
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med., 27 May 2022
Sec. Cardiovascular Imaging
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.915876
This article is part of the Research TopicCase Reports in Cardiovascular Imaging: 2022View all 28 articles
 Yuya Fukudome1
Yuya Fukudome1 Michinari Hieda2*
Michinari Hieda2* Shiho Masui2
Shiho Masui2 Taku Yokoyama2
Taku Yokoyama2 Shutaro Futami2
Shutaro Futami2 Shohei Moriyama2
Shohei Moriyama2 Kei Irie2
Kei Irie2 Mitsuhiro Fukata2
Mitsuhiro Fukata2 Tomoki Ushijima3
Tomoki Ushijima3 Akira Shiose3
Akira Shiose3 Koichi Akashi2
Koichi Akashi2A 31-year-old woman was referred to our hospital for evaluation of a cardiac mass in the right atrium. Cardiac magnetic resonance imaging indicated a cystic mass filled with fluid accumulation in the right atrium. The mass was identified as a cardiac cyst and was surgically removed. Pathological examination revealed an extremely rare bronchogenic cyst. Bronchogenic cysts are benign congenital abnormalities of primitive foregut origins that form in the mediastinum during embryonic development. There is unusual clinical dilemmas surrounding the treatment plan for cardiac surgery or biopsy of cardiac masses, especially in patients with rare cardiac cysts. The anatomical location of the cyst can be related to various clinical symptoms and complications. In cases of indeterminate cardiac cysts, direct cyst removal without prior biopsy is of utmost importance.
The incidence of primary cardiac tumors is approximately 0.02%, with three-quarters of cardiac tumors are benign. Among these benign cardiac tumors, cardiac myxoma is the most frequent. Other major types of benign cardiac tumors include fibromas, rhabdomyomas, papillomas, lipomas, papillary fibroelastomas, hemangiomas, and bronchogenic cysts (1).
Bronchogenic cysts in the atrial septum are extremely rare. Regarding the intracardiac bronchogenic cysts, they have been reported in only 22 cases, to date (2). They are benign congenital cysts of primitive foregut origin that form in the mediastinum during embryonic development (3–5). These bronchogenic cysts represent 6% to 15% of primary mediastinal masses (6), and may develop in the neck, spinal dura mater, sub-diaphragm, diaphragm (7), or retroperitoneal regions (8). The heart is derived from the mesoderm, and its development differs from that of the ectoderm-derived respiratory system. The embryonic cardiac primordium is close to the primordial bronchial tree and foregut. Therefore, it has been indicated that migration of the embryonic cardiac primordium to the cardiac muscle site during abnormal budding may be involved in cardiac cyst development (9). In addition, ectopic bronchogenic cysts are often misdiagnosed preoperatively, because they have no imaging features and have different clinical manifestations (10). Therefore, multimodality imaging is the most important approach to establish an accurate diagnosis.
A 31-year-old woman presented to a local physician 2 months before her referral with palpitations and shortness of breath. At the time, a paroxysmal atrial fibrillation was detected by a Holter-electrocardiogram examination. Thereafter, successful catheter ablation of paroxysmal atrial fibrillation was performed. Presence of a cardiac tumor in the right atrium was suspected during catheter ablation. Therefore, the patient was referred to our hospital for further evaluation.
On presentation to our hospital, the patient had no symptoms. The patient’s medical history included asthma, emergency cesarean section for pregnancy-induced hypertension, paroxysmal atrial fibrillation, gestational diabetes, and type 2 diabetes mellitus. The patient was managed with edoxaban (60 mg daily) for paroxysmal atrial fibrillation and dapagliflozin (5 mg), sitagliptin (50 mg), and metformin (500 mg) for type 2 diabetes mellitus. The patient had no other pertinent family medical history.
Her height, weight, body mass index, and body surface area were 157.8 cm, 64.6 kg, 25.94 kg/m2, and 1.66 m2, respectively. The patient had body temperature, 37.2°C; blood pressure, 130/88 mmHg; pulse rate, 108 bpm; and O2 saturation measured by pulse oximetry, 98% on room air. Normal heart and lung sounds were ausculated without any murmur. The abdomen was soft and tender. There was no edema in the lower legs. Her blood test results are shown in Table 1. A 12-lead electrocardiogram showed normal sinus rhythm. Chest radiography revealed a cardiothoracic ratio of 43.9% without pleural effusion or pulmonary congestion. Transthoracic echocardiography revealed normal left ventricular ejection fraction (71%) and diastolic function (E/e’: 9.0) without valvular diseases. Although we attempted to visualize the tumor in the right atrium using a subcostal approach, it was unclear due to obesity.
Transesophageal echocardiography revealed a 16 × 10 mm isoechoic mass lesion on the right interatrial septum. The mass had a clear border, smooth surface, and broad base without mobility and apparent feeding vessels (Figure 1; Supplementary Videos 1, 2). A plain thoracic computed tomography (CT) revealed a high-intensity nodule on the right interatrial septum at the border of the inferior vena cava (Figure 2A). The absorption value was 110 HU and size was 13 × 9 mm. Moreover, there was no change in absorption value before and after contrast enhancement. Cardiac MRI showed a 14-mm nodule attached to the inter atrial septum. The nodule showed little higher signal than its surrounding muscle on a T1-weighted image. In addition, the tumor had high signal intensity on a T2-weighted image. No significant abnormal enhancement in LV wall and nodule on a late gadolinium enhancement image. Based on these findings, the nodule was suggested to be a cystic mass including liquid component (Figures 2B,C). In addition, whole-body positron emission tomography-CT showed no fluorodeoxyglucose accumulation in the lesion.
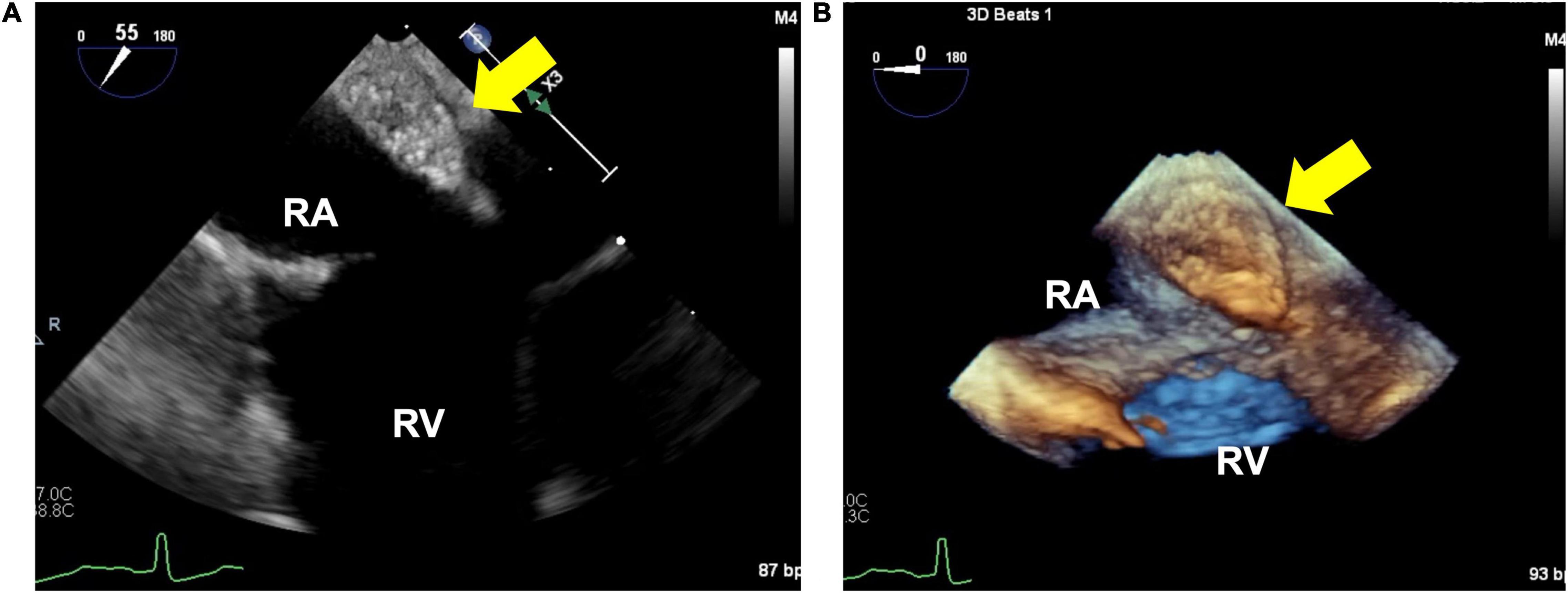
Figure 1. 2-dimensional (A) and 3-dimensional (B) image of the isoechoic mass using transesophageal echocardiography. (A) 16×10×10 mm isoechoic mass. This image shows an isoechoic mass on the right interatrial septum near the inferior vena cava. (B) Mass is well-defined, has a smooth surface, and broadly adherent to the atrial.
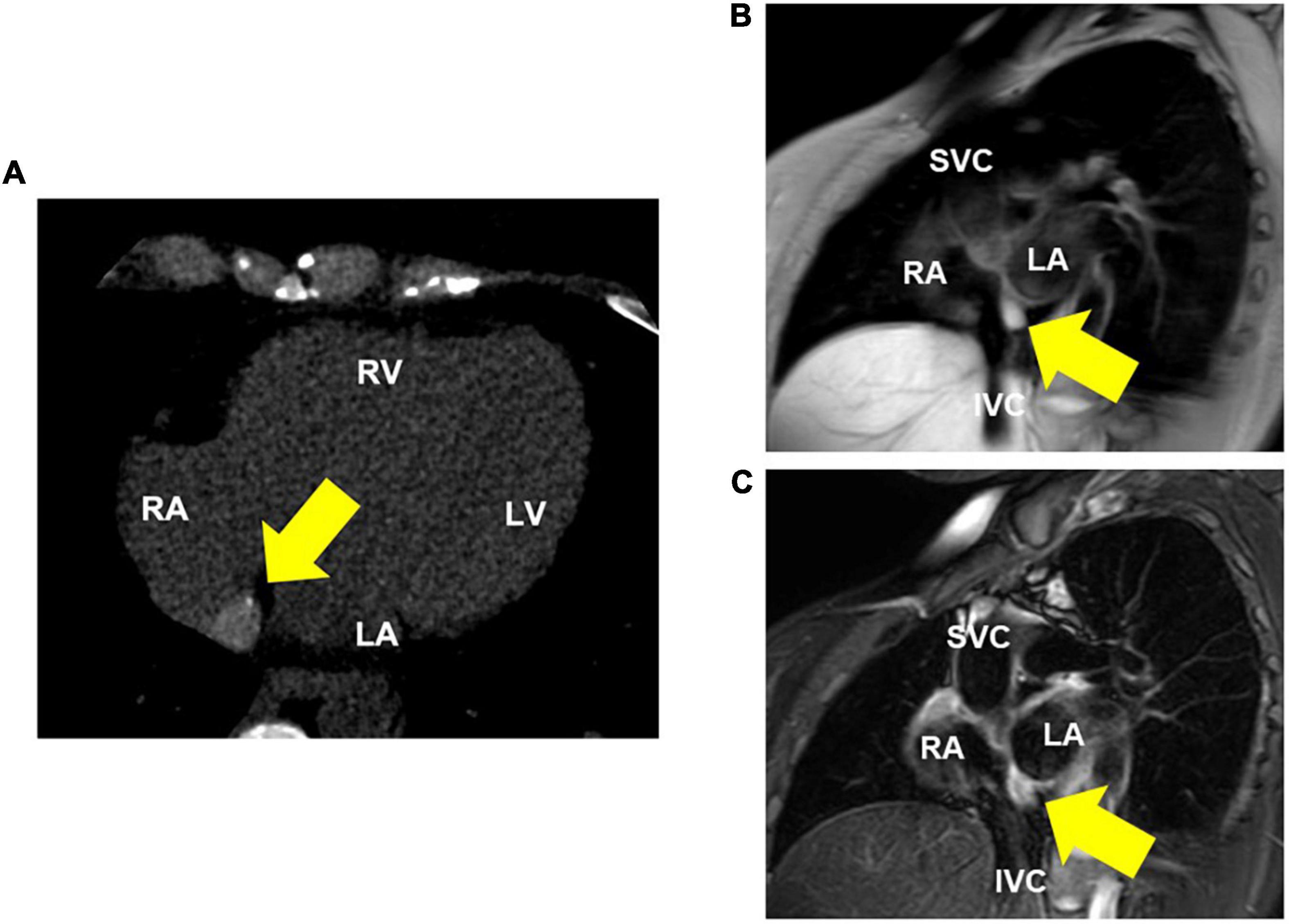
Figure 2. Thoracic computed tomography (CT) and MRI (T1 and T2-weighted) images. (A) CT shows a high-intensity nodule on the interatrial septum. (B) Cardiac MRI (T1-weighted image) reveals a nodule that is little higher signal than its surrounding muscle. (C) Cardiac MRI (T2-weighted image) reveals a high-intensity nodule on the interatrial septum, suggesting a cystic lesion with fluid components. RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle; SVC, superior vena cava; IVC, inferior vena cava.
The right atrial cyst was resected using minimally invasive cardiac surgery via right mini-thoracotomy. After aortic clamping, induction of cardiac arrest, and incision of the right atrium, a white mass with a smooth surface appeared on the posterior wall of the right atrium-inferior vena cava junction. A yellowish-white viscous mucous material was found in the mass with no thrombus (Figure 3). The mass was completely resected, and the right atrial posterior wall defect in the inferior vena cava was reconstructed using a bovine pericardial patch. Pathohistological evaluation revealed multilineage ciliated columnar epithelium and mucous glands present, resembling the respiratory epithelium in the intima of some cyst walls (Figure 4). No findings, such as nuclear irregularities or atypical cells, suggested malignancy. Therefore, the patient was diagnosed with a bronchogenic cyst (11).
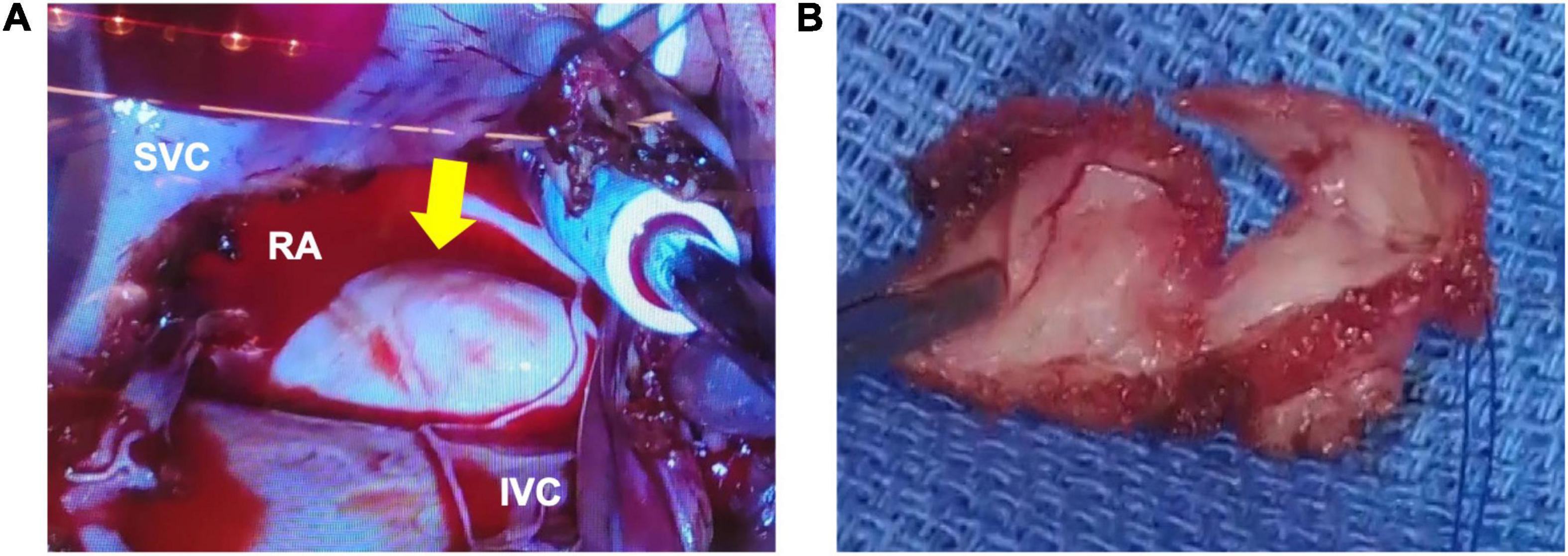
Figure 3. Macroscopic findings during surgery. (A) The outer surface of the mass is white, smooth, and soft. (B) A yellowish-white viscous mucous material is found in the mass. There are no thrombi inside or outside the cyst. RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle; SVC, superior vena cava; IVC, inferior vena cava.
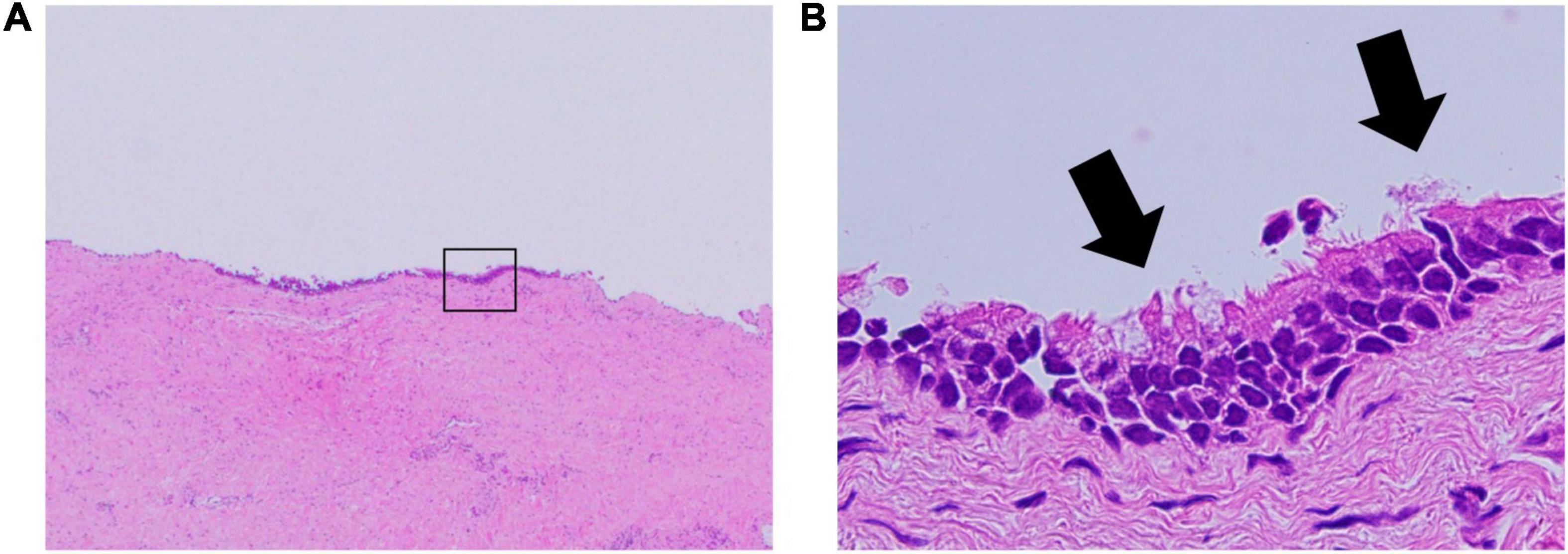
Figure 4. Pathology of the bronchogenic cyst. (A) Hematoxylin and eosin stain; original magnification, 20×. (B) The ciliated columnar epithelium suggests a bronchogenic cyst; original magnification, 400×.
The patient’s postoperative course was favorable and uneventful. She was discharged 21 days later without any complications. Currently, the patient visits the hospital as an outpatient without recurrence.
This was an extremely rare case of bronchogenic cyst occurring in a right atrium, in which establishing an accurate preoperative diagnosis was difficult. The differential diagnoses of intra-cardiac tumors include myxomas, lipomas, papillary fibroelastomas, metastatic tumors, cardiac cysts, or thrombi. Myxomas are the most frequent benign cardiac tumors occurring in adulthood: asymptomatic in 0–20% of cases (12). Lipomas are adipocyte-derived masses occurring in the epicardial myocardium, with approximately 25% of these lesions occur in the intracardiac lumen (13). These tumors are generally observed as isoechoic masses when the capillary blood flow in the stalk becomes obstructed, the mass becomes necrotic, and its echo-image is hypoechogenic (14). Papillary fibroelastomas generally occur on the valvular surface and are most commonly found in the aortic valve (15). In this present case, tumor markers were not elevated, leading to ruling out a metastatic cardiac tumor. The D-dimer level also indicated a low likelihood of thrombosis. Pericardial cysts are rare benign disease that often identified incidentally on chest X-ray or trans-thoracic echocardiography, the majority of cases of this disease are asymptomatic. Since complex type pericardial cysts are defined as the presence of solid compornents, it was difficult to distinguish between pericardial cysts and this case. However, these diseases are more likely to be located at the right cardiophrenic angle followed by the left cardiophrenic angle in mediastinal sites (16). Thus, we ruled out pericardial cysts. Although, differential diagnosis as an extra-cardiac tumors include substernal thyroid, thymic tumor, intra-thoracic cystic hygroma, serous cyst, and aortic aneurysm, all they were excluded by multimodality imaging studies. In this case, multimodality imaging suggested that the cystic lesion contained both fluid and blood. Thus, the preoperative clinical diagnosis was suspected to be a right atrial cyst.
Cardiovascular magnetic resonance (CMR) imaging is an important diagnostic tool for evaluating patients with suspected cardiac tumors. A previous study demonstrated that CMR diagnoses were 25% no mass, 16% pseudo mass, 16% thrombus, 17% benign tumor, and 23% malignant tumor (17). Compared to the final diagnosis, the CMR diagnosis was accurate in 98.4% of patients; patients with CMR diagnoses of pseudo mass and the benign tumor had mortality rates similar to those without masses, but patients with malignancy [hazard ratio (HR) 3.31 (2.40–4.57)] and thrombus [HR 1.46 (1.00–2.11) (17). The CMR diagnosis had more prognostic value than clinical factors such as left ventricular ejection fraction, coronary artery disease, or history of extracardiac malignancy (P < 0.001) (17). Therefore, we should perform CMR for evaluating patients with suspected cardiac tumors since CMR diagnosis is a powerful independent predictor of mortality over clinical risk factors (18).
We reviewed all case reports of recent intra-cardiac bronchogenic cyst published and summarized them in Table 2. Common symptoms of bronchogenic cysts include cough, fever, and dyspnea (19). Miwa et al. reported a case of a bronchogenic cyst in the lower interatrial septum that demonstrated complete atrioventricular block (20). In this case, the patient had paroxysmal atrial fibrillation and a cyst in the right interatrial septum near the inferior vena cava. Although the causal relationship between tumors and arrhythmias is not clear, arrhythmia of unknown origin in younger patients may lead clinicians to suspect a cardiac tumor. Furthermore, in some cases, obstruction of the superior vena cava (SVC) and dysphagia have been reported (21, 22). In addition, bronchogenic cysts can cause compression into the left atrium, pulmonary veins, or coronary arteries (23, 24). Regarding these reasons, it might be essential to focus on the anatomical location of the cyst accurately.
Whether to perform a surgery or biopsy first is a dilemma in diagnosing rare cardiac tumors or cysts. Puncture aspiration biopsy was an option for preoperative diagnosis. However, biopsy of a cystic lesion could rupture the trachea, thoracic cavity, or pericardial cavity. In addition, there has been a reported case of cystic infection after endobronchial ultrasonography-guided fine needle aspiration. Therefore, cystic lesions in the intra-cardiac cavity should not be considered for a needle aspiration (25). Given these complications, direct surgical resection is recommended without prior tumor biopsy (26). In addition, bronchogenic cyst is a risk of malignant transformation in adulthood, and risk of complications increase over time. Therefore, bronchogenic cysts are recommended for preferably early surgery (27). Since it could not be judged whether the tumor was benign or malignant, we decided to resect it directly. It must be emphasized that preoperative diagnosis can also be complicated by the extreme rarity of such cases. This case describes the usefulness of multimodality imaging in diagnosing bronchogenic cysts preoperatively with minimal complications.
There is a clinical dilemma surrounding the treatment plan for cardiac surgery or biopsy of cardiac masses, especially in patients with rare cardiac cysts. The anatomical location of the cyst is related to various clinical symptoms and complications. Therefore, multimodality imaging might be practical for assessing the exact anatomical location of cysts and avoiding various complications. This case describes a rare bronchogenic cyst in the right atrium and highlights the importance of multimodality imaging for appropriate diagnosis and management. In cases of indeterminate cardiac cysts, direct removal of the cyst without previous biopsy is of utmost importance.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for this study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
YF and MH contributed significantly to the writing and editing to the manuscript. MH, SMa, TY, SF, KI, SMo, and MF managed the patient. TU and AS performed the surgeries. All authors critically revised the report, commented on the drafts of the manuscript, and approved the final version for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank to acknowledge all staffs who contributed to this case diagnosis, therapy, and decision-making.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.915876/full#supplementary-material
1. Kamiya H, Yasuda T, Nagamine H, Sakakibara N, Nishida S, Kawasuji M, et al. Surgical treatment of primary cardiac tumors: 28 Years’ experience in kanazawa university hospital. Jpn Circ J. (2001) 65:315–9. doi: 10.1253/jcj.65.315
2. Kawase Y, Takahashi M, Takemura H, Tomita S, Watanabe G. Surgical treatment of a bronchogenic cyst in the interatrial septum. Ann Thorac Surg. (2002) 74:1695–7. doi: 10.1016/s0003-4975(02)03863-8
3. Maier HC. Bronchiogenic cysts of the mediastinum. Ann Surg. (1948) 127:476–502. doi: 10.1097/00000658-194803000-00010
4. Suen HC, Mathisen DJ, Grillo HC, LeBlanc J, McLoud TC, Moncure AC, et al. Surgical management and radiological characteristics of bronchogenic cysts. Ann Thorac Surg. (1993) 55:476–81. doi: 10.1016/0003-4975(93)91022-f
5. Kobza R, Oechslin E, Jenni R. An intrapericardial bronchogenic cyst. Interact Cardiovasc Thorac Surg. (2003) 2:279–80. doi: 10.1016/s1569-9293(03)00044-6
6. Gutiérrez GS, Gutiérrez FG, Bastianelli GA, Vaccarino GN. Bronchogenic cyst in an unusual location. Asian Cardiovasc Thorac Ann. (2021) 29:44–6. doi: 10.1177/0218492320960271
7. Simonetti S, Canalís E, Macías L, Carrasco MA. Clinico-pathological features of the intradiaphragmatic bronchogenic cysts: report of a case and review of the literature. Pathologica. (2018) 110:116–20.
8. Itoh H, Shitamura T, Kataoka H, Ide H, Akiyama Y, Hamasuna R, et al. Retroperitoneal bronchogenic cyst: report of a case and literature review. Pathol Int. (1999) 49:152–5. doi: 10.1046/j.1440-1827.1999.00837.x
9. Jiang H, Wang H, Wu H, Li X. Bronchogenic cyst of the interatrial septum. J Cardiothorac Surg. (2013) 8:171. doi: 10.1186/1749-8090-8-171
10. Luo Y, Chen D, Yang X. Bronchogenic cyst in the right atrium: a case report. Asian J Surg. (2022) 45:1162–64. doi: 10.1016/j.asjsur.2021.12.072
11. St-Georges R, Deslauriers J, Duranceau A, Vaillancourt R, Deschamps C, Beauchamp G, et al. Clinical spectrum of bronchogenic cysts of the mediastinum and lung in the adult. Ann Thorac Surg. (1991) 52:6–13. doi: 10.1016/0003-4975(91)91409-o
12. Yuda S, Nakatani S, Yutani C, Yamagishi M, Kitamura S, Miyatake K. Trends in the clinical and morphological characteristics of cardiac myxoma: 20-year experience of a single tertiary referral Center in Japan. Circ J. (2002) 66:1008–13. doi: 10.1253/circj.66.1008
13. da Silveira WL, Nery MW, Soares EC, Leite AF, Nazzetta H, Batista MA, et al. Lipoma of the right atrium. Arq Bras Cardiol. (2001) 77:361–8. doi: 10.1590/s0066-782x2001001000006
14. Fyke FE III, Seqard JB, Edwards WD, Miller FA Jr., Reeder GS, Schattenberg TT, et al. Primary cardiac tumors: experience with 30 consecutive patients since the introduction of two-dimensional echocardiography. J Am Coll Cardiol. (1985) 5:1465–73. doi: 10.1016/s0735-1097(85)80364-8
15. Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. (2003) 146:404–10. doi: 10.1016/s0002-8703(03)00249-7
16. Khayata M, Alkharabsheh S, Shah NP, Klein AL. Pericardial cysts: a contemporary comprehensive review. Curr Cardiol Rep. (2019) 21:64. doi: 10.1007/s11886-019-1153-5
17. Shenoy C, Grizzard JD, Shah DJ, Kassi M, Reardon MJ, Zagurovskaya M, et al. Cardiovascular magnetic resonance imaging in suspected cardiac tumour: a multicentre outcomes study. Eur Heart J. (2021) 43:71–80. doi: 10.1093/eurheartj/ehab635
18. Giusca S, Kelle S, Korosoglou G. When tissue and outcomes are the issue. cardiac magnetic resonance for patients with suspected cardiac tumours. Eur Heart J. (2021) 43:81–3. doi: 10.1093/eurheartj/ehab625
19. Lateef N, Kuniyoshi J, Latif A, Ahsan MJ, Shaikh K, DeVrieze B, et al. Cardiac Tamponade as a complication of bronchogenic cyst. Proc (Bayl Univ Med Cent). (2020) 34:172–4. doi: 10.1080/08998280.2020.1795594
20. Miwa E, Tani T, Okada Y, Furukawa YA. Rare cardiac tumor: bronchogenic cyst of interatrial septum. Echocardiography. (2017) 34:474–5. doi: 10.1111/echo.13445
21. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. (2003) 30:105–8.
22. Liu HS, Li SQ, Cao ZL, Zhang ZY, Ren H. Clinical features and treatment of bronchogenic cyst in adults. Chin Med Sci J. (2009) 24:60–3. doi: 10.1016/s1001-9294(09)60061-4
23. Han SJ, Cho HJ, Kang MW, Yu JH, Na MH, Kang SK. A life-threatening bronchogenic cyst. Korean J Thorac Cardiovasc Surg. (2018) 51:69–71. doi: 10.5090/kjtcs.2018.51.1.69
24. Azeem F, Finlay M, Rathwell C, Awad WI. A near fatal presentation of a bronchogenic cyst compressing the left main coronary artery. J Thorac Cardiovasc Surg. (2008) 135:1395–6. doi: 10.1016/j.jtcvs.2007.09.082
25. Gamrekeli A, Kalweit G, Schäfer H, Huwer H. Infection of a bronchogenic cyst after ultrasonography-guided fine needle aspiration. Ann Thorac Surg. (2013) 95:2154–5. doi: 10.1016/j.athoracsur.2012.10.071
26. Kirmani B, Kirmani B, Sogliani F. Should asymptomatic bronchogenic cysts in adults be treated conservatively or with surgery? Interact Cardiovasc Thorac Surg. (2010) 11:649–59. doi: 10.1510/icvts.2010.233114
27. Fievet L, D’Journo XB, Guys JM, Thomas PA, De Lagausie P. Bronchogenic cyst: best time for surgery? Ann Thorac Surg. (2012) 94:1695–9. doi: 10.1016/j.athoracsur.2012.06.042
28. Foley JR, Irwin RB, Abidin N. Multimodality characterization of interatrial cyst. J Am Coll Cardiol. (2012) 59:2217. doi: 10.1016/j.jacc.2011.11.071
29. Shiferaw K, Lobrinus AJ, Grabherr S, Michaud K, Mangin P, Schrag B. One case, 3 rare simultaneous findings: intramyocardial bronchogenic cyst, P.H558r variant of Scn5a gene, and granular cell tumor of the esophagus. Am J Forensic Med Pathol. (2012) 33:335–8. doi: 10.1097/PAF.0b013e318264e9ef
30. Olsen M, Mitchell TA, Percival TJ, Helsel BS. Interatrial bronchogenic cyst resection. Ann Thorac Surg. (2015) 100:709–11. doi: 10.1016/j.athoracsur.2014.10.025
31. Forcillo J, Dion D, Sauvageot C, Jeanmart H. Intraventricular bronchogenic cyst: a rare congenital anomaly. Ann Thorac Surg. (2015) 100:1101–3. doi: 10.1016/j.athoracsur.2014.11.059
32. Grozavu C, Fera A, Iliaş M, Pantile D. Intrapericardial development of a bronchogenic cyst - case report. Chirurgia (Bucur). (2016) 111:345–9.
33. Wang J, Zhu Q, Liang B, Shi H, Han P, Kong X. Left ventricular bronchogenic cyst. Ann Thorac Surg. (2016) 101:744–6. doi: 10.1016/j.athoracsur.2015.03.083
34. Smer A, Alla VM, Abuissa H. A 50-year-old man with incidental cardiac mass. Heart. (2017) 103:189. doi: 10.1136/heartjnl-2016-310337
35. Shiohira S, Sasaki T, Maeda S, Kawabata M, Goya M, Hirao K. Bronchogenic cyst of the atrioventricular septum presenting with ventricular fibrillation. HeartRhythm Case Rep. (2017) 3:389–91. doi: 10.1016/j.hrcr.2017.05.005
36. Nishida N, Hata Y, Nomoto K. Intramyocardial bronchogenic cyst: histological appearance and a review of the literature. Cardiovasc Pathol. (2017) 28:64–7. doi: 10.1016/j.carpath.2017.03.005
37. Blesneac C, Horvath E, Muntean I, Benedek T, Toganel R. Intracardiac bronchogenic cyst associated with ventricular septal defect: an extremely rare feature in children. Eur Heart J Cardiovasc Imaging. (2018) 19:1074. doi: 10.1093/ehjci/jey078
38. Van Praet KM, Stamm C, Sündermann SH, Meyer A, Unbehaun A, Montagner M, et al. Minimally invasive cardiac surgery: removal of an interatrial intraseptal bronchogenic cyst through a periareolar approach. Innovations (Phila). (2018) 13:230–2. doi: 10.1097/imi.0000000000000502
39. Gimpel D, Conway J, Meikle F, Lin Z, McCormack DJ, El-Gamel A. Acute shortness of breath due to reoccurrence of an intrapericardial bronchogenic cyst. Respirol Case Rep. (2019) 7:e00431. doi: 10.1002/rcr2.431
40. Li Z, Xiang D, Gao L, Tan J, Zeng X. Resection of a giant bronchogenic cyst in the left atrium. Can J Cardiol. (2020) 36:967.e13–15. doi: 10.1016/j.cjca.2020.02.078
41. Fukada Y, Endo Y, Nakanowatari H, Kitagawa A, Tsuboi E, Irie Y. Bronchogenic cyst of the interatrial septum. Fukushima J Med Sci. (2020) 66:41–3. doi: 10.5387/fms.2019-29
Keywords: bronchogenic cyst, cardiac mass, cardiac biopsy, cardiac MRI, imaging
Citation: Fukudome Y, Hieda M, Masui S, Yokoyama T, Futami S, Moriyama S, Irie K, Fukata M, Ushijima T, Shiose A and Akashi K (2022) Case Report: Bronchogenic Cyst in the Right Atrium of a Young Woman. Front. Cardiovasc. Med. 9:915876. doi: 10.3389/fcvm.2022.915876
Received: 08 April 2022; Accepted: 02 May 2022;
Published: 27 May 2022.
Edited by:
Grigorios Korosoglou, GRN Klinik Weinheim, GermanyReviewed by:
Alexandros Kallifatidis, St. Luke’s Hospital, GreeceCopyright © 2022 Fukudome, Hieda, Masui, Yokoyama, Futami, Moriyama, Irie, Fukata, Ushijima, Shiose and Akashi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michinari Hieda, aGllZGEubWljaGluYXJpLjI2NUBtLmt5dXNodS11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.