- 1Heart Center, Zhengzhou Ninth People's Hospital, Zhengzhou, China
- 2Department of Cardiology, Helmut-G.-Walther-Klinikum, Lichtenfels, Germany
- 3Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 4Cardiovascular Division, Department of Medicine, University of Minnesota Medical School, Minneapolis, MN, United States
- 5Clinic for General Internal Medicine and Cardiology, Catholic Medical Center Koblenz-Montabaur, Koblenz, Germany
- 6Department of Cardiology, Kunming Medical University, Kunming, China
Background: Higher CHA2DS2-VASc score is associated with an increased risk of adverse cardio-cerebrovascular events in patients with non-valvular atrial fibrillation (NVAF), regardless of oral anticoagulation (OAC) status. However, whether this association still exists in patients undergoing left atrial appendage closure (LAAC) is unknown. We evaluated the impact of CHA2DS2-VASc score on LAAC efficacy and outcomes.
Methods: A total of 401 consecutive patients undergoing LAAC were included and divided into 3 groups based on CHA2DS2-VASc score (0–2, 3–4, and ≥5). Baseline characteristics, periprocedural complications, and long-term outcomes were collected and compared across all groups.
Results: There were no significant differences in implantation success, periprocedural complications, and long-term outcomes across all score groups. Kaplan-Meier estimation showed that the cumulative ratio of freedom from all-cause mortality (P = 0.146), cardiovascular mortality (P = 0.519), and non-cardiovascular mortality (P = 0.168) did not differ significantly by CHA2DS2-VASc score group. LAAC decreased the risks of thromboembolism and major bleeding, resulting in a relative risk reduction (RRR) of 82.4% (P < 0.001) and 66.7% (P < 0.001) compared with expected risks in the overall cohort, respectively. Subgroup analysis indicated that observed risks of thromboembolism and major bleeding were significantly lower than the expected risks in score 3–4 and score ≥5 groups, respectively. The level of RRR increased with CHA2DS2-VASc score (P < 0.001 for trend) for thromboembolism but not for major bleeding (P = 0.2729 for trend).
Conclusion: Patients with higher CHA2DS2-VASc score did not experience worse outcomes, which may be partly attributed to more benefits provided by LAAC intervention in such patients compared to those with a low score.
Introduction
Atrial fibrillation (AF) has been considered a cardiovascular epidemic and is associated with an increased risk of complications such as thromboembolic stroke, heart failure, and death (1, 2). Oral anticoagulation (OAC) therapy is one of the most effective strategies for preventing stroke and reducing the incidence of complications in these patients. Previous studies showed that both traditional warfarin and direct oral anticoagulants (DOACs) decrease the risk of stroke or systemic embolic events (3), where DOACs demonstrated a favorable risk-benefit profile in risk reduction of hemorrhagic stroke, intracranial hemorrhage, and all-cause mortality, but an increased risk of gastrointestinal bleeding compared with warfarin (4). However, OACs face several limitations in their application, especially in patients at higher risk of bleeding and in patients who are not optimal candidates for long-term OAC because of OAC contraindications or non-compliance.
Intracardiac thrombus arising from the left atrial appendage (LAA) is the main cause of thromboembolic stroke in patients with non-valvular AF (NVAF) (5). Percutaneous LAA closure (LAAC) by an occluder is a non-pharmacologic approach to stroke prevention in NVAF patients. The long-term outcomes of randomized clinical trials showed that the efficacy of LAAC with the WATCHMAN device was comparable to warfarin in preventing stroke, with additional reductions in the risk of major bleeding and mortality (6). Furthermore, in patients with a high risk of stroke and bleeding, LAAC was still non-inferior to DOACs in preventing stroke and decreasing bleeding risks (7).
The CHA2DS2-VASc score has been proved to be significantly associated with the risk of cardiogenic embolism in patients with AF and is commonly used to stratify the risk of future thromboembolism or adverse outcomes in current clinical practice guidelines (8–10). Recent studies demonstrated that the risk of major adverse cardiovascular and cerebrovascular events or mortality in AF patients taking OACs still increased sequentially with increasing CHA2DS2-VASc score (9, 11). However, data on the relationship between CHA2DS2-VASc score and clinical outcomes in patients treated with percutaneous LAAC are limited. We investigated the association between CHA2DS2-VASc score groups and LAAC efficacy and outcomes.
Methods
Subjects
This is a retrospective cohort study. Patients with NVAF who consecutively underwent LAAC with the WATCHMAN device (Boston Scientific, Marlborough, MA, USA) at Helmut-G.-Walther Klinikum, Lichtenfels, Germany, from February 2012 to June 2018 and the WATCHMAN device or LAmbreTM occluder (Lifetech Scientific Corp., Shenzhen, China) at Zhengzhou Ninth People's Hospital, Zhengzhou, China from October 2016 throughout September 2021 were enrolled. The inclusion criteria for LAAC were as follows: (1) patients with known NVAF or whose NVAF was newly diagnosed during hospitalization, (2) those with a high risk of stroke or systemic embolism, (3) those with a high risk of major bleeding or contraindication for long-term anticoagulation therapy, or (4) those who were reluctant to take oral anticoagulation drugs. Patients with malignancy or multiple organ failure with a life expectancy of <1 year or patients with an intracardiac thrombus in the left atria/left atrial appendage detected by imaging were excluded. Written informed consent for LAAC was obtained from all participants. The study was carried out based on the Declaration of Helsinki and approved by the Ethics Committee of Helmut-G.-Walther Klinikum, Lichtenfels, Germany and Zhengzhou Ninth People's Hospital, Zhengzhou, China. Patients were divided into three groups based on CHA2DS2-VASc score, namely, 0–2, 3–4, and ≥5. Data on baseline characteristics, periprocedural complications within 7 days, and long-term outcomes were collected and analyzed.
Procedure
The detailed protocol of LAAC with the WATCHMAN device has been described previously (12). LAAC was performed under general anesthesia. Intra-procedural transesophageal echocardiography (TEE) and fluoroscopy were used to guide device implantation. After septum puncture, a WATCHMAN occluder was implanted in the left atrial appendage according to the device's directions for use. Implantation of LAmbre devices was generally similar to that of the WATCHMAN device. The occluder was released after the implantation met criteria, such as position, size, sealing effect, and stability. Implantation success was defined as an adequate closure of the LAA without residual peri-device leaks >5 mm in width, absence of device-related thrombus (DRT), and stable position of the device. Patients were hospitalized for 24–48 h, and those without significant procedure-related complications were then discharged.
Post-procedural antithrombotic treatments
To allow time for device endothelialization post-procedure, patients were prescribed antithrombotic drugs. The postprocedural antithrombotic regimen was at the discretion of the physician based on patients' clinical characteristics.
Follow-up
Patients were followed through with TEE evaluation and clinical visits. TEE follow-up was performed at approximately 45 days and 6 months after device implantation. Clinical follow-up was scheduled at 45 days and 6 months after the procedure as well as at the end of this study.
Outcomes
The primary outcomes were the success rate of device implantation and major adverse events during long-term follow-up. The secondary outcomes were severe periprocedural complications within 7 days. Thromboembolism (ischemic stroke/transient ischemic attack [TIA]/systemic embolism), major bleeding (intracranial hemorrhage/gastrointestinal bleeding/other major bleeding), DRT, mortality (cardiovascular mortality/non-cardiovascular mortality), and combined efficacy endpoints (thromboembolism/mortality) were defined as major adverse events. Severe periprocedural complications included ischemic stroke, TIA, systemic embolism, major bleeding, pericardial effusion/cardiac tamponade, severe vascular complication, and device-related death.
Statistical analysis
We used SPSS version 26.0 (SPSS Inc., Chicago, Illinois) for data analysis. Continuous variables are presented as means with standard deviation. Categorical variables are expressed as counts and proportions in percentage. The trends across the multiple groups were evaluated via the Cochran-Armitage test for categorical variables, the exact Cochran-Armitage test for categorical variables with rare events, or the Jonckheere-Terpstra test for metrical variables. The difference between two groups was compared with chi-square (χ2) tests for categorical variables, Mann-Whitney-Wilcoxon tests for metrical variables, or Student's t-tests for continuous variables. The mortality risk at long-term follow-up after LAAC was assessed by Kaplan-Meier curve analysis. The survival curves show cumulative ratios of freedom from all-cause mortality, cardiovascular mortality, and non-cardiovascular mortality. A log-rank test was used to compare the differences in mortality risk among groups.
The estimated annual rates of thromboembolism and major bleeding were calculated based on patient CHA2DS2-VASc and HAS-BLED scores, respectively (13, 14). The observed annual rates of thromboembolism and major bleeding were calculated as events per 100 patient-years, which were calculated as the total number of patients in whom new thromboembolism or major bleeding events developed during follow-up in a group divided by the total followed patient-years and then multiplied by 100. The efficacy of LAAC on thromboembolic or major bleeding risks was analyzed by comparing the estimated risk of thromboembolism or major bleeding events and observed risk. A chi-square test with relative risk (RR) and its 95% confidence interval (CI) was used to assess the differences between estimated and observed risks in one group. The number needed to treat (NNT) to prevent one event in thromboembolism or major bleeding by LAAC intervention was calculated in the overall cohort, score 0–2, score 3–4, and score ≥5 groups using the formula NNT = 1 / (predicted annual rate of event—observed annual rate of event).
The relative risk reduction (RRR) in thromboembolic or major bleeding events was calculated using the formula RRR = (estimated annual rate—observed annual rate)/estimated annual rate. To evaluate the differences regarding the impact of LAAC on thromboembolic and major bleeding outcomes in patients by CHA2DS2-VASc score group, the comparisons of RRR in thromboembolism or major bleeding were performed and analyzed by a chi-square test among groups, respectively. A P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
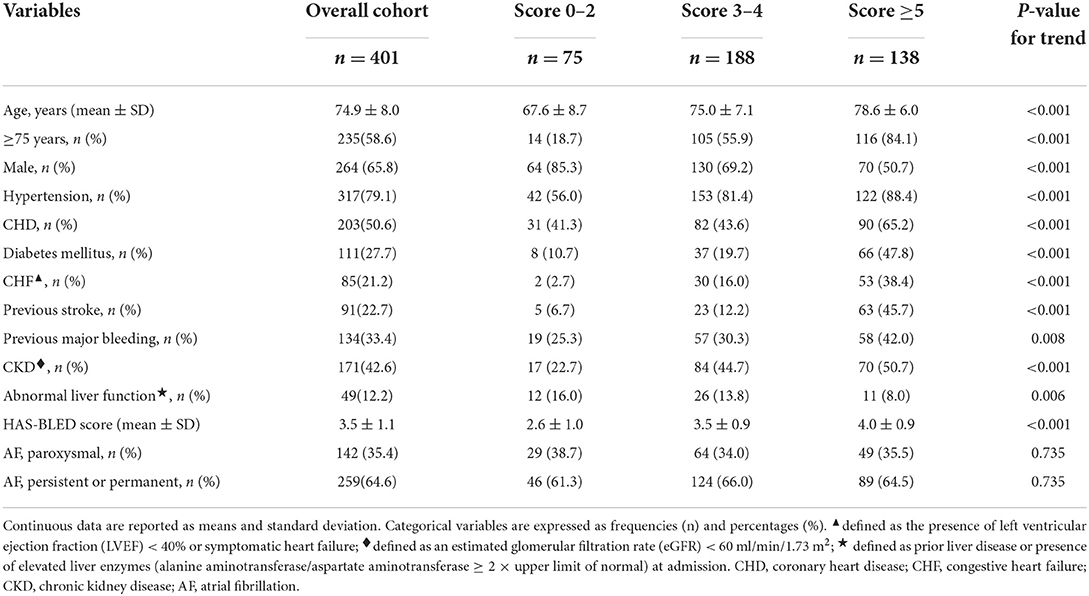
A total of 411 consecutive patients with NVAF were scheduled to undergo LAAC. LAAC was performed successfully in 401 patients (mean age 74.9 ± 8.0 years; 65.8% male) who were subsequently enrolled in this study. Among the other 10 patients, LAA morphology was not suitable for WATCHMAN in 6 cases, repeated DRT occurred in 1 case, and cardiac tamponade occurred in 2 cases at Helmut-G.-Walther Klinikum, Lichtenfels, Germany; severe iliac vein stenosis after pelvic surgery presented in 1 case at Zhengzhou Ninth People's Hospital, Zhengzhou, China. Among the overall cohort, 75 cases had scores 0–2, 188 cases had scores 3–4, and 138 cases had scores ≥5 according to CHA2DS2-VASc score. Out of the 75 patients with scores 0–2, 2 cases had 0 score whose indication for LAAC was gastrointestinal bleeding and OAC incompliance for each 1 case, and 10 cases had 1 score whose indication for LAAC was gastrointestinal bleeding in 5 cases, OAC incompliance in 2 cases, anemia in 1 case, and refusal of OAC in 2 cases. The total implantation success was 97.6%. There was no statistical difference for device implant success among groups (score 0–2: 97.3%; score 3–4: 97.8%; score ≥5: 97.5%; P = 0.965 for trend). The baseline characteristics of patients are presented in Table 1. Patients in the score ≥5 group were oldest (P < 0.001); most likely to be female; and have hypertension, coronary heart disease (CHD), diabetes mellitus, congestive heart failure (CHF), previous stroke, previous major bleeding, and chronic kidney disease (CKD) (each P < 0.01 for trend), while the proportion of patients with abnormal liver function was lowest in this group (P = 0.006 for trend). HAS-BLED score increased with increasing CHA2DS2-VASc scores (P < 0.001 for trend). However, the type of NVAF was comparable among all groups (Table 1).
Periprocedural complications
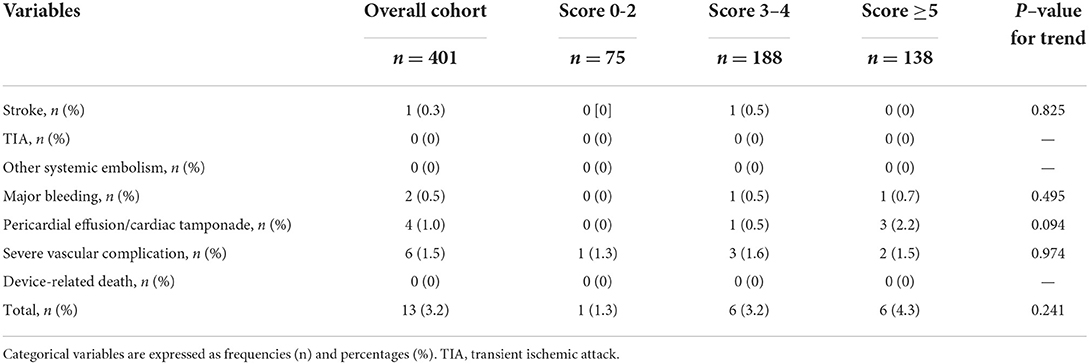
The total incidence of periprocedural complications was 3.2%, with 1.3%, 3.2%, and 4.3% in score 0–2, score 3–4, and score ≥5 groups, respectively (Table 2). No device-related death was observed in the overall cohort. There were no significant trends in the incidence of complications such as stroke, major bleeding, pericardial effusion/cardiac tamponade, and severe vascular complication among the three groups.
Antithrombotic regimen post-LAAC
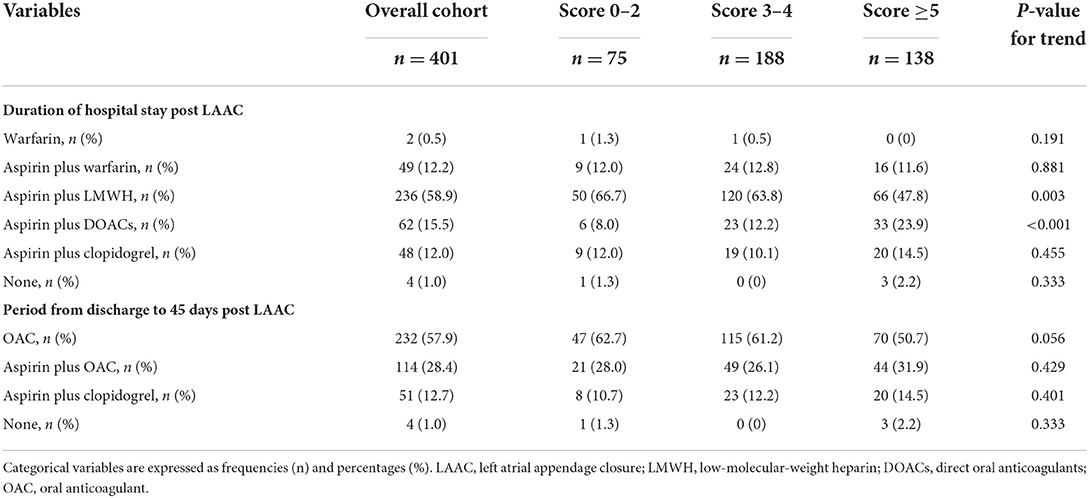
Variation in antithrombotic regimens occurred mainly at the first 45 days after the procedure. During their hospital stay post-LAAC, patients were prescribed warfarin, aspirin plus warfarin, aspirin plus low-molecular-weight heparin (LMWH), a combination of aspirin and DOACs, aspirin plus clopidogrel, or no antithrombotic medications; most patients (58.9 %) were treated with aspirin plus LMWH (Table 3). No significant trends were found for these regimens among the three groups for the duration of hospital stay post-LAAC except for aspirin plus LMWH which was more often prescribed in the score 0–2 group, and aspirin plus DOACs with the highest proportion in score ≥5 group. For the period from discharge to 45 days post-LAAC, all the antithrombotic regimens were continued except for subcutaneous injection of LMWH, which was switched to oral antithrombotics. The antithrombotic strategies for this period were also comparable among groups (Table 3). At the 45-day visit post-procedure, if TEE imaging identified adequate closure of the LAA (no residual peri-device jet >5 mm) without DRT, the antithrombotic regimen was switched to aspirin plus clopidogrel until 6 months post-procedure. Aspirin alone was administered indefinitely if TEE indicated adequate closure without DRT at the 6-month visit. If TEE showed inadequate closure or a presence of DRT, OACs were restarted until an adequate seal or disappearance of the thrombus was confirmed by TEE imaging.
Long-term outcomes
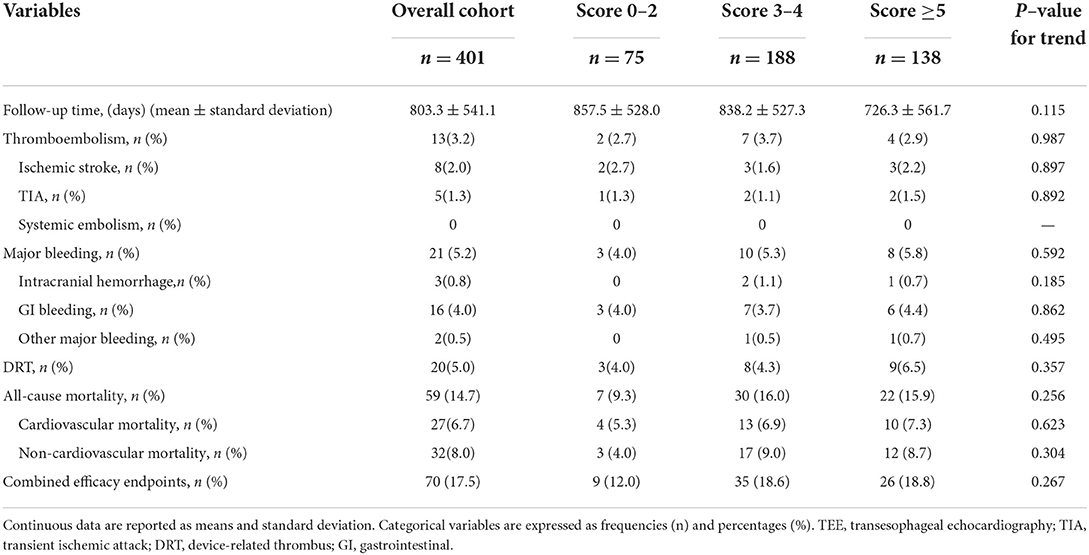
All participants were followed up. The mean follow-up duration was 803 days (2.2 years), which corresponded to 882.2 patient-years in the overall cohort, with 176.1, 431.6, and 274.5 patient-years in the score 0–2, score 3–4, and score ≥5 groups, respectively. No significant differences were found in follow-up duration among the three groups (P = 0.115 for trend).
Table 4 shows the long-term outcomes after LAAC across the three score groups. In the overall cohort, the incidence of thromboemboli was 3.2%, 2.0% for ischemic stroke, and 1.3% for TIA. Major bleeding events occurred in 21 cases (5.2%), and the majority of major bleeding events (16 cases) were attributed to gastrointestinal bleeding. DRT was observed in 20 cases (5.0%) by TEE visit. Furthermore, a total of 59 deaths (14.7%) occurred in the overall cohort, among which cardiovascular and non-cardiovascular mortality rates were 6.7% (27 cases) and 8.0% (32 cases), respectively. However, the incidence rates of thromboembolism, major bleeding, DRT, all-cause mortality, cardiovascular mortality, non-cardiovascular mortality, and combined efficacy endpoints were comparable among the three score subgroups, respectively [each P = no significance (NS) for trend] (Table 4).
To investigate the impact of CHA2DS2-VASc score on survival after LAAC, we performed a Kaplan-Meier survival curve estimation, which showed that the cumulative ratio of freedom from all-cause mortality (log-rank test for trend, P = 0.146), cardiovascular mortality (log-rank test for trend, P = 0.519), or non-cardiovascular mortality (log-rank test for trend, P = 0.168) did not differ significantly by CHA2DS2-VASc score group, respectively (Figures 1–3, respectively).
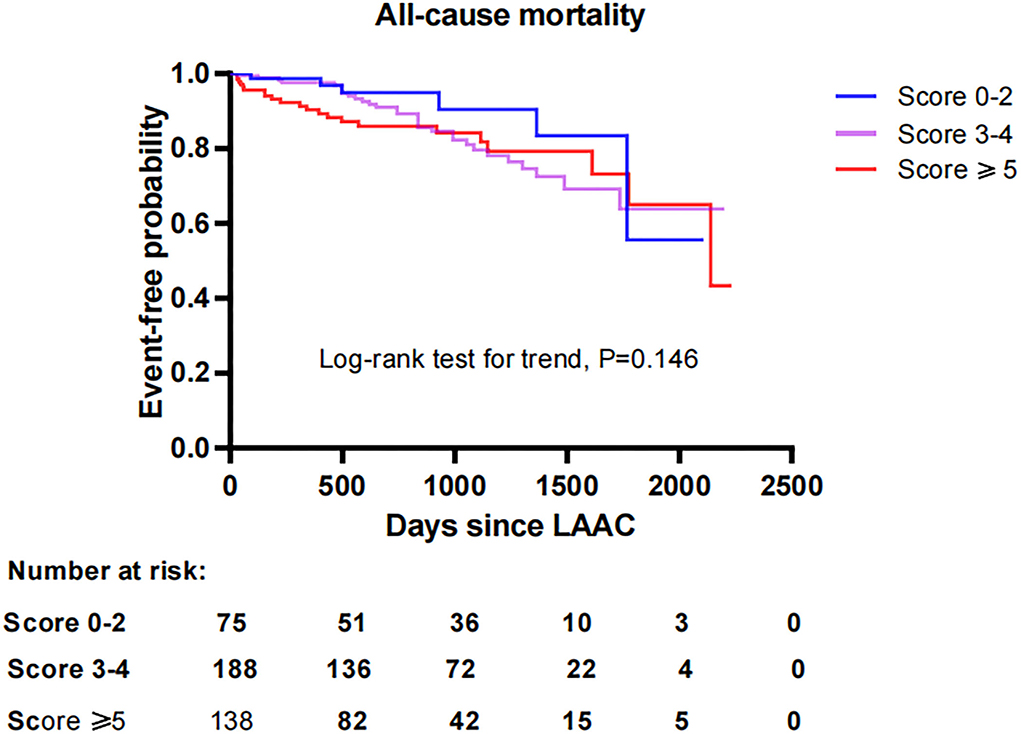
Figure 1. Comparison of the cumulative ratio of freedom from all-cause mortality in different subgroups. LAAC, left atrial appendage closure.
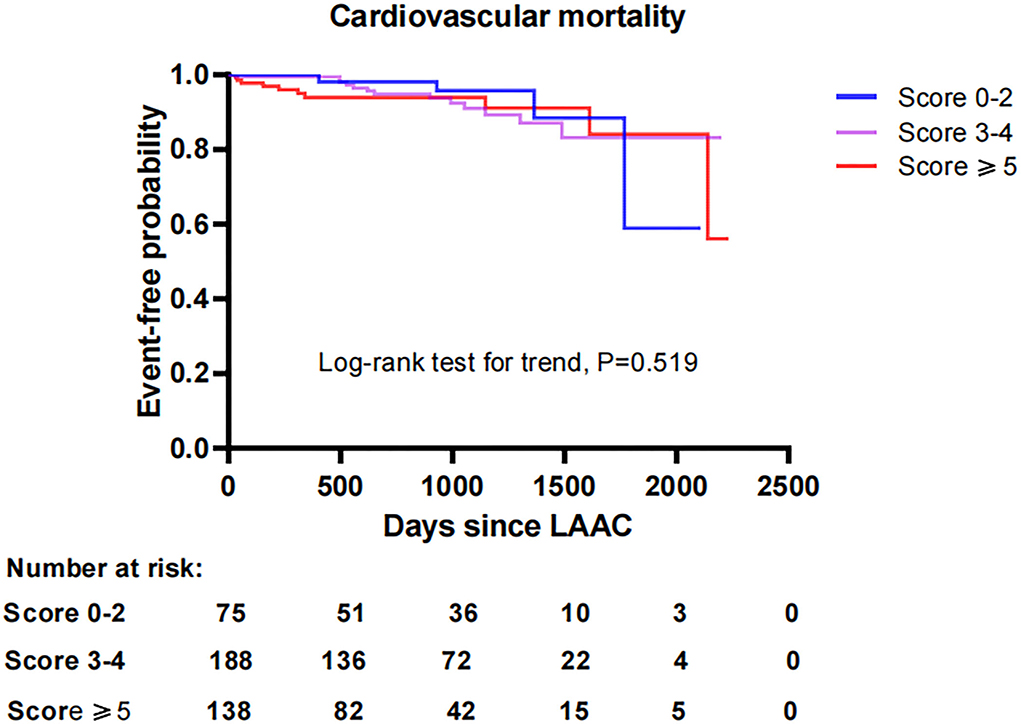
Figure 2. Comparison of the cumulative ratio of freedom from cardiovascular mortality in different subgroups. LAAC, left atrial appendage closure.
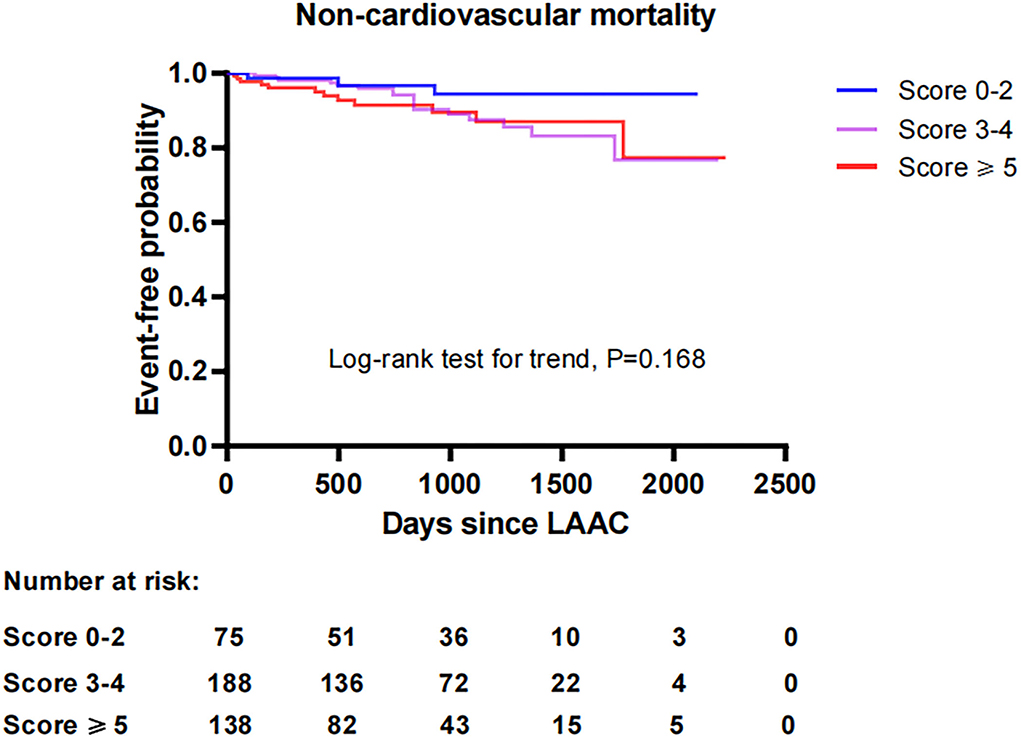
Figure 3. Comparison of the cumulative ratio of freedom from non-cardiovascular mortality in different subgroups. LAAC, left atrial appendage closure.
Effectiveness of LAAC on annual risk reduction of thromboembolism by CHA2DS2-VASc score
The estimated annualized thromboembolic rates were 8.5%, 2.9%, 6.7%, and 14.1% in the overall cohort, score 0–2, score 3–4, and score ≥5 groups, respectively. However, the observed annualized thromboembolic rates were 1.5%, 1.1%, 1.6%, and 1.5%, which yielded a RRR of 82.4% (RR: 5.667, 95% CI: 2.475–13.06, P < 0.001), 62.1% (RR: 2.000, 95% CI: 0.267–15.10, P = 1.000), 76.1% (RR 4.333, 95% CI: 1.353–14.03, P = 0.019), and 89.4% (RR 10.0, 95% CI: 2.675–38.13, P < 0.001), respectively (Figure 4). Accordingly, the NNT to prevent one thromboembolic event for LAAC was 14 (95% CI: 10–26), 75, 19 (95% CI: 10–127), and 8 (95% CI: 5–16), respectively.
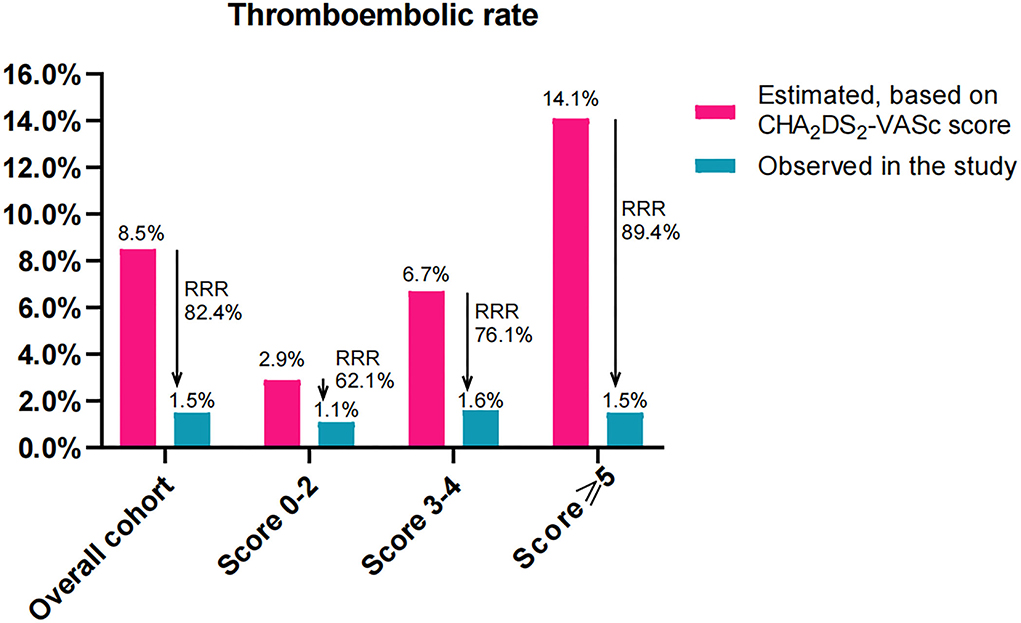
Figure 4. Effectiveness of LAAC in risk reduction of thromboembolism (100 patient-years) during the follow-up in the study groups divided according to CHA2DS2-VASc score. RRR, relative risk reduction.
For the comparison of RRR among the three subgroups, the level of RRR increased with CHA2DS2-VASc score (P < 0.001 for trend). Compared with patients in the score 0–2 group, the level of RRR was significantly increased in those with score 3–4 (RR: 0.824, 95% CI: 0.666–0.981, P = 0.033) and score ≥5 (RR: 0.703, 95% CI: 0.572–0.829, P < 0.001) groups, respectively. Meanwhile, the level of RRR was also markedly greater in patients with score ≥5 than that in those with score 3–4 (RR: 0.853, 95% CI: 0.770–0.943, P = 0.002).
Effectiveness of LAAC on annual risk reduction of major bleeding by CHA2DS2-VASc score
The estimated annualized major bleeding rates were 7.2%, 5.4%, 7.2%, and 8.3% in subjects of overall cohort, score 0–2, score 3–4, and score ≥5, respectively. However, the observed annualized major bleeding rates were 2.4%, 1.7%, 2.3%, and 2.9%, which constituted an RRR of 66.7% (RR: 3.048, 95% CI: 1.912–4.881, P < 0.001), 59.3% (RR: 3.333, 95% CI: 1.038–10.94, P = 0.078), 68.1% (RR 3.100, 95% CI: 1.594–6.09, P < 0.001), and 65.1% (RR 2.875, 95% CI: 1.366–6.128, P = 0.007), respectively (Figure 5). Accordingly, the NNT to prevent one major bleeding event for LAAC was 9 (95% CI: 7–6), 10, 9 (95% CI: 6–22), and 9 (95% CI: 5–35), respectively.
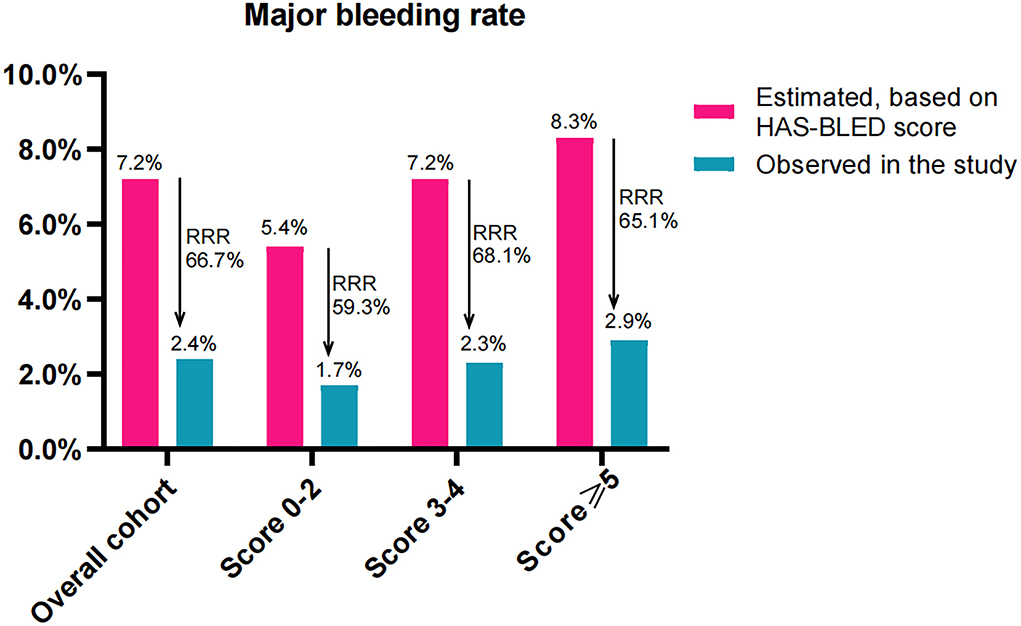
Figure 5. Effectiveness of LAAC in risk reduction of major bleeding (100 patient-years) during the follow-up in the study groups divided according to CHA2DS2-VASc score. RRR, relative risk reduction.
When comparing the RRR of major bleeding among the three subgroups, the extent of RRR did not differ significantly (P = 0.587 for trend). No statistically significant differences in the extent of RRR were found between patients score 3–4 and score 0–2 (RR: 0.881, 95% CI: 0.702–1.069, P = 0.250), and between patients score ≥5 and score 3–4 (RR: 1.044, 95% CI: 0.895–1.228, P = 0.634) or even score 0–2 (RR: 0.92, 95% CI: 0.726–1.136, P = 0.460).
Discussion
This study demonstrated the following findings. (1) For AF patients, the higher the CHA2DS2-VASc score, the higher the comorbidity; however, no statistically significant differences were observed in implantation success or periprocedural complications along all score groups after LAAC. (2) The long-term outcomes and the cumulative ratio of freedom from mortality were comparable across all score groups. (3) LAAC decreased the thromboembolic risk in the overall cohort, score 3–4, and score ≥5 groups compared with estimated risk. The level of RRR of thromboembolism increased with CHA2DS2-VASc score among the three score subgroups. (4) LAAC lowered the major bleeding risk in the overall cohort, score 3–4, and score ≥5 groups compared with estimated risk, but the extent of RRR in major bleeding did not differ among subgroups.
The baseline clinical characteristics of the overall cohort were similar to those reported in previous randomized clinical trials, except for a higher proportion of patients ≥75 years of age and a slightly lower incidence of hypertension (6). We noted that the score ≥5 group not only had the oldest patients but also had the highest percentage of women and had a significantly increased prevalence of concomitant multimorbidity, such as hypertension, CHD, diabetes, CHF, previous stroke, previous major bleeding, and CKD, as well as the highest HAS-BLED scores. This finding is expected as many of the aforementioned clinical variables are scoring criteria for the CHA2DS2-VASc scale (13). In regard to the high HAS-BLED score correlating with a higher CHA2DS2-VASc score, scoring criteria for the HAS-BLED scale are similar to those of the CHA2DS2-VASc scale (14). Previous studies showed that the clinical risk of stroke or death in patients with AF increased with increasing CHA2DS2-VASc score (15, 16). Yoon et al. reported that accumulation of risk factors with increasing CHA2DS2-VASc score may translate to greater thromboembolism risk in AF patients at 10-year follow-up (17). Therefore, patients with higher CHA2DS2-VASc score were deemed as high-risk groups in our study. Nevertheless, the device implant success rate was consistent regardless of CHA2DS2-VASc score, and the incidence rate of periprocedural complications within 7 days did not demonstrate a significant increasing trend among patients with elevated CHA2DS2-VASc scores post-LAAC. Our implantation success rate was similar to that in the EWOLUTION registry, but the total incidence of periprocedural complications through 7 days was lower (18). Overall, the results showed that LAAC was equally safe in AF patients, regardless of CHA2DS2-VASc score.
With respect to the impact of CHA2DS2-VASc score on efficacy and long-term prognosis of LAAC, Ivănescu reported that major adverse cardiovascular and cerebrovascular events, including myocardial infarction, stroke, and mortality, increased with CHA2DS2-VASc score in AF patients (11). NVAF patients with increased CHA2DS2-VASc scores are not only considered prone to thromboembolic events but also associated with an increased risk of major bleeding in patients receiving oral warfarin or rivaroxaban (19). Furthermore, a small sample study suggested the possible role of CHA2DS2-VASc score in the development of DRT after LAAC (20). Even in patients with other conditions such as chronic kidney disease or CHD regardless of the presence of AF, CHA2DS2-VASc score was also identified to be significantly associated with worse outcomes (21, 22). However, did the correlation between increased CHA2DS2-VASc score and worse prognosis still present in patients with AF treated with LAAC? Our study showed that patients with higher CHA2DS2-VASc score did not experience worse outcomes in the incidences of thromboembolism, major bleeding, DRT, mortality risk, and combined efficacy endpoints in comparison to those with a lower score after LAAC during long-term follow-up. The higher incidences of adverse clinical outcomes attributed to higher CHA2DS2-VASc score were not observed in patients with higher scores undergoing LAAC in this study. Especially for the difference in mortality by CHA2DS2-VASc score, survival curves calculated by Kaplan-Meier estimation demonstrated that the cumulative ratio of freedom from all-cause mortality, cardiovascular mortality, or non-cardiovascular mortality was similar across the all score groups after LAAC, respectively. These results were not in accordance with those in other intervention studies that increases in CHA2DS2-VASc score were correlated with poor outcomes in AF patients or those receiving anticoagulation therapy (11, 19, 23). Previous studies also revealed that patients with AF, as well as those treated with oral anticoagulants who were at higher risk for major adverse cardiovascular and cerebrovascular events, could be identified by CHA2DS2-VASc score (11, 24). However, it was noteworthy that our study demonstrated that the CHA2DS2-VASc score did not significantly affect the risks of major adverse outcomes in AF patients who underwent LAAC during long-term follow-up. This might be due to the substantial differences in efficacy derived from different intervention strategies. LAAC vs. DOACs may have favorable effectiveness in reducing the risk of major bleeding and mortality among high-risk patients with AF (25). Furthermore, patients with NVAF who previously underwent LAAC had better neurological outcomes after acute ischemic stroke events in comparison with patients on warfarin (26). Therefore, these significant advantages of LAAC intervention over OAC therapy in high-risk AF patients may lead to the inconsistency regarding the influence of CHA2DS2-VASc score on outcomes among NVAF patients treated with LAAC and OAC. In fact, our findings suggest that LAAC may eliminate or normalize the difference of long-term adverse outcomes by CHA2DS2-VASc score in AF patients.
In the overall cohort, the observed yearly thromboembolic rate was as low as 1.5%, representing a significant RRR of 82.4% in thromboembolism after LAAC compared with the expected risk by CHA2DS2-VASc score, which was in line with findings in LAAC registries with a risk reduction of 84% in the composite endpoint of ischemic stroke/TIA/systemic embolism (27). Despite the higher annual rate of expected thromboembolism due to an increase in CHA2DS2-VASc score in AF patients, subgroup analysis showed that the observed annual thromboembolic rates did not significantly increase with CHA2DS2-VASc score point. Conversely, the RRR in thromboembolism increased significantly with CHA2DS2-VASc score across all score groups with an additional significant RRR in score 3–4 and ≥5 groups. In fact, patients with the highest CHA2DS2-VASc scores experienced the greatest RRR in thromboembolism post-LAAC. To gain a better understanding of the clinical relevance to this thromboembolism reduction, the NNTs for LAAC intervention were calculated. The NNT for LAAC intervention in the score ≥5 group was particularly low-−8 (95% CI: 5–16) over a mean 2.2 years of follow-up—which was notable in comparison with the NNTs in the 0–2 and 3–4 groups of 75 and 19, respectively. These results demonstrated that the higher the CHA2DS2-VASc score, the greater the benefit of LAAC intervention in risk reduction of thromboembolism in AF patients. Our conclusions were different from other reports, in which AF patients with higher CHA2DS2-VASc score were still at higher risk and did not benefit more from optimal anticoagulation or ablation in risk reduction of the thromboembolic event or other adverse outcomes (28, 29). So, our findings highlighted the greater efficacy of LAAC intervention in the RRR of thromboembolism in higher score groups but especially in patients with the highest CHA2DS2-VASc score. This may be one of the reasons why no worse outcomes were found in patients with vs. without a higher CHA2DS2-VASc score after LAAC. Further study with large sample size is needed to verify the conclusions in such a special group.
Several studies reported that CHA2DS2-VASc score is also associated with the risk of major bleeding in patients who underwent anticoagulation therapy (19, 30). Therefore, the expected annualized major bleeding rates based on HAS-BLED score were evidently higher in patients with higher CHA2DS2-VASc scores in our study. However, the observed annual major bleeding rate after LAAC was statistically lower than the expected risk in the overall cohort group with a RRR of 66.7% in major bleeding, which was similar to the risk reduction of 70% of major bleeding in AF patients treated with combined LAAC and catheter ablation (27). Recent randomized control trials confirmed that LAAC could yield a favorable outcome in major bleeding compared with treatment by warfarin or DOACs in AF patients (6, 31). Our subgroup analysis also indicated that patients with higher CHA2DS2-VASc score rather than those with the score 0–2 exhibited lower observed annual major bleeding rates compared with the HAS-BLED predicted rates. Interestingly, unlike RRR in thromboembolism, the level of RRR in major bleeding did not increase statistically with CHA2DS2-VASc score across the three groups. This suggests that LAAC may offer major bleeding benefits in high-risk patients and that the efficacy of LAAC on the extent of RRR in major bleeding does not appear to be influenced by CHA2DS2-VASc score.
There are several limitations in this study. First, this is a non-randomized observational study that may exist a possible selection bias and have a decreased power in assessing LAAC efficacy and long-term outcomes by CHA2DS2-VASc score. Second, we investigated the effect of LAAC on thromboembolism and major bleeding events using comparisons with historically expected risks instead of risks of control groups, which limits the scope of our conclusions as risk scores do not contain all individual patient factors. Finally, the relatively small number of patients may not be enough to evaluate the LAAC effectiveness by CHA2DS2-VASc score.
In summary, despite NVAF patients with higher CHA2DS2-VASc score being susceptible to an increased risk of major adverse cardio-cerebrovascular events, they did not exhibit lower implantation success, more periprocedural complications, or worse long-term outcomes after LAAC compared to those with lower CHA2DS2-VASc score. LAAC resulted in significant decreases in the risks of thromboembolism and major bleeding over the expected risks in patients with higher scores, and the level of RRR in thromboembolism rather than in major bleeding increased with CHA2DS2-VASc score among the three score groups. This study suggests that LAAC may eliminate the difference in long-term adverse outcomes by CHA2DS2-VASc score, which may be partly attributed to more benefits provided by LAAC intervention in AF patients with a higher CHA2DS2-VASc score versus those with a lower score.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Helmut-G.-Walther Klinikum, Lichtenfels, Germany and Zhengzhou Ninth People's Hospital, Zhengzhou, China. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MiZ designed the study, performed the data collection and data analysis, interpreted the patient data, and wrote the manuscript. MeZ performed data collection, statistical analysis of data, production of the statistical graph, and revision of the manuscript. CH, FP, and NH performed a critical revision of the manuscript. JW, QY, and ZM contributed to the data analysis and discussion of the results. JY contributed to the study design, interpretation of data, and critical revision of the manuscript. All authors read and approved the final version of the manuscript.
Conflict of interest
JY is a consultant to Boston Scientific and LifeTech Scientific.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lloyd-Jones DM, Wang TJ, Leip EP. Lifetime risk for development of atrial fibrillation: the Framingham heart study. Circulation. (2004) 110:1042–6. doi: 10.1161/01.CIR.0000140263.20897.42
2. Bosch NA, Cimini J, Walkey AJ. Atrial fibrillation in the ICU. Chest. (2018) 154:1424–34. doi: 10.1016/j.chest.2018.03.040
3. Själander S, Sjögren V, Renlund H, Norrving B, Själander A. TDabigatran, rivaroxaban and apixaban vs. high TTR warfarin in atrial fibrillation. Thromb Res. (2018) 167:113–18. doi: 10.1016/j.thromres.2018.05.022
4. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383:955–62. doi: 10.1016/S0140-6736(13)62343-0
5. Marsico F, Cecere M, Parente A, Paolillo S, de Martino F, Dellegrottaglie S, et al. Effects of novel oral anticoagulants on left atrial and left atrial appendage thrombi: an appraisal. J Thromb Thrombolysis. (2017) 43:139–48. doi: 10.1007/s11239-016-1421-9
6. Reddy VY, Doshi SK, Kar S, Gibson DN, Price MJ, Huber K, et al. 5-year outcomes after left atrial appendage closure: from the prevail and protect AF trials. J Am Coll Cardiol. (2017) 70:2964–75. doi: 10.1016/j.jacc.2017.10.021
7. Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol. (2020) 75:3122–35. doi: 10.1016/j.jacc.2020.04.067
8. Borre E, Goode A, Raitz G, Shah B, Lowenstern A, Chatterjee R, et al. Predicting thromboembolic and bleeding event risk in patients with non-valvular atrial fibrillation: a systematic review. Thromb Haemost. (2018) 118:2171–87. doi: 10.1055/s-0038-1675400
9. Proietti M, Rivera-Caravaca JM, Esteve-Pastor MA, Marín F, Lip GYH. Stroke and thromboembolism in warfarin-treated patients with atrial fibrillation: comparing the CHA2DS2-VASc and GARFIELD-AF risk scores. Thromb Haemost. (2021) 121:1107–14. doi: 10.1055/a-1333-4448
10. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collabo-ration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2020) 42:373–498. doi: 10.1093/eurheartj/ehaa798
11. Ivănescu AC, Buzea CA, Delcea C, Dan GA. Stroke risk scores as predictors of severe outcomes in atrial fibrillation: a comprehensive review. Am J Ther. (2021) 28:e319–34. doi: 10.1097/MJT.0000000000001357
12. Fountain RB, Holmes DR, Chandrasekaran K, Packer D, Asirvatham S, Van Tassel R, et al. The PROTECT AF (WATCHMAN left atrial appendage system for embolic PROTECTion in patients with atrial fibrillation) trial. Am Heart J. (2006) 151:956–61. doi: 10.1016/j.ahj.2006.02.005
13. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial fibrillation cohort study. Eur Heart J. (2012) 33:1500–10. doi: 10.1093/eurheartj/ehr488
14. Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, stroke, bleeding history or Predisposition, Labile INR, elderly, drugs/alcohol concomitantly) score. J Am Coll Cardiol. (2011) 57:173–80. doi: 10.1016/j.jacc.2010.09.024
15. Saliba W, Gronich N, Barnett-Griness O, Rennert G. Usefulness of CHADS2 and CHA2DS2-VASc VASc scores in the prediction of new-onset atrial fifibrillation: a population-based study. Am J Med. (2016) 129:843–49. doi: 10.1016/j.amjmed.2016.02.029
16. Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY. Assessment of the CHA2DS2-VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA. (2015) 314:1030–8. doi: 10.1001/jama.2015.10725
17. Yoon M, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. Dynamic changes of CHA2DS2-VASc score and the risk of ischaemic stroke in asian patients with atrial fibrillation: a Nationwide Cohort Study. Thromb Haemost. (2018) 118:1296–304. doi: 10.1055/s-0038-1651482
18. Boersma LV, Schmidt B, Betts TR, Sievert H, Tamburino C, Teiger E, et al. EWOLUTION investigators. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. (2016) 37:2465–74. doi: 10.1093/eurheartj/ehv730
19. Peacock WF, Tamayo S, Patel M, Sicignano N, Hopf KP, Yuan Z. CHA2DS2-VASc scores and major bleeding in patients with nonvalvular atrial fibrillation who are receiving rivaroxaban. Ann Emerg Med. (2017) 69:541–50. doi: 10.1016/j.annemergmed.2016.09.032
20. Kaneko H, Neuss M, Weissenborn J, Butter C. Predictors of thrombus formation after percutaneous left atrial appendage closure using the WATCHMAN device. Heart Vessels. (2017) 32:1137–43. doi: 10.1007/s00380-017-0971-x
21. Vodošek Hojs N, Ekart R, Bevc S, Piko N, Hojs R. CHA2DS2-VASc score as a predictor of cardiovascular and all-cause mortality in chronic kidney disease patients. Am J Nephrol. (2021) 52:404–11. doi: 10.1159/000516121
22. Parfrey S, Teh AW, Roberts L, Brennan A, Clark D, Duffy SJ, et al. The role of CHA2DS2-VASc score in evaluating patients with atrial fibrillation undergoing percutaneous coronary intervention. Coron Artery Dis. (2021) 32:288–94. doi: 10.1097/MCA.0000000000000987
23. Raatikainen MJP, Penttilä T, Korhonen P, Mehtälä J, Lassila R, Lehto M. The quality of warfarin therapy and CHA2DS2-VASc score associate with the incidence of myocardial infarction and cardiovascular outcome in patients with atrial fibrillation: data from the nationwide FinWAF Registry. Eur Heart J Cardiovasc Pharmacother. (2018) 4:211–9. doi: 10.1093/ehjcvp/pvy009
24. Jover E, Roldán V, Gallego P, Hernández-Romero D, Valdés M, Vicente V, et al. Predictive value of the CHA2 DS2-VASc score in atrial fibrillation patients at high risk for stroke despite oral anticoagulation. Rev Esp Cardiol (Engl Ed). (2012) 65:627–33. doi: 10.1016/j.rec.2012.02.016
25. Nielsen-Kudsk JE, Korsholm K, Damgaard D, Valentin JB, Diener HC, Camm AJ, et al. Clinical outcomes associated with left atrial appendage occlusion versus direct oral anticoagulation in atrial fibrillation. JACC Cardiovasc Interv. (2021) 14:69–78. doi: 10.1016/j.jcin.2020.09.051
26. Lee OH, Kim YD, Kim JS, Pak HN, Hong GR, Shim CY, et al. Favorable neurological outcome after ischemic cerebrovascular events in patients treated with percutaneous left atrial appendage occlusion compared with warfarin. Catheter Cardiovasc Interv. (2019) 94:E23–E9. doi: 10.1002/ccd.27913
27. Phillips KP, Romanov A, Artemenko S, Folkeringa RJ, Szili-Torok T, Senatore G, et al. Combining left atrial appendage closure and catheter ablation for atrial fibrillation: 2-year outcomes from a multinational registry. Europace. (2020) 22:225–31. doi: 10.1093/europace/euz286
28. Zhu WG, Xiong QM, Hong K. Meta-analysis of CHADS2 versus CHA2DS2-VASc for predicting stroke and thromboembolism in atrial fibrillation patients independent of anticoagulation. Tex Heart Inst J. (2015) 42:6–15. doi: 10.14503/THIJ-14-4353
29. Jacobs V, May HT, Bair TL, Crandall BG, Cutler M, Day JD, et al. The impact of risk score (CHADS2 versus CHA2DS2-VASc) on long-term outcomes after atrial fibrillation ablation. Heart Rhythm. (2015) 12:681–6. doi: 10.1016/j.hrthm.2014.12.034
30. Roldan V, Marin F, Manzano-Fernandez S, Gallego P, Vilchez JA, Valdes M, et al. The HAS-BLED score has better prediction accuracy for major bleeding than CHADS2 or CHA2DS2-VASc scores in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. (2013) 62:2199–204. doi: 10.1016/j.jacc.2013.08.1623
Keywords: atrial fibrillation, CHA2DS2-VASc score, left atrial appendage closure, outcomes, stroke, major bleeding
Citation: Zhao M, Zhao M, Hou CR, Post F, Herold N, Walsleben J, Yuan Q, Meng Z and Yu J (2022) Comparative analysis of left atrial appendage closure efficacy and outcomes by CHA2DS2-VASc score group in patients with non-valvular atrial fibrillation. Front. Cardiovasc. Med. 9:905728. doi: 10.3389/fcvm.2022.905728
Received: 27 March 2022; Accepted: 28 June 2022;
Published: 22 July 2022.
Edited by:
Shaojie Chen, Cardioangiological Center Bethanien (CCB), GermanyReviewed by:
Dongming Hou, Boston Scientific, United StatesMamoo Nakamura, Cedars Sinai Medical Center, United States
Copyright © 2022 Zhao, Zhao, Hou, Post, Herold, Walsleben, Yuan, Meng and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangtao Yu, ai55dUBray1rbS5kZQ==
†These authors have contributed equally to this work and share first authorship
 Mingzhong Zhao
Mingzhong Zhao Mengxi Zhao
Mengxi Zhao Cody R. Hou4
Cody R. Hou4 Zhaohui Meng
Zhaohui Meng Jiangtao Yu
Jiangtao Yu