
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med., 29 July 2022
Sec. Coronary Artery Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.916616
This article is part of the Research TopicInsights in Coronary Artery Disease: 2022View all 20 articles
 Nino Cocco1*
Nino Cocco1* Rosalinda Madonna2
Rosalinda Madonna2 Valeria Cammalleri1
Valeria Cammalleri1 Giulio Cocco3
Giulio Cocco3 Domenico De Stefano1
Domenico De Stefano1 Danilo Ricciardi1
Danilo Ricciardi1 Francesco Grigioni1
Francesco Grigioni1 Gian Paolo Ussia1
Gian Paolo Ussia1An anomalous aortic origin of a coronary artery (AAOCA) from the opposite sinus, with an interarterial course, has been associated with an increased risk of myocardial ischemia and sudden death. As the exact pathophysiology of AAOCA is not well understood, the clinical management is also not well defined. With increased use of non-invasive imaging, the diagnosis of AAOCA is increasing and the association of anomalous origin and atherosclerotic disease is becoming a more important topic. We report a rare case of AAOCA chronic total occlusion (CTO). A 40-year-old Caucasian man was referred for invasive coronary angiography (ICA) due to typical chest pain and positive myocardial scintigraphy. ICA demonstrated CTO of an anomalous right coronary artery (ARCA) originating from the left side of the ascending aorta with an interarterial course. There was no lesion in the left coronary artery. During the procedure, unexpected rupture of the coronary artery occurred after dilatation with a small balloon at low pressure. The complication in this case was handled with good procedural final result but was an occasion for a food for thought. Coronary artery perforations are rare but life-threatening procedural complications that are usually caused by predisposing anatomical and procedural factors. We issue a warning on the risk of complications during complex percutaneous coronary intervention of these arteries, and we reconsidered the pathophysiology of the anomaly in a way that could change the approach to the disease. Based on this complication, we hypothesized that the wall of the artery could be fragile due to histopathological alterations, which could have a role in the pathophysiology of coronary malignancy. Future autopsy studies should be focused on the analysis of the arterial wall of the patient affected by sudden death with this anomaly.
An anomalous aortic origin of a coronary artery (AAOCA) from the opposite sinus, with an interarterial course between the pulmonary artery and aorta, has been associated with an increased risk of myocardial ischemia and sudden cardiac death (SCD) (1–4). The degree of risk (5, 6) and the pathophysiology of myocardial ischemia (7, 8) are debatable. Guidelines are limited and recommendations are mainly based on expert opinions (9, 10). Moreover, with increased use of non-invasive imaging to evaluate coronary artery disease, an increase in the absolute numbers of AAOCA is expected, with a concomitant increase in the prevalence of coronary artery disease. The management of patients affected by anomalous origin and atherosclerotic disease is becoming a more important topic in the field of coronary artery disease (11). We report a rare case of an anomalous right coronary artery (ARCA) originating from the left side of the ascending aorta with symptomatic chronic total occlusion (CTO). An unexpected complication led us to reconsider the pathophysiological basis of ARCA and to suspect histological involvement of the arterial wall.
The goals of this paper are three-fold: (1) to attempt an estimation of SCD specific for right AAOCA (ARCA) based on extrapolations of incidence numbers of SCD in the population and report the prevalence of right AAOCA, (2) to review the presumed pathophysiology of SCD in ARCA and present new insights, and (3) to issue a warning on the risk of complications during percutaneous coronary intervention (PCI) for ARCA CTO.
A 40-year-old Caucasian man, without any remarkable risk factors, was hospitalized for investigation and treatment of a several-month-history of class II angina. Myocardial scintigraphy, three weeks prior, showed moderate reversible perfusion defects involving the inferoposterior wall area. The patient was previously healthy and participated in non-competitive soccer. A 12-lead electrocardiogram and baseline echocardiography showed no abnormal wall motion and well-functioning valves. A baseline angiogram was performed from the right radial artery. The left coronary artery, originating from the left sinus, did not exhibit significant stenosis. There was Rentrop III collaterality towards the right coronary artery (RCA). The RCA, originating high and anteriorly over the left sinus, showed CTO in the middle tract (Figure 1). A Jr4 guide-catheter was chosen and a 1,5 × 8 mm anchoring balloon was placed in the conus artery. The occlusion was smoothly crossed with a tapered polymeric guidewire supported by a 1.8 Fr microcatheter. The attempt to bring the microcatheter beyond the occlusion failed because of the friction encountered, so we performed a predilatation with 1.5 × 12 mm compliant balloon. Good anterograde flow was restored (Figure 2). A second dilatation with a 2 × 15 mm balloon at 10 atm was performed to better prepare the lesion. A few seconds after this dilatation, the patient experienced chest pain. Angiography revealed a type II Ellis perforation in the midline and an extensive type D dissection distally to the ostium (Figure 3; Supplementary Video 1). A 3 × 26 mm zotarolimus eluting stent was implanted with long inflation (5 min). After deployment, the effusion disappeared. Four additional stents were implanted to cover the dissection. The final angiography showed a good result (Figure 4; Supplementary Video 2). Echocardiography showed negligible pericardial effusion. The patient was clinically stable and asymptomatic. Troponin levels remained stable on two occasions. A coronary computed tomography scan (CTA) confirmed the anomalous antero-left high origin of the RCA 17 mm from the sinotubular junction, and showed the angled ostium and interarterial course, about 2 cm between the aorta and pulmonary trunk (Figure 5), with modest effusion (10 mm) on CTA.
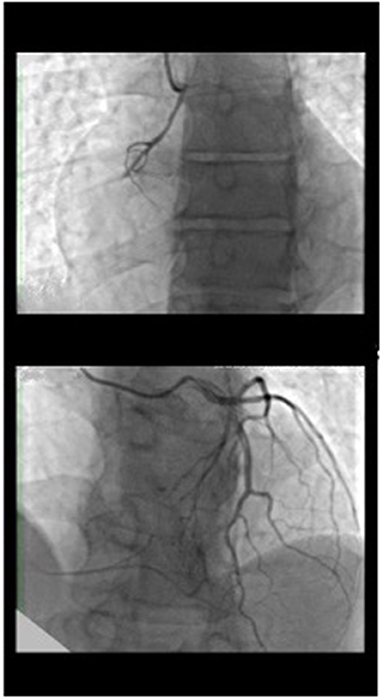
Figure 1. Coronary angiography of the left system showed left coronary artery normally positioned without atherosclerotic disease with rentrop III heterocoronary collateral circulation toward the right coronary artery, which has an anomalous origin on the left side of the ascending aorta and chronic occlusion in the proximal atrioventricular tract.
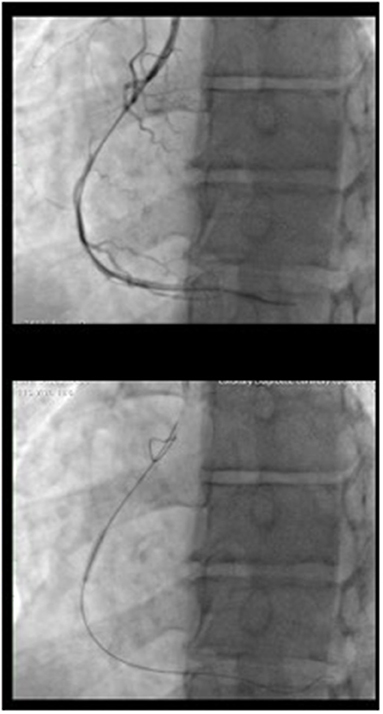
Figure 2. CTO treatment procedure: The occlusion was crossed with a tapered polymeric guidewire supported by microcatheter. The flow was restored after a dilatation of the occlusion with 1.5 x 12 balloon. Than a dilatation with a 2 x 15 balloon at 10 atm was performed.
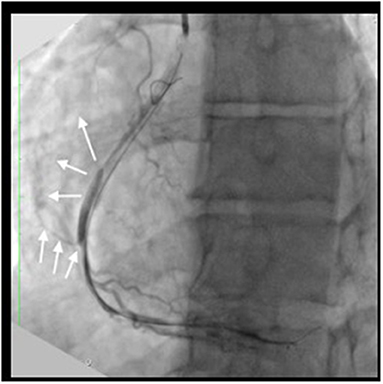
Figure 3. Procedural complication: The angiography after CTO dilatation with compliant 2 x 15 mm balloon at 10 atm showed coronary Ellis II perforation (white arrow) and diffuse type D dissection.
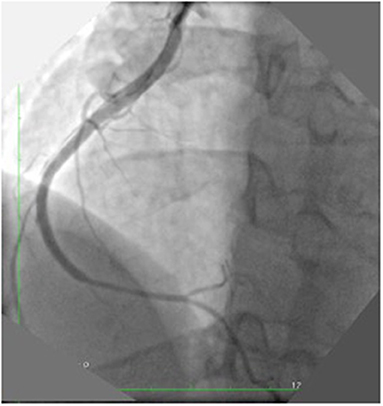
Figure 4. Post PCI final result: A first stent was implanted in the ruptured tract with long inflation (5 min). After the deployment the effusion disappeared. Four additional stents were implanted to cover the dissection. The final angiography showed a good result.
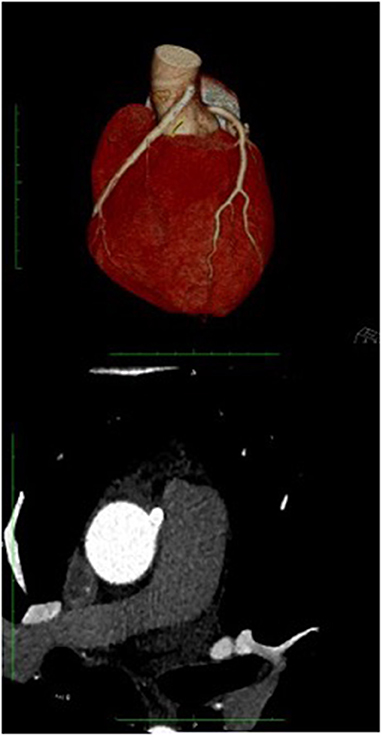
Figure 5. Coronary TC: The coronary TC confirmed the anomalous origin from opposite site of ascending aorta 17 mm from the sinotubular junction, with interarterial course. Modest effusion (10 mm).
Coronary artery anomalies have been reported as the second most frequent cause of SCD in young athletes, accounting for 12% of deaths (12, 13). Coronary artery anomalies arising from the pulmonary artery or the wrong sinus with an interarterial course between the pulmonary artery and aorta, referred to as AAOCA, are more often related to sudden death (SD) (14, 15). The most common subtype is an ARCA arising from the left side (prevalence 0.23%), followed by an anomalous left coronary artery (ALCA) arising from the right side (prevalence 0.03%) (1, 16). ALCA from the pulmonary artery is extremely rare (11, 15). Although evidence demonstrates that interarterial ALCA and ARCA are associated with an increased risk of SCD, their absolute risk in the general population is unknown. Thus, controversy remains regarding the optimal approach to risk stratify and manage these patients. With increased use of non-invasive imaging, the prevalence is increasing (1, 16) and could become a serious public health hazard.
The first data on the rates of SCD in AAOCA came almost exclusively from autopsy series, which reported high, yet discrepant, mortality rates between studies (0-50% for ARCA and 30-100% for ALCA) (1, 4, 12). In the first series, 50% of SD cases had anomalous coronary arteries (30% right and 80% left) (1, 4, 12). From this data, the estimated risk of SCD in AAOCA was very high (50% for ALCA and 25% for ARCA) (17, 18) and many authors agreed that surgical correction should have been the first line of therapy in these patients (19–22). However, this autopsy statistic report has been proven wrong. It reported on the chance of a person who dies suddenly having an anomalous coronary artery, not the risk of SD from an anomalous coronary artery, only reflecting the prevalence of AAOCA in those who died. Subsequent studies indicated that the risk of SD was far lower, but discrepancy in the data remains. The rate of death from AAOCA has been reported as being between 0.07 and 0.61 per 100,000 person years (5, 12, 16, 20, 22). From this data, it was determined that the cumulative risk of death over a 20-year period from the age of 15–34 years in patients with AAOCA was 6.3% for ALCA and 0.2% for ARCA (23). Assuming the prevalence of AAOCA is 0.1-0.2% (24–27), the risk of death for AAOCA is 0.12–0.15% (1:860–1:652), involving above all young people engaged in frequent vigorous exercise and patients with ALCA (5, 28). A more recent review of a database of 5,100 cases of SCD referred to a specialist cardiac pathology center between January 1994 and March 2017, and identified a subgroup of 30 cases (0.6%) with AAOCA (15). The number of SCD from ARCA and ALCA were compared, and, although ALCA is considered more malignant, the SCD numbers for ARCA were slightly higher (11 patients, 37% vs. 10 patients 37%, respectively). ALCA from the pulmonary artery occurred in seven cases (23%). Exercise-induced SCD was associated more frequently with ALCA than ARCA, where death occurred often at rest or during sleep. The mean age was much higher, compared to previous studies (28+/−17 years); there were patients in the fifth and sixth decades of life, most with ARCA.
The risk of SCD in patients with AAOCA are inconclusive due to the conflicting results. We believe this discrepancy could have stemmed from the fact that ARCA and ALCA have different clinical features and the identification of a unique risk of AAOCA (29) could have been the original reason for the conflicting results in the literature. SCD was more likely to occur in ALCA than ARCA; in fact, SCD in young, trained patients are mainly caused by ALCA. But, when we consider the populations of each age group, the number of SCD cases were equivalent or even greater for ARCA (15), since ARCA was more prevalent (0.23 vs. 0.03%) (6, 11, 30). In patients of older age, death often occurs at rest or during sleep (15). Thus, ARCA is not a minor disease but a different disease, most likely with a different pathophysiological pathway as well. The risk of SD in a patient with ARCA has not been properly analyzed. We attempted to estimate the risk of SD based on the data from previous studies.
The age-adjusted national incidence of SCD is 60 per 100,000 (95% confidence interval; 54–66 per 100,000) (31, 32) and 0.6% thereof are caused by AAOCA (15), translating to an incidence of 3,6 per 1,000,000 per year. ARCA are found in 36% of SCD caused by AAOCA (15), translating to an incidence of 1,3 per 1,000,000 per year. Since the prevalence of ARCA is 0.23%, we can derive that 1,3 per 2300 persons a year with ARCA experience SCD. Therefore, the incidence of SD in a patient with ARCA is about 1:1800. This is the first attempt to estimate the incidence of SCD in a patient specifically affected by ARCA.
Assuming the pathophysiological understanding of the disease is key to predict the risk of SCD in AAOCA; several studies have attempted to isolate a key primary factor, but no single anatomical component of the coronary artery has been isolated. Several studies have been done to find a true “denominator” causing ischemia and SCD. Several putative mechanisms have been proposed to account for the association between SCD and an anomalous origin of a coronary artery from the opposite sinus. The downstream event is presumed to be ischemia (8, 33–37). Historically, the interarterial course was thought to be the crucial abnormality, assuming a scissor-like mechanism created by the close proximity of the aorta and pulmonary artery, especially during exertion (1, 2, 8). However, the pulmonary artery is unlikely to exert sufficient pressure to occlude a coronary artery in the absence of pulmonary hypertension. Therefore, the interarterial course may act only as a surrogate for other anatomical high-risk features, including a slit-like ostium, acute take off angle, and proximal narrowing (also referred to as hypoplasia) with an elliptical vessel shape and intramural course. The anatomical high-risk feature of a slit-like ostium at the ectopic origin is defined as a ≥50% reduction in the minimal lumen diameter compared to the normal distal reference diameter (34). Thus, the deformed coronary ostium with a decreased cross-sectional area acts as an ostial stenosis. Due to the slit-like ostium opening, during exercise and systolic expansion of the aortic root, the orifice can collapse in a valve-like manner (34, 35). An acute take-off angle (less than 45°) is defined as an axial course of the proximal segment tangential to the great vessel circumference. Kinking of an anomalous coronary artery during exercise was proposed as a contributing ischemia-inducing mechanism (8, 34). The intramural course length is considered the most relevant feature in terms of hemodynamic repercussion (6, 35–37). The intramural coronary artery is slit-like and is characterized by thin inner and outer aortic wall layers and lateral luminal compression that undergoes phasic increases with each systole (38). This anatomical feature is considered by many authors as being related to SCD, especially if associated with the other features mentioned above (37). Unfortunately, in clinical practice, none of these have been shown to be useful in the prognostic assessment of SD and there are no prognostically distinguishing pathological features that could be prospectively defined (7–10). Finally, another theory postulates that compression and kinking of the anomalous artery could cause intimal damage and a propensity to spasm and ischemia (39, 40), which was described more than 20 years ago, but considered implausible by the majority (8, 41).
We describe a rare case of anterograde CTO of the RCA with anomalous origin from the opposite side of the ascending aorta. Data regarding complex PCI, especially for CTO, in this setting are very limited (24, 26, 27, 42–48). The procedure was unexpectedly complicated by perforation and extensive type D dissection after predilatation with a small balloon at low pressure. Coronary perforation is a rare, but life-threatening, complication. It is usually due to high pressure balloon dilatation or the use of an oversized balloon in the context of predisposing factors such as tortuosity, calcification, old age, CTO, or small vessel caliber (24). The myocardial bridge is also an important predisposing factor (25). A 2 mm diameter balloon inflated at 10 atm in a 3.5 mm diameter artery causing perforation is almost inexplicable, even in a CTO procedure. The rupture could be explained by structural fragility of the artery. An anomalous course with acute angulation may favor the complication but there could also be a histopathological explanation. In the myocardial bridge, for example, the anomalous course of the artery is associated with intimal and endothelial differences in the tunneled artery that predispose to a greater risk of rupture during PCI. The intimal layer is thinner, and the endothelial cells are spindle shaped. This alteration and compression carry a major risk for perforation (25). Bunji et al. (39) hypothesized that the RCA located between the aorta and the pulmonary artery is prone to compression or kinking phenomena, resulting in intimal disruption and subsequent predisposition to vasospasm that correlates with ischemia and SCD. The histopathological alteration causing wall fragility could also be an explanation for the complication in our case.
PCI in an anomalous coronary artery is difficult, particularly with CTO, due to technical complexities, from engaging the coronary ostium to delivery of hardware through the vessel. Only a few cases have been reported in the literature (24, 26, 27, 42–48) (Table 1).
Nine cases of ARCA CTO have been reported in the literature. Three major complications, two of which were not related to catheter manipulation, were mentioned. A case of aorto-coronary dissection due to balloon dilatation and rupture was described by Abdou and Wu (27). In this case, the type D dissection started from the proximal ARCA and extended retrograde, involving the aorta. Estimation of the risk of complication is not the purpose of this paper, considering the small number of patients, but this trend is unusual. Furthermore, analysis of the cases showed a common tract of occlusion in most cases. Nine out of ten cases involved the proximal atrioventricular part of the artery, in the area between the cone branch and the first branch for the acute margin (27, 42–48). Due to the difference in anatomy of the anomalous artery, we divided it into four segments. We considered the proximal segment to be the tract from the origin to the origin of the conus or sinoatrial branches, the proximal atrioventricular segment the tract from the conus branch to halfway to the acute margin, the middle segment the tract from this halfway point to the acute margin, and a distal segment from the acute margin to the base of the heart at the junction of the atrial and ventricular septum. Analyzing the non-CTO PCI cases in the literature, we confirmed that the tract of disease in ARCA was almost always the proximal atrioventricular segment. A review of 18 cases with imaging showed that 16 had proximal atrioventricular segment involvement (49–62), five of six had SCA- STEMI (58–62), and 11 of 12 had chronic critical stenosis (49–58). Furthermore, Bruls et al. (63) described a case of resuscitated SD in a 37-year-old man with ARCA in whom they performed coronary artery bypass grafting. After surgical examination of the artery, several initial branches in its first segment (infundibular branch or the sinus nodal artery) and a fibrously thickened wall were noted. These data suggest that histological alteration may play an important role in ARCA malignancy (Figure 6).
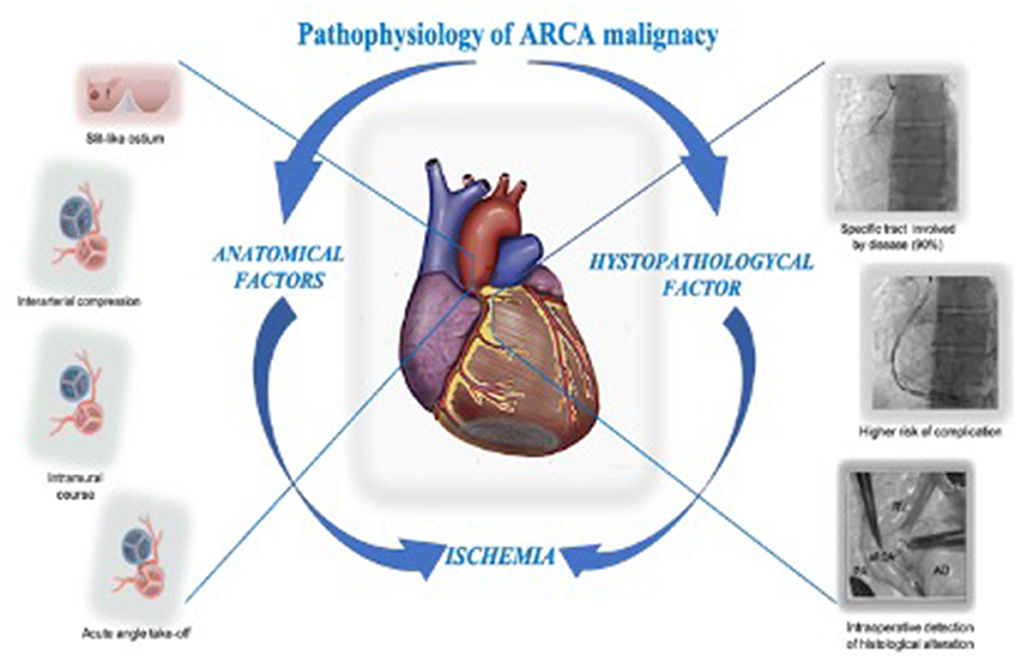
Figure 6. ARCA presumed ischemia pathophysiology: The downstream event in the pathophysiology of ARCA malignancy in the contest of SCD is presumed to be ischemic. The anatomical feature like the interarterial passage causing compression in a scissor-like mechanism, the slit-like ostium acting as an ostial stenosis, the acute take-off angle causing kinking, and the intramural course causing compression, are historically considered involved in the pathogenesis of ischemia. Unfortunately, in clinical practice none of these have been shown to be useful in the prognostic assessment of SD. The higher incidence of procedural complications in the context of ARCA CTO treatment, the presence of a specific tract of the artery involved by the disease (90% of the case reported in literature), and the intraoperative detection of histological alteration of the anomalous artery wall in a patient who suffered from a SCA call us to reconsider the histopathological alteration in the pathogenesis of ischemia and ARCA malignancy. ARCA, anomalous right anomalous coronary originating from the left side; CTO, chronic total occlusion; SCD, sudden cardiac death.
There is no greater tragedy in medicine than SD in an apparently healthy person. AAOCA is the second leading cause of SCD in athletes, and with increased use of non-invasive imaging, the prevalence is expected to increase. The interarterial course is believed to be the factor of malignancy in this context and a lot of effort has been made to find a true denominator in understanding the pathophysiological pathway. After almost 50 years, none of the suspected anatomical factors have been shown to be useful in the prognostic assessment of SD and there are no prognostically distinguishing pathological features that could be prospectively defined. Therefore, the clinical management is still not defined. The strategy of the common pathway causing malignancy has failed, thus, the interarterial course may act only as a surrogate. ARCA and ALCA have very different clinical features. ALCA is rare but more malignant and, among AAOCA patients, causes most SD cases in young patients. ARCA is relatively common and less malignant, but when populations of each age group are evaluated, the number of SCDs it causes are equal, or even greater than ALCA. These patients are older, and deaths often occur at rest. Therefore, ARCA is not a minor disease, but a different disease. Based on this assumption, the pathophysiological basis of malignancy could also be different. In our case, an unexpected complication led to reconsideration of a possible parietal structural involvement in ARCA malignancy. The theory of histological alteration of the arterial wall playing a role in the pathophysiology of ARCA malignancy is plausible based on three observations: (1) the higher incidence of coronary periprocedural major dissection; (2) the evidence of a specific tract of the artery being affected by disease (90% of the cases reported), which may render it particularly prone to stress, torsion, or kinking, and thus more prone to histological alteration; and (3) the intraoperative detection of wall alteration of the anomalous artery that was demonstrated during surgical examination of the first tract of ARCA in a 36-year-old patient.
The istologyical alteration causing spasm and ischemia, suspected by some authors but considered implausible by the community, especially after studies performed with intracoronary acetylcholine, would seem to disprove this hypothesis. We hypothesized that this alteration could involve ARCA in specific segments and that spasm may not be the only factor for ischemia. This alteration could render arteries more prone to compression, kinking, atherosclerosis, or thrombus. We also hypothesized that each patient may have a different involvement and that the few patients with more important histological distortion could be those at most risk. The histological pattern of the anomalous arterial wall was never evaluated.
In conclusion, 50 years since the identification of the relationship between SCD and AAOCA, the risk of SCD in a carrier patient has still not been defined and the pathophysiological causes of the disease are unclear. For the first time in the literature, we issue a warning on the risk of complications during percutaneous treatment of a CTO in an anomalous right coronary artery and we reconsidered the pathophysiological basis of the anomaly in a way that could change the approach to the disease.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.916616/full#supplementary-material
AAOCA, Anomalous aortic origin of a coronary artery; ALCA, Anomalous left coronary artery; ARCA, Anomalous right coronary artery; CTO, Chronic total occlusion; ICA, Invasive coronary angiography; PCI, Percutaneous coronary intervention; RCA, Right coronary artery; SD, Sudden death; SCD, Sudden cardiac death.
1. Cheitlin MD, De Castro CM, McAllister HA. Sudden death as a complication of anomalous left coronary origin from the anterior sinus of valsalva, A not-so-minor congenital anomaly. Circulation. (1974) 50:780–7. doi: 10.1161/01.CIR.50.4.780
2. Roberts WC, Siegel RJ, Zipes DP. Origin of the right coronary artery from the left sinus of valsalva and its functional consequences: analysis of 10 necropsy patients. Am J Cardiol. (1982) 49:863–8. doi: 10.1016/0002-9149(82)91970-1
3. Kragel AH, Roberts WC. Anomalous origin of either the right or left main coronary artery from the aorta with subsequent coursing between aorta and pulmonary trunk: analysis of 32 necropsy cases. Am J Cardiol. (1988) 62:771–7. doi: 10.1016/0002-9149(88)91220-9
4. Frescura C, Basso C, Thiene G, Corrado D, Pennelli T, Angelini A, et al. Anomalous origin of coronary arteries and risk of sudden death: a study based on autopsy population of congenital heart disease. Hum Pathol. (1998) 29:689–95. doi: 10.1016/S0046-8177(98)90277-5
5. Peñalver JM, Mosca RS, Weitz D, Phoon CK. Anomalous aortic origin of coronary arteries from the opposite sinus: a critical appraisal of risk. BMC Cardiovasc Disord. (2012) 12:83. doi: 10.1186/1471-2261-12-83
6. Cheezum MK, Liberthson RR, Shah NR, Villines TC, O'Gara PT, Landzberg MJ, et al. Anomalous aortic origin of a coronary artery from the inappropriate sinus of valsalva. J Am Coll Cardiol. (2017) 69:1592–608. doi: 10.1016/j.jacc.2017.01.031
7. Taylor AJ, Byers JP, Cheitlin MD, Virmani R. Anomalous right or left coronary artery from the contralateral coronary sinus: “high-risk” abnormalities in the initial coronary artery course and heterogeneous clinical outcomes. Am Heart J. (1997) 133:428–35. doi: 10.1016/S0002-8703(97)70184-4
8. Bigler MR, Ashraf A, Seiler C, Praz F, Ueki Y, Windecker S, et al. Hemodynamic relevance of anomalous coronary arteries originating from the opposite sinus of Valsalva-in search of the evidence. Front CardioVasc Med. (2020) 7:591326. doi: 10.3389/fcvm.2020.591326
9. Baumgartner H, De Backer J. The ESC clinical practice guidelines for the management of adult Congenital Heart Disease 2020. Eur Heart J. (2020) 41:4153–4. doi: 10.1093/eurheartj/ehaa701
10. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll. (2018). doi: 10.1016/j.jacc.2018.08.1028
11. Cheezum MK, Ghoshhajra B, Bittencourt MS, Hulten EA, Bhatt A, Mousavi N, et al. Anomalous origin of the coronary artery arising from the opposite sinus: prevalence and outcomes in patients undergoing coronary CTA. Eur Heart J Cardiovasc Imaging. (2017) 18:224–35. doi: 10.1093/ehjci/jev323
12. Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. (1996) 276:199–204. doi: 10.1001/jama.1996.03540030033028
13. Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. (2009) 119:1085–92. doi: 10.1161/CIRCULATIONAHA.108.804617
14. Hill SF, Sheppard MN. A silent cause of sudden cardiac death especially in sport: congenital coronary artery anomalies. Br J Sports Med. (2014) 48:1151–6. doi: 10.1136/bjsports-2013-092195
15. Finocchiaro G, Behr ER, Tanzarella G, Papadakis M, Malhotra A, Dhutia H, et al. Anomalous coronary artery origin and sudden cardiac death: clinical and pathological insights from a national pathology registry. JACC Clin Electrophysiol. (2019) 5:516–22. doi: 10.1016/j.jacep.2018.11.015
16. Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. (2006) 296:1593–601. doi: 10.1001/jama.296.13.1593
17. Liberthson RR, Dinsmore RE, Fallon JT. Aberrant coronary artery origin from the aorta. Report of 18 patients, review of literature and delineation of natural history and management. Circulation. (1979) 59:748–54. doi: 10.1161/01.CIR.59.4.748
18. Jaggers J, Lodge AJ. Surgical therapy for anomalous aortic origin of the coronary arteries. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. (2005) 8:122–7. doi: 10.1053/j.pcsu.2005.01.004
19. Erez E, Tam VK, Doublin NA, Stakes J. Anomalous coronary artery with aortic origin and course between the great arteries: improved diagnosis, anatomic findings, and surgical treatment. Ann Thorac Surg. (2006) 82:973–7. doi: 10.1016/j.athoracsur.2006.04.089
20. Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. (2004) 141:829–34. doi: 10.7326/0003-4819-141-11-200412070-00005
21. Gulati R, Reddy VM, Culbertson C, Helton G, Suleman S, Reinhartz O, et al. Surgical management of coronary artery arising from the wrong coronary sinus, using standard and novel approaches. J Thorac Cardiovasc Surg. (2007) 134:1171–8. doi: 10.1016/j.jtcvs.2007.02.051
22. Vouhé PR. Anomalous aortic origin of a coronary artery is always a surgical disease. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. (2016) 19:25–9. doi: 10.1053/j.pcsu.2015.12.007
23. Brothers J, Carter C, McBride M, Spray T, Paridon S. Anomalous left coronary artery origin from the opposite sinus of valsalva: evidence of intermittent ischemia. J Thorac Cardiovasc Surg. (2010) 140:e27–9. doi: 10.1016/j.jtcvs.2009.06.029
24. Patel VG, Brayton KM, Tamayo A, Mogabgab O, Michael TT, Lo N, et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Intv. (2013) 6:128–36. doi: 10.1016/j.jcin.2012.10.011
25. Pourhoseini S, Bakhtiari M, Babaee A, Ostovan MA, Eftekhar-Vaghefi SH, Ostovan N, et al. Increased risk of coronary perforation during percutaneous intervention of myocardial bridge: what histopathology says. J Cardiovasc Thorac Res. (2017) 9:108–12. doi: 10.15171/jcvtr.2017.18
26. Patra S, Halder A, Chakraborty R, Dey S. Percutaneous coronary intervention in chronic total occlusion of anomalous right coronary artery: an onerous journey. Am J Cardiovasc Dis. (2021) 11:624−7.
27. Abdou SM, Wu C-J. Treatment of aortocoronary dissection complicating anomalous origin right coronary artery and chronic total intervention with intravascular ultrasound guided stenting. Catheter Cardiovasc Interv. (2011) 78:914–9. doi: 10.1002/ccd.23021
28. Mirchandani S, Phoon CK. Management of anomalous coronary arteries from the contralateral sinus. Int J Cardiol. (2005) 102:383–9. doi: 10.1016/j.ijcard.2004.10.010
29. Gentile F, Castiglione V, De Caterina R. Coronary artery anomalies. Circulation. (2021) 144:983–96. doi: 10.1161/CIRCULATIONAHA.121.055347
30. Davis JA, Cecchin F, Jones TK, Portman MA. Major coronary artery anomalies in a pediatric population: incidence and clinical importance. J Am Coll Cardiol. (2001) 37:593–7. doi: 10.1016/S0735-1097(00)01136-0
31. Stecker EC, Reinier K, Marijon E, Narayanan K, Teodorescu C, Uy-Evanado A, et al. Public health burden of sudden cardiac death in the United States. Circ Arrhythm Electrophysiol. (2014) 7:212–7. doi: 10.1161/CIRCEP.113.001034
32. Kuriachan VP, Sumner GL, Mitchell LB. Sudden cardiac death. Curr Probl Cardiol. (2015) 40:133–200. doi: 10.1016/j.cpcardiol.2015.01.002
33. Maron BJ. Triggers for sudden cardiac death in the athlete. Cardiol Clin. (1996) 14:195–210. doi: 10.1016/S0733-8651(05)70273-3
34. Virmani R, Chun PK, Goldstein RE, Robinowitz M, McAllister HA. Acute takeoffs of the coronary arteries along the aortic wall and congenital coronary ostial valve-like ridges: association with sudden death. J Am Coll Cardiol. (1984) 3:766–71. doi: 10.1016/S0735-1097(84)80253-3
35. Angelini P, Walmsley R, Cheong BY, Ott DA. Left main coronary artery originating from the proper sinus but with acute angulation and an intramural course, leading to critical stenosis. Tex Heart Inst J. (2010) 37:221–5.
36. Kaushal S, Backer CL, Popescu AR, Walker BL, Russell HM, Koenig PR, et al. Intramural coronary length correlates with symptoms in patients with anomalous aortic origin of the coronary artery. Ann Thorac Surg. (2011) 92:986–91. doi: 10.1016/j.athoracsur.2011.04.112
37. Jegatheeswaran A, Devlin PJ, Mccrindle BW, Williams WG, Jacobs ML, Blackstone EH, et al. Features associated with myocardial ischemia in anomalous aortic origin of a coronary artery: a congenital heart surgeons' society study. J Thorac Cardiovasc Surg. (2019) 158:822–834.e3. doi: 10.1016/j.jtcvs.2019.02.122
38. Angelini P. Coronary artery anomalies – current clinical issues, definitions, classification, incidence, clinical relevance, and treatment guidelines. Tex Heart Inst J. (2002) 29:271–8.
39. Bunji K, kanaya H, Ikeda M, Uno Y, Fujita S, Kato F, et al. Acute inferior myocardial infarction and coronary spasm in a patient with an anomalous origin of the right coronary artery from the left sinus of Valsalva. Jpn Circ J. (2000) 64:641–3. doi: 10.1253/jcj.64.641
40. Maddoux GL, Goss JE, Ramo BW, Raff GL, Heuser RR, Shadoff N, et al. Angina and vasospasm at rest in a patient with an anomalous left coronary system het. Cardiovasc Diagn. (1989) 16:95–8. doi: 10.1002/ccd.1810160205
41. Grani C, Kaufmann PA, Windecker S, Buechel RR. Diagnosis and management of anomalous coronary arteries with a malignant course. Inter Cardiol. (2019) 14:83–8. doi: 10.15420/icr.2019.1.1
42. Yamada R, Hirohata A, Kume T, Neishi Y, Uemura S. Retrograde coronary intervention for chronic total occlusion of RCA ostium with anomalous origin: a case report. J Cardiol Cases. (2019) 19:182–5. doi: 10.1016/j.jccase.2019.01.003
43. Senguttuvan NB, Sharma SK, Kini A. Percutaneous intervention of chronic total occlusion of anomalous right coronary artery originating from left sinus - use of mother and child technique using guideliner. Indian Heart J. (2015) 67 Suppl 3:S41–2. doi: 10.1016/j.ihj.2015.10.300
44. Porwal SC, Vishwanath H, Tasgaonkar R, Sitapara T, Thakkar A. Percutaneous coronary intervention in chronic total occlusion of anomalous right coronary artery. Int J Clin Med. (2014) 05:567–71. doi: 10.4236/ijcm.2014.510078
45. Kaneda H, Takahashi S, Saito S. Successful coronary intervention for chronic total occlusion in an anomalous right coronary artery using the retrograde approach via a collateral vessel. J Invasive Cardiol. (2007) 19:E1–4.
46. Gasparini G, Oreglia J, Reimers B. Successful retrograde recanalization of a very rare anomalous origin right coronary artery chronic total occlusion. J Cardiovasc Dis Diagn. (2017) 5:10.00263–1000264
47. Fang HY, Wu CC, Wu CJ. Successful transradial antegrade coronary intervention of a rare right coronary artery high anterior downward takeoff anomalous chronic total occlusion by double anchoring technique and retrograde guidance. Int Heart J. (2009) 50:531–8) 29:271–8. doi: 10.1536/ihj.50.531
48. Young L, Harb SC, Puri R, Khatri J. Percutaneous coronary intervention of an anomalous coronary chronic total occlusion: the added value of three-dimensional printing. Catheter Cardiovasc Interv. (2020) 96:330–5. doi: 10.1002/ccd.28625
49. Bigler MR, Huber AT, Räber L, Gräni C. A case report of a symptomatic right anomalous coronary artery with concomitant atherosclerotic disease: the benefit of a sequential comprehensive non-invasive and invasive diagnostic approach. European Heart. Case Rep. (2021) 5:ytab081. doi: 10.1093/ehjcr/ytab081
50. Musial B, Schob A, De Marchena E, Kessler KM. Percutaneous transluminal coronary angioplasty of anomalous right coronary artery. Cathet Cardiovasc Diagn. (1991) 22:39–41. doi: 10.1002/ccd.1810220109
51. Charney R, Spindola-Franco Serge C, Grose R. Coronary angioplasty of anomalous Righ coronary arteries. Cathet Cardiovasc Diagn. (1993) 29:23–235. doi: 10.1002/ccd.1810290312
52. Çalişkan M, Çiftçi Z, Güllü H, Alpaslan M. Anomalous right coronary artery from the left sinus of valsalva presenting a challenge for percutaneous coronary intervention Türk Kardiyol Dern Arş - Arch Turk. Soc Cardiol. (2009) 37:44–7.
53. Cohen MG, Tolleson TR, Peter RH, Harrison JK, Sketch MH. Successful percutaneous coronary intervention with stent implantation in anomalous right coronary arteries arising from the left sinus of Valsalva: A report of two cases. Catheter Cardiovasc Interv. (2002) 55:105–8. doi: 10.1002/ccd.10062
54. Sun D, Bogart D. A technique to perform PCI and stenting in an anomalous RCA from the left sinus of Valsalva. Case Rep Cardiol. (2012) 2012:article ID 801423. doi: 10.1155/2012/801423
55. Yumoto K, Aoki H, Shirai Y, Shinoda Y, Kato K. Successful coronary stenting in anomalous right coronary artery by using an inner catheter with mother and child technique under multislice CT guidance. Cardiovasc Interv Ther. (2013) 28:106–10. doi: 10.1007/s12928-012-0124-1
56. Uthayakumaran K, Subban V, Lakshmanan A, Pakshirajan B, Solirajaram R, Krishnamoorthy J, et al. Coronary intervention in anomalous origin of the right coronary artery (ARCA) from the left sinus of Valsalva (LSOV): a single center experience. Indian Heart J. (2014) 430e:434 doi: 10.1016/j.ihj.2014.05.029
57. Suryanarayana P, Lee JZ, Abidov A, Lotun K. Anomalous right coronary artery: case series and review of literature. Cardiovasc Revasc Med. (2015) 16:362–6. doi: 10.1016/j.carrev.2015.03.006
58. Chakraborty B, Chan CNS, Tan AF. Percutaneous transluminal coronary angioplasty of an anomalous right coronary artery arising from a separate ostium in the left sinus of valsalva a case report. Angiology. (1995) 46:629–32 doi: 10.1177/000331979504600711
59. Conde-Vela CS, Sabate M, Jime'Nez P, Quevedo, Herna R, Ndez-Antoli'N. Primary percutaneous coronary intervention of an anomalous right coronary artery originating from the left sinus of valsalva acute. Cardiac Care J. (2006) 8:229–32. doi: 10.1080/17482940600973018
60. Matchison JC, Shavelle DM. Primary percutaneous coronary intervention of an anomalous right coronary artery arising from the left coronary cusp using an undersized Judkins catheter: A case report. Int J Angiol. (2007) 16:33–5. doi: 10.1055/s-0031-1278243
61. Azzarelli S, Amico F, Giacoppo M, Argentino V, Di Mario C, Fiscella A. Primary coronary angioplasty in a patient with anomalous origin of the right coronary artery from the left sinus of Valsalva. J Cardiovasc Med (Hagerstown). (2007) 8:943–5. doi: 10.2459/JCM.0b013e328012b0e7
62. Aznaouridis K, Alahmar A. Transradial primary angioplasty of anomalous right coronary artery from the left sinus of valsalva. Indian Heart J. (2017) 69:411–3. doi: 10.1016/j.ihj.2017.05.001
Keywords: anomalous right coronary artery, sudden death, anomalous aortic origin of a coronary artery, chronic total occlusion, percutaneous coronary intervention, PCI complication
Citation: Cocco N, Madonna R, Cammalleri V, Cocco G, De Stefano D, Ricciardi D, Grigioni F and Ussia GP (2022) Percutaneous treatment of a CTO in an anomalous right coronary artery: A rupture paved the way for new insights. Front. Cardiovasc. Med. 9:916616. doi: 10.3389/fcvm.2022.916616
Received: 09 April 2022; Accepted: 01 June 2022;
Published: 29 July 2022.
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Christoph Gräni, Universitätsspital Bern, SwitzerlandCopyright © 2022 Cocco, Madonna, Cammalleri, Cocco, De Stefano, Ricciardi, Grigioni and Ussia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nino Cocco, bmlub2NvY2NvQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.