- 1Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
- 2Libin Cardiovascular Institute, University of Calgary, Calgary, AB, Canada
- 3Faculty of Kinesiology and Recreation Management, University of Manitoba, Winnipeg, MB, Canada
- 4Institute of Cardiovascular Sciences, St. Boniface Hospital Albrechtsen Research Centre, Winnipeg, MB, Canada
- 5Alberta Kidney Disease Network, Calgary, AB, Canada
- 6Alberta Children's Hospital Research Institute, University of Calgary, Calgary, AB, Canada
- 7O'Brien Institute for Public Health, University of Calgary, Calgary, AB, Canada
Background: Postmenopausal hormone therapy (HT) is associated with increased cardiovascular risk. Although the route of estrogen administration may play a role in mediating risk, previous studies have not controlled for concomitant progestin use.
Objective: To investigate the association between the route of estrogen therapy (oral or non-oral) HT use, without concomitant progestin, and blood pressure and arterial stiffness in postmenopausal women.
Methods: Systolic blood pressure [SBP], diastolic blood pressure [DBP]), arterial stiffness (aortic pulse wave velocity [aPWV] and augmentation index at 75 beats per minute [AIx]) were measured using a validated automated brachial cuff-based oscillometric approach (Mobil-O-Graph) in a community-dwelling sample of 328 women.
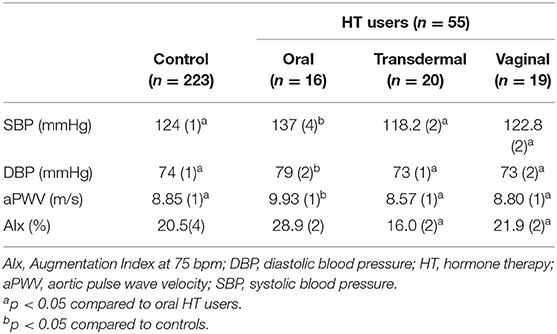
Results: Fifty-five participants (16.8%) were ever users (current and past use) of estrogen-only HT (oral [n = 16], transdermal [n = 20], vaginal [n = 19]), and 223 were never HT users (control). Ever use of oral estrogen was associated with increased SBP and DBP (Oral: SBP: 137 ± 4 mmHg, DBP: 79 ± 2 mmHg) compared to use of non-oral estrogen (transdermal: SBP: 118 ± 2 mmHg, DBP: 73 ± 1 mmHg; p < 0.01 & p = 0.012, respectively; vaginal: SBP: 123 ± 2 mmHg DBP: 73 ± 2 mmHg; p = 0.02 & p = 0.01, respectively.) and controls (SBP: 124 ± 1 mmHg, DBP: 74 ± 1 mmHg, p = 0.03, p = 0.02, respectively) after adjustment for covariates. aPWV was higher in oral estrogen ever users (9.9 ± 1 m/s) compared to non-oral estrogen (transdermal: 8.6 ± 0.3 m/s, p < 0.01; vaginal: 8.8 ± 0.7 m/s, p = 0.03) and controls (8.9 ± 0.5 m/s, p = 0.03) but these associations were no longer significant after adjustment for covariates. AIx was higher in oral estrogen (29 ± 2 %) compared to non-oral estrogen (transdermal: 16 ± 2 %; vaginal: 22 ± 1.7 %) but this association was no longer significant after adjustment for covariates (p = 0.92 vs. non-oral; p = 0.74 vs. control).
Conclusion: Ever use of oral estrogen was associated with increased SBP and DBP compared to non-oral estrogen use and no use. Given the cardiovascular risk associated with both menopause and increased blood pressure, further studies are required exploring the potential benefits of non-oral estrogen in postmenopausal women.
Introduction
The population of postmenopausal women will reach over 1 billion globally by the year 2025 (1). The hallmark of the menopausal transition is the presence of vasomotor symptoms such as hot flashes and/or night sweats, which are experienced by over 75% of women and may persist for over 7 years (2). Hormone therapy (HT) remains the most effective treatment for menopausal symptoms, but the decision to use HT is complex and requires individualized balancing of the risks and benefits. Previous studies have indicated an increase in adverse cardiovascular events with HT use (3). Data suggests that the route of estrogen administration may play a role in mediating the effects of exogenous estrogen on cardiovascular risk (4–11), with non-oral routes being associated with lower measures of blood pressure and other cardiovascular risk factors (5, 7, 12, 13).
Previous studies have examined the impact of different routes of estrogen administration on vascular measures and have shown conflicting results (6, 8, 14–19), which is most likely due to differences in study populations, age and cause of menopause, timing of postmenopausal hormone initiation, and notably, the presence of progestin. The Women's Health Initiative (WHI), a trial in which postmenopausal women with a uterus were randomized to both oral conjugated equine estrogen (CEE) and medroxyprogesterone acetate (MPA) or placebo was stopped early due to negative cardiovascular outcomes in the intervention arm (3). However, the WHI trial in which postmenopausal women without a uterus were randomized to only oral CEE without concomitant progestin compared to placebo was conducted to completion and showed no difference in cardiovascular outcomes (20), suggesting that MPA, and progestins in general, play a role independent of estrogen on cardiovascular health (21). The objective of this study was to investigate the association between the route of administration of estrogen exposure, in the absence of concomitant progestin use, on blood pressure and arterial stiffness in postmenopausal women.
Methods
Study Design
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for the cross-sectional studies were followed to develop this manuscript (22). This study was a cross-sectional, secondary analysis of the Women's Advanced Risk-assessment in Manitoba (WARM) Hearts Study (Clinical Trial: NCT03938155) (23). The initial study was a prospective cohort study, designed to investigate novel cardiovascular prognostic tools to identify women at elevated risk for experiencing an adverse cardiovascular event over a 5-year period (23). The WARM Hearts Study was approved by the University of Manitoba Health Research Ethics Board and St. Boniface Hospital Research Review Committee (HS22576, H2019:063). The secondary analysis was approved by the Conjoint Health Research Ethics Board of the University of Calgary (REB20-1456). Written informed consent was obtained from all study participants in accordance with the Declaration of Helsinki.
Participants
The initial observational cohort study was conducted at the St. Boniface Hospital Asper Clinical Research Institute and University of Manitoba Active Living Centre in Winnipeg, Manitoba, Canada. Participants were recruited using a convenience sampling approach through word of mouth, newspaper articles, an online webpage, media interviews, poster advertisements or presentations at community events. Females aged 55 years and older with a Manitoba Personal Health Information Number (PHIN) were included in the study. Participants were excluded if they had previously been hospitalized for the following cardiovascular conditions: ischemic heart disease, acute myocardial infarction, stroke/transient ischemic attack, percutaneous coronary intervention, coronary artery bypass surgery, congestive heart failure, peripheral artery disease, congenital heart disease and arrhythmias. Participants were also excluded if they had received medical advice against participating in physical activity.
All participants underwent a medical history, physical examination, and laboratory screening. All demographic information (e.g., age, sex assigned at birth, current gender identity, race/ethnicity) was determined by self-report. Data on menopausal status (e.g., age at menopause, vasomotor symptoms), previous contraceptive use and reproductive history were obtained using a standardized questionnaire.
Hormone Therapy Exposure
Participants were categorized based on self-reported current or past HT use (ever use) or controls (no HT use). HT use was categorized based on formulation: (1) estrogen-only; (2) progestin only; (3) combined estrogen and progestin. For the purposes of this study, data on estrogen-only HT use was analyzed to investigate the effect of estrogen, independent of concomitant progestin use, on surrogate cardiovascular markers. Estrogen HT was stratified by route of administration, including oral, transdermal (transdermal patch and gel) and vaginal use.
Outcomes
The primary outcomes of this exploratory study were the difference in SBP and DBP between (1) oral and non-oral ever users; (2) oral ever users and controls; (3) non-oral ever users and controls; and (4) transdermal and vaginal ever users. The secondary outcomes were the differences in aortic pulse wave velocity (aPWV) and augmentation index (AIx) standardized to 75 beats per minute between (1) oral and non-oral ever users; (2) oral ever users and controls; (3) non-oral ever users and controls; and (4) transdermal and vaginal ever users. aPWV and AIx are measures of arterial stiffness and are validated measures of cardiovascular risk (24). Seated blood pressure measurements were taken at the start of the study visit by placing a standard BP cuff on the left upper arm as per guidelines (25) using the validated Mobil-O-Graph (IEM, Stolberg, Germany). The Mobil-O-Graph is an ambulatory automated BP monitor validated for the non-invasive assessment of brachial blood pressure (26, 27) and estimates of aPWV (28, 29), and AIx simultaneously indirectly using a mathematical transformation of the brachial cuff waveform (30). The device has been validated in healthy individuals and patients with essential hypertension (30, 31), and estimates of aPWV has an acceptable accuracy similar to intra-aortic readings (28) and cardiac magnetic resonance imaging (29). Measurements were taken in triplicate and the second and third measurements were averaged.
Study Protocol
Participants were studied in the fasting state. Blood was drawn and immediately centrifuged at 2,000 × g for 10 min after collection. Plasma was then aliquoted into 500 μL samples and stored at −80°C. The plasma total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, and fasting blood glucose levels of the participants were measured enzymatically.
A pre-exercise BP and heart rate clearance was conducted for participant safety (32). Participants whose measurements exceeded a SBP of 160 mmHg, a diastolic BP of 90 mm Hg or a heart rate of 100 beats per minute were given 5 min to rest before the measurement was retaken. If any of the participant's measurements remained above these values for two consecutive measures, the study day was terminated, and participants were advised to visit their primary healthcare provider for assessment.
Statistical Analysis
General characteristics of the study population by route of estrogen HT use were presented as the mean ± SE or median and interquartile range (IQR) as appropriate for continuous variables and proportions for dichotomous variables. Separate analyses of current estrogen-only use participants and former estrogen-only use were conducted. Differences in baseline characteristics across categories for HT were compared using a one-way ANOVA. Bonferroni post-hoc test was performed to adjust for multiple comparisons and identify significant differences between groups. Associations were evaluated using linear regression analysis and presented with the beta coefficient and 95% confidence intervals (CI). A forward stepwise multivariate regression analysis was used to assess the contribution of covariates. Models were adjusted based on factors known to be associated with outcome and exposure. The primary outcome (SBP and DBP) was adjusted for age and body mass index. aPWV was adjusted for age, mean arterial pressure, age at menopausal onset, history of vasomotor symptoms, and history of hypertension. As the augmentation index is dependent on age (33), sex (33), and heart rate (34), no additional adjustments were made for any of the above-mentioned variables for the following reasons: (1) the calculation for AIx already adjusts for age; (2) the study population was entirely female; and (3) AIx is standardized to 75 beats per minute. The AIx was adjusted for age at menopausal onset (35, 36), history of vasomotor symptoms (37), and history of hypertension (38). Due to significant collinearity among hypertension, diabetes, chronic kidney disease, and myocardial infarction identified by increased variance inflation factors, only hypertension was included as a covariate in the model. Separate analyses were conducted with either controls or oral estrogen ever use as the referent group. Assumptions for both linear and logistic regression models were tested. Sensitivity analyses were conducted by stratifying participants by former or current estrogen-only HT use status. All analyses were conducted using STATA (version 9.13; College Station, TX, USA) with a two-tailed significance level of <0.05.
Results
Study Cohort
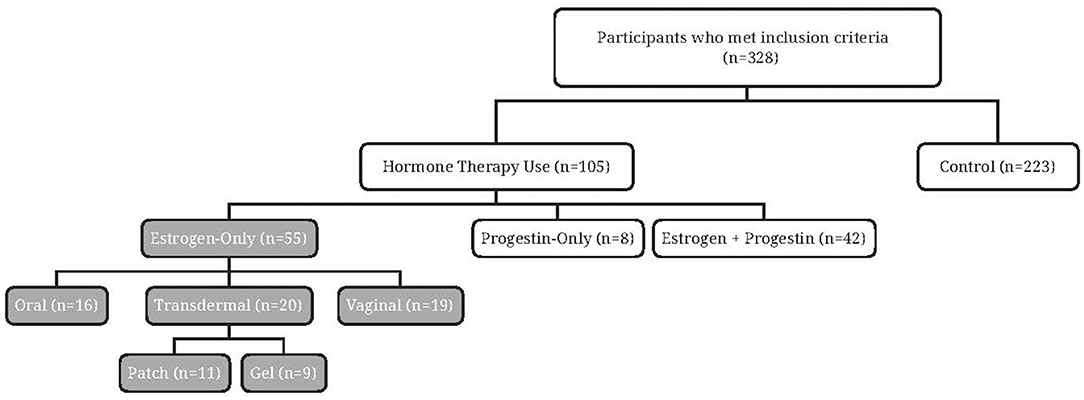
Of the 328 participants recruited (Figure 1), the majority (n = 223) reported never using HT. Approximately one-third (n = 105) reported current or past use of HT and just over half reported estrogen-only HT (n = 55). Among the oral estrogen-only HT users, few participants were currently using oral estrogen (n = 2), with most reporting past use (n = 14). Similar numbers of current (n = 20) and past estrogen use (n = 19) were reported by non-oral (transdermal and vaginal) estrogen-only HT users.
Baseline Characteristics
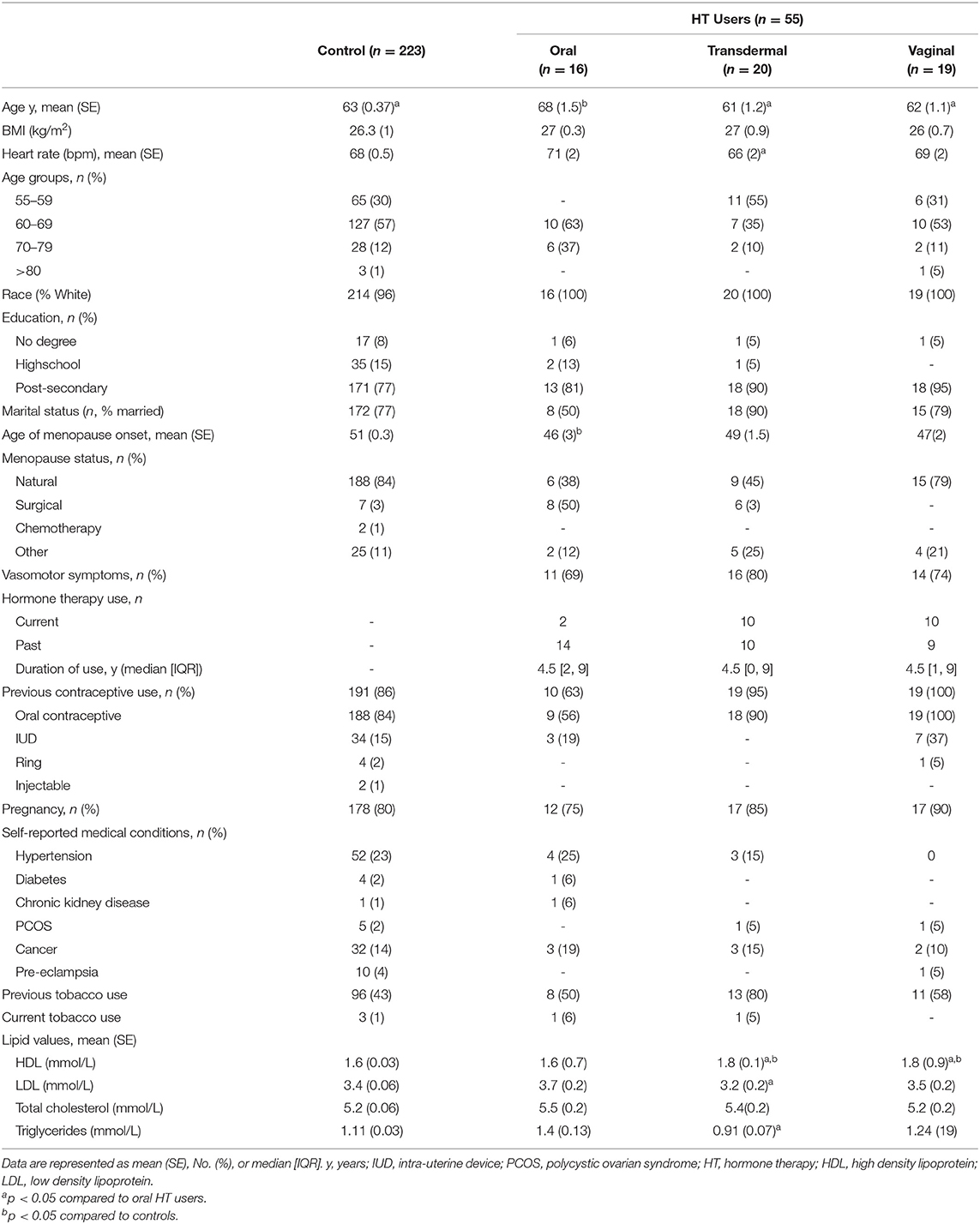
All participants reported being assigned female sex at birth and self-identified as women. The majority of participants self-reported as white (96%) and were between 60 and 69 years of age (Table 1). Participants who reported use of oral estrogen were older than non-oral estrogen users and controls (p < 0.001 and p = 0.01, respectively). Duration of estrogen use was similar across the groups (median, [IQR]. Oral: 4.5 [2, 9] years; transdermal: 4.5 [0, 9] years; vaginal: 4.5 [1, 9] years). Oral users reported reaching menopause at a younger age compared to controls (p < 0.001); however, there was no difference in age of menopausal onset between oral and non-oral users (p = 0.8). Most oral estrogen HT users reported surgical menopause, while all non-oral estrogen HT users and control achieved menopause naturally. The majority of non-oral users of estrogen had a previous history of tobacco use.
Blood Pressure
All study participants demonstrated BP readings within the normotensive range and there were no differences in BP measures between estrogen users and controls (p = 0.8). However, SBP and DBP were increased in oral estrogen users compared to non-oral estrogen users (transdermal: SBP p <0.01; DBP, p = 0.012; vaginal: SBP p = 0.02; DBP, p = 0.01) and controls (SBP p = 0.03; DBP, p = 0.02; Table 2 and Figure 2). These associations remained significant after adjustment for age and BMI (Tables 3, 4). No differences in SBP or DBP were observed between transdermal and vaginal estrogen users (p = 0.79), or transdermal and vaginal estrogen users and controls (p = 0.83). Separate sensitivity analyses of current estrogen-only use participants and former estrogen-only use participants did not show any differences in SBP or DBP across different routes of estrogen (Tables 3, 4).
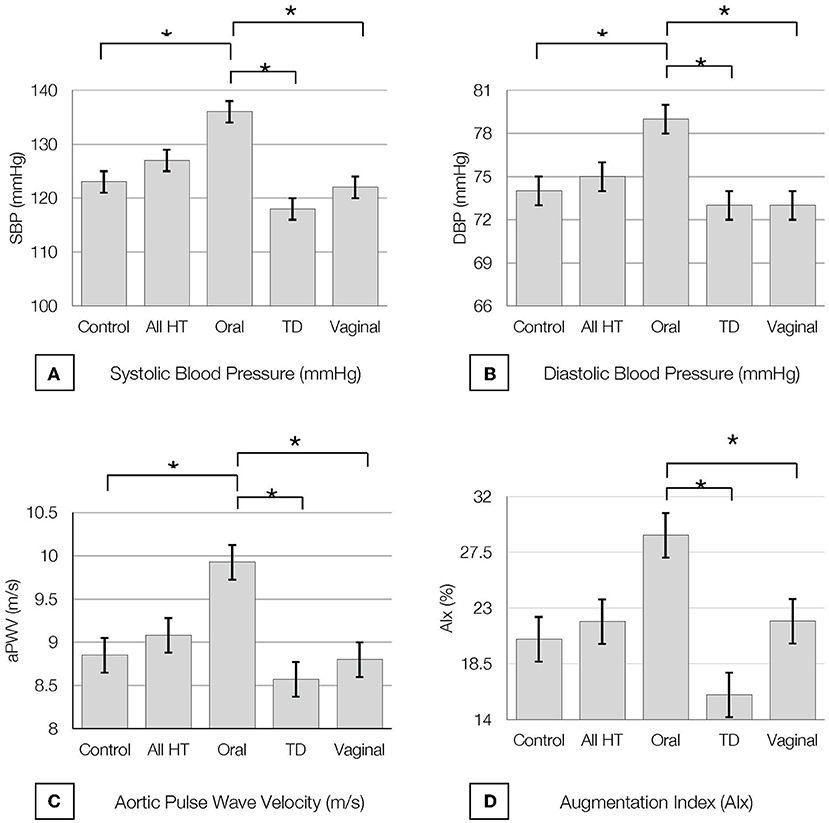
Figure 2. Baseline (A) Systolic Blood Pressure (SBP), (B) Diastolic Blood Pressure (DBP), (C) Pulse Wave Velocity (PWV) and (D) Augmentation Index at 75 bpm (AI@75) by hormone therapy type. All values are presented as mean ± SE.* indicates P < 0.05. All HT, all hormone therapy users; TD, transdermal.
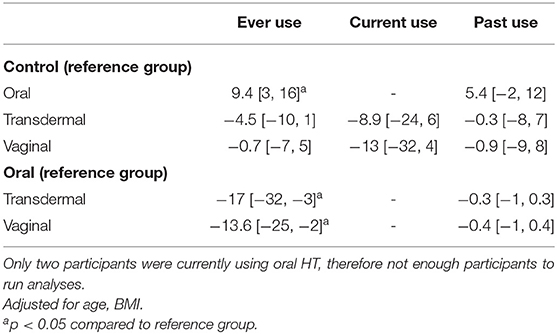
Table 3. Multi-variate analysis of SBP by route of administration of hormone therapy type presented as beta coefficients, [95% CI].
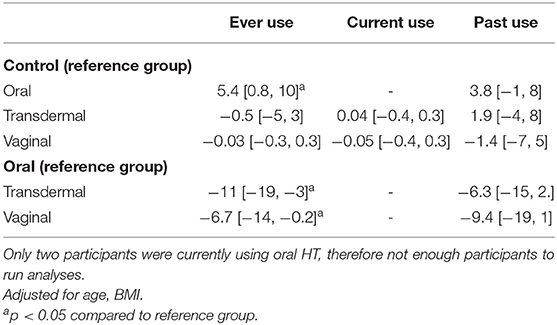
Table 4. Multi-variate analysis of DBP by route of administration of hormone therapy type presented as beta coefficients, [95% CI].
Pulse Wave Velocity
All study participants had aPWV measurements in the normal range (24, 39) and there were no differences in aPWV measures between estrogen users and controls (p = 0.7). Oral estrogen users demonstrated increased aPWV compared to both transdermal (p < 0.01) and vaginal (p = 0.03) estrogen users as well as controls (p = 0.03) (Table 2 and Figure 2), but after adjustment for covariates, route of estrogen delivery was not associated with aPWV (Supplementary Table 1). No differences were observed within non-oral estrogen user groups or between non-oral (transdermal and vaginal) estrogen users and controls. Separate sensitivity analyses of current estrogen-only use participants and former estrogen-only use participants did not show any differences in aPWV across different routes of estrogen delivery (Supplementary Table 1).
Augmentation Index
All study participants had AIx measurements in the normal range (39) and there were no differences in AIx measures between estrogen users and controls (p = 0.6). Oral estrogen users demonstrated increased AIx compared to non-oral estrogen users (transdermal: p < 0.01; vaginal: p = 0.04) (Table 2 and Figure 2), but after adjustment for covariates, route of estrogen delivery was not associated with AIx (Supplementary Table 2). No differences were observed between transdermal and vaginal users, or non-oral estrogen users and controls. Separate sensitivity analyses of current estrogen-only use participants and former estrogen-only use participants did not show any differences in AIx across different routes of estrogen delivery (Supplementary Table 2).
Discussion
To the best of our knowledge, this is the largest observational study examining the association between the route of administration of estrogen HT in the absence of a concomitant progestin on measures of BP and arterial stiffness in postmenopausal women. The key findings of this study are as follows: (1) ever use of oral estrogen was associated with increased SBP and DBP compared to ever use of non-oral estrogen (transdermal and vaginal) and never use (control), even after adjustment for covariates; (2) ever use of non-oral estrogen was associated with similar SBP and DBP compared to never use (control); (3) ever use of transdermal and vaginal estrogen was associated with similar measures of SBP and DBP; (4) arterial stiffness measures (aPWV and AIx) were similar between oral, non-oral and never use (control). The data suggests that oral estrogen use alone, without concomitant progestin, is associated with increased SBP and DBP compared to non-oral estrogen use and controls. Putting the difference in SBP into clinical context, compared with either non-oral estrogen or never use of estrogen, the 30 year risk of cardiovascular disease associated with this increase in blood pressure amongst oral estrogen users is almost 30% higher (40) - roughly the remaining lifespan after menopause (41).
Oral estrogens undergo hepatic first-pass metabolism, which has been associated with activation of the renin-angiotensin-aldosterone system (RAAS) and increased levels of circulating angiotensin II (6, 7, 42, 43). In contrast, non-oral delivery methods, such as transdermal, injectable, or transvaginal estrogen bypass the liver and are not associated with upregulated RAAS activity. Through bypass of the digestive tract and liver, transdermal estrogen formulations have the advantage of delivering unmetabolized estradiol directly to the bloodstream and thus require lower doses compared with oral agents. The Women's Health Initiative Observational Study reported that the odds of incident treated hypertension after 3 years did not vary according to dose of estrogen (44), and estradiol levels have not been shown to be associated with cardiovascular risk (45), at least in users of oral estrogen, suggesting different metabolic pathways likely play a role in the different risk profiles seen between oral and non-oral routes of estrogen administration (46).
Blood pressure is a major modifiable risk factor for cardiovascular disease (47). Blood pressure often increases in the menopausal transition, and while it has been suggested that this is due to decreases in estrogen levels (48), this has not been definitively established (49, 50). Studies on the effect of postmenopausal estrogen therapy on blood pressure are conflicting. The Heart and Estrogen/progestin Replacement Study (HERS), a randomized placebo-controlled trial of 2,763 postmenopausal women with a history of cardiovascular disease, showed a 2 mmHg increase in mean SBP with oral CEE + MPA use, although this was not the primary outcome (14). Similarly, the WHI examined the effect of these same interventions on primary prevention of cardiovascular disease and reported that in postmenopausal women, oral CEE and CEE+MPA at conventional doses both increased mean SBP compared to placebo, an effect that was most pronounced in younger women and those who were Hispanic or White (15). In contrast, a 3-year, multicenter, randomized, double-blind, placebo-controlled trial of 875 healthy postmenopausal women aged 45 to 64 years showed that CEE alone or in combination with progestin had no effect on blood pressure (16). Similarly, a four-year randomized trial of 727 younger postmenopausal women randomized to either transdermal estradiol or oral CEE (with both groups taking cyclical micronized progesterone) showed similar changes in BP (8). Of note, in addition to the use of progesterone, the participants in this study were recently menopausal and on average more than a decade younger than those in our study population, which may account for the lack of differences in blood pressure with the use of different routes of estrogen administration.
Previous work has shown differential changes in blood pressure with oral and non-oral routes of estrogen administration. In a randomized trial of postmenopausal women, 28 participants received continuous oral CEE plus cyclic MPA, 28 received a continuous transdermal estradiol patch plus cyclic MPA, and 27 did not receive either therapy (51). After 12 months, there were no differences in BP across groups, although the transdermal group demonstrated a decrease in arterial stiffness. Our study population differs from these in that participants were taking estrogen-only hormone therapy to exclude any potential effects of progestins on the outcomes of interest. A non-randomized, prospective study of 90 normotensive, oophorectomized women, aged 30–59 years on either oral (n = 50) or transdermal (n = 40) estrogen therapy demonstrated a decrease in blood pressure after 6 months in the transdermal group, with no change in the oral group (17). However, there was significant variability in individual responses with BP increasing in more than one-third of the women on either treatment, in keeping with recent findings from the Study of Women's Health Across the Nation (SWAN) cohort demonstrating distinct BP trajectories over the menopause transition that were independent of estradiol levels or hormone therapy use (49). A randomized trial of 38 younger (averages ages 54.6 and 55.5 years) normotensive women with natural menopause compared the effects of transdermal estradiol and oral CEE and MPA (6), and reported a decrease in DBP (−3 mmHg) and mean BP (−3.2 mmHg) after 12 months in the transdermal group, whereas the oral group did not demonstrate any changes in BP. A randomized crossover placebo-controlled study in 12 normotensive postmenopausal women (53 ± 2 years of age, 10 ± 3 years after the last menstrual period) examined the effects of 8 weeks of transdermal estradiol (200 microgram/d), oral conjugated estrogens (0.625 mg/d), or placebo (52). After 8 weeks of transdermal estrogen, ambulatory diastolic BP fell by 5 ± 2 mm Hg (p = 0.0003) but no change was observed with oral CEE or placebo. The reported discrepancies across studies of the effect of route of estrogen administration on blood pressure likely reflect differences in estrogen dose, formulation, populations, age, cause, and age at menopause as well as timing of initiation of hormone therapy. Many of these studies examined the effects of combined estrogen and progestins, with may have also impacted outcomes. A strength of the present study is the inclusion of participants with no concomitant progestin use.
Arterial stiffness is a validated predictor of cardiovascular morbidity and mortality (24, 53). There is limited data on the effect of postmenopausal hormone therapy on arterial stiffness. A study of 52 postmenopausal women demonstrated greater arterial compliance in hormone therapy users compared to non-users, and greater arterial compliance in estrogen-only users compared to combined estrogen and progestin-users (18). Interestingly, arterial compliance increased significantly in hormone users after 4 weeks of cessation, but the authors did not report the route of estrogen administration or concomitant progestin use (18). Conversely, a recent cross-sectional study (19) of 36 menopausal women reported that hormone therapy (six oral, two transdermal estrogen)-users had higher aPWV, consistent with greater cardiovascular risk, than postmenopausal non-users (n = 26), although the authors did not report whether hormone therapy included a progestin. Similar to studies examining the effect of postmenopausal hormone therapy on blood pressure, it is possible that factors other than the route of estrogen administration contributed to the reported outcomes, including differing methodologies to measure arterial stiffness (54).
This study has strengths and limitations. First, because of the self-reported nature of HT exposure in this study, recall bias leading to misclassification of hormone exposure is a possibility. However, most studies comparing self-reports of HT with physician/pharmacy records have shown moderate to good agreement (55). Next, most oral estrogen users in this study underwent surgical menopause while in contrast, non-oral estrogen users and controls underwent natural menopause. Recall of age at menopause has been shown to be better for surgical menopause than for natural menopause (56, 57). Because estrogen therapy is often started around menopause, it is reasonable to assume that recall of age at first use and duration would be similarly affected. Overall, the agreement between self-report and the medical record on the use of oral estrogens is at least moderate (58). Since participants using oral estrogen in our study were more likely to undergo surgical menopause, this suggests that they were more likely to initiate estrogen at the time of menopause, which is associated with improved cardiovascular outcomes (59). It is important to note that the type of estrogen used was not reported. The most commonly used oral estrogen formulations, at least in the United States, for systemic treatment of menopausal symptoms are estradiol and conjugated equine estrogens (CEE) (60), whereas transdermal and vaginal forms of estrogen are exclusively estradiol (61); estradiol exposure is associated with better cardiovascular outcomes compared to CEE (62) and it is possible that type of estrogen also played a role in our outcomes of interest. Non-oral HT users were younger and prescribed fewer cardiovascular medications than their oral estrogen counterparts (63). As such, we included age and medical comorbidities in our linear regression analyses to mitigate these potential differences. Of note, history of tobacco use was higher in the non-oral estrogen group; it is thus possible that the risk demonstrated with oral estrogen use in our study may actually be an underestimate. Lastly, the study population was restricted to ever users of estrogen-only menopausal HT with the majority being past, rather than current, users. The results may thus not be applicable to women who use combined estrogen and progestin HT or who currently use estrogen only HT. However, an important strength of this study is that limiting our study population to estrogen-only users, we were able to investigate the independent associations of route of administration estrogen exposure on measures of blood pressure and arterial stiffness while minimizing confounding factors.
In this community-dwelling postmenopausal population, oral estrogen HT use, without concomitant progestin, was associated with increased SBP and DBP. Menopause is associated with increased cardiovascular risk (64). Vasomotor symptoms (65) are common and the guideline-recommended treatment is estrogen with or without concomitant progestin (66). Therefore, an understanding of the potential cardiovascular benefits and risks of different routes of administration of estrogen HT will allow individuals and their health care providers to make informed decisions regarding therapy during this important transition time. Although it is unclear if the effects of different routes of administration of estrogen use on blood pressure in the absence of a progestin are limited to a particular time period, or whether the timing of initiation or duration of use plays a role, the association between oral estrogen use and increased blood pressure in our study warrants attention in the growing postmenopausal population.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Conjoint Health Research Ethics Board of the University of Calgary (REB20-1456) and University of Manitoba Health Research Ethics Board and St. Boniface Hospital Research Review Committee (HS22576, H2019:063). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CK and SA prepared the concept, designed the study, performed statistical analysis, and prepared the manuscript. JH, KB, and TD recruited participants and collected the data. All authors were involved in the interpretation of the results and the revision of the manuscript and approved the submitted version of the manuscript.
Funding
CK was funded by the Libin Cardiovascular Institute Doctoral Scholarship in Women's Cardiovascular Health and the Canadian Institute of Health Research-Leaders in Medicine MD/PhD award. JH was funded by the Pawan K. Singal Graduate Scholarship in Cardiovascular Sciences and Kappa Kappa Gamma Foundation of Canada Scholarship. KB was funded by a Frederick Banting & Charles Best Canada Graduate Scholarships – Doctoral Award. TD holds a St. Boniface Hospital Research Foundation Molson Women's Heart Health Research grant to conduct the WARM Hearts study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.913609/full#supplementary-material
References
1. Shifren JL, Gass ML. The North American menopause society recommendations for clinical care of midlife women. Menopause. (2014) 21:1038–62. doi: 10.1097/GME.0000000000000319
2. Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. (2015) 175:531–9. doi: 10.1001/jamainternmed.2014.8063
3. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women's health initiative randomized controlled trial. JAMA. (2002) 288:321–33. doi: 10.1001/jama.288.3.321
4. von Schoultz B. Oestrogen therapy: oral vs. non-oral administration. Gynecol Endocrinol. (2009) 25:551–3. doi: 10.1080/09513590902836551
5. Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Levesque H, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the Esther study. Circulation. (2007) 115:840–5. doi: 10.1161/CIRCULATIONAHA.106.642280
6. Ichikawa J, Sumino H, Ichikawa S, Ozaki M. Different effects of transdermal and oral hormone replacement therapy on the renin-angiotensin system, plasma bradykinin level, and blood pressure of normotensive post-menopausal women. Am J Hypertens. (2006) 19:744–9. doi: 10.1016/j.amjhyper.2005.10.006
7. Odutayo A, Cherney D, Miller J, Ahmed SB, Lai V, Dunn S, et al. Transdermal contraception and the renin-angiotensin-aldosterone system in pre-menopausal women. Am J Physiol Renal Physiol. (2015) 308:F535–40. doi: 10.1152/ajprenal.00602.2014
8. Miller VM, Naftolin F, Asthana S, Black DM, Brinton EA, Budoff MJ, et al. The Kronos early estrogen prevention study (keeps): what have we learned? Menopause. (2019) 26:1071–84. doi: 10.1097/GME.0000000000001326
9. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. (2003) 348:645–50. doi: 10.1056/NEJMsb022365
10. Mohammed K, Abu Dabrh AM, Benkhadra K, Al Nofal A, Carranza Leon BG, Prokop LJ, et al. Oral vs. transdermal estrogen therapy and vascular events: a systematic review and meta-analysis. J Clin Endocrinol Metabol. (2015) 100:4012–20. doi: 10.1210/jc.2015-2237
11. Seeland U, Demuth I, Regitz-Zagrosek V, Steinhagen-Thiessen E, König M Prokop differences in arterial wave reflection and the role of exogenous and endogenous sex hormones: results of the berlin aging study Ii. J Hypertens. (2020) 38:1040–6. doi: 10.1097/HJH.0000000000002386
12. Shufelt CL, Merz CNB, Prentice RL, Pettinger MB, Rossouw JE, Aroda VR, et al. Hormone therapy dose, formulation, route of delivery, and risk of cardiovascular events in women: findings from the whi observational study. Menopause. (2014) 21:260. doi: 10.1097/GME.0b013e31829a64f9
13. Shufelt CL, Manson JE. Menopausal hormone therapy and cardiovascular disease: the role of formulation, dose, and route of delivery. J Clin Endocrinol Metab. (2021) 106:1245–54. doi: 10.1210/clinem/dgab042
14. Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and estrogen/progestin replacement study (Hers) research group JAMA. (1998) 280:605–13. doi: 10.1001/jama.280.7.605
15. Shimbo D, Wang L, Lamonte MJ, Allison M, Wellenius GA, Bavry AA, et al. The effect of hormone therapy on mean blood pressure and visit-to-visit blood pressure variability in postmenopausal women: results from the women's health initiative randomized controlled trials. J Hypertens. (2014) 32:2071. doi: 10.1097/HJH.0000000000000287
16. Miller VT, LaRosa J, Barnabei V, Kessler C, Levin G, Smith-Roth A, et al. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women: the post-menopausal estrogen/progestin interventions (Pepi) trial. JAMA. (1995) 273:199–208. doi: 10.1001/jama.1995.03520270033028
17. Akkad AA, Halligan AW, Abrams K, Al-Azzawi F. Differing responses in blood pressure over 24 h in normotensive women receiving oral or transdermal estrogen replacement therapy. Obst Gynecol. (1997) 89:97 doi: 10.1016/s0029-7844(97)84258-5
18. Rajkumar C, Kingwell BA, Cameron JD, Waddell T, Mehra R, Christophidis N, et al. Hormonal therapy increases arterial compliance in post-menopausal women. J Am Coll Cardiol. (1997) 30:350–6. doi: 10.1016/S0735-1097(97)00191-5
19. Laakkonen EK, Karppinen JE, Lehti S, Lee E, Pesonen E, Juppi HK, et al. Associations of sex hormones and hormonal status with arterial stiffness in a female sample from reproductive years to menopause. Front Endocrinol. (2021) 6:1622. doi: 10.3389/fendo.2021.765916
20. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the women's health initiative randomized controlled trial. JAMA. (2004) 291:1701–12. doi: 10.1001/jama.291.14.1701
21. Stanczyk FZ, Hapgood JP, Winer S, Mishell Jr DR. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. (2013) 34:171–208. doi: 10.1210/er.2012-1008
22. Von Elm E, Altman DG, Egger M, Pocock SJ Gies, intracellular actions, and and clstrengthening the reporting of observational studies in epidemiology (strobe) statement: guidelines for reporting observational studies. Ann Intern Med. (2007) 147:573–7. doi: 10.7326/0003-4819-147-8-200710160-0001
23. Rose AV, Boreskie KF, Hay JL, Thompson L, Arora RC, Duhamel TA. Protocol for the warm hearts study: examining cardiovascular disease risk in middle-aged and older women-a prospective, observational cohort study. BMJ Open. (2021) 11:e044227. doi: 10.1136/bmjopen-2020-044227
24. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. (2010) 55:1318–27. doi: 10.1016/j.jacc.2009.10.061
25. Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, Dawes M, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Canadian J Cardiol. (2016) 32:1263–82. doi: 10.1016/j.cjca.2016.07.510
26. Franssen PM, Imholz BP. Evaluation of the mobil-o-graph new generation abpm device using the esh criteria. Blood Press Monit. (2010) 15:229–31. doi: 10.1097/MBP.0b013e328339be38
27. Wei W Tess Monit generation abpm device using the esh critmobil-o-graph: 24 h-blood pressure measurement device. Blood Press Monit. (2010) 15:225–8. doi: 10.1097/MBP.0b013e328338892f
28. Hametner B, Wassertheurer S, Kropf J, Mayer C, Eber B, Weber T. Oscillometric estimation of aortic pulse wave velocity: comparison with intra-aortic catheter measurements. Blood Press Monit. (2013) 18:173–6. doi: 10.1097/MBP.0b013e3283614168
29. Feistritzer HJ, Reinstadler SJ, Klug G, Kremser C, Seidner B, Esterhammer R, et al. Comparison of an oscillometric method with cardiac magnetic resonance for the analysis of aortic pulse wave velocity. PLoS ONE. (2015) 10:e0116862. doi: 10.1371/journal.pone.0116862
30. Wassertheurer S, Kropf J, Weber T, Van der Giet M, Baulmann J, Ammer M, et al. A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens. (2010) 24:498–504. doi: 10.1038/jhh.2010.27
31. Weiss W, Gohlisch C, Harsch-Gladisch C, Tnalysis M, Baulmann J, Ammer Mof aortic pulse waestimation of central blood pressure: validation of the mobil-o-graph in comparison with the sphygmocor device. Blood Press Monit. (2012) 17:128–31. doi: 10.1097/MBP.0b013e328353ff63
32. Thomas SG, Goodman JM, Burr JF. Evidence-based risk assessment and recommendations for physical activity clearance: established cardiovascular disease. App Physiol, Nutri, Metabol. (2011) 36:S190–213. doi: 10.1139/h11-050
33. McEniery CM, Yasmin N, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR, et al. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the anglo-cardiff collaborative trial (Acct). J Am College Cardiol. (2005) 46:1753–60. doi: 10.1016/j.jacc.2005.07.037
34. Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. (2000) 525(Pt 1):263. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x
35. Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. (2016) 1:767–76. doi: 10.1001/jamacardio.2016.2415
36. Zaydun G, Tomiyama H, Hashimoto H, Arai T, Koji Y, Yambe M, et al. Menopause is an independent factor augmenting the age-related increase in arterial stiffness in the early postmenopausal phase. Atherosclerosis. (2006) 184:137–42. doi: 10.1016/j.atherosclerosis.2005.03.043
37. Tjoe B, Fell B, LeVee A, Wei J, Shufelt C. Current perspective on menopause hormone therapy and cardiovascular risk. Curr Treat Options Cardiovasc Med. (2021) 23:137–42. doi: 10.1007/s11936-021-00917-2
38. Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. (2003) 107:2864–9. doi: 10.1161/01.CIR.0000069826.36125.B4
39. Paiva AM, Mota-Gomes MA, Brandao AA, Silveira FS, Silveira MS, Okawa RT, et al. Reference values of office central blood pressure, pulse wave velocity, and augmentation index recorded by means of the mobil-o-graph Pwa monitor. HypertenRes. (2020) 43:1239–48. doi: 10.1038/s41440-020-0490-5
40. Ji H, Niiranen TJ, Rader F, Henglin M, Kim A, Ebinger JE, et al. Sex differences in blood pressure associations with cardiovascular outcomes. Circulation. (2021) 143:761–3. doi: 10.1161/CIRCULATIONAHA.120.049360
41. Bushnik T, Tjepkema M, Martel L. Health-Adjusted Life Expectancy in Canada: Statistics Canada Ottawa (2018).
42. Krattenmacher R, Knauthe R, Parczyk K, Walker A, Hilgenfeldt U, Fritzemeier K. Estrogen action on hepatic synthesis of angiotensinogen and Igf-I: direct and indirect estrogen effects. J Steroid Biochem Mol Biol. (1994) 48:207–14. doi: 10.1016/0960-0760(94)90146-5
43. Deschepper CF. Angiotensinogen: hormonal regulation and relative importance in the generation of angiotensin Ii. Kidney Int. (1994) 46:1561–3. doi: 10.1038/ki.1994.446
44. Wild RA, Larson JC, Crandall CJ, Shadyab AH, Allison M, Gass M, et al. Hormone therapy formulation, dose, route of delivery, and risk of hypertension: findings from the women's health initiative observational study (Whi-Os). Menopause. (2021) 28:1108–16. doi: 10.1097/GME.0000000000001828
45. Rexrode KM, Manson JE, Lee I-M, Ridker PM, Sluss PM, Cook NR, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. (2003) 108:1688–93. doi: 10.1161/01.CIR.0000091114.36254.F3
46. Kopper NW, Gudeman J, Thompson DJ. Transdermal hormone therapy in postmenopausal women: a review of metabolic effects and drug delivery technologies. Drug Des Devel Ther. (2008) 2:193. doi: 10.2147/DDDT.S4146
47. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. (2020) 75:285–92. doi: 10.1161/HYPERTENSIONAHA.119.14240
48. Issa Z, Seely EW, Rahme M, Fuleihan GE-H. Effects of hormone therapy on blood pressure. Menopause. (2015) 22:456–68. doi: 10.1097/GME.0000000000000322
49. Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Thurston RC, et al. Trajectories of blood pressure in midlife women: does menopause matter? Circ Res. (2022) 130:312–22. doi: 10.1161/CIRCRESAHA.121.319424
50. Tikhonoff V, Casiglia E, Gasparotti F, Spinella P. The uncertain effect of menopause on blood pressure. J Hum Hypertens. (2019) 33:421–8. doi: 10.1038/s41371-019-0194-y
51. Sumino H, Ichikawa S, Kasama S, Takahashi T, Kumakura H, Takayama Y, et al. Different effects of oral conjugated estrogen and transdermal estradiol on arterial stiffness and vascular inflammatory markers in postmenopausal women. Atherosclerosis. (2006) 189:436–42. doi: 10.1016/j.atherosclerosis.2005.12.030
52. Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. (2001) 103:2903–8. doi: 10.1161/01.CIR.103.24.2903
53. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. (2001) 37:1236–41. doi: 10.1161/01.HYP.37.5.1236
54. Benas D, Kornelakis M, Triantafyllidi H, Kostelli G, Pavlidis G, Varoudi M, et al. Pulse wave analysis using the mobil-o-graph, arteriograph and complior device: a comparative study. Blood Press. (2019) 28:107–13. doi: 10.1080/08037051.2018.1564236
55. Paganini-Hill A, Clark LJ. Comparison of patient recall of hormone therapy with physician records. Menopause. (2007) 14:230–4. doi: 10.1097/01.gme.0000235364.50028.b5
56. Bean JA, Leeper JD, Wallace RB, Sherman BM, Jagger H. Variations in the reporting of menstrual histories. Am J Epidemiol. (1979) 109:181–5. doi: 10.1093/oxfordjournals.aje.a112673
57. Hahn RA, Eaker E, Rolka H. Reliability of reported age at menopause. Am J Epidemiol. (1997) 146:771–5. doi: 10.1093/oxfordjournals.aje.a009353
58. HILL AP, Ross R. Reliability of recall of drug usage and other health-related information. Am J Epidemiol. (1982) 116:114–22. doi: 10.1093/oxfordjournals.aje.a113386
59. Kalantaridou SN, Naka KK, Papanikolaou E, Kazakos N, Kravariti M, Calis KA, et al. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metabol. (2004) 89:3907–13. doi: 10.1210/jc.2004-0015
60. Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. (2015) 126:859. doi: 10.1097/AOG.0000000000001058
61. Hill DA, Crider M, Hill SR. Hormone therapy and other treatments for symptoms of menopause. Am Fam Physician. (2016) 94:884–9.
62. Smith NL, Blondon M, Wiggins KL, Harrington LB, van Hylckama Vlieg A, Floyd JS, et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. (2014) 174:25–34. doi: 10.1001/jamainternmed.2013.11074
63. Simon JA, Laliberte F, Duh MS, Pilon D, Kahler KH, Nyirady J, et al. Venous thromboembolism and cardiovascular disease complications in menopausal women using transdermal vs. oral estrogen therapy. Menopause. (2016) 23:600–10. doi: 10.1097/GME.0000000000000590
64. El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the american heart association. Circulation. (2020) 142:e506–e32. doi: 10.1161/CIR.0000000000000912
65. Avis NE, Crawford SL, Green R. Vasomotor symptoms across the menopause transition: differences among women. Obstetrics Gynecol Clin. (2018) 45:629–40. doi: 10.1016/j.ogc.2018.07.005
Keywords: hormone therapy (HT), estrogen, blood pressure, arterial stiffness, augmentation index, pulse wave velocity, menopause, women
Citation: Kalenga CZ, Hay JL, Boreskie KF, Duhamel TA, MacRae JM, Metcalfe A, Nerenberg KA, Robert M and Ahmed SB (2022) The Association Between Route of Post-menopausal Estrogen Administration and Blood Pressure and Arterial Stiffness in Community-Dwelling Women. Front. Cardiovasc. Med. 9:913609. doi: 10.3389/fcvm.2022.913609
Received: 05 April 2022; Accepted: 09 May 2022;
Published: 10 June 2022.
Edited by:
Georgios Kararigas, University of Iceland, IcelandReviewed by:
Ronee Harvey, Mayo Clinic, United StatesMaaike Schilperoort, Columbia University Irving Medical Center, United States
Copyright © 2022 Kalenga, Hay, Boreskie, Duhamel, MacRae, Metcalfe, Nerenberg, Robert and Ahmed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sofia B. Ahmed, sofia.ahmed@albertahealthservices.ca
 Cindy Z. Kalenga
Cindy Z. Kalenga