
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med., 02 August 2022
Sec. Hypertension
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.915470
Background: Although pulmonary arterial hypertension (PAH) is a fatal disease, specific drugs have been used to treat PAH. These drugs predominantly target these three pathobiological pathways: Endothelin receptor antagonist (ERA), nitric oxide (NO), and prostanoids pathways. In this review, we aimed to analyze the efficacy and safety of oral targeted treatments for PAH.
Methods: The national library of medicine (MEDLINE), excerpta medica database (EMBASE), and Cochrane Central Register of Controlled Trials databases were searched. Randomized controlled trials that compared the oral targeted drugs with placebos were selected. We calculated odds ratios (ORs) with 95% confidence intervals (CIs) for variables with dichotomous outcomes, and standardized mean differences with continuous outcomes variables. Additionally, the mean of the differences for the 6-min walk distance (6MWD) was analyzed.
Results: In total, 23 studies involving 7,121 patients were included in this study. These studies show that orally PAH-specific drugs could decrease the risk of clinical worsening events, with an OR of 0.55 (p < 0.001). Furthermore, these drugs could improve exercise capacity, showing a 21.74-m increase in 6MWD (95% CI: 17.53–25.95 m) and cause a greater amelioration of functional class (OR = 0.60, 95% CI: 0.47–0.76). Additionally, subgroup analysis indicated that compared with placebo, ERAs, and drugs in the NO pathway were most effective and safe, which are associated with an improvement in exercise capacity, 6MWD, and worsening events-free survival rate.
Conclusion: Nitric oxide exhibited the most prominent clinical effect on exercise tolerance. However, in the subgroup analysis, oral targeted drugs of different pathways show applicability to different populations, which highlights the need for precise treatment in the clinical setting.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=297946], identifier [CRD 42022297946].
Pulmonary arterial hypertension (PAH) is a fatal disease that exhibits pulmonary vascular remodeling. It is characterized by progressively increasing pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR). PAH eventually leads to hypoxia, right-sided heart failure, and death (1). According to guidelines, PAH is defined as a mean baseline PAP of less than 25 mm Hg, associated with pulmonary artery wedge pressure ≤15 mm Hg and elevated PVR ≥ 3 Wood units via right heart catheterization. PAH is generally classified into the following: (1) idiopathic or heritable; (2) drug or toxin-induced (aminorex, benfluorex, and toxic rapeseed oil); and (3) associated condition-induced, such as connective tissue disease, human immunodeficiency virus infection, congenital heart disease, and schistosomiasis (1). Since the 1990s, various drugs have been used to treat specific-PAH types, predominantly focusing on three pathobiological pathways: endothelin receptor antagonist (ERA), nitric oxide (NO), and prostanoids pathways (2), which significantly improve exercise capacity and cardiopulmonary parameters. These agents may be administered through the following: ingestion, inhalation, subcutaneous injection, and intravenous injection (2, 3). However, previous studies have indicated that intravenous injection can lead to embolism and thrombosis. Additionally, local pain with subcutaneous injection is a common occurrence (4–6). Although inhalation has proven to be effective and safe, only a few drugs have been recommended by the American Heart Association/European Society of Hypertension. Additionally, the inhaled drugs are often short-acting and require multiple daily doses (7). Comparatively, ingestion is commonly used and patients usually show good compliance. In 2016, a quantitative meta-analysis found that, compared to a placebo, oral drugs can ameliorate exercise intolerance, increase pulmonary vascular remodeling, and improve quality of life (8). However, sitaxsentan, an ERA that was eradicated from the market due to fatal hepatotoxicity in 2010 (9), and imatinib, a PAH medication terminated due to severe side effects (subdural hematoma), were included in the study. As a guideline for PAH, in 2018, it was proposed that the following targeted treatments be recommended: bosentan, ambrisentan, and macitentan in the ERA pathway; sidenafil, tadalafil, vardenafil, and udenafil in the NO pathway; and iloprost, beraprost, selexipag, and treprostinil in the prostanoids pathway (10). With studies [Fu et al. (11); etc.] indicating more proof about the efficacy and safety of targeted drugs of each type, including ingestion, inhalation, subcutaneous injection, and intravenous injection, focus has been put on the oral drugs as these are important treatments for PAH therapy. Early in 2018, Zheng et al. (12) meta-analyzed 25 randomized controlled trial (RCTs) about oral targeted therapies in PAH, showing the benefits of oral treatments on clinical worsening events (CWEs) in PAH. Accordingly, to provide the most suitable strategy for PAH populations with specific clinical characteristics for precision treatment, more detailed subgroup analyses are required. Hence, we conducted a meta-analysis and pooled data from clinical trials comparing oral PAH-specific drugs with placebo to assess the efficacy and safety of these treatments in different age groups and different functional classes (FCs).
This study complied with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines. Two investigators (H-RZ and QL) independently searched PubMed, EMBASE, and Cochrane Central Register of Controlled Trials databases up to 10 December 2021. The full strategy is available in Table 1. Related manuscripts were manually searched to identify other eligible citations. The same authors independently screened the citations at the title and abstract level. Studies on the following were included: (1) randomized, placebo-controlled clinical trials; (2) patients diagnosed with PAH; (3) PAH-specific therapies with oral administration [oral prostanoids, ERAs, phosphodiesterase-5 inhibitor, prostacyclin receptor agonists, receptor tyrosine kinase inhibitors (RTK), and soluble guanylate cyclase stimulators]; and (4) one of the following outcomes: (a) safety outcomes (CWEs and adverse drug responses; b) exercise capacity outcomes (6-min walk distance, FC, and cardiopulmonary hemodynamics). These trials were then excluded if: (1) a combination therapy with other forms of drugs for patients with PAH was used; (2) the data included the use of a prohibited drug; (3) studies included persistent pulmonary hypertension in newborns; (4) acute hemodynamic responses to drugs occurred, and (5) sample size > 50. The full texts of the potentially eligible studies were then reviewed. Disagreements were resolved by a consensus with a third investigator (X-JJ). Patient and public involvement statements and ethical approval were not necessary for a secondary study.
The following data were extracted from each article: first author, year of publication, duration of medication administration, number of participants in each group, treatment regimen, daily dosage, clinical characteristics of participants, CWEs, effects on a 6-min walk distance (6MWD), and hemodynamic values [mean pulmonary arterial pressure (mPAP), PVR, mean right atrial pressure, cardiac index, and pulmonary capillary wedge pressure (PCWP)]. CWEs were defined as all-cause mortality, lung or heart-lung transplantation, hospitalization for PAH, and escalation of treatment, which could be identified based on the original data documented from the articles or calculated from the events reported. The quality of the included trials was gaged according to a previous systematic review by using the Cochrane Collaboration’s risk-of-bias tool (13), including the following six domains: (1) allocation sequence generation, (2) allocation concealment, (3) blinding of participants, personnel, and outcome assessors, (4) completeness of participant follow-up, (5) handling of incomplete outcome data, and (6) protection against selective outcome reporting. Contact the author by email if necessary.
Review Manager version 5.3 was used for statistical analysis. For all treatment comparisons, the random or fixed-effect model was applied according to the heterogeneity among studies, using the χ2 test the I2 statistic, with values > 50% (or p of χ2 test < 0.01), indicating substantial heterogeneity. Multi-arm studies were assessed by comparing each active arm with the control group separately. We calculated odds ratios (ORs) with 95% confidence intervals (CIs) for dichotomous outcomes, and calculated standardized mean differences (SMDs) for continuous outcomes, with mean of differences (MD) for 6MWD. To assess publication bias, we examined the funnel plot for evidence of small-study effects.
Overall, 5,460 records were identified by literature search (PubMed 1,611 articles, EMBASE 2,102 articles, and the Cochrane Library 1,747 articles), and 3,448 records were recorded after 2,012 duplicates were removed. A total of 3,416 records were excluded after a review of titles and abstracts, which included duplications, reviews, case reports, animal studies, or studies irrelevant to our analysis. Additionally, four articles about sitaxsentan and imatinib were removed (14–17). The remaining 29 citations underwent detailed assessment at the full-text level, where one RCT evaluated the acute vascular effects (18), one trial did not record CWEs (19), and four trials did not meet sample size requirements (20–23). Eventually, 23 trials (24–46) meeting the inclusion criteria were pooled in this meta-analysis. The screening process is illustrated in Figure 1. These studies were conducted from 2002 to 2021 and encompassed 7,121 patients, most of which were multicenter studies. The treatment duration of oral targeted drugs in all studies ranged from 12 to 115 weeks, with a median treatment duration of 12 weeks. The FC of patients was evaluated at baseline and was mostly in II or III according to World Health Organization criteria, and details are shown in Table 2.
Data on the safety of the overall oral targeted treatment for PAH were obtained from 23 articles, where no heterogeneity existed (I2 = 0.0%). The fixed effects model showed that oral targeted therapy could improve mortality compared with the control group (OR = 0.55, 95% CI, range: 0.49–0.62, p < 0.001). Furthermore, three approaches lowered the occurrence of CWEs: with OR = 0.49 (95% CI, range: 0.37–0.66, p < 0.001) in ERA, OR = 0.46 (95% CI, range: 0.34–0.62, p < 0.001) in NO, and OR = 0.58 (95% CI, range: 0.49–0.69, p < 0.001) in prostanoids. The frequency of adverse drug responses to these oral agents for PAH was detected in 23 citations, and the three most common adverse event (AEs) were headache (37.3%, 95% CI, range: 36.1–38.5%), diarrhea (19.9%, 95% CI, range: 18.7–21.1%), and nausea (20.1%, 95% CI, range: 18.8–21.4%). Subgroup analysis showed that the three most common AEs in ERA were headache (12.5%, 95% CI, range: 10.7–14.4%), peripheral edema (13.9%, 95% CI, range: 11.7–16.0%), and upper respiratory tract infection (16.4%, 95% CI, range: 13.6–19.3%); headache (31.4%, 95% CI, range: 28.9–34.0%), diarrhea (9.8%, 95% CI, range: 8.1–11.5%), and dyspepsia (8.6%, 95% CI, range: 6.9–10.2%) in NO; and headache (72.1%, 95% CI, range: 70.1–74.2%), diarrhea (49.6%, 95% CI, range: 47.4–51.9%), and nausea (40.3%, 95% CI, range: 38.1–42.5%) in prostanoids.
Twenty-seven studies reported a change in 6MWD from baseline to the endpoints (a short-to medium-term treatment of no more than 2 years; Table 3). Outcomes of meta-analysis in 6MWD demonstrated that PAH-specific drugs in oral administration improve 6MWD by 21.74 m (95% CI from 17.53 to 25.95 m, p < 0.001). Consequently, in different approaches, compared with placebo, oral drugs in ERA elevated 6MWD by 23.31 m (95% CI, range: 15.25–31.37, p < 0.001; I2 = 0.0%), and a 29.16-m improvement in 6MWD was detected in the NO way (95% CI, range: 23.13–35.20, p < 0.001; I2 = 0.0%), of 10.33 m in the prostanoids way (95% CI, range: 4.03–16.63, p < 0.001, I2 = 0.0%). The details are presented in Figure 2. Changes in FC were detected in each citation, showing improved or remained state in 92.0% of enrolled patients (95% CI, range: 91.0–93.1%), and only worsened in 8.0% (95% CI, range: 6.9–9.0%). Hence, the data showed that a short-medium term of oral PAH-specific medication administration was statistically associated with an obvious improvement in FC (OR = 0.60, 95% CI, range: 0.47–0.76, p < 0.001; I2 = 31%), where an apparent amelioration was only found in drugs when targeting prostanoids (p = 0.04) and NO (p < 0.001), while no significant change targeting ERAs (p = 0.12).
We investigated the correlation between AEs and exercise tolerance in participants treated with active drugs. In the meta-regression analysis, we found few relationships between the change in 6MWD from baseline (Δ6MWD) and CWEs (p = 0.032; Figure 3). We also found no relationship between CWEs and deterioration rate of FC (p = 0.775; Figure 4).
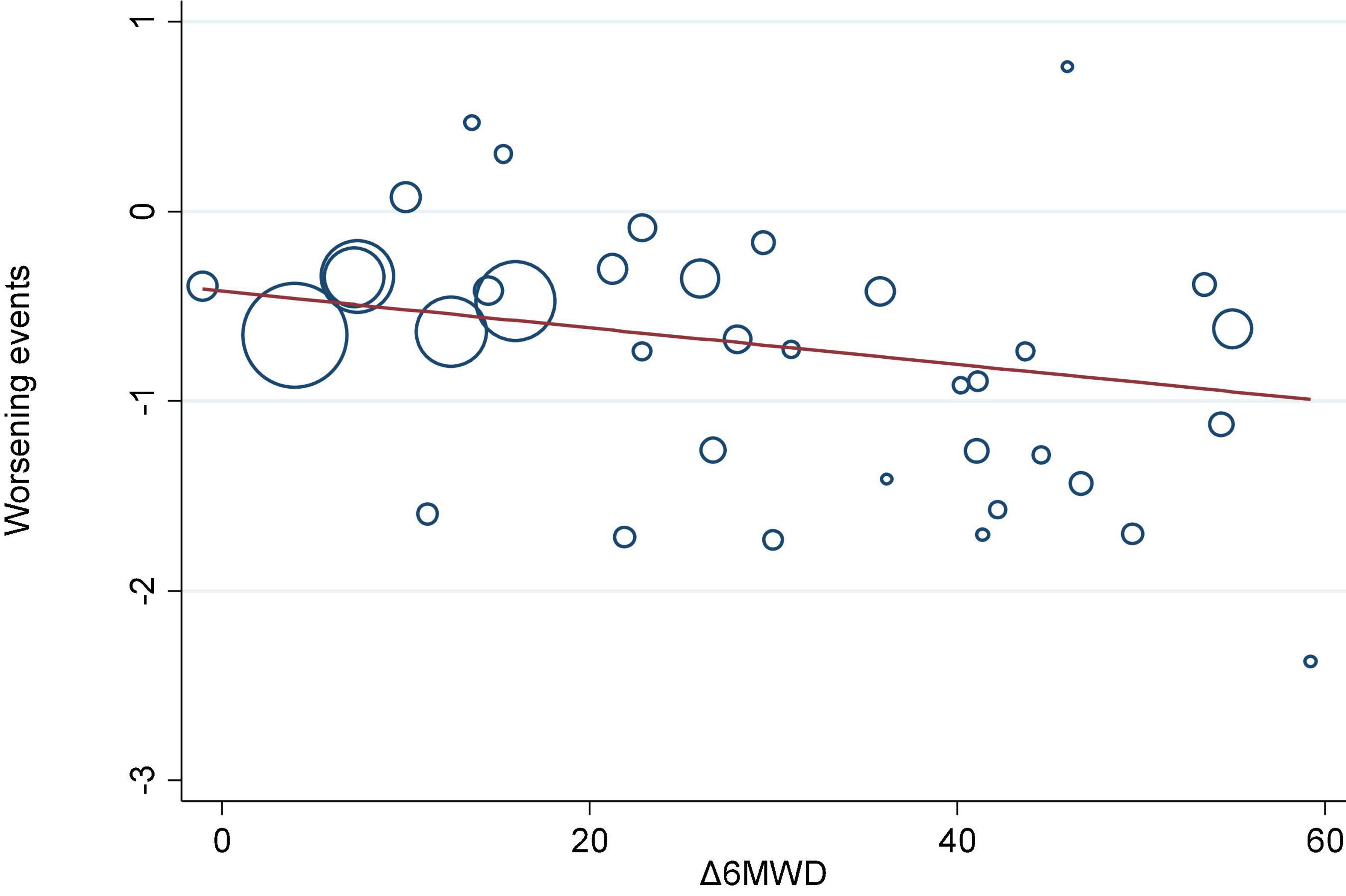
Figure 3. Meta-regression analysis of 6-min walk distance from baseline (Δ6MWD) and clinical worsening events (CWEs).
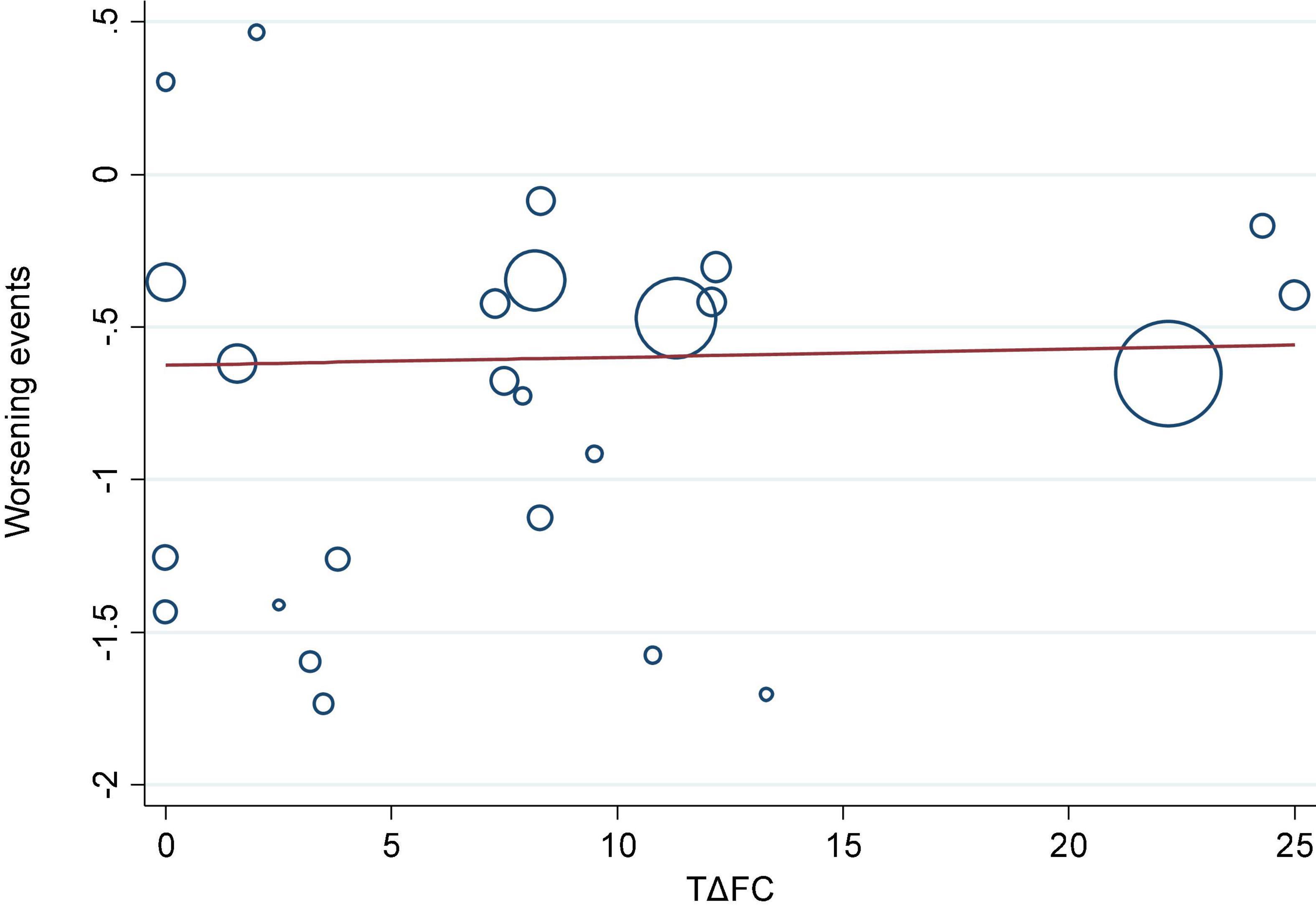
Figure 4. Meta-regression analysis of change in functional class from baseline (ΔFC) and clinical worsening events (CWEs).
Cardiopulmonary hemodynamics have been achieved in some studies (27, 28, 30, 33, 34, 37–39, 44, 46). With regard to mPAP, 8 articles (30, 33, 34, 37–39, 44, 46) have shown a decrease compared to that of oral targeted drugs with placebo (SMD = −0.42, 95% CI, range: −0.63 to −0.21, p < 0.001; I2 = 72.0%), and the changes were associated with a similar trend in PVR (SMD = −0.63, 95% CI, range: −0.76 to −0.50, p < 0.001; I2 = 0.0%) and mRAP (SMD = −0.06, 95% CI, range: −0.35 to −0.23, p = 0.67; I2 = 83.0%), but there were rare improvements in PCWP (SMD = 0.09, 95% CI, range: −0.08–0.25, p = 0.32; I2 = 0.0%). For systemic circulation, oral drugs targeting PAH could improve CI with an SMD of 0.45 (95% CI, range: 0.25–0.66, p < 0.001; I2 = 70.0%). The details of the different approaches are shown in Table 4.
Participants in the study were classified as grades I–II or III–IV based on their initial FC ratings. We performed subgroup analysis based on the FC grade of the treatment group.
As shown in Table 5, patients with FC I–II improved by 21.60 m after oral treatment (95% CI, range: 12.86–30.34, p < 0.001), but only ERAs (MD: 19.74, 95% CI, range: 9.42–30.06, p < 0.001) increased among the three classical pathway targeted drugs. In patients with FC III–IV, oral targeted drugs of three classical pathways, ERAs, NO, and prostanoids, all showed improvement at 6MWD of 28.88 m (95% CI: 15.99–41.78), 27.38 m (95% CI, range: 21.08–33.69), and 10.08 m (95% CI, range: 3.24–16.92), respectively.
The OR estimate for CWEs was a reduction of 50% (95% CI, range: 0.36–0.70, p < 0.001) in FC I–II; only ERAs reduced the OR of CWEs by 0.52 (p = 0.002), while NO and prostanoids decreased by 0.40 and 0.50, respectively, which was not statistically significant. However, the CWES results of oral treatment of FC III-IV patients showed the same trend as that of the 6MWD. The ORs of ERA, NO, and prostanoids were 0.47, 0.46, and 0.57, respectively.
As shown in Table 5, we divided the treatment group into two groups (<50 years and ≥50 years) according to the age of patients to observe the therapeutic effect of the different types of drugs in various age stages.
In younger populations (<50 years), agents of the NO pathway reduced the risk of CWEs, with OR = 0.50 (95% CI, range: 0.20–1.27, p = 0.14; I2 = 21.0%), and an OR of 0.48 in ERAs (95% CI, range: 0.34–0.69, p < 0.001; I2 = 35.0%), whereas prostanoids reduced the OR of CWEs by 43% (95% CI, range: 0.47–0.68, p < 0.001; I2 = 0.0%). ERAs lowered the risk of CWEs, with an OR of 0.47 (95% CI, range: 0.25–0.88, p = 0.02; I2 = 50.0%), associated with an OR of 0.45 in NO (95% CI, range: 0.32–0.63, p < 0.001; I2 = 0.0%) and 0.68 in prostanoids (p = 0.11) in the older population (≥50 years).
As shown in 6MWD, NO increased by 38.37 m in 6MWD (95% CI, range: 18.79–57.95 m, p < 0.001; I2 = 0.0%), and ERAs and prostanoids also improved by 22.23 m (95% CI, range: 12.30–32.15 m, p < 0.001; I2 = 0.0%) and 17.08 m of 6MWD (95% CI, range: 4.25–29.92 m, p = 0.009; I2 = 0.0%), respectively, in younger patients (<50 years). In the older populations, NO increased 6MWD by 28.20 m (95% CI, range: 21.85–34.54, p < 0.001; I2 = 0.0%), and a 25.41-m elevation of 6MWD was detected in ERAs (95% CI, range: 11.61–39.21, p < 0.001; I2 = 0.0%), with 8.20-m elevation in prostanoids (95% CI, range: 0.47, 15.94, p = 0.04; I2 = 0.0%).
As demonstrated above, PAH-targeted drugs could significantly reduce the incidence of worsening events in ERAs, NO, and prostanoids. In the condition, subgroup analysis further indicated that when compared to placebo, macitentan lowered the risk of worsening events, with an OR of 0.61 (p < 0.001), associated with an OR of 0.54 in bosentan (p < 0.001) and 0.27 in ambrisentan (p < 0.001). In the NO pathway, tadalafil, riociguat, and vardenafil contributed to a decreased risk of CWEs, with an OR of 0.47 (p < 0.001), 0.28 (p = 0.02), and 0.09 (p = 0.04), respectively, but a non-obvious outcome was found in patients treated with sildenafil and udenafil. Furthermore, in prostanoids pathway, only treprostinil with oral administration and selexipag were detected to lead to a low risk of mortality, with an OR of 0.67 and 0.52, respectively.
As data presented in 6MWD, subgroup analysis demonstrated an obvious improvement when patients were treated with ERAs, where bosentan increased 6MWD by 23.46 m (95% CI, range: 11.42–35.49, p < 0.001), and 43.84-m elevation of 6MWD were detected in ambrisentan, with 19.46-m elevation in macitentan (95% CI, range: 7.66–31.25, p = 0.001). In the NO pathway, except for udenafil, other drugs (tadalafil, sidenafil, riociguat, and vardenafil) detected an obvious elevation of 6MWD, and an opposite trend was found in prostanoids; only treprostinil showed an improvement in 6MWD (MD = 10.34 m, 95% CI, range: 2.85–17.82 m, p = 0.007; details in Table 6).
For the meta-analysis of all oral targeted drugs on worsening events and 6MWD (Figure 5), there was no evidence of publication bias by inspection of the funnel plot. The details of the quality assessment of all pooled studies are shown in Figure 6.
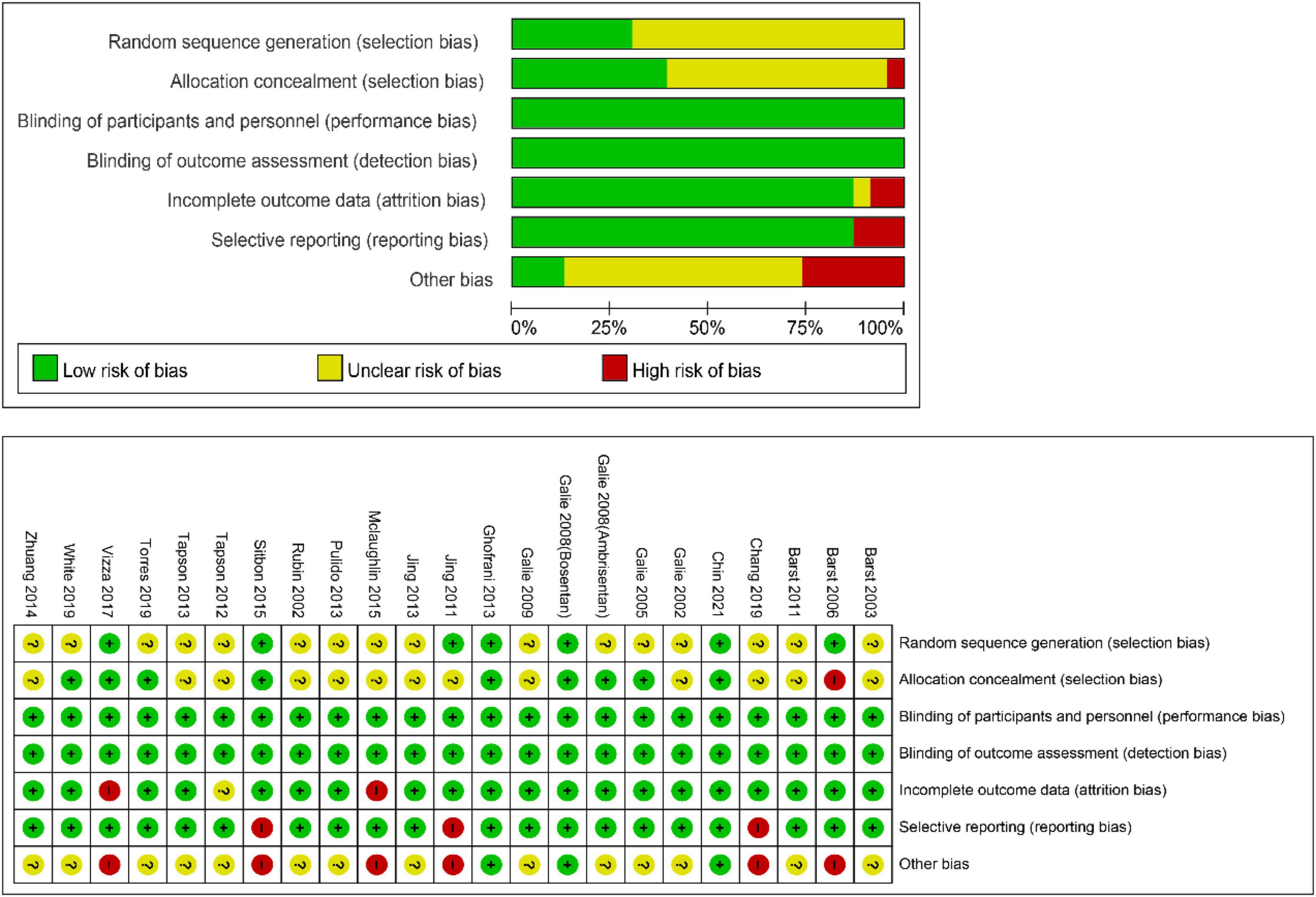
Figure 6. Cochrane collaboration’s risk-of-bias tool and risk of bias graph for the pooled studies in this meta-analysis.
Pulmonary arterial hypertension is a life-threatening disorder, and the 5-year survival rate is less than 60% (47). An approved drug could lengthen the lifespan of patients, but it cannot reverse the development of the disease. Targeted drugs, such as those that affect ERA, NO, and prostanoids pathways, are still the recommended therapy for PAH patients (2). As such, they have been shown to slow PAH progression and improve exercise capacity and cardiopulmonary hemodynamics. Furthermore, oral administration may be the common preference for patients with FC I–II. Although some drugs have yielded side effects (48), the symptoms are often mild and eventually clear without treatment. In 2015, the outcomes of a meta-analysis demonstrated that the oral administration of PAH-specific drugs is a safe and effective treatment. However, this study incorporated sitaxsentan and imatinib, which are no longer in clinical use. Meanwhile, there is no detailed description of the precise treatment of PAH patients of different ages and FC grades with oral drugs.
Prostanoids, ERAs, and NO signaling pathway drugs are all used to achieve therapeutic goals through vasodilation, while RTK drugs can theoretically treat PAH by inhibiting the pathogenesis pathway of PAH, but their drug imatinib is not approved for the treatment of PAH due to side effects, such as subdural hemorrhage. Therefore, the three major classical pathway drugs are still mainly used clinically to treat PAH, and these traditional methods still have a high mortality rate, although they alleviate the symptoms to some extent. Nanomedicines and gene therapy are emerging approaches for the treatment of PAH in the future, but a higher level of evidence is needed to reach the goal of individualized treatment (49). In the meta-regression analysis, there is a correlation between CWEs and the change in 6MWD (p = 0.032; Figure 3), which possibly emphasizes the need to monitor CWEs.
Most NO drugs (including tadalafil, vardenafil, and riociguat) were identified to be effective. Indeed, Galie et al. (30), Vizza et al. (36), and Chang et al. (37) have found that oral udenafil and sildenafil might not improve the exercise capacity or pulmonary vascular remodeling in short-term treatment. Even treprostinil, a type of prostanoids, was detected as the only agent with a significant amelioration in exercise capacity, where it showed an obvious change in hemodynamics of prostanoids. AEs reported in the three classical methods were mild to moderate, with a high prevalence of headache. Additionally, the most common severe adverse drug response in oral ERAs was right-sided heart failure, accounting for approximately 14.0% (95% CI, range: 10.9–17.1%), dyspnea in the NO pathway (approximately 5.7%, 95% CI, range: 4.2–7.2%), and prostanoids (16.0%, 95% CI, range: 13.3–18.6%), respectively.
Age-treatment subgroup analysis indicated that the oral administration of NO pathway agents improved exercise capacity, cardiopulmonary hemodynamics, and worsening events-free survival rate across all age groups (<50 years and ≥50 years). In the younger populations (<50 years), NO revealed a significant improvement in 6MWD (MD = 38.37, p < 0.001) and a reduction of 0.50 in the OR of CWEs (p = 0.14). Additionally, the data of NO demonstrated excellent clinical efficacy in the older patients, increasing 6MWD by 28.20 m (p < 0.001), and significantly reducing CWEs, with an OR of 0.45 (p < 0.001). Age-related vasculature alteration is an obvious risk factor for PAH (50). Furthermore, clinical registries (51) have mentioned age-related differences in functional status and hemodynamics in PAH where the mean age of patients was reported as 50–65 years old. The Scottish composite index constructed a multivariate Cox model based on data from 182 PAH patients, using age, sex, etiology, right atrial pressure, cardiac output, and 6MWD to predict survival (52). Yi et al. found that older patients (≥50 years) were more likely to die than younger patients (<50 years) based on PAH registries in the United Kingdom and Ireland (48). This means that age is a predominant factor affecting patient prognosis. ERAs and NO significantly reduced the OR of CWEs by 53% (p = 0.02) and 54% (p < 0.001), respectively. ERAs showed the greatest efficacy on CWEs and could be used as the first targeted oral agent in elderly patients (≥50 years). Additionally, PAH is mainly a disease in women, according to data from registries (53). The study did not perform a subgroup analysis of sex and PAH classification because most of the 23 studies included women, and most of the patients had primary PAH.
In the subgroup analysis, subdivided by FC, ERAs demonstrated superior clinical efficacy in patients with FC I–II, 6MWD increasing by 19.74 m and reducing the OR of CWEs by 52%. ERAs also showed the best clinical efficacy compared to that of placebo in patients with FC III–IV, with a 6MWD 28.88-m improvement and a 53% reduction in the OR of CWEs. Although FC was not included in the Cox model mentioned above, FC and age were also included in the prognostic prediction as relevant parameters in some prediction equations or models proposed (54). By subgroup analysis of age and FC, patients were stratified using evidence-based medicine to aid doctors in choosing more appropriate drugs to improve patient outcomes.
Currently, patients with pulmonary hypertension are clinically classified into the following five groups: (1) those with predominantly pulmonary artery disease; (2) those consisting of pulmonary hypertension associated with left heart disease; (3) those with PAH associated with pulmonary disease and hypoxia; (4) those with PAH due to chronic thromboembolism; and (5) those due to unclear multifactorial mechanisms. This classification is widely accepted and used in clinical practice, but this approach is not ideal for some patients. Precision therapy, which can also be referred to as stratified therapy, intervenes based on the greatest benefit and least harm for most patients (55). We build on this by again subgrouping patients using age and FC class, narrowing the patient population so that the majority of patients in the group are susceptible to the drugs used, and improving prognosis. The NO drugs showed excellent efficacy in all age groups, but not in patients with FC I–II. However, in the subgroup analysis of FC, ERAs showed the best clinical efficacy. In clinical practice, doctors can choose the corresponding treatment plan based on basic patient information. First, we determine the age of the patient and select the appropriate drug. Then, consider the FC grade of the patient and decide whether to add other drugs to form a dual or triple treatment. In general, the clinical conditions of patients taking oral drugs are not serious, which is why most of the patients involved in this study were in FC II–III. When the patient does not fall under any of these conditions, other treatments can be used, such as inhaled or intravenous drugs. When the level of individualized treatment is not reached, age and FC grade can be first subdivided into a small number of patients to achieve accurate treatment for this small population.
The limitations of this study are as follows: (1) the sample size of some studies was too small and there was great uncertainty; (2) only studies in English were included in this meta-analysis, and studies not included in other languages may result in partial data omission; (3) since this was a secondary study, it was difficult to obtain individual patient data, which may cause uncertainty in the data; (4) only some studies have reported hemodynamic changes; and (5) fewer studies were pooled in the subgroup analysis.
In this meta-analysis, we assessed the efficacy and safety of orally targeted drugs for PAH. This study compared the clinical efficacy of three classical oral targeted drugs through subgroup analysis of age and FC grade, providing evidence-based medicine for precise clinical treatment.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
H-RZ and QL collected the data. H-RZ and H-YK analyzed the data. H-RZ wrote the manuscript. H-YK modified the manuscript. X-JJ provided the idea. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Coons J, Pogue K, Kolodziej A, Hirsch G, George M. Pulmonary arterial hypertension: a pharmacotherapeutic update. Curr Cardiol Rep. (2019) 21:141. doi: 10.1007/s11886-019-1235-4
2. Montani D, Chaumais M, Guignabert C, Günther S, Girerd B, Jaïs X, et al. Targeted therapies in pulmonary arterial hypertension. Pharmacol Ther. (2014) 141:172–91. doi: 10.1016/j.pharmthera.2013.10.002
3. Klinger J, Elliott C, Levine D, Bossone E, Duvall L, Fagan K, et al. Therapy for pulmonary arterial hypertension in adults: update of the chest guideline and expert panel report. Chest. (2019) 155:565–86. doi: 10.1016/j.chest.2018.11.030
4. O’Connell C, Amar D, Boucly A, Savale L, Jaïs X, Chaumais MC, et al. Comparative safety and tolerability of prostacyclins in pulmonary hypertension. Drug Saf. (2016) 39:287–94. doi: 10.1007/s40264-015-0365-x
5. Kurzyna M, Małaczyńska-Rajpold K, Koteja A, Pawlak A, Chrzanowski L, Furdal M, et al. An implantable pump Lenus pro§in the treatment of pulmonary arterial hypertension with intravenous treprostinil. BMC Pulm Med. (2017) 17:162. doi: 10.1186/s12890-017-0474-7
6. Lombardi S, Kingman M, Duncan M, Berngard S, Fernandes T. Titration of pulmonary arterial hypertension therapeutics: experience-based recommendations. Respir Med. (2018) 143:139–46. doi: 10.1016/j.rmed.2018.09.002
7. Lang I, Gaine SP. Recent advances in targeting the prostacyclin pathway in pulmonary arterial hypertension. Eur Respir Rev. (2015) 24:630–41. doi: 10.1183/16000617.0067-2015
8. Zhang H, Zhang R, Jiang X, Sun K, Wu D, Jing Z. Effects of oral treatments on clinical outcomes in pulmonary arterial hypertension: a systematic review and meta-analysis. Am Heart J. (2015) 170:96–103; 103.e1–14. doi: 10.1016/j.ahj.2015.04.003
9. Owen K, Cross D, Derzi M, Horsley E, Stavros FL. An overview of the preclinical toxicity and potential carcinogenicity of sitaxentan (Thelin§), a potent endothelin receptor antagonist developed for pulmonary arterial hypertension. Regul Toxicol Pharmacol. (2012) 64:95–103. doi: 10.1016/j.yrtph.2012.05.017
10. Nair A. Pharmacologic therapy for pulmonary artery hypertension. Curr Opin Cardiol. (2020) 35:643–56. doi: 10.1097/HCO.0000000000000796
11. Fu W, He W, Li Y, Chen Y, Liang J, Lei H, et al. Efficacy and safety of novel-targeted drugs in the treatment of pulmonary arterial hypertension: a Bayesian network meta-analysis. Drug Deliv. (2021) 28:1007–19. doi: 10.1080/10717544.2021.1927243
12. Zheng YG, Ma H, Chen L, Jiang XM, Zhou L, Lin S, et al. Efficacy and safety of oral targeted therapies in pulmonary arterial hypertension: a meta-analysis of randomized clinical trials. Pulm Circ. (2018) 8:2045894018798183. doi: 10.1177/2045894018798183
13. Higgins J, Altman D, Gøtzsche P, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
14. Barst R, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. (2004) 169:441–7. doi: 10.1164/rccm.200307-957OC
15. Sandoval J, Torbicki A, Souza R, Ramírez A, Kurzyna M, Jardim C, et al. Safety and efficacy of sitaxsentan 50 and 100 mg in patients with pulmonary arterial hypertension. Pulm Pharmacol Ther. (2012) 25:33–9. doi: 10.1016/j.pupt.2011.10.002
16. Hoeper MM, Barst RJ, Bourge RC, Feldman J, Frost AE, Galié N, et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation. (2013) 127:1128–38.
17. Ghofrani H, Morrell N, Hoeper M, Olschewski H, Peacock AJ, Barst RJ, et al. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med. (2010) 182:1171–7. doi: 10.1164/rccm.201001-0123OC
18. Chang S, Kim H, Chang H, Kim DK. Acute hemodynamic changes after single administration of udenafil in pulmonary arterial hypertension: a phase IIa study. Korean Circ J. (2019) 49:353–60. doi: 10.4070/kcj.2018.0281
19. Wilkins M, Ali O, Bradlow W, Wharton J, Taegtmeyer A, Rhodes CJ, et al. Simvastatin as a treatment for pulmonary hypertension trial. Am J Respir Crit Care Med. (2010) 181:1106–13. doi: 10.1164/rccm.2009111-699OC
20. Channick R, Badesch DB, Sitbon O, Robbins IM, Frost A, Tapson VF, et al. Effects of the dual endothelin receptor antagonist bosentan in patients with pulmonary hypertension: a placebo-controlled study. Lancet. (2001) 358:1119–23. doi: 10.1016/S0140-6736(01)06250-X
21. Galiè N, Müller K, Scalise A, Grünig E. PATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertension. Eur Respir J. (2015) 45:1314–22. doi: 10.1183/09031936.00105914
22. Langleben D, Christman BW, Barst RJ, Dias VC, Galiè N, Higenbottam TW, et al. Effects of the thromboxane synthetase inhibitor and receptor antagonist terbogrel in patients with primary pulmonary hypertension. Am Heart J. (2002) 143:E4. doi: 10.1067/mhj.2002.121806
23. Simonneau G, Torbicki A, Hoeper MM, Delcroix M, Karlócai K, Galiè N, et al. Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur Respir J. (2012) 40:874–80. doi: 10.1183/09031936.00137511
24. Rubin L, Badesch D, Barst R, Galie N, Black CM, Keogh A, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. (2002) 346:896–903. doi: 10.1056/NEJMoa012212
25. Barst R, Langleben D, Badesch D, Frost A, Lawrence EC, Shapiro S, et al. Treatment of pulmonary arterial hypertension with the selective endothelin-A receptor antagonist sitaxsentan. J Am Coll Cardiol. (2006) 47:2049–56.
26. Galiè N, Olschewski H, Oudiz R, Torres F, Frost A, Ghofrani HA, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. (2008) 117:3010–9. doi: 10.1161/CIRCULATIONAHA.107.742510
27. Galiè N, Rubin L, Hoeper M, Jansa P, Al-Hiti H, Meyer G, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. (2008) 371:2093–100. doi: 10.1016/S0140-6736(08)60919-8
28. Pulido T, Adzerikho I, Channick R, Delcroix M, Galiè N, Ghofrani HA, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. (2013) 369:809–18. doi: 10.1056/NEJMoa1213917
29. McLaughlin V, Channick R, Ghofrani H, Lemarié JC, Naeije R, Packer M, et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J. (2015) 46:405–13. doi: 10.1183/13993003.02044-2014
30. Galiè N, Ghofrani H, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. (2005) 353:2148–57. doi: 10.1056/NEJMoa050010
31. Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. (2009) 119:2894–903. doi: 10.1161/CIRCULATIONAHA.108.839274
32. Barst RJ, Oudiz RJ, Beardsworth A, Brundage BH, Simonneau G, Ghofrani HA, et al. Tadalafil monotherapy and as add-on to background bosentan in patients with pulmonary arterial hypertension. J Heart Lung Transplant. (2011) 30:632–43. doi: 10.1016/j.healun.2010.11.009
33. Jing ZC, Yu ZX, Shen JY, Wu BX, Xu KF, Zhu XY, et al. Vardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. (2011) 183:1723–9. doi: 10.1164/rccm.201101-0093OC
34. Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. (2013) 369:330–40. doi: 10.1056/NEJMoa1209655
35. Zhuang Y, Jiang B, Gao H, Zhao W. Randomized study of adding tadalafil to existing ambrisentan in pulmonary arterial hypertension. Hypertens Res. (2014) 37:507–12. doi: 10.1038/hr.2014.28
36. Vizza C, Jansa P, Teal S, Dombi T, Zhou D. Sildenafil dosed concomitantly with bosentan for adult pulmonary arterial hypertension in a randomized controlled trial. BMC Cardiovasc Disord. (2017) 17:239. doi: 10.1186/s12872-017-0674-3
37. Chang HJ, Song S, Chang SA, Kim HK, Jung HO, Choi JH, et al. Efficacy and safety of udenafil for the treatment of pulmonary arterial hypertension: a placebo-controlled, double-blind, Phase IIb clinical trial. Clin Ther. (2019) 41:1499–507. doi: 10.1016/j.clinthera.2019.05.006
38. Galiè N, Humbert M, Vachiéry J, Vizza CD, Kneussl M, Manes A, et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. (2002) 39:1496–502. doi: 10.1016/S0735-1097(02)01786-2
39. Barst R, McGoon M, McLaughlin V, Tapson V, Rich S, Rubin L, et al. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol. (2003) 41:2119–25. doi: 10.1016/S0735-1097(03)00463-7
40. Tapson V, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest. (2012) 142:1383–90. doi: 10.1378/chest.11-2212
41. Tapson V, Jing Z, Xu K, Pan L, Feldman J, Kiely DG, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest. (2013) 144:952–8. doi: 10.1378/chest.12-2875
42. Jing ZC, Parikh K, Pulido T, Jerjes-Sanchez C, White RJ, Allen R, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. (2013) 127:624–33. doi: 10.1161/CIRCULATIONAHA.112.124388
43. Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. (2015) 373:2522–33. doi: 10.1056/NEJMoa1503184
44. Torres F, Farber H, Ristic A, McLaughlin V, Adams J, Zhang J, et al. Efficacy and safety of ralinepag, a novel oral IP agonist, in PAH patients on mono or dual background therapy: results from a phase 2 randomised, parallel group, placebo-controlled trial. Eur Respir J. (2019) 54:1901030. doi: 10.1183/13993003.01030-2019
45. White R, Jerjes-Sanchez C, Bohns Meyer G, Pulido T, Sepulveda P, Wang KY, et al. Combination therapy with oral treprostinil for pulmonary arterial hypertension. A double-blind placebo-controlled clinical trial. Am J Respir Crit Care Med. (2020) 201:707–17.
46. Chin K, Sitbon O, Doelberg M, Feldman J, Gibbs JSR, Grünig E, et al. Three- Versus Two-Drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J Am Coll Cardiol. (2021) 78:1393–403. doi: 10.1016/j.jacc.2021.07.057
47. Mandras S, Mehta H, Vaidya A. Pulmonary hypertension: a brief guide for clinicians. Mayo Clin Proc. (2020) 95:1978–88. doi: 10.1016/j.mayocp.2020.04.039
48. Vishal P, Bhardwaj A, Nair A. Pharmacotherapy for pulmonary arterial hypertension. J Thorac Dis. (2019) 11:S1767–81. doi: 10.21037/jtd.2019.09.14
49. Dhoble S, Patravale V, Weaver E, Lamprou DA, Patravale T. Comprehensive review on novel targets and emerging therapeutic modalities for pulmonary arterial Hypertension. Int J Pharm. (2022) 621:121792. doi: 10.1016/j.ijpharm.2022.121792
50. Raucci A, Macrì F, Castiglione S, Badi I, Vinci MC, Zuccolo E, et al. MicroRNA-34a: the bad guy in age-related vascular diseases. Cell Mol Life Sci. (2021) 78:7355–78. doi: 10.1007/s00018-021-03979-4
51. DesJardin JT, Kolaitis NA, Kime N, Kronmal RA, Benza RL, Elwing JM, et al. Age-related differences in hemodynamics and functional status in pulmonary arterial hypertension: baseline results from the Pulmonary Hypertension Association Registry. J Heart Lung Transplant. (2020) 39:945–53. doi: 10.1016/j.healun.2020.05.005
52. Benza R, Miller D, Foreman A, Frost AE, Badesch DB, Benton WW, et al. Prognostic implications of serial risk score assessments in patients with pulmonary arterial hypertension: a Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) analysis. J Heart Lung Transplant. (2015) 34:356–61. doi: 10.1016/j.healun.2014.09.016
53. Ling Y, Johnson M, Kiely D, Condliffe R, Elliot CA, Gibbs JSR, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. (2012) 186:790–6. doi: 10.1164/rccm.201203-0383OC
54. D’Alonzo G, Barst R, Ayres S, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. (1991) 115:343–9. doi: 10.7326/0003-4819-115-5-343
Keywords: pulmonary arterial hypertension, prostanoids, endothelin receptor antagonists, phosphodiesterase 5 inhibitors, precision therapy
Citation: Zhu H-r, Kuang H-y, Li Q and Ji X-j (2022) Effects of oral targeted treatments in pulmonary arterial hypertension: A systematic review and meta-analysis. Front. Cardiovasc. Med. 9:915470. doi: 10.3389/fcvm.2022.915470
Received: 08 April 2022; Accepted: 08 July 2022;
Published: 02 August 2022.
Edited by:
Ryou Tanaka, Tokyo University of Agriculture and Technology, JapanReviewed by:
Chi Zhang, Shanghai Jiao Tong University, ChinaCopyright © 2022 Zhu, Kuang, Li and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-juan Ji, aml4aWFvanVhbjIwMDNAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.