
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 30 August 2022
Sec. Cardiovascular Epidemiology and Prevention
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.913888
Background: Depression, as an independent risk factor, can lead to a substantially increased risk of coronary heart disease (CHD). The overall body of evidence involving depression and CHD is not consistent. Therefore, we performed an update meta-analysis to evaluate the association between depression and the risk of patients with CHD.
Methods: Studies were identified through a comprehensive literature search of the PubMed, Embase, and the Cochrane Library database from its inception to 28 September 2021 for titles/abstracts with restricted to English language articles. The literature was screened according to the inclusion and exclusion criteria. Along with data extraction, we evaluated the quality of eligible studies using the Newcastle-Ottawa Scale (NOS). The primary outcome was fatal or non-fatal CHD. We calculated relative risk (RR) with 95% confidence intervals (CIs) using a random-effects models. The protocol was registered in the PROSPERO registration (registration number CRD42021271259).
Results: From 9,151 records, we included 26 prospective cohort studies published from 1998 to 2018, consisting of 402,597 patients. Either in depression-exposured group or non-depression-exposured group, the mean age of all participants ranged from 18 to 99 years. Moreover, the NOS scores of these studies are eventually indicated that the quality of these eligible studies was reliable. In general, the pooled results showed that patients with depression had a higher risk of CHD compared to patients without depression (RR = 1.21, 95% CI: 1.14–1.29). Additionally, the funnel plot appeared to be asymmetry, indicating there existing publication bias for the pooled results between depression and CHD. A sensitivity analysis was used to assess the stability of the relationship between depression and CHD that indicating the results robust (RR = 1.15, 95% CI: 1.09–1.21).
Conclusion: Depression may increase risk of CHD. Future studies on the share pathogenic mechanisms of both depression and CHD may develop novel therapies.
Coronary heart disease (CHD) is the most common heart disease worldwide, accounting for an estimated 200 million people (1). In 2019, the World Health Organization estimated that approximately 17.9 million of worldwide deaths were due to CHD (2). It is a chronic and complex disease that refers to a general term for disease narrowing of the coronary artery wall owing to fatty material accumulation (3). Its primary clinical symptoms represented chest pain, chest tightness, syncope, and so on, posing a severe effect to human health and the quality of life (4, 5). The growing burden of CHD has been a main public health problem in China (6). Despite the pathogeny of CHD is not entirely clear, risk factors such as age, gender, obesity, diabetes, high cholesterol, high blood pressure, and unhealthy living habits like smoking, could explain about 50–60% of the etiology of CHD (7–9).
Depressive is a leading and growing cause of disability, a main contributor to the total global burden of disease, with more than an estimated 280 million people affected worldwide (10). Depression is considered a chronic disease affecting 26% women and 18% men (11). The psychological symptoms that patients with depression experience are the primary contributing factors to its heavy disease burden (12). The burden posed by depression could be alleviated by increasing access to timely treatment. Depression results from a complex interaction of various factors. At present, the relationship between depression and CHD has attracted increasing academic attention. According to position papers of the American Heart Association and the European Society of Cardiology, depression may be a modifiable prognostic factor for CHD (13, 14). Apart from those, it has been reported that depression, as an independent risk factor, can lead to a substantially increased risk of CHD, as it not only diminished the quality of life in patients with CHD, but also increased the occurrence rate of main adverse cardiac events (15, 16).
Although a number of studies showed a specific link between depression and CHD (17–19), the overall body of evidence is not consistent (20, 21). Meta-analysis overcomes the shortcomings of traditional literature review, and has the characteristics of quantitative synthesis; it can provide a systematic, repeatable and objective synthesis method for the same problem (22). In the previous meta-analysis (23) an increased risk of myocardial infarction (MI) and coronary death was found with the presence of depression in people. The study however assessed 19 studies including two diseases of MI and CHD from 1996 to 2014. Therefore, in this present study, we aim to perform an update the meta-analysis and further the nature of the relationship between depression and CHD morbidity.
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to perform this updated systematic review and meta-analysis to determine the relationship between depression and CHD.(24) The protocol was registered in the PROSPERO registration (registration number CRD42021271259). Studies were identified through a comprehensive literature search of the PubMed, Embase, and the Cochrane Library database from its inception to 28 September 2021 for titles/abstracts with restricted to English language articles, by two authors independently. The following search term of the Medical Subject Headings (MeSH)/Emtree combined with free text words as well as all known spellings were used to search: depression/depression disorder and Coronary Disease, which could be adjusted in different databases. Detailed full-search strategies were provided in Supplementary Table 1. Moreover, no restriction in region, publication years and types were in this literature search. A further complementary screening of the references of eligible published studies and meta-analysis was tracked manually as a supplementation.
Published studies were included if they simultaneously could meet the following criteria. These studies: (1) recruited subjects who were depression without CHD at baseline visit; (2) they were exposed to depression (yes or not); moreover, depression was defined by diagnostic interview, self-completed scaled questionnaire, anti-depressant medication, physician diagnosis, or self-reported diagnosis; (3) studies provided relative risk (RR), hazard ratio (HR), or odds ratio (OR) with 95% confidence interval (CI); and (4) they were adopted prospective cohort study design with predictors’ assessment at baseline. When one study met one of the following criteria, it was excluded: (1) they were unable to obtain original text, such as conference abstract, review article etc.; (2) they included other types of diseases not as a single outcome; (3) the required data cannot be calculated; and (4) only non-desired outcomes were reported. For studies with the same data in at least two studies, we give priority to including the most recent and detailed studies. If there were divergences between two authors, these studies were discussed in further detail or arbitrated by third author until agreement was reached.
The following information was extracted independently by two authors detailing with a predesigned Excel spreadsheet carefully from each study, including the first author’s name, publication year, study design, population size, country, age, female (%), race, diabetes, hypertension, history of smoking, hyperlipidemia, BMI categories, depression measurement, outcomes, the adjusted and unadjusted effect estimates (HR, RR, or OR) with CI, adjustment variables included in the final model, and length of follow-up. For studies provided both adjusted and unadjusted effect estimates (HR, RR, or OR), the adjusted one was used to further analysis.
Along with data extraction, we evaluated the quality of eligible studies using the Newcastle-Ottawa Scale (NOS) (25), which contains eight items classified into three domains: selection, comparability, and outcome. The NOS scores ranged from 1 to 9 “★,” with 9 “★” representing the best quality. Data extraction and quality assessment were conducted by two authors independently, and if there exists any disagreement, a third author resolved and provided an arbitration.
The primary outcome was the confounder adjusted HR, OR, and RR with the corresponding 95% CI. The original HRs obtained in the text were considered as equivalent estimations for RRs.(26) For a study only reported OR, particular formulae were used to convert OR to RR.(27) We calculated the pooled RR with the corresponding 95% CI for the association between depression and the risk of CHD. The heterogeneity between studies was quantified using Cochrane Q-test and the I2 statistic. The statistically significant refers to Q ≤ 0.1 or I2 > 50% (28). When there existed significant heterogeneity, a random-effects model was selected, if not, we chose the fixed-effect model to perform the pooled analyses. In addition, subgroup analyses were conducted to explore potential sources of heterogeneity based on age, sex, publication year, outcome indicator, and duration of follow-up. Subsequently, we performed sensitivity analysis by excluding one study in turn to assess the robustness of the meta-analysis results. Furthermore, funnel plots, Begg’s test and Egger’s test were used to evaluate potential publication bias, which was defined as P < 0.05. All mentioned above data analyses were performed with Stata 14.0 software (STATA Corp., College Station, TX, United States).
The database search and literature selection are shown in Figure 1. Overall, 9,153 papers were retrieved via the initial literature search, among which 1,071 were removed due to duplication. Subsequently, after title and abstract screening, 8,000 papers were further excluded, remaining 82 potentially eligible papers for full-text review. After reviewing the full-text articles, a total of 25 papers were included into this present meta-analysis since 17 of them include other types of disease, 12 cannot calculated the required data, 17 did not report desired outcomes, and 11 were unable to obtain original text. Moreover, we also manually tracked the references of retrieved papers and meta-analyses, and an additional paper has been added. Finally, a total of 26 articles (20, 21, 29–52) were included in the systematic review and meta-analysis.
Among included 26 prospective cohort studies, the publication year ranged from 1998 to 2018 and the sample capacity included 402,597 subjects. With respect to the regions that conducted these studies, the majority of them was conducted in one country or region, two of them in several countries, and another study that did not indicate where it was done. Either in depression-exposured group or non-depression-exposured group, the mean age of all participants ranged from 18 to 99 years. The measurement of depression mainly involved Center for Epidemiological Studies-Depression (CES-D), International Classification of Diseases, Seventh, Eighth, Ninth, Tenth Revision (ICD-7, -8, -9, -10), Geriatric Depression Scale (GDS), Beck Depression Inventory (BDI), Mental Health Index-5 (MHI-5), Zung Self-rating Depression Scale (ZSDS), Minnesota Multiphasic Personality Inventory (MMPI-2 D), Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Non-patient edition (SCID-I/NP), and diagnostic and statistical manual of mental disorders, third edition (DSMMD). Nineteen studies reported outcome indicators using HR, whereas five reported using RR, and two reported using OR. Notably, the follow-up time for the prospective cohort study were reported, ranging from 4 to 37 years. All included studies evaluated outcomes using multiple adjustment variables, however, one study only have adjusted for age.
As described previously, we used the NOS to evaluate the 26 included studies. Detailed scores are presented in Supplementary Table 2, in which “★” representing 1 point, “×” representing 0 point, and “—” representing uncertain points. Moreover, the quality scores of these studies are eventually presented in Table 1, the scores were 6 to 9 “★,” indicating that the quality of these studies was reliable.
The impact of depression on CHD was evaluated in 28 cohort studies, due to fatal and non-fatal being divided into 2 separate studies. Figure 2 illustrated the results of pooled RR with a random-effects model. In general, the pooled results showed that patients with depression had a higher risk of CHD compared to patients without depression (RR = 1.21, 95% CI: 1.14–1.29). Simultaneously, the heterogeneity was significant (I2 = 76.8%, P = 0.000). Additionally, we also performed a sensitivity analysis to assess the stability of our results regarding the relationship between depression and CHD. Omitting one study at a time by sequentially, we found no significant changes, indicating the results robust (RR = 1.15, 95% CI: 1.09–1.21) (shown in Supplementary Figure 1).
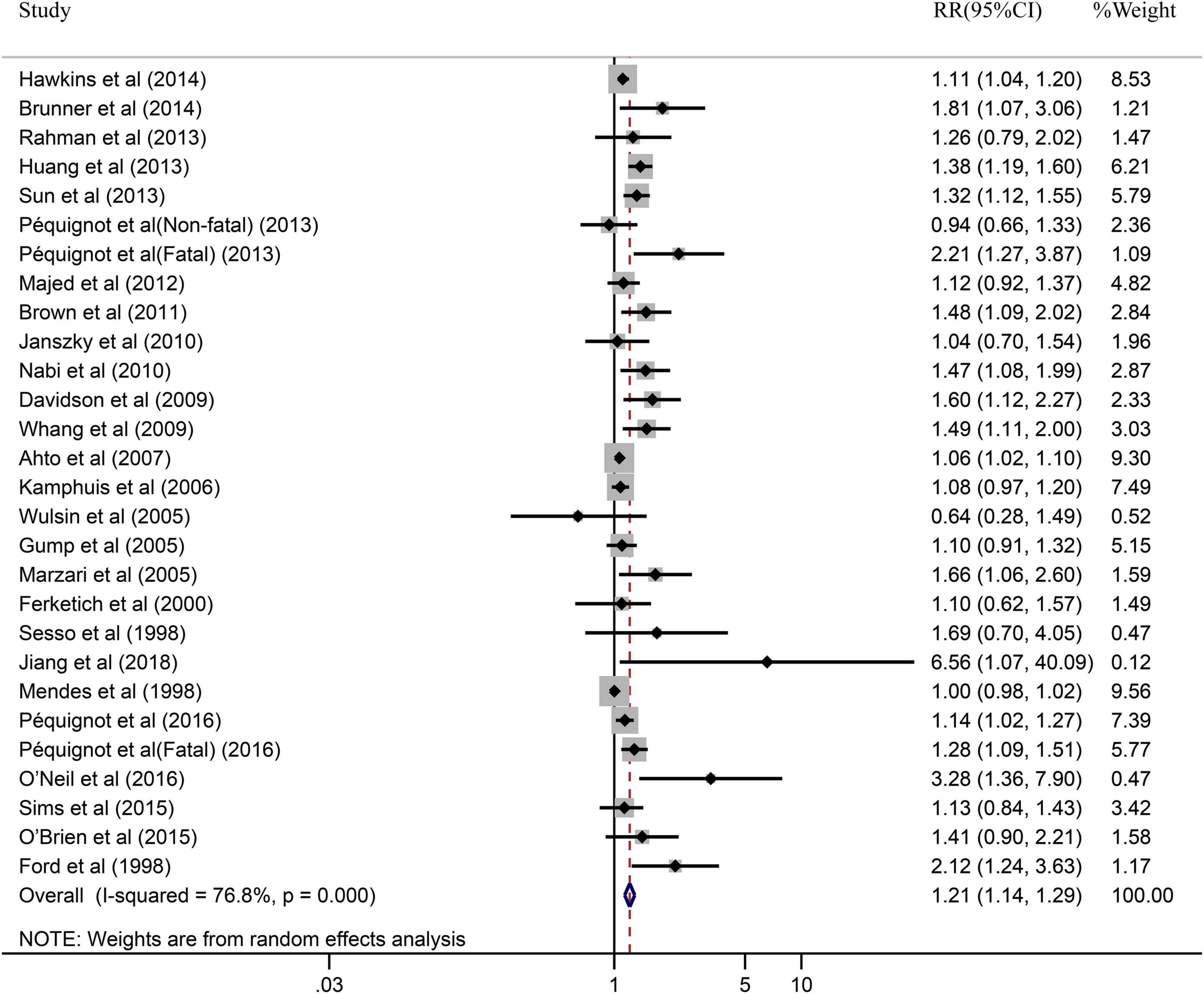
Figure 2. Forest plot presents the association between depression and the risk of CHD in prospective cohort study.
Due to the significant heterogeneity of the pooled results, subgroup analysis was further conducted to explore the source of the heterogeneity. Results of the subgroup analysis by age (≥65 or <65) indicated that the pooled RR were 1.10 (95% CI: 1.05–1.15) in ≥65 studies and 1.37 (95% CI: 1.20–1.55) in <65 studies. However, the intragroup heterogeneity did not decrease significantly (Figure 3). In addition, based on either sex (men, women, or combined) or publication (≥2005 or <2005), no statistically significant association was observed (present respectively in Figures 4, 5). Furthermore, a stratified subgroup analysis based on evaluation index was found that the pooled results were 1.18 (95% CI: 1.12–1.25) in HR group, 3.74 (95% CI: 1.70–8.26) in OR group, and 1.08 (95% CI: 0.99–1.18) in RR group. Besides, the intragroup heterogeneity decreased to a low level of 0% in OR group (P = 0.05) (Figure 6). Similarly, the statistically significant association was observed in ≤5 follow-up group (Figure 7).
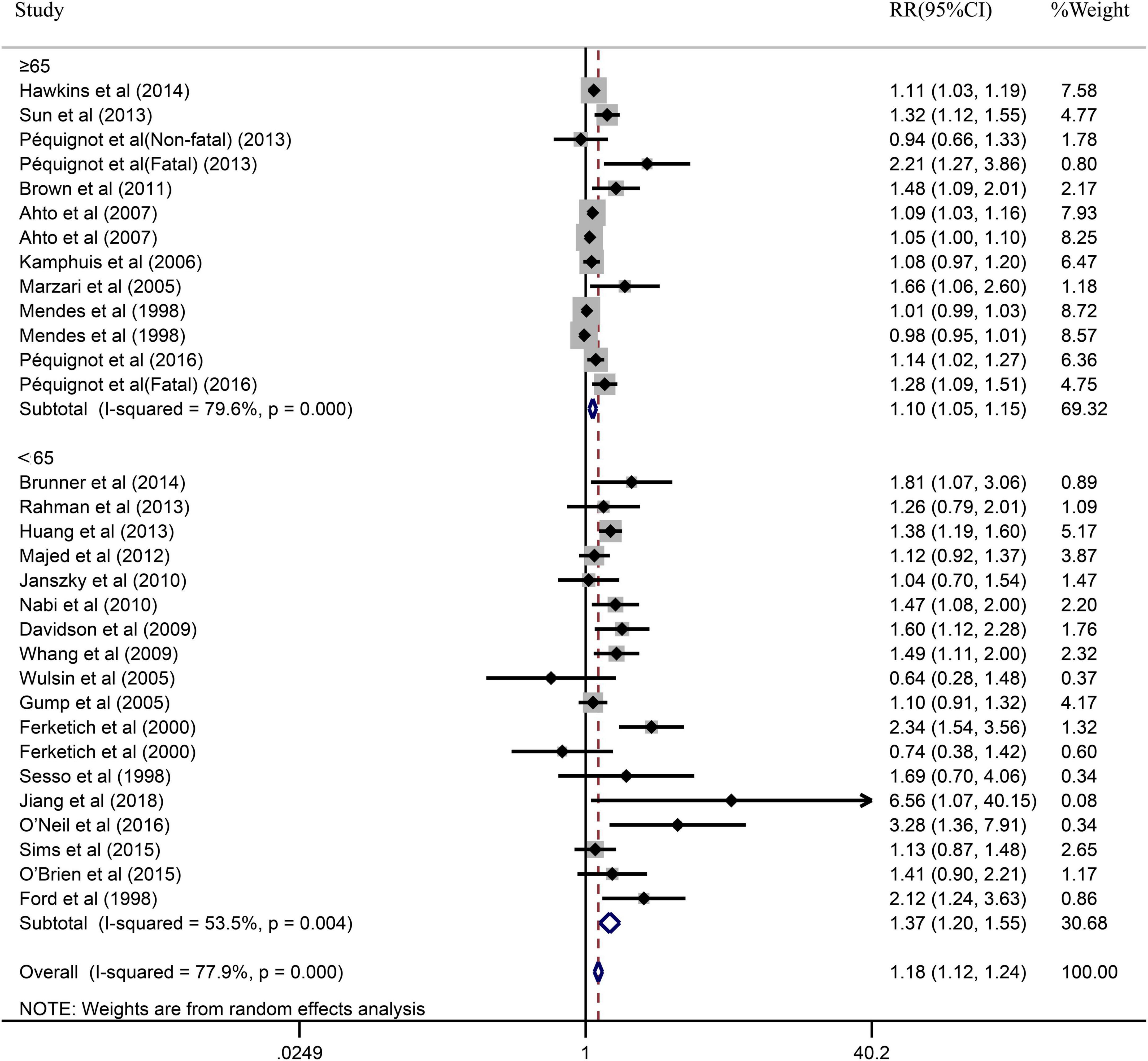
Figure 3. Subgroup analysis with age (≥65 or <65) presents the association between depression and the risk of coronary heart disease in a prospective cohort study.
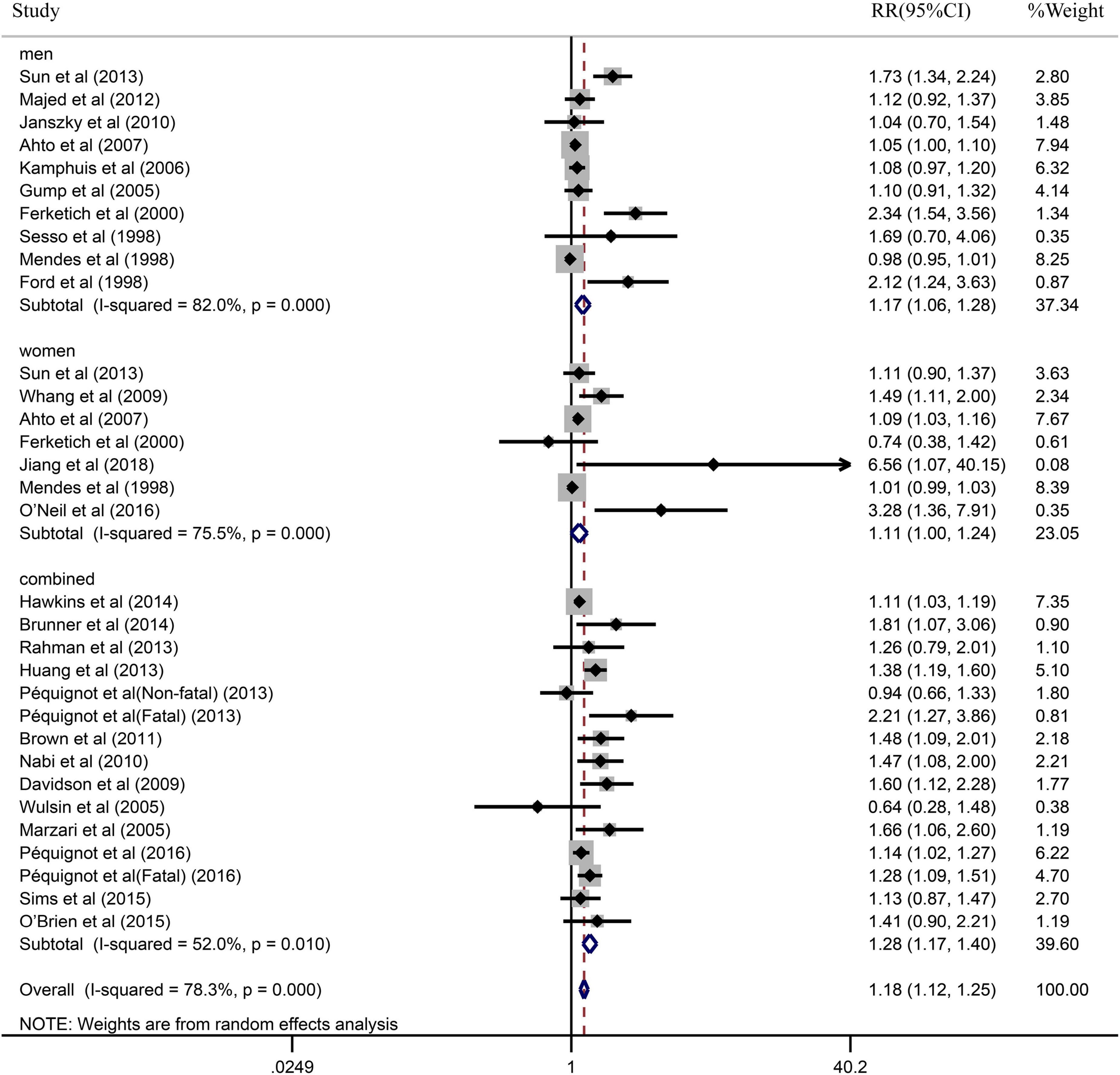
Figure 4. Subgroup analysis with sex (men, women, or combined) presents the association between depression and the risk of coronary heart disease in a prospective cohort study.
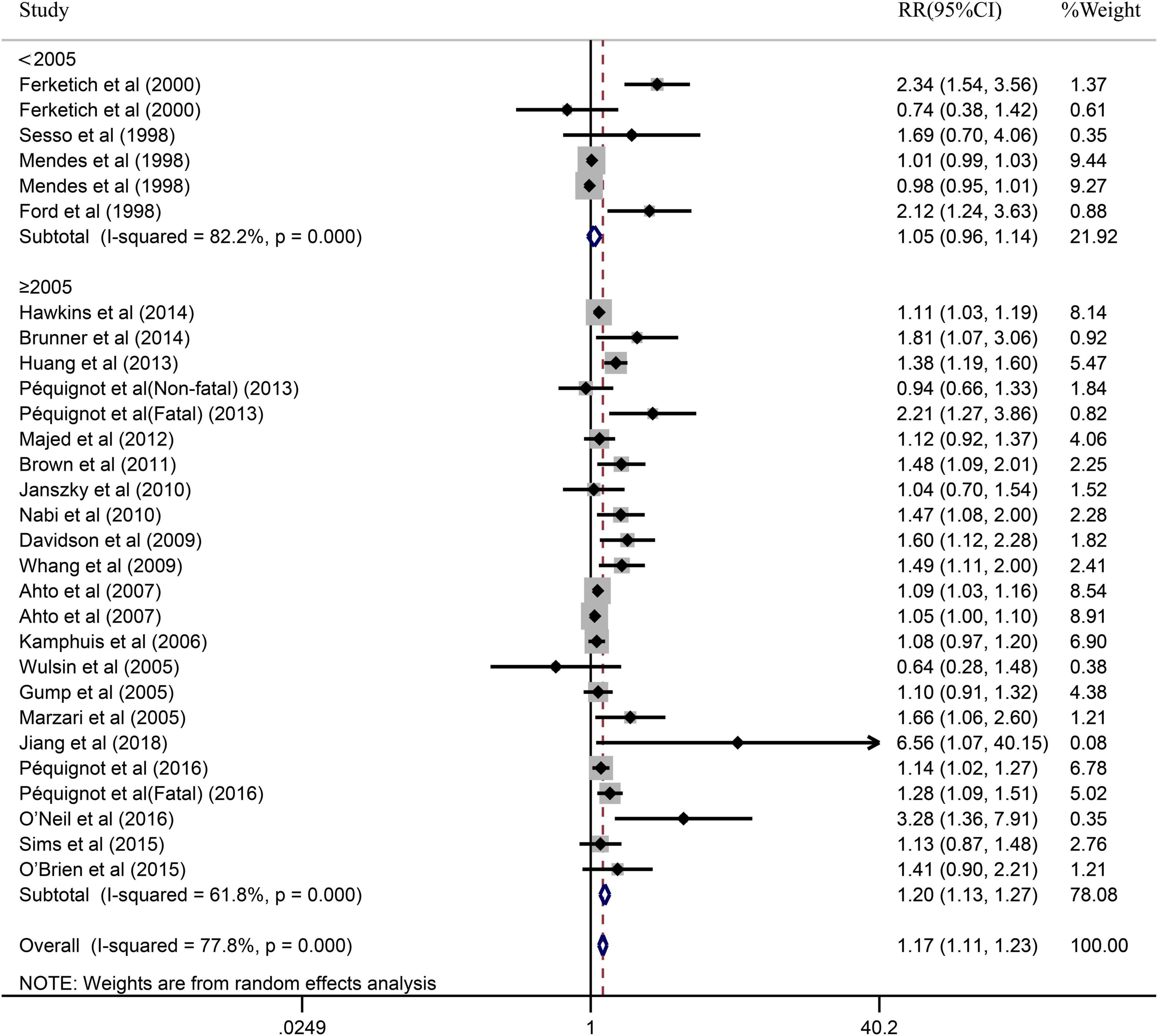
Figure 5. Subgroup analysis with publication year (<2005 or ≥2005) presents the association between depression and the risk of coronary heart disease in a prospective cohort study.
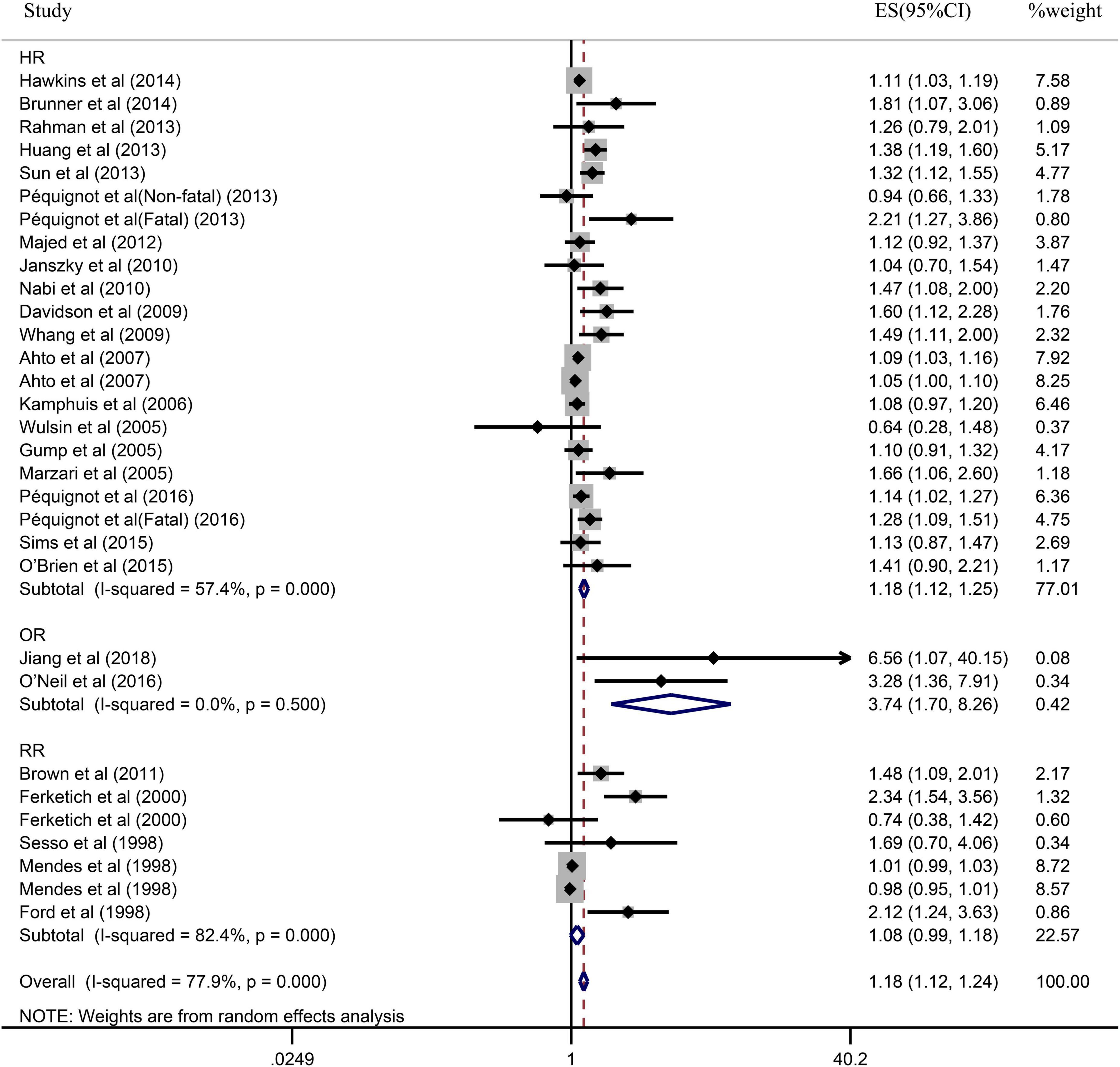
Figure 6. Subgroup analysis with evaluation index (HR, OR, or RR) presents the association between depression and the risk of coronary heart disease in a prospective cohort study.
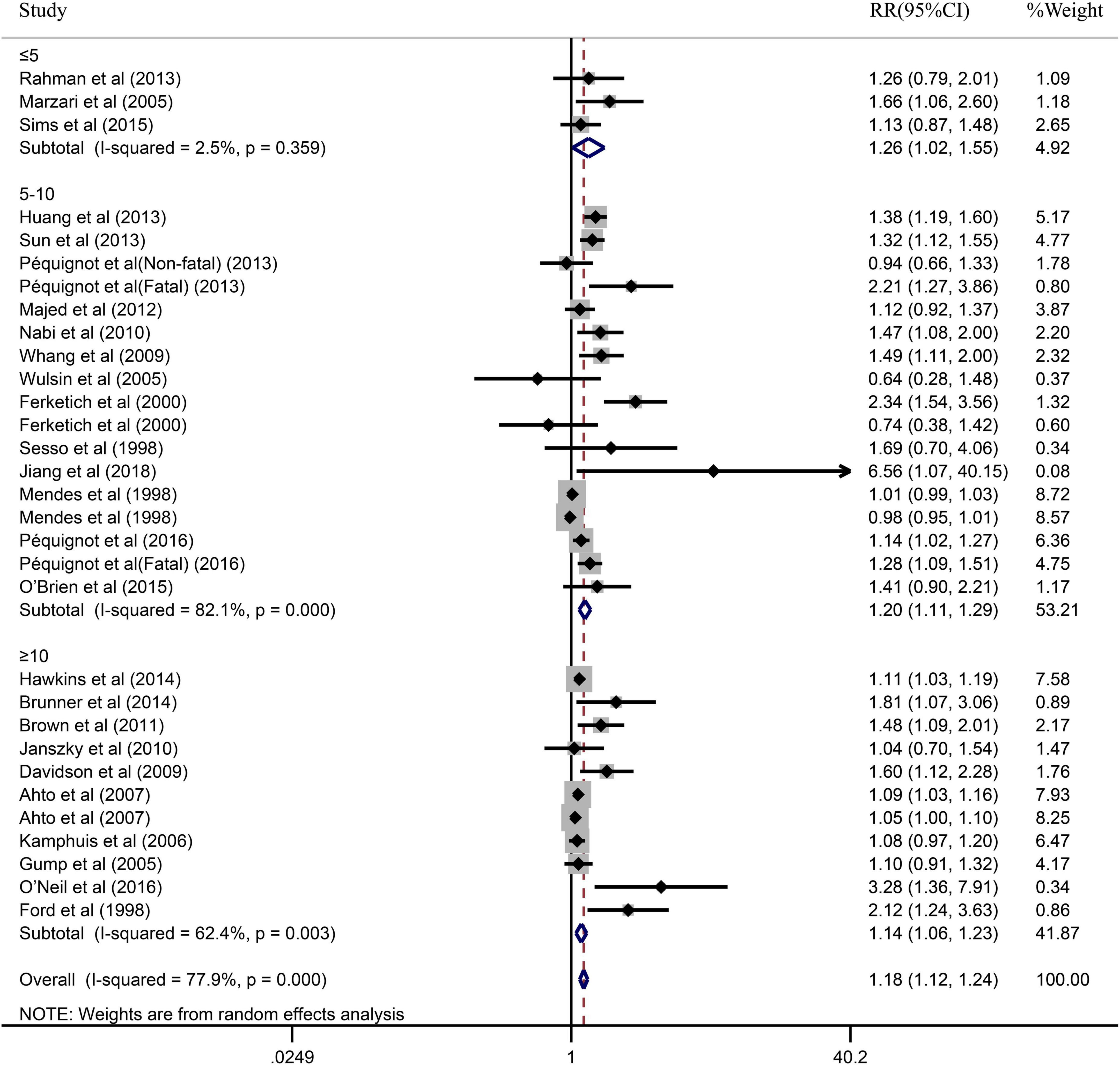
Figure 7. Subgroup analysis with the duration of follow-up (≤5, 5–10 or ≥10) presents the association between depression and the risk of coronary heart disease in a prospective cohort study.
The funnel plot appeared to be asymmetry, indicating there existing publication bias for the pooled results between depression and CHD, as indicated Figure 8. In the meanwhile, the Begg’s rank correlation and Egger linear regression test also documented significant (P < 0.05), as shown in Supplementary Figures 2, 3.
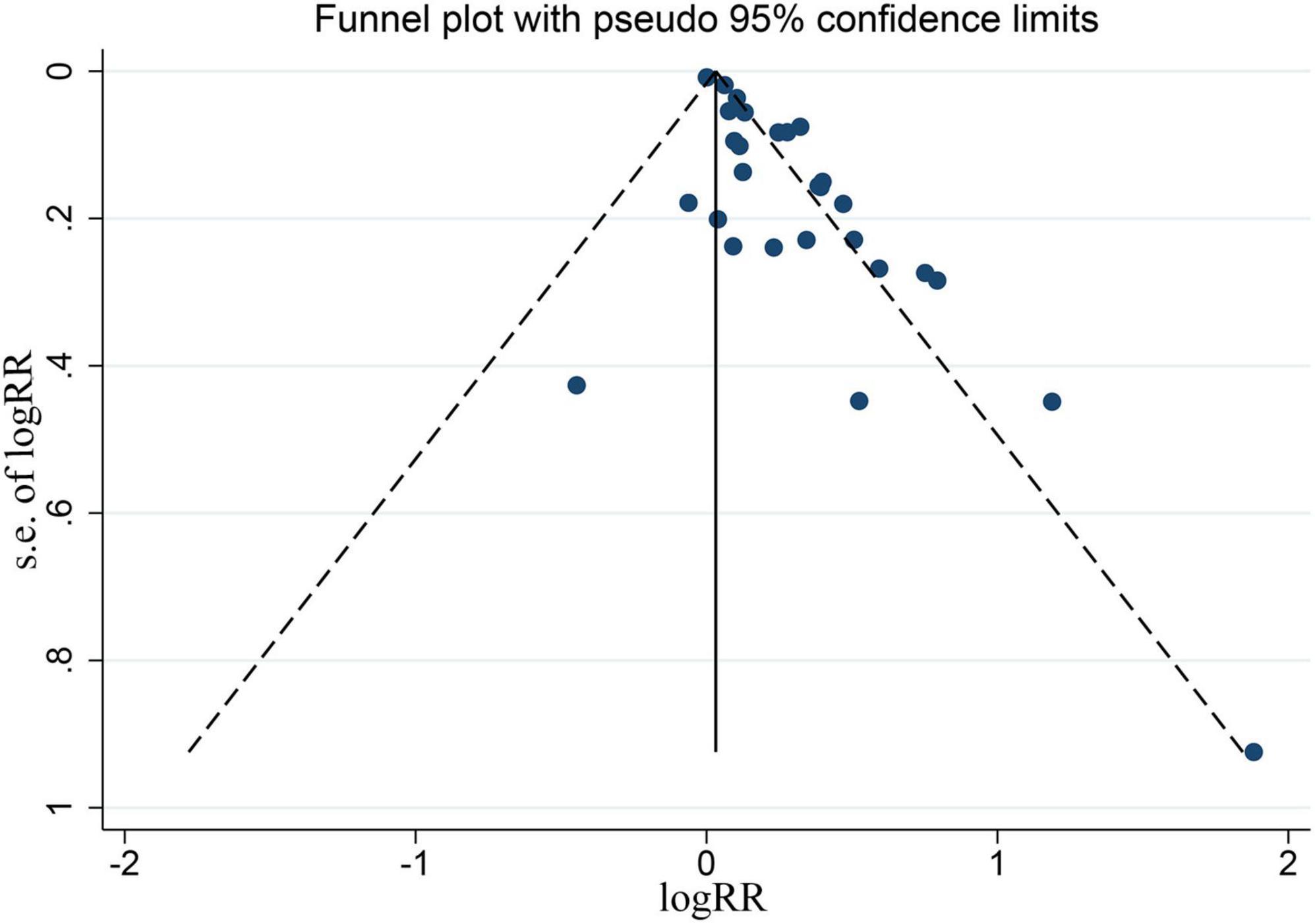
Figure 8. Funnel plot for all the eligible studies that provided ORs, RRs, or HRs for depression and the risk of CHD.
This present study updated the meta-analysis of the risk association between depression and CHD based on a broad range of studies involving 402,597 participants. Our results are limited to prospective cohort studies suggest that depression may be modifiable risk factor for CHD. Moreover, the increased risk related with depression persisted and remained statistically significant in sensitivity analysis when eliminating the studies one by one as well as in all subgroup analysis stratified by participant age, sex, and follow-up. In the meanwhile, we also found that the publication bias of this study was significant. Due to the worldwide prevalence of depression, our findings of the meta-analysis have significant meanings for global public health.
Increased risk between depression and CHD was found in the previous several meta-analyses, which is consistent with our study findings (17, 19, 23). However, these studies were published relatively early, which could not include more recent literatures, which is likely to decrease the credibility of the evidence. In addition, of which, one study (23) also included MI not just simple CHD. Therefore, on the basis of previous meta-analyses, our study also included literatures of recent years, indicating that depression was an important risk factor for CHD, which further makes the results more credible.
In our meta-analysis, there exist different degrees of heterogeneity for the relationship between depression and CHD. According to the subgroup analyses, some significant and valuable results were identified. A major finding was that depression could increase the risk of CHD in the group of evaluation index using RR, whereas was significant in the group of evaluation index using HR and OR. The RR did not take time into account when assessing depression increased risk of CHD, and as well known, depression could be treatable and is strongly correlated with the duration of follow-up. In the meantime, we also found that there was no statistically significant in the group of studies published 2005 year and later, but did not have statistically significant relationship for studies published 2005 year and before. One possible explanation to this finding was that might be due to the small number of included studies published before 2005.
The specific mechanisms connecting depression to increased CHD risk are complex and multifactorial, which were still incompletely clear until now (53). One possible explanation is chronic dysregulation of autonomic function, refers to an imbalance of the sympathetic and parasympathetic systems, which is considered to be a crucial mechanism (53). Previous animal study has shown that depression may result with cardiovascular and autonomic imbalance presenting elevating heart rate, reducing heart rate variability (54), and elevating cardiac sympathetic tone (55, 56). Furthermore, the majority studies of patients with CHD have found that compared with those without depression, patients with depression have lower HRV and higher heart rate, and decreased baroreceptor sensitivity as well as increased QT interval variability and heart rate turbulence (53, 57). Another hypothesized explanation for the increased risk of CHD related with depression is chronic inflammation that is a well-known risk factor for CHD occurrence and development (58). In addition to the mechanisms mentioned above, previous studies also have proposed the following underlying mechanisms, such as changed brain and neuronal function affecting neuroendocrine pathways, immune responses, life behavior, platelet activation and thrombosis, as well as cardiac metabolic risk factors (14, 57). The available evidence have indicated that depression consistently associated with increased risk of CHD (17, 23, 57) and vice versa (59, 60). Not only that, but related studies have reported that two conditions are highly comorbid (61, 62). Psychopathy regulation could obviously improve the quality of life of patients with CHD and decrease the incidence rate of acute cardiovascular events (63).
Certainly, there are also a few limitations should be acknowledged in this current meta-analysis, despite our results are promising. Firstly, in this meta-analysis, we only included studies mainly in English, which may cause publication bias. Therefore, we adopted the search strategy of the Mesh combined free keyword to make the retrieval as comprehensive as possible to eliminate publication bias as much as possible. Secondly, another possible limitation of the current meta-analysis is the use of various methods to assess depression. We try to ensure included eligible studies with the same evaluation method to reduce heterogeneity. Thirdly, considerable heterogeneity existed in our study, to which we applied a random-effects model when a significant heterogeneity is existing; therefore, the increased risk of depression for CHD may be underestimated. Finally, confounding factors of outcome evaluation indexes were inconsistent, which may produce biases. Despite risk factors have been adjusted using multivariate, the possibility of other inadequate adjustment for unmeasured or imprecisely measured confounding factors that increase the risk of CHD cannot be excluded.
In summary, in a meta-analysis of 402,597 participants in 26 prospective cohort studies, depression may increase risk of CHD. Future studies on the share pathogenic mechanisms of both depression and CHD may develop novel therapies. The clinical significance lies in raising vigilance against patients with depression and CHD, and early detection and timely medical treatment for CHD patients through a multiple approach.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
HC and LS: conceptualization, methodology, and software. HC and HZ: data curation and writing—original draft preparation. LS: visualization, investigation, supervision, validation, and writing—reviewing and editing. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Nos. 82074398 and 81873276) and the Fundamental Research Funds for the Central Public Welfare Research Institutes (YZ-202125).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.913888/full#supplementary-material
Supplementary Figure 1 | Sensitivity analysis for all the eligible studies that provided ORs, RRs, or HRs for depression and the risk of CHD.
Supplementary Figure 2 | Begg’s funnel plot for all the eligible studies that provided ORs, RRs, or HRs for depression and the risk of CHD.
Supplementary Figure 3 | Egger’s publication bias plot for all the eligible studies that provided ORs, RRs, or HRs for depression and the risk of CHD.
Supplementary Table 1 | Detailed full-search strategies in different databases.
Supplementary Table 2 | Detailed scores of NOS for all eligible studies.
1. Lotta LA, Wittemans LBL, Zuber V, Stewart ID, Sharp SJ, Luan J, et al. Association of genetic variants related to gluteofemoral vs abdominal fat distribution with type 2 diabetes, coronary disease, and cardiovascular risk factors. JAMA. (2018) 320:2553–63. doi: 10.1001/jama.2018.19329
2. World Health Organization [WHO].Cardiovascular diseases (CVDs). Geneva: World Health Organization (2019).
3. Ulbricht TL, Southgate DA. Coronary heart disease: seven dietary factors. Lancet. (1991) 338:985–92. doi: 10.1016/0140-6736(91)91846-m
4. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive summary: heart disease and stroke statistics–2016 update: a report from the American heart association. Circulation. (2016) 133:447–54. doi: 10.1161/cir.0000000000000366
5. Blais C, Rochette L, Ouellet S, Huynh T. Complex evolution of epidemiology of vascular diseases, including increased disease burden: from 2000 to 2015. Can J Cardiol. (2020) 36:740–6. doi: 10.1016/j.cjca.2019.10.021
6. Ma LY, Chen WW, Gao RL, Liu LS, Zhu ML, Wang YJ, et al. China cardiovascular diseases report 2018: an updated summary. J Geriatr Cardiol. (2020) 17:1–8. doi: 10.11909/j.issn.1671-5411.2020.01.001
7. Cheng YC, Sheen JM, Hu WL, Hung YC. Polyphenols and oxidative stress in atherosclerosis-related ischemic heart disease and stroke. Oxid Med Cell Longev. (2017) 2017:8526438. doi: 10.1155/2017/8526438
8. Li C, Ma R, Zhang X, Ma J, Wang X, He J, et al. Risk of coronary heart disease in the rural population in Xinjiang: a nested case-control study in China. PLoS One. (2020) 15:e0229598. doi: 10.1371/journal.pone.0229598
9. Roberts R, Campillo A, Schmitt M. Prediction and management of CAD risk based on genetic stratification. Trends Cardiovasc Med. (2020) 30:328–34. doi: 10.1016/j.tcm.2019.08.006
10. World Health Organization [WHO] Depression: Fact Sheet. Geneva: World Health Organisation (2021). Available online at: http://www.who.int/mediacentre/factsheets/fs369/en/ (accessed November 28, 2021).
11. Shim RS, Baltrus P, Ye J, Rust G. Prevalence, treatment, and control of depressive symptoms in the United States: results from the National health and nutrition examination survey (NHANES), 2005-2008. J Am Board Fam Med. (2011) 24:33–8. doi: 10.3122/jabfm.2011.01.100121
12. Peng R, Wang Y, Huang Y, Liu Z, Xu X, Ma Y, et al. The association of depressive symptoms with disability among adults in China. J Affect Disord. (2022) 296:189–97. doi: 10.1016/j.jad.2021.09.030
13. Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. (2014) 129:1350–69. doi: 10.1161/cir.0000000000000019
14. Vaccarino V, Badimon L, Bremner JD, Cenko E, Cubedo J, Dorobantu M, et al. Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. Eur Heart J. (2020) 41:1687–96. doi: 10.1093/eurheartj/ehy913
15. Su SF, Chang MY, He CP. Social support, unstable angina, and stroke as predictors of depression in patients with coronary heart disease. J Cardiovasc Nurs. (2018) 33:179–86. doi: 10.1097/jcn.0000000000000419
16. de Heer EW, Palacios JE, Adèr HJ, van Marwijk HWJ, Tylee A, van der Feltz-Cornelis CM. Chest pain, depression and anxiety in coronary heart disease: consequence or cause? A prospective clinical study in primary care. J Psychosom Res. (2020) 129:109891. doi: 10.1016/j.jpsychores.2019.109891
17. Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. (2006) 27:2763–74. doi: 10.1093/eurheartj/ehl338
18. Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. (2007) 22:613–26. doi: 10.1002/gps.1723
19. Gan Y, Gong Y, Tong X, Sun H, Cong Y, Dong X, et al. Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiatry. (2014) 14:371. doi: 10.1186/s12888-014-0371-z
20. O’Brien EC, Greiner MA, Sims M, Hardy NC, Wang W, Shahar E, et al. Depressive symptoms and risk of cardiovascular events in blacks: findings from the jackson heart study. Circ Cardiovasc Qual Outcomes. (2015) 8:552–9. doi: 10.1161/circoutcomes.115.001800
21. Péquignot R, Dufouil C, Prugger C, Pérès K, Artero S, Tzourio C, et al. High level of depressive symptoms at repeated study visits and risk of coronary heart disease and stroke over 10 years in older adults: the three-city study. J Am Geriatr Soc. (2016) 64:118–25. doi: 10.1111/jgs.13872
22. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London: The Cochrane Collaboration (2011).
23. Wu Q, Kling JM. Depression and the risk of myocardial infarction and coronary death: a meta-analysis of prospective cohort studies. Medicine. (2016) 95:e2815. doi: 10.1097/md.0000000000002815
24. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/m18-0850
25. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
26. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. (1987) 9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298
27. Xu W, Tan CC, Zou JJ, Cao XP, Tan L. Sleep problems and risk of all-cause cognitive decline or dementia: an updated systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. (2020) 91:236–44. doi: 10.1136/jnnp-2019-321896
28. Song F, Sheldon TA, Sutton AJ, Abrams KR, Jones DR. Methods for exploring heterogeneity in meta-analysis. Eval Health Prof. (2001) 24:126–51. doi: 10.1177/016327870102400203
29. Ford DE, Mead LA, Chang PP, Cooper-Patrick L, Wang NY, Klag MJ. Depression is a risk factor for coronary artery disease in men: the precursors study. Arch Intern Med. (1998) 158:1422–6. doi: 10.1001/archinte.158.13.1422
30. Mendes De Leon CF, Krumholz HM, Seeman TS, Vaccarino V, Williams CS, Kasl SV, et al. Depression and risk of coronary heart disease in elderly men and women: new haven EPESE, 1982-1991. Arch Intern Med. (1998) 158:2341–8. doi: 10.1001/archinte.158.21.2341
31. Sesso HD, Kawachi I, Vokonas PS, Sparrow D. Depression and the risk of coronary heart disease in the Normative Aging Study. Am J Cardiol. (1998) 82:851–6. doi: 10.1016/s0002-9149(98)00491-3
32. Ferketich AK, Schwartzbaum JA, Frid DJ, Moeschberger ML. Depression as an antecedent to heart disease among women and men in the NHANES I study. National Health and Nutrition Examination Survey. Arch Intern Med. (2000) 160:1261–8. doi: 10.1001/archinte.160.9.1261
33. Gump BB, Matthews KA, Eberly LE, Chang YF. Depressive symptoms and mortality in men: results from the Multiple Risk Factor Intervention Trial. Stroke. (2005) 36:98–102. doi: 10.1161/01.STR.0000149626.50127.d0
34. Marzari C, Maggi S, Manzato E, Destro C, Noale M, Bianchi D, et al. Depressive symptoms and development of coronary heart disease events: the Italian longitudinal study on aging. J Gerontol A Biol Sci Med Sci. (2005) 60:85–92. doi: 10.1093/gerona/60.1.85
35. Wulsin LR, Evans JC, Vasan RS, Murabito JM, Kelly-Hayes M, Benjamin EJ. Depressive symptoms, coronary heart disease, and overall mortality in the Framingham Heart Study. Psychosom Med. (2005) 67:697–702. doi: 10.1097/01.psy.0000181274.56785.28
36. Kamphuis MH, Kalmijn S, Tijhuis MA, Geerlings MI, Giampaoli S, Nissinen A, et al. Depressive symptoms as risk factor of cardiovascular mortality in older European men: the Finland, Italy and Netherlands Elderly (FINE) study. Eur J Cardiovasc Prev Rehabil. (2006) 13:199–206. doi: 10.1097/01.hjr.0000188242.64590.92
37. Ahto M, Isoaho R, Puolijoki H, Vahlberg T, Kivelä SL. Stronger symptoms of depression predict high coronary heart disease mortality in older men and women. Int J Geriatr Psychiatry. (2007) 22:757–63. doi: 10.1002/gps.1735
38. Davidson KW, Schwartz JE, Kirkland SA, Mostofsky E, Fink D, Guernsey D, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. (2009) 103:755–61. doi: 10.1016/j.amjcard.2008.11.035
39. Whang W, Kubzansky LD, Kawachi I, Rexrode KM, Kroenke CH, Glynn RJ, et al. Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses’. Health Study. J Am Coll Cardiol. (2009) 53:950–8. doi: 10.1016/j.jacc.2008.10.060
40. Janszky I, Ahnve S, Lundberg I, Hemmingsson T. Early-onset depression, anxiety, and risk of subsequent coronary heart disease: 37-year follow-up of 49,321 young Swedish men. J Am Coll Cardiol. (2010) 56:31–7. doi: 10.1016/j.jacc.2010.03.033
41. Nabi H, Kivimäki M, Suominen S, Koskenvuo M, Singh-Manoux A, Vahtera J. Does depression predict coronary heart disease and cerebrovascular disease equally well? The health and social support prospective cohort study. Int J Epidemiol. (2010) 39:1016–24. doi: 10.1093/ije/dyq050
42. Brown JM, Stewart JC, Stump TE, Callahan CM. Risk of coronary heart disease events over 15 years among older adults with depressive symptoms. Am J Geriatr Psychiatry. (2011) 19:721–9. doi: 10.1097/JGP.0b013e3181faee19
43. Majed B, Arveiler D, Bingham A, Ferrieres J, Ruidavets JB, Montaye M, et al. Depressive symptoms, a time-dependent risk factor for coronary heart disease and stroke in middle-aged men: the PRIME Study. Stroke. (2012) 43:1761–7. doi: 10.1161/strokeaha.111.645366
44. Huang CJ, Hsieh MH, Hou WH, Liu JC, Jeng C, Tsai PS. Depression, antidepressants, and the risk of coronary heart disease: a population-based cohort study. Int J Cardiol. (2013) 168:4711–6. doi: 10.1016/j.ijcard.2013.07.173
45. Péquignot R, Tzourio C, Péres K, Ancellin ML, Perier MC, Ducimetière P, et al. Depressive symptoms, antidepressants and disability and future coronary heart disease and stroke events in older adults: the three city study. Eur J Epidemiol. (2013) 28:249–56. doi: 10.1007/s10654-013-9765-3
46. Rahman I, Humphreys K, Bennet AM, Ingelsson E, Pedersen NL, Magnusson PK. Clinical depression, antidepressant use and risk of future cardiovascular disease. Eur J Epidemiol. (2013) 28:589–95. doi: 10.1007/s10654-013-9821-z
47. Sun WJ, Xu L, Chan WM, Lam TH, Schooling CM. Are depressive symptoms associated with cardiovascular mortality among older Chinese: a cohort study of 64,000 people in Hong Kong? Am J Geriatr Psychiatry. (2013) 21:1107–15. doi: 10.1016/j.jagp.2013.01.048
48. Brunner EJ, Shipley MJ, Britton AR, Stansfeld SA, Heuschmann PU, Rudd AG, et al. Depressive disorder, coronary heart disease, and stroke: dose-response and reverse causation effects in the Whitehall II cohort study. Eur J Prev Cardiol. (2014) 21:340–6. doi: 10.1177/2047487314520785
49. Hawkins MAW, Callahan CM, Stump TE, Stewart JC. Depressive symptom clusters as predictors of incident coronary artery disease: a 15-year prospective study. Psychosom Med. (2014) 76:38–43. doi: 10.1097/PSY.0000000000000023
50. Sims M, Redmond N, Khodneva Y, Durant RW, Halanych J, Safford MM. Depressive symptoms are associated with incident coronary heart disease or revascularization among blacks but not among whites in the reasons for geographical and racial differences in stroke study. Ann Epidemiol. (2015) 25:426–32. doi: 10.1016/j.annepidem.2015.03.014
51. O’Neil A, Fisher AJ, Kibbey KJ, Jacka FN, Kotowicz MA, Williams LJ, et al. Depression is a risk factor for incident coronary heart disease in women: an 18-year longitudinal study. J Affect Disord. (2016) 196:117–24. doi: 10.1016/j.jad.2016.02.029
52. Jiang X, Asmaro R, O’Sullivan DM, Modi J, Budnik E, Schnatz PF. Depression may be a risk factor for coronary heart disease in midlife women <65 years: a 9-year prospective cohort study. Int J Cardiol. (2018) 271:8–12. doi: 10.1016/j.ijcard.2018.05.085
53. Penninx BW. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. (2017) 74:277–86. doi: 10.1016/j.neubiorev.2016.07.003
54. Jangpangi D, Mondal S, Bandhu R, Kataria D, Gandhi A. Alteration of heart rate variability in patients of depression. J Clin Diagn Res. (2016) 10:CM04–06. doi: 10.7860/jcdr/2016/22882.9063
55. Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. (2003) 78:703–10. doi: 10.1016/s0031-9384(03)00050-7
56. Shi S, Liang J, Liu T, Yuan X, Ruan B, Sun L, et al. Depression increases sympathetic activity and exacerbates myocardial remodeling after myocardial infarction: evidence from an animal experiment. PLoS One. (2014) 9:e101734. doi: 10.1371/journal.pone.0101734
57. Carney RM, Freedland KE. Depression and coronary heart disease. Nat Rev Cardiol. (2017) 14:145–55. doi: 10.1038/nrcardio.2016.181
58. Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J. (2015) 36:482c–9c. doi: 10.1093/eurheartj/ehu403
59. Anda R, Williamson D, Jones D, Macera C, Eaker E, Glassman A, et al. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of U.S. adults. Epidemiology. (1993) 4:285–94. doi: 10.1097/00001648-199307000-00003
60. Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. (1996) 93:1976–80. doi: 10.1161/01.cir.93.11.1976
61. Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. (2014) 35:1365–72. doi: 10.1093/eurheartj/eht462
62. Khandaker GM, Zuber V, Rees JMB, Carvalho L, Mason AM, Foley CN, et al. Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatry. (2020) 25:1477–86. doi: 10.1038/s41380-019-0395-3
Keywords: depression, coronary heart disease, relative risk, meta-analysis, prospective cohort studies
Citation: Cao H, Zhao H and Shen L (2022) Depression increased risk of coronary heart disease: A meta-analysis of prospective cohort studies. Front. Cardiovasc. Med. 9:913888. doi: 10.3389/fcvm.2022.913888
Received: 06 April 2022; Accepted: 08 August 2022;
Published: 30 August 2022.
Edited by:
Carmine Pizzi, University of Bologna, ItalyReviewed by:
Luca Bergamaschi, University of Bologna, ItalyCopyright © 2022 Cao, Zhao and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Shen, c2hlbmxpMTExNkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.