is drawn out to achieve full oxygenation and then injected into the artery at a certain flow rate to ensure that the patient has an adequate oxygen supply and mean arterial pressure. An intra-aortic balloon pump (IABP) could increase diastolic pressure, augment coronary perfusion, and decrease systolic afterload, in addition to increasing the forward flow by inflating and deflating the balloon which decreases myocardial oxygen demand and provides hemodynamic support (6). ECMO and IABP can play positive roles in the treatment of CS. Intervention studies on ECMO with or without IABP are needed to improve the clinical treatment and survival rates of CS patients. The combination of these two methods has been gradually applied when rescuing patients with CS, and studies on patient survival or death have been published. However, there was no meta-analysis to evaluate the efficacy of ECMO combined with IABP and ECMO alone. In this study, we intend to systematically compare the outcomes of ECMO combined with IABP and ECMO alone in the treatment of patients with CS.
Data and Methods
Literature Search Strategy
PubMed, Embase, Cochrane Library, Web of Science, and Clinical Trials databases were used in the retrieval process. A combination of ECMO, intra-aortic balloon pump, and cardiogenic shock were the medical subject headings (MeSH) keywords that were searched in the English language articles. All literature in the database was searched until March 2022. The search strategy for PubMed and other databases is described in the Supplementary Material. This meta-analysis was conducted according to the PRISMA recommendation (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) (7).
Literature Inclusion and Exclusion Criteria
Literature inclusion criteria: (1) patients diagnosed with CS; (2) patients treated with ECMO alone vs. ECMO plus IABP; and (3) intervention studies. Exclusion criteria: (1) meta-analyses, reviews, comments, and letters; (2) literature without basic data; (3) literature with duplicate data; and (4) articles on animal experiments. This systematic review was performed by two authors who have previously published multiple meta-analyses. Each author independently judged whether the retrieved literature could be included in the study, and an additional judgment by a third author was provided in case of disagreement.
Literature Quality Evaluation Criteria
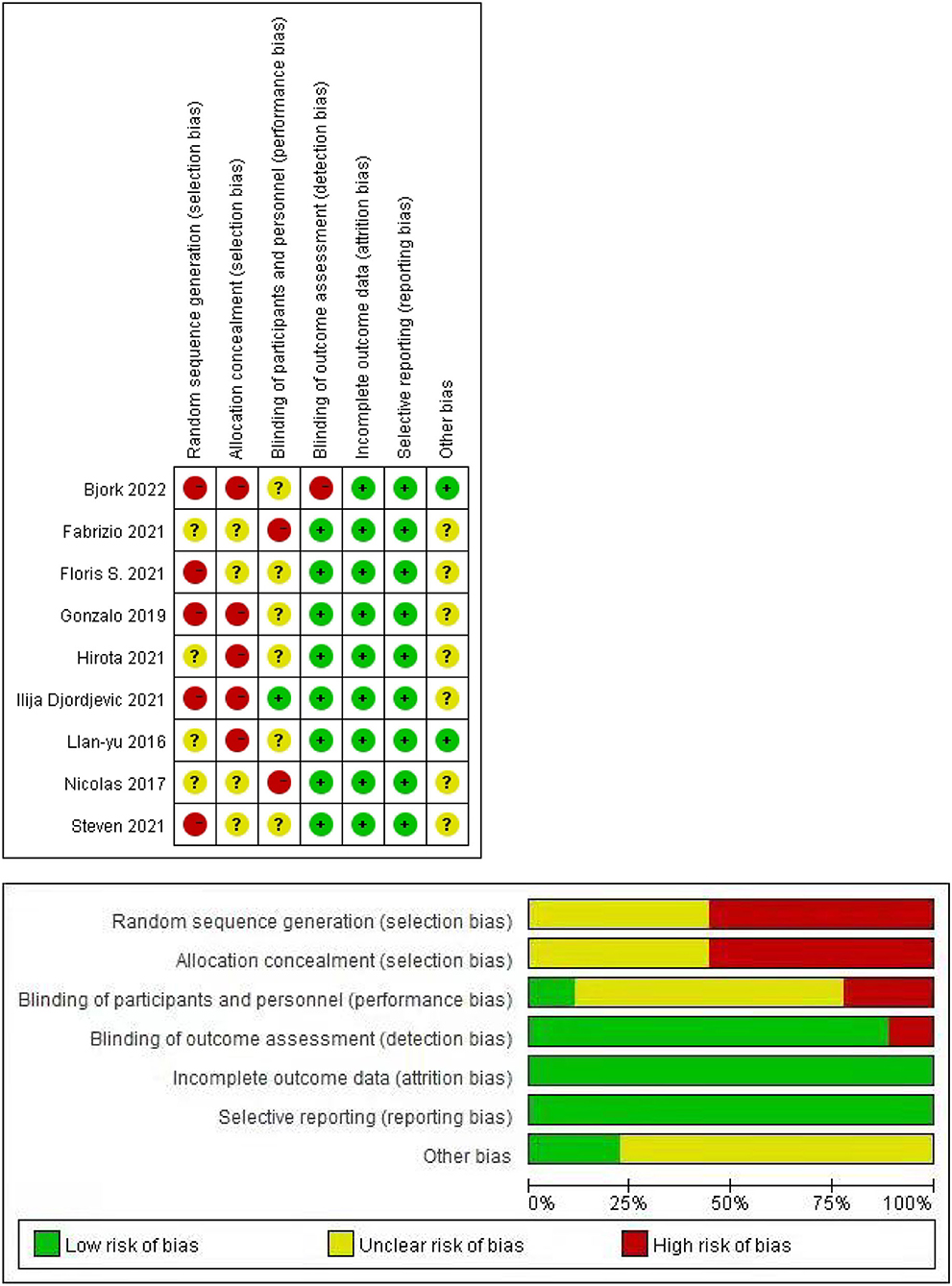
The methodological index for nonrandomized studies (MINORS) was applied for an in-depth assessment of the quality of the research (8). The index was used to evaluate the quality of the literature by assigning values to 12 items, each of which was scored 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The total MINORS scores were obtained by adding all the scores of the 12 items. Moreover, two authors independently evaluated the MINORS scores for each included study (Table 1).
Data Extraction
We extracted the baseline information of the studies, including authors, publication year, country, age, sex composition, body mass index (BMI), patients with diabetes, hypertension, chronic obstructive pulmonary disease (COPD), prior PCI, prior myocardial infarction (MI), a priori coronary artery grafting (CABG), and several other items. Data that was not directly extracted was obtained by data transformation.
Statistical Methods
Statistical analysis of the data was performed using RevMan 5.3 software. We used the fixed or random-effects meta-analysis model and a forest plot to present the pooled estimates of the odds ratio (OR) or mean difference (MD) and 95% confidence interval (CI). The heterogeneity was evaluated using the I-square (I2) statistic. A funnel map was established to assess publication bias, and STATA 13.0 software was used for sensitivity analysis. P-value < 0.05 was considered a statistically significant difference.
Results
Literature Search Results
Seven hundred and ninety-eight articles were obtained by searching with the proposed criteria. A total of 491 duplicate articles and 187 review-type articles were then excluded by reading the titles and abstracts. A total of 120 articles were included in the next retrieval step, and 101 articles were eliminated after carefully reading the full texts. Ultimately, a total of 19 articles were carefully investigated, and 10 of them were excluded because they were not intervention studies (Figure 1). The flowchart of the study selection process was conducted according to the PRISMA recommendation (7).
Basic Characteristics of the Included Literature
A total of nine studies (9–17) were ultimately included, including studies from New York, Japan, Taiwan, and cities in Europe, with a total of 2,573 patients (Table 1). The basic characteristics of the participants were as follows: the proportion of men was between 66 and 95; the mean BMI (body mass index) ranged from 22.8 to 29.1 kg/m2; 9–43% had diabetes; 4–66% had hypertension; 1–14% had COPD (chronic obstructive pulmonary disease), and 8–65% had MI (myocardial infarction). The indications for ECMO/IABP included myocarditis, dilated heart disease, acute heart failure, and so on (Table 1).
Quality of Evidence and Risk of Bias Across Studies
The quality of evidence according to the MINORS is presented in the last line of Table 1. The total score of each article was more than 16, which indicated that the quality of the articles was relatively high. In addition, all the included studies may have publication biases. We did not include RCTs, so there are many red dots and long red bars in Figure 2, which represents a high risk of random sequence generation and allocation concealment.
Outcomes
In-hospital Survival
ECMO combined with IABP treatment was associated with a higher rate of in-hospital survival in patients with CS (OR = 1.58, 95% CI = 1.26–1.98, P < 0.0001; Figure 3A). STATA software was used to verify the sensitivity analysis, and the result was stable after a single exclusion of RCTs (Figure 3B). The funnel map was stacked, and all points were under the funnel, indicating that there was no publication bias in the included studies when evaluating the result of in-hospital survival (Figure 3C).
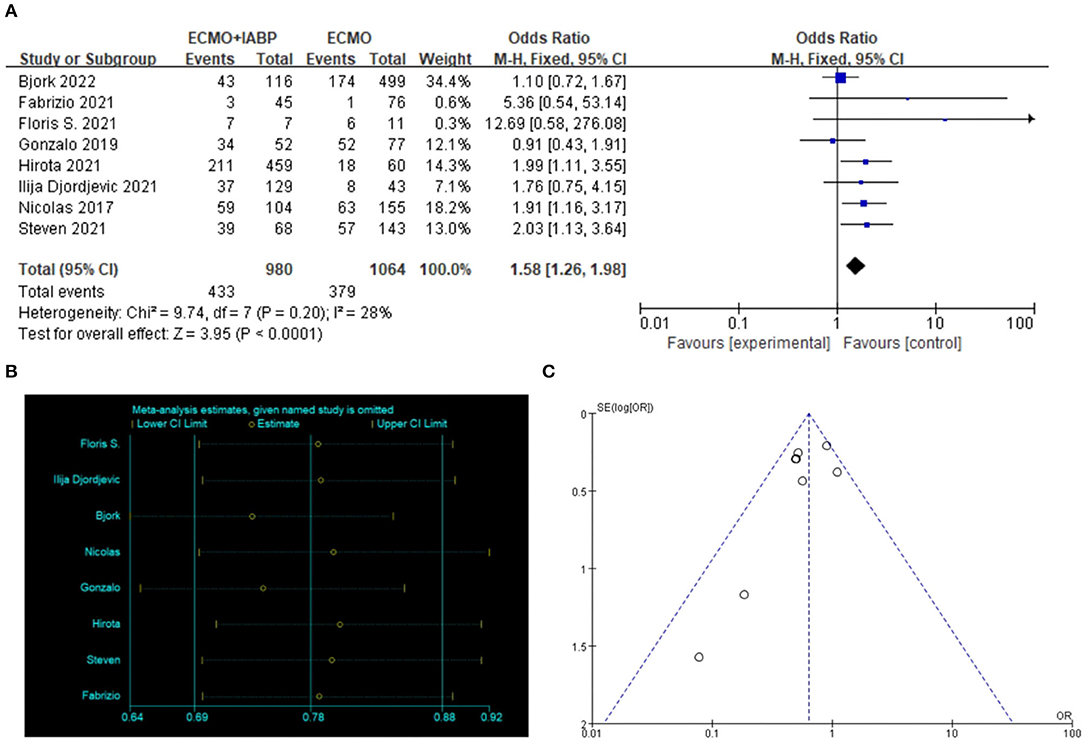
Figure 3. In-hospital survival. Forest plot of in-hospital survival in patients treated with ECMO with IABP vs. ECMO alone (A), the sensitivity analysis was tested in STATA (B). The symmetry of the funnel diagram indicates that there is no publication bias (C). ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump.
Duration of ECMO and ICU Admission
The treatment of ECMO combined with IABP did not significantly reduce the duration of ECMO (MD = 0.36, 95% CI = −0.12–0.84, P = 0.14; Figure 4A) or ICU stay (MD = −1.95, 95% CI = −4.05–0.15, P = 0.07) in the patients with CS (Figure 4B).
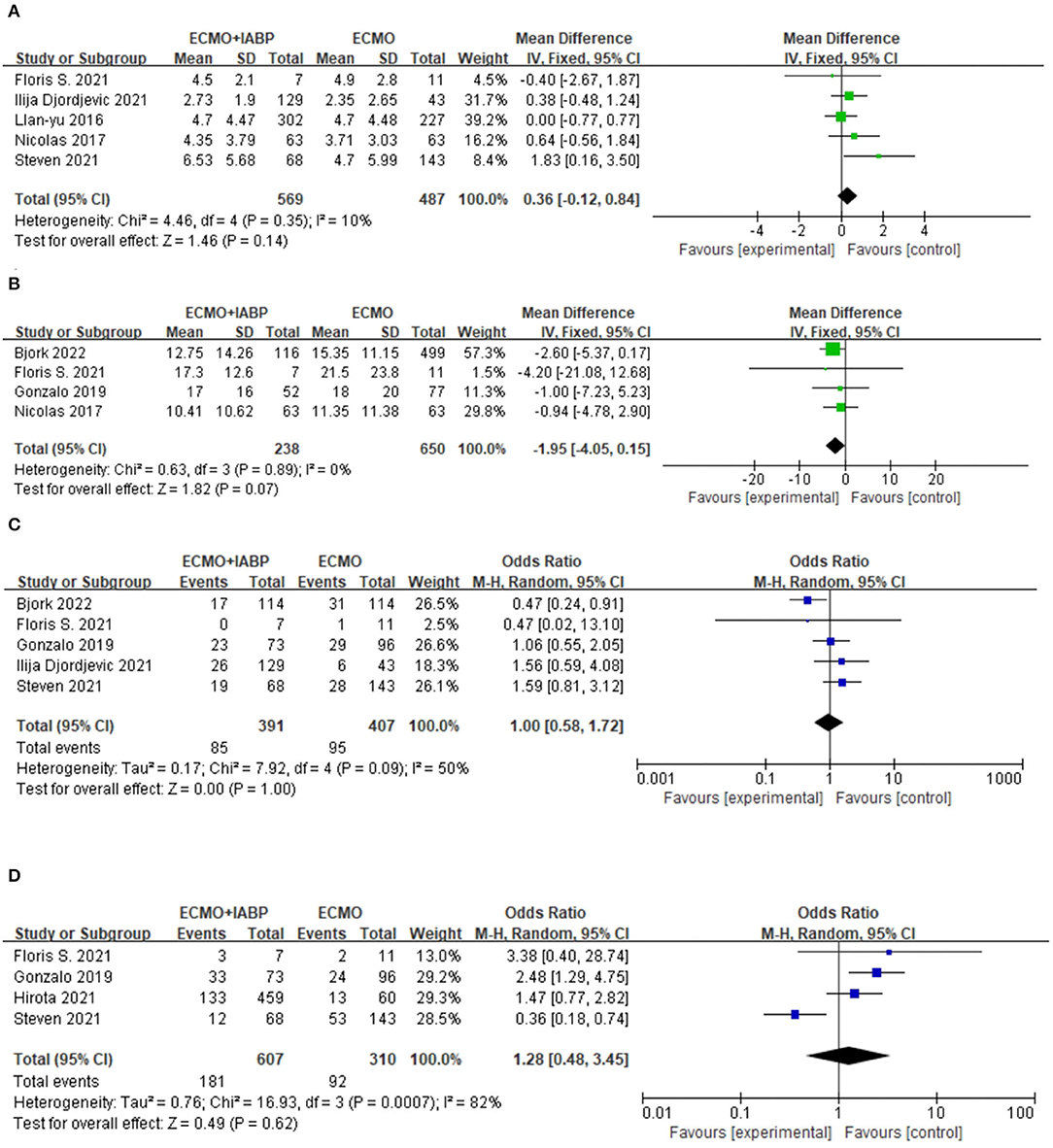
Figure 4. Forest plots of this meta-analysis. Duration of ECMO (A), Duration of ICU (B), Infection/sepsis (C), and Bleeding (D). ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump.
Incidence of Infection/Sepsis
There was no difference in the incidence of infection or sepsis between the two groups (OR = 1.0, 95% CI = 0.58–1.72, P = 1.0; Figure 4C).
Bleeding
There was no significant difference in the bleeding results between the two intervention methods (OR = 1.28, 95% CI = 0.48–3.45, P = 0.62; Figure 4D).
Discussion
We conducted a systematic meta-analysis of the studies on ECMO with IABP vs. ECMO alone in patients with CS. After conducting the meta-analysis, we found that ECMO combined with IABP was beneficial in saving lives. We found that there was a significant increase in the in-hospital survival rate in CS patients who received ECMO combined with IABP when compared with those who received ECMO alone. There was no obvious discrepancy in the duration of ECMO and ICU stay, the incidence of infection/sepsis, or bleeding between the two groups. This meta-analysis was the first to analyze intervention studies on ECMO with IABP and ECMO alone in CS patients. The results revealed that a combined therapy of ECMO and IABP was critical to improving the in-hospital survival rate. This meta-analysis provided a reference for the clinical application of cardiac auxiliary devices.
In-hospital survival is a direct index used to evaluate the therapeutic effect of rescue measures for severe cardio-respiratory failure. IABP acted as an assisting circulatory support device that could enhance cardiac diastolic function and improve coronary (18), cerebral (19), and systemic circulation. Regardless of the cause of shock, ECMO and IABP are guaranteed to save lives. Madershahian et al. proved that additional IABP therapy was shown to increase coronary bypass graft flow (ECMO alone 46.869.6 ml/min vs. ECMO+IABP 56.4612.1 ml/min; p < 0.005) (20). A 10-year clinical study found that IABP alone could significantly improve the survival rate of CS patients when compared with ECMO alone (IABP = 49.5%, ECMO = 30.5%) (21). Multiple studies have proven that IABP plays an important role in improving the survival rate of patients. In our meta-analysis, we also found that IABP, as an additional therapy, can significantly improve the in-hospital survival rate in CS patients.
The duration of ECMO and ICU stay were related to the prognosis of CS patients. Omar et al. proved that the duration of ECMO was an independent predictor of intracranial hemorrhage during ECMO support. They believed that the longer the ECMO duration, the higher the probability of intracranial hemorrhage (22). Data from a large multicenter database suggested that a longer duration of ECMO support after pediatric cardiac surgery was associated with poor outcomes (23). Glenn et al. (24) found an improved survival rate but no decrease in ECMO duration (25). In our meta-analysis, we also found no shortening of ECMO or ICU duration between these two groups, with low heterogeneity among the included studies. We believe that for patient survival, the shorter the duration of ECMO and ICU stay, the better the treatment effect.
Infection or sepsis may lead to fever and increase the burden on the circulatory system. Allou et al. found that 17.7% of patients developed cannula-related infections, including Enterobacteriaceae (38%), and Staphylococcus spp. (28.2%), and Pseudomonas aeruginosa (18.3%) (25). Yun et al. found that patients with gram-negative rods died more frequently and earlier than those with gram-positive cocci (26). However, there was also evidence that proved that an acquired infection was not independently associated with mortality (27) and that a prolonged duration of ECMO was an independent risk factor for nosocomial infection (28). In our meta-analysis, we found that there was no significant difference in infection or sepsis when patients were treated with ECMO and IABP or ECMO alone. This may be related to the aseptic operation and the care of the pipeline area during and after catheterization rather than the treatment difference.
Bleeding and thrombotic complications during ECMO have a significant impact on patient outcomes (29). Optimal anticoagulation, such as antithrombin supplementation, was needed while the cardiac assist device was used after analyzing activated partial thromboplastin time (APTT), antithrombin (AT) activity, platelet count, and fibrinogen concentration (30). In our meta-analysis, there was no significant difference in bleeding between ECMO combined with IABP and ECMO alone. Bleeding might be related to the use of anticoagulants and not independently affected by the treatment measures. However, different modes of ECMO and primary diagnoses were proven to be related to total bleeding (31). There was no adequate information on thrombosis to conduct the meta-analysis in our study. Moreover, it should be noted that removal of ECMO was associated with thrombosis (31). It is important to remove the ECMO cannula first and then the IABP cannula second to prevent the showering of thrombi.
Potential limitations existed in the process of this meta-analysis. First, all the patients had pre-existing conditions, with different diseases and different levels of lactic acid, which may have affected the outcomes of the study. Moreover, there was no particular order in which the IABP and ECMO were placed, which resulted in heterogeneity. Second, the nine articles were authored from different continents like America, Europe, and Asia, which may have affected the outcomes for the CS patients. Different race populations may have different responses to the same treatment. Third, all patients had different causes of CS and various degrees of myocardial injury and elevated blood lactic acid that occurred before treatment with ECMO or IABP. Fourth, the definition of bleeding may vary in different trials. We did not perform a subgroup analysis of bleeding because of insufficient data. Fifth, the nine studies were not randomized controlled trials but were same-period nonrandom control trials, which may have had an impact on the outcomes. Although there were no RCTs in the clinical intervention study of the combination of ECMO and IABP, we believe that it was reasonable to choose additional IABP therapy according to the patient's clinical needs rather than random selection.
Conclusion
This study showed that ECMO combined with IABP could be more effective in improving survival in patients with CS than ECMO alone.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
This study was designed by JZ and PZ. PZ and CY contributed data to the paper and statistical analysis. Interpretation of data were performed by PZ. All authors were involved in the drafting and revision of the manuscript and the final version to be published.
Funding
This work was supported by the National Natural Science Foundation of China (82070372 and 82170418) and Hubei Province's Outstanding Medical Academic Leader Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.917610/full#supplementary-material
References
1. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. (2017) 136:e232–68. doi: 10.1161/CIR.0000000000000525
2. Berg DD, Bohula EA, Morrow DA. Epidemiology and causes of cardiogenic shock. Curr Opin Crit Care. (2021) 27:401–8. doi: 10.1097/MCC.0000000000000845
3. Vallabhajosyula S, Arora S, Lahewala S, Kumar V, Shantha G, Jentzer JC, et al. Temporary mechanical circulatory support for refractory cardiogenic shock before left ventricular assist device surgery. J Am Heart Assoc. (2018) 7:e10193. doi: 10.1161/JAHA.118.010193
4. Le Gall A, Follin A, Cholley B, Mantz J, Aissaoui N, Pirracchio R. Veno-arterial-ECMO in the intensive care unit: from technical aspects to clinical practice. Anaesth Crit Care Pain Med. (2018) 37:259–68. doi: 10.1016/j.accpm.2017.08.007
5. Zhang Q, Han Y, Sun S, Zhang C, Liu H, Wang B, et al. Mortality in cardiogenic shock patients receiving mechanical circulatory support: a network meta-analysis. BMC Cardiovasc Disord. (2022) 22:48. doi: 10.1186/s12872-022-02493-0
6. Huu AL, Shum-Tim D. Intra-aortic balloon pump: current evidence & future perspectives. Future Cardiol. (2018) 14:319–28. doi: 10.2217/fca-2017-0070
7. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
8. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. Anz J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
9. van den Brink FS, Zivelonghi C, Vossenberg TN, Bleeker GB, Winia VL, Sjauw KD, et al. VA-ECMO with IABP is associated with better outcome than VA-ECMO alone in the treatment of cardiogenic shock in ST-Elevation myocardial infarction. J Invasive Cardiol. (2021) 33:E387–92. doi: 10.1093/ehjci/ehaa946.1790
10. Djordjevic I, Deppe AC, Sabashnikov A, Kuhn E, Eghbalzadeh K, Merkle J, et al. Concomitant ECMO and IABP support in postcardiotomy cardiogenic shock patients. Heart Lung Circ. (2021) 30:1533–9. doi: 10.1016/j.hlc.2021.03.276
11. Bjornsdottir B, Biancari F, Dalen M, Dell'Aquila AM, Jonsson K, Fiore A, et al. Postcardiotomy venoarterial extracorporeal membrane oxygenation with and without Intra-Aortic balloon pump. J Cardiothorac Vasc Anesth. (2022) 36:2876–83. doi: 10.1053/j.jvca.2022.02.006
12. Brechot N, Demondion P, Santi F, Lebreton G, Pham T, Dalakidis A, et al. Intra-aortic balloon pump protects against hydrostatic pulmonary oedema during peripheral venoarterial-extracorporeal membrane oxygenation. Eur Heart J Acute Cardiovasc Care. (2018) 7:62–9. doi: 10.1177/2048872617711169
13. Barge-Caballero G, Castel-Lavilla MA, Almenar-Bonet L, Garrido-Bravo IP, Delgado JF, Rangel-Sousa D, et al. Venoarterial extracorporeal membrane oxygenation with or without simultaneous intra-aortic balloon pump support as a direct bridge to heart transplantation: results from a nationwide Spanish registry. Interact Cardiovasc Thorac Surg. (2019) 29:670–7. doi: 10.1093/icvts/ivz155
14. Kida H, Sotomi Y, Hikoso S, Nakatani D, Mizuno H, Suna S, et al. Prognostic significance of intra-aortic balloon pumping support in patients with acute myocardial infarction and veno-arterial extracorporeal membrane oxygenation therapy. J Cardiol. (2022) 79:179–85. doi: 10.1016/j.jjcc.2021.10.011
15. Char S, Fried J, Melehy A, Mehta S, Ning Y, Kurlansky P, et al. Clinical efficacy of direct or indirect left ventricular unloading during venoarterial extracorporeal membrane oxygenation for primary cardiogenic shock. J Thorac Cardiovasc Surg. (2021). doi: 10.1016/j.jtcvs.2021.06.024
16. Lin LY, Liao CW, Wang CH, Chi NH Yu HY, Chou NK, et al. Effects of additional intra-aortic balloon Counter-Pulsation therapy to cardiogenic shock patients supported by extra-corporeal membranous oxygenation. Sci Rep. (2016) 6:23838. doi: 10.1038/srep23838
17. Monaco F, Ajello S, Calabrò MG, Melisurgo G, Landoni G, Arata A, et al. Left ventricular unloading with an IABP in patients undergoing ventricular tachycardia ablation with ECMO support. J Cardiothor Vasc an. (2021) 35:2686–93. doi: 10.1053/j.jvca.2020.12.049
18. De Silva K, Lumley M, Kailey B, Alastruey J, Guilcher A, Asrress KN, et al. Coronary and microvascular physiology during intra-aortic balloon counterpulsation. JACC Cardiovasc Interv. (2014) 7:631–40. doi: 10.1016/j.jcin.2013.11.023
19. Pfluecke C, Christoph M, Kolschmann S, Tarnowski D, Forkmann M, Jellinghaus S, et al. Intra-aortic balloon pump (IABP) counterpulsation improves cerebral perfusion in patients with decreased left ventricular function. Perfusion. (2014) 29:511–6. doi: 10.1177/0267659114525218
20. Madershahian N, Liakopoulos OJ, Wippermann J, Salehi-Gilani S, Wittwer T, Choi YH, et al. The impact of intraaortic balloon counterpulsation on bypass graft flow in patients with peripheral ECMO. J Card Surg. (2009) 24:265–8. doi: 10.1111/j.1540-8191.2009.00807.x
21. Lang CN, Kaier K, Zotzmann V, Stachon P, Pottgiesser T, von Zur MC, et al. Cardiogenic shock: incidence, survival and mechanical circulatory support usage 2007-2017-insights from a national registry. Clin Res Cardiol. (2021) 110:1421–30. doi: 10.1007/s00392-020-01781-z
22. Omar HR, Mirsaeidi M, Mangar D, Camporesi EM. Duration of ECMO is an independent predictor of intracranial hemorrhage occurring during ECMO support. ASAIO J. (2016) 62:634–6. doi: 10.1097/MAT.0000000000000368
23. Gupta P, Robertson MJ, Beam B, Gossett JM, Schmitz ML, Carroll CL, et al. Relationship of ECMO duration with outcomes after pediatric cardiac surgery: a multi-institutional analysis. Minerva Anestesiol. (2015) 81:619–27.
24. Glenn IC, Abdulhai S, Lally PA, Schlager A. Early CDH repair on ECMO: improved survival but no decrease in ECMO duration (A CDH Study Group Investigation). J Pediatr Surg. (2019) 54:2038–43. doi: 10.1016/j.jpedsurg.2019.01.063
25. Allou N, Lo PH, Persichini R, Bouchet B, Braunberger E, Lugagne N, et al. Cannula-Related infection in patients supported by peripheral ECMO: clinical and microbiological characteristics. ASAIO J. (2019) 65:180–6. doi: 10.1097/MAT.0000000000000771
26. Yun JH, Hong SB, Jung SH, Kang PJ, Sung H, Kim MN, et al. Epidemiology and clinical characteristics of bloodstream infection in patients under extracorporeal membranous oxygenation. J Intensive Care Med. (2021) 36:1053–60. doi: 10.1177/0885066620985538
27. Cashen K, Reeder R, Dalton HJ, Berg RA, Shanley TP, Newth C, et al. Acquired infection during neonatal and pediatric extracorporeal membrane oxygenation. Perfusion. (2018) 33:472–82. doi: 10.1177/0267659118766436
28. Wang J, Wang L, Jia M, Du Z, Hou X. Extracorporeal membrane Oxygenation-Related nosocomial infection after cardiac surgery in adult patients. Braz J Cardiovasc Surg. (2021) 36:743–51. doi: 10.21470/1678-9741-2020-0068
29. Reed RC, Rutledge JC. Laboratory and clinical predictors of thrombosis and hemorrhage in 29 pediatric extracorporeal membrane oxygenation nonsurvivors. Pediatr Dev Pathol. (2010) 13:385–92. doi: 10.2350/09-09-0704-OA.1
30. Silva R, Grabowski EF. Flow devices to assess platelet function: historical evolution and current choices. Semin Thromb Hemost. (2019) 45:297–301. doi: 10.1055/s-0039-1683840
Keywords: extracorporeal membrane oxygenation (ECMO), intra-aortic balloon pump (IABP), cardiogenic shock, survival (MeSH), meta-analysis
Citation: Zeng P, Yang C, Chen J, Fan Z, Cai W, Huang Y, Xiang Z, Yang J, Zhang J and Yang J (2022) Comparison of the Efficacy of ECMO With or Without IABP in Patients With Cardiogenic Shock: A Meta-Analysis. Front. Cardiovasc. Med. 9:917610. doi: 10.3389/fcvm.2022.917610
Received: 11 April 2022; Accepted: 24 May 2022;
Published: 07 July 2022.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Faizus Sazzad, National University of Singapore, SingaporePaul C. Tang, University of Michigan, United States
Copyright © 2022 Zeng, Yang, Chen, Fan, Cai, Huang, Xiang, Yang, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Yang, eWFuZ2ppYW5AY3RndS5lZHUuY24=; Jing Zhang, emhhbmdqaW5nQGN0Z3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Ping Zeng
Ping Zeng Chaojun Yang1†
Chaojun Yang1† Jing Chen
Jing Chen Zhixing Fan
Zhixing Fan Jun Yang
Jun Yang Jian Yang
Jian Yang