
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cardiovasc. Med., 11 August 2022
Sec. Heart Failure and Transplantation
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.910957
This article is part of the Research TopicNovel Phenotyping and Risk Stratification Strategies for Heart FailureView all 16 articles
The importance of the left atrium (LA) has been emphasized in recent years as the features of heart failure (HF), especially with regard to variability in patient and pathology phenotypes, continue to be uncovered. Of note, among the population with HF with preserved ejection fraction (HFpEF), pressure or size of the LA have become a target for advanced monitoring and a therapeutic approach. In the case of diastolic dysfunction or pulmonary hypertension, which are often observed in patients with HFpEF, a conventional approach with clinical symptoms and physical signs of decompensation turned out to have a poor correlation with LA pressure. Therefore, to optimize HF treatment for these populations, several devices that are applied directly to the LA have been developed. First, two LA pressure (LAP) sensors (Heart POD and V-LAP Device) were developed and may enable patient self-management remotely with LAP-guided and physician-directed style. Second, there are device-based approaches that aim to decompress the LA directly. These include: (1) interatrial shunt devices; (2) left ventricular assist devices with LA cannulation; and (3) the left atrial assist device. While these novel device-based therapies are not yet commercially available, there is expected to be a rise in the proposition and adoption of a wider range of choices for monitoring or treating LA using device-based options, based on LA dimensional reduction and optimization of the clinically significant pressure relief. Further development and evaluation are necessary to establish a more favorable management strategy for HF.
In the treatment of chronic heart failure (HF), left atrial (LA) function has been identified as one of the most important parameters affecting the quality of life and potential deterioration or improvement of left atrial unloading. In ~90% of hospitalizations for exacerbation of HF, there is pulmonary congestion related to an increase in LA pressure (LAP) (1–3). In daily medical care, however, current management strategies for ambulatory HF patients generally rely on clinical symptoms and physical signs of decompensation, even though these indicators have a poor correlation with LAP (4).
For patients with HF with preserved ejection fraction (HFpEF) or pulmonary hypertension, diastolic dysfunction or right HF are the main pathophysiological elements responsible for the clinical representation and overall course of the disease. These types of HF tend to be resistant to simple volume reduction or vasodilators, since these approaches do not directly reduce the LAP in case left ventricular (LV) systolic function is preserved. Even though patients with HFpEF account for nearly half of the entire HF population (5), there is still an unmet need for effective therapeutic options.
To achieve more dedicated HF control for this population, it is essential to understand the specific clinical requirements in order to have effective options for monitoring LAP, and accurately adjusted, effective LA decompression. In this mini-review, we describe several novel devices that are currently in the development pipeline to treat HF that specifically address the LA function and parameters. The features, advantages and limitations of these device platforms are discussed.
Changes in volume status or ventricular function, followed by decompensation and severe symptoms in HF patients, often require hospitalization and invasive monitoring of the patient's clinical status to achieve optimal medication and volume control. To limit the number of hospitalizations, strong efforts have been made for several decades to develop an accurate and remote monitoring system for early detection of exacerbation of chronic HF conditions (6). The unprecedented era of the COVID-19 pandemic has emphasized the necessity of reliable remote monitoring of HF patients more than ever.
Currently, continuous and remote monitoring of pulmonary artery pressure (PAP) have been confirmed to be associated with a reduced number of hospitalizations and mortality rates in the HF with reduced ejection fraction (HFrEF) population (7, 8). Also, PAP-guided therapies (PAP sensors) are the only commercially available option for CHF management so far. However, PAP does not always reflect left-sided ventricular filling pressures (9, 10) as seen in advanced HF patients with increased pulmonary vascular resistance or patients with pulmonary hypertension or acute HF.
Under these circumstances, LAP direct monitoring systems (intra-cardiac pressure readings) were developed to provide more sensitive and important information, including the evaluation of diastolic function and atrial arrhythmias.
The Heart POD (Abbott, Abbott Park, IL) was developed as the first attempt to place in a human a permanently implantable direct LAP monitoring device (11). This is a pacemaker-shaped device with a coil antenna implanted in the subcutaneous pocket and a sensor lead placed across the atrial septum (Figure 1A). A patient advisor module is used to communicate with the implanted sensor lead. In a prospective, multicenter, non-randomized, open-label feasibility clinical trial [the Hemodynamically Guided Home Self-Therapy in Severe Heart Failure Patients (HOMEOSTASIS) trial], the Heart POD was implanted in eight patients with established HF (11). At 12 weeks of follow-up, the device measurements were as accurate as within ±5 mm Hg of simultaneous pulmonary capillary wedge pressure readings, and no complications were reported.
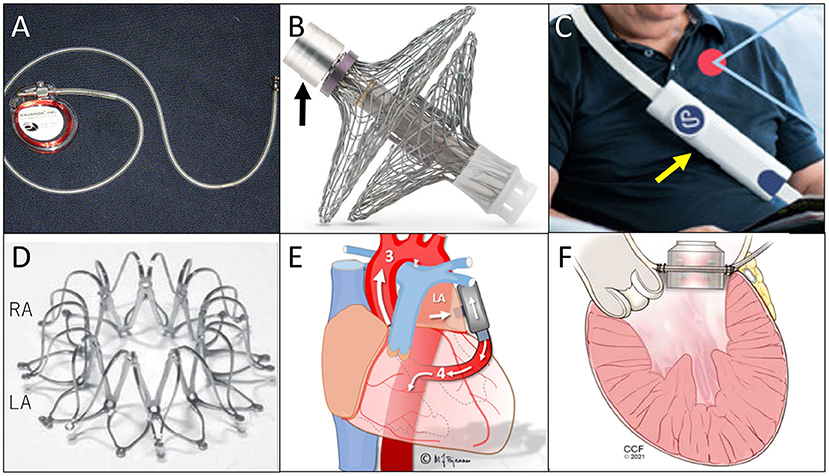
Figure 1. Illustrations of each device. (A) Heart POD, quoted from (6); (B) Sensory implant of V-LAP System (black arrow is the pressure-sensing assembly connected to electronic circuitry), quoted from (6); (C) Sensor of V-LAP (yellow arrow), quoted from (15); (D) Corvia Atrial Shunt Device, quoted from (19); (E) PulseVAD, quoted from (30), and; (F) Left Atrial Assist Device, quoted from (32).
As a follow-up to the HOMEOSTASIS trial, a prospective and observational study of a physician-directed patient self-management system targeting LAP was conducted, enrolling 40 patients with HFrEF and HFpEF and a history of acute decompensation (12). During pressure-guided therapy, mean daily LAP fell in the first 3 months from 17.6 to 14.8 mm Hg (p = 0.003), and the frequency of LAP elevation higher than 25 mm Hg was reduced by 67% (p< 0.001). In addition, improvements in New York Heart Association (NYHA) functional Class, LV ejection fraction, and pharmacological profiles were observed.
Following this, the Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy (LAPTOP-HF) study was initiated (13). The LAPTOP-HF was a prospective, multicenter, randomized, controlled clinical trial, which was designed to enroll up to 730 patients with NYHA functional class II and either of a history of hospitalization for HF in past 12 months or an elevated B-type natriuretic peptide level, regardless of the LV ejection fraction. The enrollment was terminated early, however, because of a large number of procedure-related complications by trans-septal punctures. The analysis of 486 patients that were enrolled prior to the termination showed that the HF therapy, with LAP-guided, physician-directed, and patient self-management, was associated with a 41% reduction in HF hospitalizations at 12 months (p = 0.005) (14). These efforts were followed by the development of the V-LAP, a more advanced LAP sensor.
The V-LAP System (Vectorious Medical Technologies, Tel Aviv, Israel), is a wireless remote monitoring system that measures LAP directly (2, 15). The system includes a sensory implant (Figure 1B) placed at the interatrial septum and an external unit (reader, Figure 1C). The implant is leadless, has no battery, and receives all its power from the reader. All procedures can be done percutaneously. A hermetically sealed tube encases the sensing elements and electronics, and bidirectional communications with a reader is enabled.
After ex vivo and in vivo animal experiences (16), the V-LAP Left Atrium Monitoring system for Patients With Chronic sysTOlic & Diastolic Congestive heart Failure (VECTOR-HF) study (ClinicalTrials.gov: NCT3775161) was recently initiated. This is a prospective, multicenter, single-arm, and open-label, first-in-human clinical study that aims to assess the safety, performance, and usability of the device in patients with NYHA class III HF. So far, 24 patients have received the device implants, which are transmitting accurate pressure measurements of LAP with no device-related complications or sensor failure events (2).
In general, the most serious concern with these LAP sensors is a higher rate of procedure-related complication. The V-LAP system seems to have less risk of thrombosis than the Heart POD because of its shape, but information about long-term biocompatibility and effectiveness are still needed.
Decompression of the LA is the most ideal therapeutic approach to relief the symptoms and vicious circle of exacerbated HF. Especially in cases of HF with diastolic dysfunction, as represented by patients with HFpEF, pharmacological treatments have not been as feasible as they have been for HFrEF. Use of LV assist devices (LVADs) is also controversial because of limited experience and concerns over the risk of ventricular suction events. Although sodium glucose cotransporter 2 (SGLT2) inhibitors were recently suggested to be beneficial for HFpEF (17, 18), their efficacy is still unclear after fluid overload is appropriately managed. SGLT2 inhibitors brought a lower risk of hospitalization for HF, but there was no improvement in death rates. Therefore, new device-based therapies that address LA decompression have been getting more attention in the area of HFpEF treatment (19, 20).
There are three options for decompressing the LA with a device: (1) fenestrate the interatrial wall; (2) use LVADs with LA cannulation; and (3) pump blood directly from the LA to the LV. High LAP and large LA size are two of the most important features of HFpEF pathology, and reducing LA size and pressure have been set as important therapeutic targets (21, 22). Here, we summarize recent findings for each type of device.
Interatrial shunt devices are the most widely applied option for the HFpEF population. There are three different devices, used as artificial interatrial shunts (23): the Corvia Atrial Shunt Device (IASD System II, Corvia Medical Inc., Tewksbury, MA), the V-Wave device (V-Wave Ltd., Caesarea, Israel), and the Atrial Flow Regulator (AFR, Occlutech, Helsingborg, Sweden). They employ the same concept of creating a shunt between the LA and the right atrium (RA) and reducing LAP by generating left-to-right flow artificially. Their materials and shapes vary, but typically, a 5-to-10 mm shunt is made and fixed at the interatrial wall by a self-expanding prosthesis, and all procedures can be done percutaneously.
Among them, the Corvia Atrial Shunt Device (Figure 1D) has already undergone several randomized clinical trials. A randomized, multicentre (international), blinded, sham-controlled trial (REDUCE LAP-HF II) (24), enrolled 1,072 participants; 314 were assigned to the device-implanted group. The placement of an atrial shunt did not reduce the total HF event rates at 12 or 24 months after implantation. The authors stated that the strategy of excluding pulmonary vascular disease might have not been adequate. Thus, with better patient selection, atrial shunt devices still have a chance to be beneficial. Nevertheless, as an HF treatment, the efficacy of this device is limited to symptom relief, and it can never be used as a causal treatment nor to stop progression of the disease.
As noted, LVADs are not considered to be as beneficial for patients with HF with diastolic dysfunction as for those with systolic dysfunction, because the LV wall in diastolic dysfunction is often too thick and the LV cavity too narrow for the LVAD inflow cannulas. The concept of applying LVADs with LA cannulation emerged, and some case reports depicted the potential efficacy of LVAD implantation in hearts with diastolic dysfunction, such as hypertrophic cardiomyopathy (25–27).
There are some novel devices that employ a similar concept of drawing blood from the LA and returning it to the aorta or subclavian artery. For example, the CircuLite Synergy Micro-Pump Device (Medtronic, Minneapolis, MN) (28) is a micropump-based form of mechanical circulatory support device, with a pump the size of an AA battery (29). The implantation can be done with a right mini-thoracotomy without using cardiopulmonary bypass, and the outflow graft is anastomosed to the subclavian artery. Although the development of the Synergy Micro-Pump Device and related project had been abandoned several years ago, this concept was successfully migrated to the new pumps, such as the VADovations cardiac assist system (VADovations, Oklahoma City, OK) or the PulseVAD (Northern Development, Strandhaugen, Oslo).
These two devices are at the beginning of the development process, and very limited information is available. Briefly, VADovations is a small pump the size of a AAA battery and is placed between the LA and the ascending aorta without inflow/outflow cannulas. The experimental results have not yet been documented. The PulseVAD is a pulsatile heart assist device and pumps blood from the LA to the descending aorta with minimally invasive surgery without cardiopulmonary bypass (Figure 1E). Gude and Fiane recently published the results of in vivo studies with the PulseVAD using ovine, and reported survival for 11 days after implantation without any complication (30).
Draining blood directly from the LA seems to be the most reasonable method of decreasing LAP, but these devices make an alternative bloodstream by shortcutting the LV. Even if they are intended as partial circulatory support, stagnation in the LV and a risk of LV thrombosis are inevitable. Also, pulsatility should be decreased as the pump support becomes larger.
Sharing the concept of draining blood from the LA, the Left Atrial Assist Device (LAAD) has a unique feature of being implanted at the mitral position and pumping blood directly to the LV (Figure 1F) (31). The target of this pump is mainly HFpEF or diastolic dysfunction with normal EF, since the systolic function of the heart needs to be maintained for the native LV to pump blood by itself. For use in hearts with preserved EF, the LAAD can decrease LAP and support the LV filling, maintaining physiological blood pathway and pulsatility.
Our progress with the LAAD was reported with in vitro and in vivo studies with calves (32, 33). The intraoperative image of the LAAD implanted at the mitral position is seen as Figure 2. The effect of reducing LAP has been successfully demonstrated with introduced diastolic HF models. The work on reduction of the device profile, which would allow shrinking of the device dimensions and favor the implantability and technology footprint inside the heart, is ongoing. Driveline exteriorization strategies are pending evaluation, and would provide more information on the most appropriate anatomical considerations and surgical options for its pass through cardiac structures. The evaluation with long-term safety and efficacy is needed, as well as the development of animal models that can simulate diastolic HF with more fidelity.
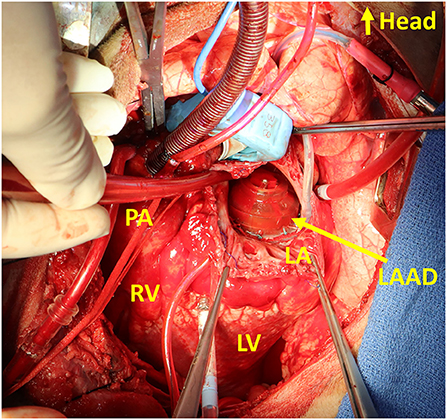
Figure 2. Intraoperative photo of the Left Atrial Assist Device (LAAD) implanted at the mitral position. LA, left atrium; LV, left ventricle; PA, pulmonary artery; RV, right ventricle.
The importance of LA function has been gradually and steadily emphasized as the concept of diastolic dysfunction has become more popular in the HF field. Decreased LA compliance and mechanics are believed to be associated with an increased risk for new onset atrial fibrillation in HFpEF (34), and reducing the LA size and pressure has become the key treatment strategy. However, considering LA as a therapeutic target requires tremendous effort, since obtaining the precise value of LAP requires invasive catheterization in the hospital.
Whether performed percutaneously or surgically, there is an inevitable risk of systemic thrombosis when any prosthetic is introduced into the LA, and so anticoagulation and/or antiplatelet therapies are usually prescribed (6, 35). Therefore, for these LA devices, patient selection is very important. Patients with diastolic dysfunction as represented by HFpEF or right HF, including HF with pulmonary hypertension, would be good candidates, since LA function plays larger roles.
The devices introduced here are all in development, and none has obtained approval from the U.S. Food and Drug Administration (FDA). Their effects on early detection or prevention of LA arrhythmia have not been demonstrated, and it's also unclear if they have any therapeutic effect on presenting arrhythmia. However, under the social, medical, and economic crises brought on by the COVID-19 pandemic, a case was reported in which constant tele-monitoring of LAP and subsequent adjustment of medication prevented possible decompensation of HF and hospitalization in a patient who was in self-isolation (15). With more evidence and the need for remote care, continuous remote monitoring by invasive sensors is expected to play a larger role in HF care.
As for the LA decompression devices, the Corvia Atrial Shunt Device received FDA breakthrough device designation in 2019, but it failed to show a long-term efficacy in a randomized trial. The pump-based devices should have promise for decompressing LA, but their development is still at the animal experiment stage, and needs more time before a first-in-human trial. Also, pump devices-implantation tends to be more invasive.
With the new device-based options, there is a wider range of choices for monitoring or treating LAP. Further development and evaluation are required to establish a more favorable management strategy for HF.
CM performed the literature search, designed the study, and prepared the manuscript. KF, JK, and TK provided methodological support in the study design and revised the manuscript. All authors have read and approved the manuscript before submission.
The LAAD study was supported by funding from National Heart, Lung and Blood Institute, National Institutes of Health (NIH), NIH Center for Accelerated Innovation at Cleveland Clinic (NCAI-CC), (NIH-NHLBI 1UH54HL119810; NCAI-19-12-APP-CCF).
Authors KF and JK are co-inventors of the LAAD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Adamson PB, Magalski A, Braunschweig F, Böhm M, Reynolds D, Steinhaus D, et al. Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system. J Am Coll Cardiol. (2003) 41:565–71. doi: 10.1016/s0735-1097(02)02896-6
2. Perl L, Meerkin D, D'Amario D, Avraham BB, Gal TB, Weitsman T, et al. The V-LAP system for remote left atrial pressure monitoring of patients with heart failure: remote left atrial pressure monitoring. J Card Fail. (2022) 28:963–72. doi: 10.1016/j.cardfail.2021.12.019
3. Adams KF Jr., Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the united states: rationale, design, and preliminary observations from the first 100,000 cases in the acute decompensated heart failure national registry (adhere). Am Heart J. (2005) 149:209–16. doi: 10.1016/j.ahj.2004.08.005
4. Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. (1989) 261:884–8.
5. Xanthopoulos A, Triposkiadis F, Starling RC. Heart failure with preserved ejection fraction: classification based upon phenotype is essential for diagnosis and treatment. Trends Cardiovasc Med. (2018) 28:392–400. doi: 10.1016/j.tcm.2018.01.001
6. Radhoe SP, Veenis JF, Brugts JJ. Invasive devices and sensors for remote care of heart failure patients. Sensors. (2021) 21:2014. doi: 10.3390/s21062014
7. Abraham J, Bharmi R, Jonsson O, Oliveira GH, Artis A, Valika A, et al. Association of ambulatory hemodynamic monitoring of heart failure with clinical outcomes in a concurrent matched cohort analysis. JAMA Cardiol. (2019) 4:556–63. doi: 10.1001/jamacardio.2019.1384
8. Givertz MM, Stevenson LW, Costanzo MR, Bourge RC, Bauman JG, Ginn G, et al. Pulmonary artery pressure-guided management of patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. (2017) 70:1875–86. doi: 10.1016/j.jacc.2017.08.010
9. Campbell P, Drazner MH, Kato M, Lakdawala N, Palardy M, Nohria A, et al. Mismatch of right- and left-sided filling pressures in chronic heart failure. J Card Fail. (2011) 17:561–8. doi: 10.1016/j.cardfail.2011.02.013
10. Drazner MH, Velez-Martinez M, Ayers CR, Reimold SC, Thibodeau JT, Mishkin JD, et al. Relationship of right- to left-sided ventricular filling pressures in advanced heart failure: insights from the escape trial. Circ Heart Fail. (2013) 6:264–70. doi: 10.1161/CIRCHEARTFAILURE.112.000204
11. Ritzema J, Melton IC, Richards AM, Crozier IG, Frampton C, Doughty RN, et al. Direct left atrial pressure monitoring in ambulatory heart failure patients: initial experience with a new permanent implantable device. Circulation. (2007) 116:2952–9. doi: 10.1161/CIRCULATIONAHA.107.702191
12. Ritzema J, Troughton R, Melton I, Crozier I, Doughty R, Krum H, et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation. (2010) 121:1086–95. doi: 10.1161/CIRCULATIONAHA.108.800490
13. Maurer MS, Adamson PB, Costanzo MR, Eigler N, Gilbert J, Gold MR, et al. Rationale and design of the left atrial pressure monitoring to optimize heart failure therapy study (LAPTOP-HF). J Card Fail. (2015) 21:479–88. doi: 10.1016/j.cardfail.2015.04.012
14. Abraham WT, Adamson PB, Costanzo MR, Eigler N, Gold M, Klapholz M, et al. Hemodynamic monitoring in advanced heart failure: results from the LAPTOP-HF trial. J Card Fail. (2016) 22:940. doi: 10.1016/j.cardfail.2016.09.012
15. Feickert S, D'Ancona G, Murero M, Ince H. Intra-cardiac microcomputer allows for innovative telemedicine in chronic heart failure during coronavirus disease-2019 pandemic: a case report. Eur Heart J Case Rep. (2020) 4:1–6. doi: 10.1093/ehjcr/ytaa501
16. Perl L, Soifer E, Bartunek J, Erdheim D, Köhler F, Abraham WT, et al. A novel wireless left atrial pressure monitoring system for patients with heart failure, first ex-vivo and animal experience. J Cardiovasc Transl Res. (2019) 12:290–8. doi: 10.1007/s12265-018-9856-3
17. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385:1451–61. doi: 10.1056/NEJMoa2107038
18. Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, et al. The sglt2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. (2021) 27:1954–60. doi: 10.1038/s41591-021-01536-x
19. Miyagi C, Miyamoto T, Karimov JH, Starling RC, Fukamachi K. Device-based treatment options for heart failure with preserved ejection fraction. Heart Fail Rev. (2021) 26:749–62. doi: 10.1007/s10741-020-10067-5
20. Burlacu A, Simion P, Nistor I, Covic A, Tinica G. Novel percutaneous interventional therapies in heart failure with preserved ejection fraction: an integrative review. Heart Fail Rev. (2019) 24:793–803. doi: 10.1007/s10741-019-09787-0
21. Issa O, Peguero JG, Podesta C, Diaz D, De La Cruz J, Pirela D, et al. Left atrial size and heart failure hospitalization in patients with diastolic dysfunction and preserved ejection fraction. J Cardiovasc Echogr. (2017) 27:1–6. doi: 10.4103/2211-4122.199064
22. Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. (2011) 124:2491–501. doi: 10.1161/CIRCULATIONAHA.110.011031
23. Emani S, Burkhoff D, Lilly SM. Interatrial shunt devices for the treatment of heart failure. Trends Cardiovasc Med. (2021) 31:427–32. doi: 10.1016/j.tcm.2020.09.004
24. Shah SJ, Borlaug BA, Chung ES, Cutlip DE, Debonnaire P, Fail PS, et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HR II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. (2022) 399:1130–40. doi: 10.1016/S0140-6736(22)00016-2
25. Kiamanesh O, Rankin K, Billia F, Badiwala MV. Left ventricular assist device with a left atrial inflow cannula for hypertrophic cardiomyopathy. JACC Case Reports. (2020) 2:2090–4. doi: 10.1016/j.jaccas.2020.10.006
26. Topilsky Y, Pereira NL, Shah DK, Boilson B, Schirger JA, Kushwaha SS, et al. Left ventricular assist device therapy in patients with restrictive and hypertrophic cardiomyopathy. Circ Heart Fail. (2011) 4:266–75. doi: 10.1161/CIRCHEARTFAILURE.110.959288
27. Wynne E, Bergin JD, Ailawadi G, Kern JA, Kennedy JL. Use of a left ventricular assist device in hypertrophic cardiomyopathy. J Card Surg. (2011) 26:663–5. doi: 10.1111/j.1540-8191.2011.01331.x
28. Burkhoff D, Maurer MS, Joseph SM, Rogers JG, Birati EY, Rame JE, et al. Left atrial decompression pump for severe heart failure with preserved ejection fraction: theoretical and clinical considerations. JACC Heart Fail. (2015) 3:275–82. doi: 10.1016/j.jchf.2014.10.011
29. Mohite PN, Sabashnikov A, Simon AR, Weymann A, Patil NP, Unsoeld B, et al. Does circulite synergy assist device as partial ventricular support have a place in modern management of advanced heart failure? Expert Rev Med Devices. (2015) 12:49–60. doi: 10.1586/17434440.2015.985208
30. Gude E, Fiane AE. Can mechanical circulatory support be an effective treatment for hfpef patients? Heart Fail Rev. (2021). doi: 10.1007/s10741-021-10154-1
31. Fukamachi K, Horvath DJ, Karimov JH, Kado Y, Miyamoto T, Kuban BD, et al. Left atrial assist device to treat patients with heart failure with preserved ejection fraction: initial in vitro study. J Thorac Cardiovasc Surg. (2021) 162:120–6. doi: 10.1016/j.jtcvs.2019.12.110
32. Miyagi C, Fukamachi K, Kuban BD, Gao S, Miyamoto T, Flick CR, et al. Left atrial circulatory assistance in simulated diastolic heart failure model: first in vitro and in vivo. J Card Fail. (2022) 28:789–98. doi: 10.1016/j.cardfail.2021.11.024
33. Miyagi C, Kuban BD, Flick CR, Polakowski AR, Miyamoto T, Karimov JH, et al. Left atrial assist device for heart failure with preserved ejection fraction: initial results with torque control mode in diastolic heart failure model. Heart Fail Rev. (2021). doi: 10.1007/s10741-021-10117-6
34. Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. (2020) 76:1051–64. doi: 10.1016/j.jacc.2020.07.009
Keywords: device-based treatment, mechanical circulatory support, left ventricular assist device (LVAD), interatrial shunt device, diastolic dysfunction (DD), heart failure with preserved ejection fraction (HFpEF), left atrial monitoring, left atrial assist
Citation: Miyagi C, Kuroda T, Karimov JH and Fukamachi K (2022) Novel approaches for left atrial pressure relief: Device-based monitoring and management in heart failure. Front. Cardiovasc. Med. 9:910957. doi: 10.3389/fcvm.2022.910957
Received: 27 April 2022; Accepted: 26 July 2022;
Published: 11 August 2022.
Edited by:
Tong Liu, Tianjin Medical University, ChinaReviewed by:
Gani Bajraktari, University of Pristina, AlbaniaCopyright © 2022 Miyagi, Kuroda, Karimov and Fukamachi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kiyotaka Fukamachi, ZnVrYW1ha0BjY2Yub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.