- 1Department of Psychiatry, Jiangxi Mental Hospital, Affiliated Mental Hospital of Nanchang University, Nanchang, China
- 2Jiangxi Provincial Clinical Research Center on Mental Disorders, Nanchang, China
- 3Department of Medical, Queen Mary School, Nanchang University, Nanchang, China
- 4Jiangxi Key Laboratory of Molecular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 5Department of Cardiology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 6Department of Critial Care Medicine, The First Affiliated Hosptial of Gannan Medical University, Ganzhou, China
Background: Depression is a possible influence factor for the increased risk of incident atrial fibrillation (AF). Although several investigations have assessed their association, the results are still controversial. Therefore, we conducted a meta-analysis to evaluate the association between depression or using antidepressants and AF.
Methods: We systemically performed the literature retrieval from two electronic databases PubMed and EMBASE until March 2022 to extract relevant data. The hazard ratios (HRs) and odds ratios (OR) from included studies with 95% confidence intervals (CIs) were adjusted into the risk ratio (RR) and pooled by using the random-effects model.
Results: Totally 9 studies about the associations between depression or antidepressants and incident AF risk were included in this meta-analysis. Among them, 5 studies specifically analyzed the impact of antidepressants on the risk of AF. The outcomes of our analysis indicated that depression or depressive symptoms could increase AF risk (RR = 1.15, 95% CI, 1.03–1.27, P < 0.01). In addition, the use of antidepressants can also increase AF risk (RR = 1.16, 95% CI, 1.07–1.25, P < 0.001). These results remained unchanged when we remove the source of heterogeneity or adjust the analysis model into the fixed-effects model.
Conclusions: Based on existing investigations, both depression and the use of antidepressants are closely related to the increase of incident AF risk.
Introduction
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia with an age-related increase in incidence (1). It is strongly associated with stroke, heart failure morbidity (2–4), and increased mortality (5, 6). Early identification of high AF risk population is crucial for avoiding the adverse consequences related to AF. There are several factors have been identified to be related to the etiology of AF, including genetic factors, environmental factors, and other complications (7). However, more than one-third of the potential risk factor contributing to AF is still unexplained. Therefore, further investigations for additional AF risk factors including smoking, cardio-metabolic factors, and several psychological factors (8–10) need to be conducted.
In recent years, the association between depression and AF has been confirmed in several basic and epidemiological studies (11, 12). Theoretically, depression is closely related to the dysregulation of the hypothalamus-pituitary-adrenal (HPA) axis and inflammation. The hyperactivation of HPA axis could induce the consistent release of cortisol, which is also a marker of cortisol resistance (13). Cortisol resistance stimulates immune activation, then the expression level of some proinflammatory cytokines, including IL-2, IL-6, IL-12, and TNF-a will be increased. These cytokines act on the brain, developing some symptoms of depression in susceptible populations (14, 15), and are capable of producing systemic inflammation. The influenced HPA axis determines that depression is often accompanied by hypertension, metabolic syndrome, and obesity (16–18), which aggravate oxidative stress and inflammation in the body. Systemic inflammation increases the risk of AF by changing the electrophysiology (i.e., affecting calcium flowing), conduction and structural substrates of the atrial (19, 20). Moreover, depression may alter the sympathetic and parasympathetic balance to induce the decreased arrhythmic threshold (1), which also influences the atrial conductivity and structural integrity (14). Smoking as an accepted risk factor of AF, is more common in people with depression, since high negative affect and low positive affect in depression might raise the patients' dependence on nicotine (21). Nicotine has been reported with the function of promoting atrial structural remodeling and interstitial fibrosis (22). Therefore, depression is a potential factor for inducing new-onset AF.
As for antidepressants which are divided into three categories including selective serotonin reuptake inhibitor (SSRI), tricyclic antidepressants and monoamine oxidase inhibitor, their cardiotoxicity has also been reported in previous studies (23, 24). Tricyclic antidepressant mainly affects intraventricular conduction, which is characterized by prolonged PR, QRS and QT intervals on the electrocardiogram (ECG) (25). SSRI tends to increase serum serotonin, which then induces the elevation of intracellular calcium level (26, 27). As a result, the amplitude of the pacemaker is increased and potentially influences the heart rhythm (27). However, on the other hand, the use of antidepressants is capable of ameliorating the imbalance conditions of proinflammatory cytokines in depression (28–30), which may reduce the risk of depression-induced AF to a certain extent.
Considering that whether depression and the use of antidepressants could increase the risk of AF remains a controversial issue in previous studies, herein, we performed a meta-analysis including all of the existing studies to detect the association between AF risk and depression.
Methods
This meta-analysis was based on the preferred reporting items for systematic review and meta-analysis (PRISMA) 2020 guidelines. Ethical approval was not provided since all data included in this study was from the published studies. The data, techniques, and materials that support the findings of this study will be available from the corresponding author according to reasonable requests.
Literature Retrieval
The PubMed, and EMBASE electronic databases were selected for systemic search in this study. Two independent reviewers identified potentially eligible studies that reported the relationship between depression and the risk of AF. There were no language restrictions in the retrieval process. Terms used in screening include (atrial fibrillation OR atrial flutter) AND (depression OR depressive symptom OR antidepressant). The literature search strategy is resented in Supplementary Table 1, and the last retrieval was conducted in March 2022.
Eligibility Criteria
Literatures meeting the following criteria were included in this study: (1) Studies that reported the relationship between depression, depressive symptoms or the use of antidepressants and the risk of incident AF; (2) Cohort or case-control studies included both comparison and control groups, and data were obtained through follow-up; (3) Studies defined the depression and depressive symptoms according to definite criteria. There was no limitation on the follow-up period. Specific literature forms including reviews, case reports, case series, editorials and meeting abstracts were excluded from this study. In addition, studies with insufficient clinical data were also decided for exclusion.
Study Selection and Data Extraction
Two authors extracted data independently through screening the literature titles and abstracts. Then the full-text screening was conducted to detect whether the literature met the inclusion criteria. All discrepancies were resolved by discussing with the third researcher. If multiple studies from the same data source were suitable for this meta-analysis, only the study that best matched the eligibility criteria were included. Studies with later publication years and longer follow-up periods were preferentially included.
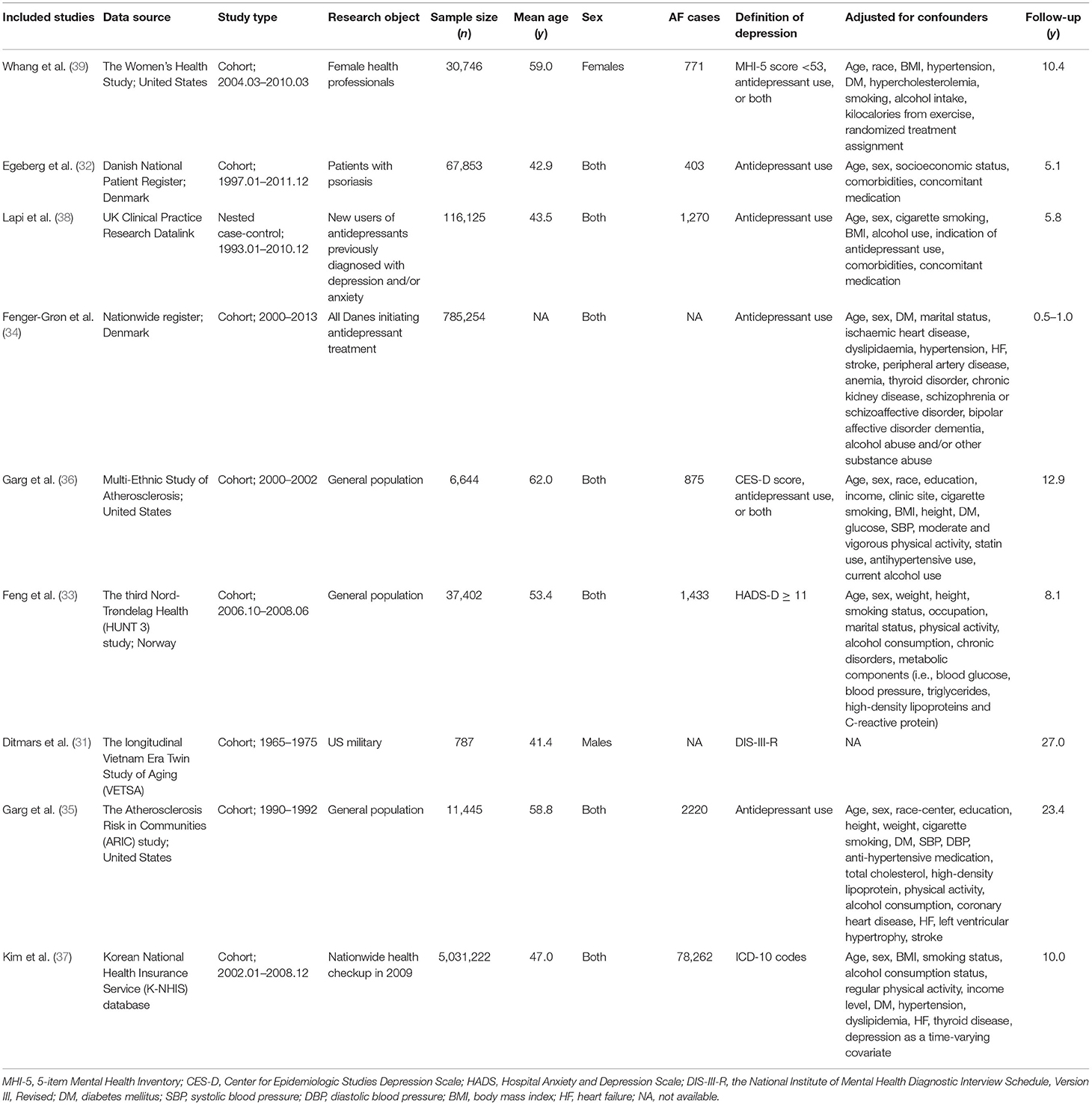
The relevant information of each study was recorded, including the first author, publication year, data source, information of participants (sample size, age, and sex), the definition of depression, adjusted confounders, and follow-up period. For the included studies that reported the adjusted RRs by using multiple models, only the most adjusted data was used in this meta-analysis.
Study Quality Assessment
The quality of eligible studies was assessed by using the Newcastle-Ottawa Scale tool, which covers three aspects, ranging from 0 to 9 stars: the cohort selection (0–4 stars), cohort comparability (0–2 stars), and the evaluation of study outcomes (0–3 stars). Studies with NOS results of <6 points were considered as low quality.
Statistical Analysis
The statistical analyses were performed by using the Review Manager version 5.4 software (The Cochrane Collaboration 2020, Nordic Cochrane Centre Copenhagen, Denmark; https://community.cochrane.org/). The statistical heterogeneity was evaluated by I2 statistic and the Cochrane Q-test. Either P < 0.1 for the Q-test or I2 ≥ 50% was considered as the indication of substantial heterogeneity. For the results, P < 0.05 was considered to have statistical significance. To the extent possible, we used the same treatment effect indicators in cohort and case-control studies. Reported maximally adjusted hazard ratios (HRs) or odds ratios (ORs), and 95% confidence intervals (CIs) were extracted. For studies that reported multiple categories of psychological factors (e.g., the degree of depressive symptoms), HR in the most severe category was used. In the results section, we refer to all relative effects metrics as “risk ratios” (RRs), which do not affect the study results or their interpretation. The corresponding natural logarithm (Ln[RR]) and standard error (SE) of each investigation were used for calculation. Given the intrinsic heterogeneity of these included studies, the inverse-variance weighted random-effects model was applied to pool the Ln [RR] and its SE. The publication bias was assessed by funnel plots as well as Egger's and Begg's tests.
Results
Study Selection
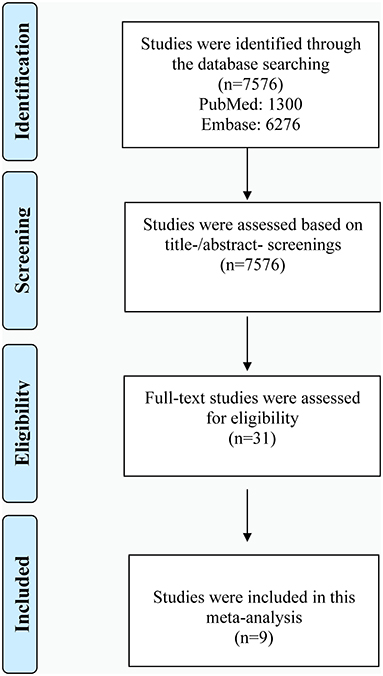
Shown in Figure 1 is the literature retrieval process. Totally, 7,576 studies were obtained from initial online searching. Among them, 1,300 studies were from PubMed and 6,276 studies from Embase database. All of these studies were assessed based on title/abstract screening. Then 31 studies underwent full-text review for eligibility assessment. Based on predefined criteria, finally, 9 eligible studies were included in our meta-analysis (31–39). The diagnostic basis of depression and AF were clearly defined in all included articles. Exhibited in Table 1 is the baseline information of participants, while the diagnostic methods for AF in included studies are presented in Supplementary Table 2. As shown in Supplementary Table 3, all of the included studies in our investigation were considered as moderate to high quality.
Relationship Between Depression and AF
All of our eligible studies examined the association between depression and the risk of incident AF. Among them, most of the studies indicated that depression was related to the increase of AF risk. Only studies from Whang et al. and Feng et al. reported that there was no evidence of an association between the increased AF risk and depression (33, 39). Presented in Figure 2 is the outcome of our meta-analysis, which indicates that depression or depressive symptoms could increase AF risk (RR = 1.15, 95% CI, 1.03–1.27, P < 0.01). However, this result represented relatively high heterogenicity (I2 = 88%). In order to detect the source of heterogeneity, we screened and analyzed all of the data in the included studies by the exclusive method. As shown in Supplementary Figure 1, after removing the data from Kim et al., the heterogeneity is acceptable and the final outcome was not influenced (RR = 1.12, 95%CI, I2 = 42, P < 0.01). Also, this result was not changed when we adjusted the analysis into the fixed-effects model (RR = 1.27, 95%CI, 1.24–1.30, P < 0.001; Supplementary Figure 2).
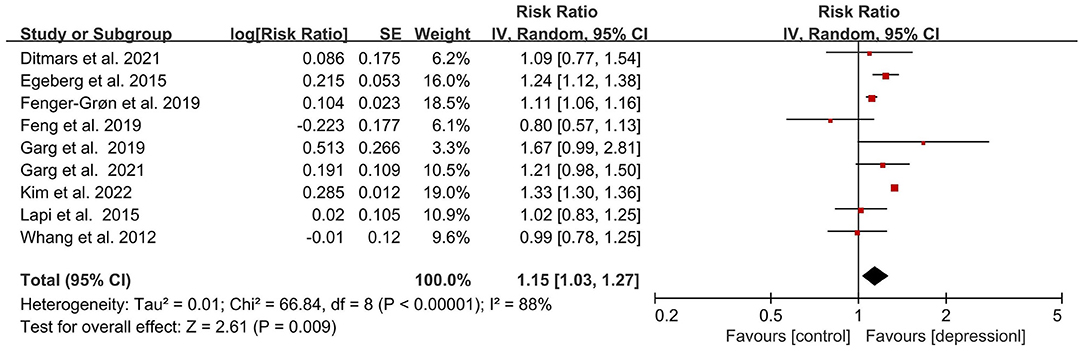
Figure 2. Forest plot for association of depression with atrial fibrillation risk. SE, standard error; CI, confidence interval; IV, inverse of the variance.
Relationship Between Antidepressants and AF
Five eligible studies reported the associations between the use of antidepressants and AF risk. Four of them reported that the risk of incident AF in antidepressant users was substantially increased. Only the study from Lapi et al. indicated that exposure to antidepressants is not associated with the increased risk of AF (38). As shown in Figure 3, the outcome of our meta-analysis support that the risk of incident AF was significantly increased in the antidepressant using population (RR = 1.16, 95% CI, 1.07–1.25, P < 0.001). The heterogenicity of included studies was acceptable (I2 = 42%). This result remained unchanged when we adjusted the analysis model into the fixed-effects model (RR = 1.13, 95%CI, 1.09–1.18, P < 0.001; Supplementary Figure 3).
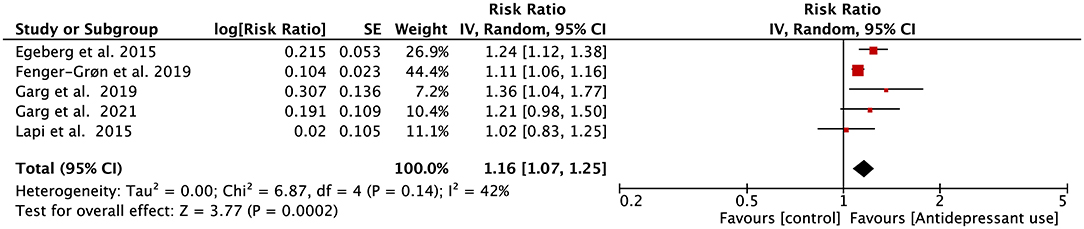
Figure 3. Forest plot for association of antidepressant use with atrial fibrillation risk. SE, standard error; CI, confidence interval; IV, inverse of the variance.
Publication Bias
As for bias risk assessment, the corresponding funnel plots for depression and antidepressant related studies were included in Supplementary Figures 4, 5. We also adopted Egger's and Begg's test to detect the presence of publication biases, which were presented in Supplementary Figures 6, 7. The results showed that the P-values of the two groups were ≥ 0.1, indicating that the bias risk of included studies in our meta-analysis was relatively lower.
Discussion
Based on previous studies, we conducted this meta-analysis to evaluate the association between depression, antidepressants and the risk of AF. After pooling the data from 9 included observational studies, the primary outcomes of our investigation indicate that both depression and the use of antidepressants are capable of increasing the risk of incident AF.
Previous studies suggested that the occurrence of depression or depressive symptoms is closely related to some immune signaling, especially proinflammatory cytokines IL-2, IL-6, IL-12 and TNF-a (14, 40, 41). These cytokines are capable of inducing systemic inflammation, which is a potential risk factor of AF since the inflammatory cell infiltration has been observed in the atrial of AF patients (42, 43). According to the studies investigating the relationship between depression and AF, these two symptoms are in a comorbid state (44, 45). However, earlier meta-analysis pointed out that depression is related to the increased risks of sudden cardiac death, ventricular tachycardia/ventricular fibrillation, and AF recurrence (19), but the association between depression and incident AF was not considered to exist (10, 19). After reanalysis, we considered that some non-negligible limitations might affect their accuracy. As for the study from Shi et al., only two studies specific for depression and AF were included. In the study from Whang et al., the selection of the included population, such as only women or mainly white people may lead to the deviation of outcomes. In addition, people identified as depression through questionnaires were also included in this meta-analysis, which may induce a bias in the diagnosis of depression. Fu et al. only included 5 studies in their study, the majority of the study participants were from the US or Europe, the ethnic interference of study outcome also cannot be fully eliminated.
In addition, whether the use of antidepressants could impact AF risk was also a controversial issue in the previous studies (38, 46). Theoretically, tricyclic antidepressants affect cardiac conduction and cardiotoxicity (25). As for the use of SSRI antidepressants, patients' serum serotonin levels are elevated during medication. Serotonin promotes calcium overload, which may trigger focal atrial extrasystoles and increase the risk of AF (27, 47). Both of these two types of drugs are associated with prolonged QTc and increased risk of arrhythmias (48), but studies focusing on the risk of incident AF increased by monoamine oxidase inhibitors are relatively fewer. However, studies have suggested that treating with antidepressants may alter the imbalance conditions of inflammatory cytokines in depression (28–30). Our findings provide strong evidence for the view that antidepressants increase the risk of incident AF, which indicates that the prevention of AF in patients with depression deserves further research in the future.
After incorporating all of the latest relevant studies, the outcome of our analysis confirms the theoretical link between depression and incident AF. However, the substantial heterogeneity of our investigation still exists. After removing the study from Kim et al. (37), the outcome was not influenced and the heterogeneity is acceptable. The sources of heterogeneity are speculated as follows: (1) The race difference between this study and other included studies was non-negligible. Kim et al. obtained data from the Korean National Health Insurance Service database, whose study population was Asian. Except for this study, the investigation participants of other included studies in our meta-analysis were from the United States or Europe. The risk of incident AF has shown differences among different ethnic groups (49, 50). (2) This study was based on insurance claim data of ICD-10 codes for depression and AF, rather than incident AF diagnosis during follow-up. The different outcome definitions between this study and the rest studies may also lead to the existence of heterogeneity.
Previous investigations have pointed out the molecular mechanisms by which depression and the use of antidepressants might increase the risk of incident AF. These mechanisms laid a theoretical foundation for our research. After pooling all of the data from existing investigations, the results of our study quantitatively confirm that patients with depression and antidepressant users have an increased risk of new-onset AF by 15 and 16%, respectively. These data suggest that the cardiovascular health of patients with depression deserves special attention, and it is necessary to strengthen the cooperation between cardiologists and psychiatrists in the process of depression treatment. For future study, the effects of different types of antidepressants on incident AF deserves further exploration, which is helpful to formulate a more reasonable management plan for patients with depression.
Limitations
Although our study has included as much data as possible and tried to avoid the influence of confounding factors, several potential limitations still exist. First of all, the substantial heterogeneity is relatively high in our study. This may be induced by analysis strategies and participant features. However, due to the limited data, subgroup analysis based on these factors cannot be carried out. Secondly, the evaluation criteria for depressive symptoms and incident AF were inconsistent, which might induce the existence of small deviations in the diagnosis of depression. Thirdly, most of the data were obtained from observational cohort studies. Although most of them were adjusted for multivariable confounding factors, the corresponding information of each included literature was not completely consistent, and the residual confounding factors cannot be completely excluded. Future studies can use the method of propensity score matching to make the baseline data of participants more comparable. Finally, the number of existing studies in the antidepressant group was relatively limited, which does not support the subgroup analysis of different types of antidepressants. Future studies can assess the effects of different types of antidepressants on incident AF after incorporating more eligible data.
Conclusion
Based on existing investigations, both depression and the use of antidepressants are related to the increased risk of incident AF. Further study is needed to conduct more subgroup analysis and confirm our findings.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by the National Natural Science Foundation of China [No. 31960146] and Special fund for postgraduate innovation in Jiangxi Province (YC2012-B011).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.897622/full#supplementary-material
References
1. Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet (London, England). (2012) 379:648–61. doi: 10.1016/S0140-6736(11)61514-6
2. Jørgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS. Acute stroke with atrial fibrillation. Copenhagen Stroke Study Stroke. (1996) 27:1765–9. doi: 10.1161/01.STR.27.10.1765
3. Hart RG, Palacio S, Pearce LA. Atrial fibrillation, stroke, and acute antithrombotic therapy: analysis of randomized clinical trials. Stroke. (2002) 33:2722–7. doi: 10.1161/01.STR.0000035735.49388.4A
4. Fuster V, Rydén LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in Collaboration With the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol. (2001) 38:1231–66. doi: 10.1016/S0735-1097(01)01587-X
5. Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol. (2007) 49:986–92. doi: 10.1016/j.jacc.2006.10.062
6. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. (2002) 113:359–64. doi: 10.1016/S0002-9343(02)01236-6
7. Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. (2009) 119:606–18. doi: 10.1161/CIRCULATIONAHA.108.825380
8. Zhu W, Yuan P, Shen Y, Wan R, Hong K. Association of smoking with the risk of incident atrial fibrillation: A meta-analysis of prospective studies. Int J Cardiol. (2016) 218:259–66. doi: 10.1016/j.ijcard.2016.05.013
9. Gaman M-A, Dobrica E-C, Pascu E, Cozma M-A, Epingeac M-E, Gaman A, et al. Cardio metabolic risk factors for atrial fibrillation in type 2 diabetes mellitus: Focus on hypertension, metabolic syndrome and obesity. J Mind Med Sci. (2019) 157–61. doi: 10.22543/7674.61.P157161
10. Fu Y, He W, Ma J, Wei B. Relationship between psychological factors and atrial fibrillation: a meta-analysis and systematic review. Medicine (Baltimore). (2020) 99:e19615. doi: 10.1097/MD.0000000000019615
11. Uchmanowicz I, Lomper K, Gros M, Kałuzna-Oleksy M, Jankowska EA, Rosińczuk J, et al. Assessment of frailty and occurrence of anxiety and depression in elderly patients with atrial fibrillation. Clin Interv Aging. (2020) 15:1151–61. doi: 10.2147/CIA.S258634
12. Rewiuk K, Wizner B, Klich-Raczka A, Wiecek A, Mossakowska M, Chudek J, et al. Atrial fibrillation independently linked with depression in community-dwelling older population. Results from the nationwide PolSenior project. Exp Gerontol. (2018) 112:88–91. doi: 10.1016/j.exger.2018.09.006
13. Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. (2008) 31:464–8. doi: 10.1016/j.tins.2008.06.006
14. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. (2008) 9:46–56. doi: 10.1038/nrn2297
15. Taler M, Gil-Ad I, Lomnitski L, Korov I, Baharav E, Bar M, et al. Immunomodulatory effect of selective serotonin reuptake inhibitors (SSRIs) on human T lymphocyte function and gene expression. Eur Neuropsychopharmacol. (2007) 17:774–80. doi: 10.1016/j.euroneuro.2007.03.010
16. Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. (2019) 24:18–33. doi: 10.1038/s41380-018-0017-5
17. Nebhinani N, Sharma P, Suthar N, Pareek V, Kunwar D, Purohit P, et al. Correlates of metabolic syndrome in patients with depression: a study from north-western India. Diabetes Metab Syndr. (2020) 14:1997–2002. doi: 10.1016/j.dsx.2020.10.013
18. Scalco AZ, Scalco MZ, Azul JB, Lotufo Neto F. Hypertension and depression. Clinics (São Paulo, Brazil). (2005) 60:241–50. doi: 10.1590/S1807-59322005000300010
19. Shi S, Liu T, Liang J, Hu D, Yang B. Depression and risk of sudden cardiac death and arrhythmias: a meta-analysis. Psychosom Med. (2017) 79:153–61. doi: 10.1097/PSY.0000000000000382
20. Munk PS, Isaksen K, Brønnick K, Kurz MW, Butt N, Larsen AI. Symptoms of anxiety and depression after percutaneous coronary intervention are associated with decreased heart rate variability, impaired endothelial function and increased inflammation. Int J Cardiol. (2012) 158:173–6. doi: 10.1016/j.ijcard.2012.04.085
21. Mathew AR, Hogarth L, Leventhal AM, Cook JW, Hitsman B. Cigarette smoking and depression comorbidity: systematic review and proposed theoretical model. Addiction (Abingdon, England). (2017) 112:401–12. doi: 10.1111/add.13604
22. Goette A, Lendeckel U, Kuchenbecker A, Bukowska A, Peters B, Klein HU, et al. Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart (British Cardiac Society). (2007) 93:1056–63. doi: 10.1136/hrt.2005.087171
23. Andrade C. Antidepressants and atrial fibrillation: the importance of resourceful statistical approaches to address confounding by indication. J Clin Psychiatry. (2019) 80:19f12729. doi: 10.4088/JCP.19f12729
24. Remick RA, Froese C. Unnecessary delay in tricyclic antidepressant treatment of a patient with atrial fibrillation. Can Fam Physician. (1989) 35:1101–2.
25. Pacher P, Ungvari Z, Nanasi PP, Furst S, Kecskemeti V. Speculations on difference between tricyclic and selective serotonin reuptake inhibitor antidepressants on their cardiac effects. Is there any? Curr Med Chem. (1999) 6:469–80. doi: 10.2174/0929867306666220330184544
26. Bruggeman C, O'Day CS. Selective Serotonin Reuptake Inhibitor Toxicity. StatPearls. Treasure Island (FL): StatPearls Publishing, Copyright © 2022, StatPearls Publishing LLC (2022).
27. Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. (2003) 14:209–14. doi: 10.1046/j.1540-8167.2003.02381.x
28. Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. (2000) 22:370–9. doi: 10.1016/S0893-133X(99)00134-7
29. Maes M. The immunoregulatory effects of antidepressants. Hum Psychopharmacol. (2001) 16:95–103. doi: 10.1002/hup.191
30. Leonard BE. The immune system, depression and the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. (2001) 25:767–80. doi: 10.1016/S0278-5846(01)00155-5
31. Ditmars HL, Logue MW, Toomey R, McKenzie RE, Franz CE, Panizzon MS, et al. Associations between depression and cardiometabolic health: a 27-year longitudinal study. Psychol Med. (2021) 12:1–11. doi: 10.1017/S003329172000505X
32. Egeberg A, Khalid U, Gislason GH, Mallbris L, Skov L, Hansen PR. Association between depression and risk of atrial fibrillation and stroke in patients with psoriasis: a Danish nationwide cohort study. Br J Dermatol. (2015) 173:471–9. doi: 10.1111/bjd.13778
33. Feng T, Malmo V, Laugsand LE, Strand LB, Gustad LT, Ellekjaer H, et al. Symptoms of anxiety and depression and risk of atrial fibrillation-The HUNT study. Int J Cardiol. (2020) 306:95–100. doi: 10.1016/j.ijcard.2019.11.107
34. Fenger-Gron M, Vestergaard M, Pedersen HS, Frost L, Parner ET, Ribe AR, et al. Depression, antidepressants, and the risk of non-valvular atrial fibrillation: A nationwide Danish matched cohort study. Eur J Prev Cardiol. (2019) 26:187–95. doi: 10.1177/2047487318811184
35. Garg PK, Claxton JS, Soliman EZ, Chen LY, Lewis TT, Mosley T, et al. Associations of anger, vital exhaustion, anti-depressant use, and poor social ties with incident atrial fibrillation: the atherosclerosis risk in communities study. Eur J Prev Cardiol. (2021) 28:633–40. doi: 10.1177/2047487319897163
36. Garg PK, O'Neal WT, Diez-Roux AV, Alonso A, Soliman EZ, Heckbert S. Negative Affect and Risk of Atrial Fibrillation: MESA. J Am Heart Assoc. (2019) 8:e010603. doi: 10.1161/JAHA.118.010603
37. Kim YG, Lee KN, Han KD, Han KM, Min K, Choi HY, et al. Association of Depression With Atrial Fibrillation in South Korean Adults. JAMA Netw Open. (2022) 5:e2141772. doi: 10.1001/jamanetworkopen.2021.41772
38. Lapi F, Azoulay L, Kezouh A, Benisty J, Matok I, Mugelli A, et al. The use of antidepressants and the risk of chronic atrial fibrillation. J Clin Pharmacol. (2015) 55:423–30. doi: 10.1002/jcph.435
39. Whang W, Davidson KW, Conen D, Tedrow UB, Everett BM, Albert CM. Global Psychological Distress and Risk of Atrial Fibrillation Among Women: The Women's Health Study. J Am Heart Assoc. (2012) 1:e001107. doi: 10.1161/JAHA.112.001107
40. Han QQ, Yu J. Inflammation: a mechanism of depression? Neurosci Bull. (2014) 30:515–23. doi: 10.1007/s12264-013-1439-3
41. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. (2010) 67:446–57. doi: 10.1016/j.biopsych.2009.09.033
42. Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. (2003) 108:3006–10. doi: 10.1161/01.CIR.0000103131.70301.4F
43. Engelmann MD, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. (2005) 26:2083–92. doi: 10.1093/eurheartj/ehi350
44. McCabe PJ. Psychological distress in patients diagnosed with atrial fibrillation: the state of the science. J Cardiovasc Nurs. (2010) 25:40–51. doi: 10.1097/JCN.0b013e3181b7be36
45. Patel D, Mc Conkey ND, Sohaney R, Mc Neil A, Jedrzejczyk A, Armaganijan L, et al. systematic review of depression and anxiety in patients with atrial fibrillation: the mind-heart link. Cardiovasc Psychiatry Neurol. (2013) 2013:159850. doi: 10.1155/2013/159850
46. Coupland C, Hill T, Morriss R, Moore M, Arthur A, Hippisley-Cox J. Antidepressant use and risk of cardiovascular outcomes in people aged 20 to 64: cohort study using primary care database. BMJ (Clinical research ed). (2016) 352:i1350. doi: 10.1136/bmj.i1350
47. Pino R, Cerbai E, Calamai G, Alajmo F, Borgioli A, Braconi L, et al. Effect of 5-HT4 receptor stimulation on the pacemaker current I(f) in human isolated atrial myocytes. Cardiovasc Res. (1998) 40:516–22. doi: 10.1016/S0008-6363(98)00198-9
48. Jasiak NM, Bostwick JR. Risk of QT/QTc prolongation among newer non-SSRI antidepressants. Ann Pharmacother. (2014) 48:1620–8. doi: 10.1177/1060028014550645
49. Nanda A, Kabra R. Racial differences in atrial fibrillation epidemiology, management, and outcomes. Curr Treat Options Cardiovasc Med. (2019) 21:85. doi: 10.1007/s11936-019-0793-5
Keywords: atrial fibrillation, depression, antidepressants, risk factor, meta-analysis
Citation: Fu Y, Feng S, Xu Y, Yang Y, Chen H, He W, Zhu W, Yin K, Xue Z and Wei B (2022) Association of Depression, Antidepressants With Atrial Fibrillation Risk: A Systemic Review and Meta-Analysis. Front. Cardiovasc. Med. 9:897622. doi: 10.3389/fcvm.2022.897622
Received: 16 March 2022; Accepted: 25 April 2022;
Published: 11 May 2022.
Edited by:
Rajeev Gupta, Medicilinic, United Arab EmiratesReviewed by:
Vito Maurizio Parato, Marche Polytechnic University, ItalyJayadevan Sreedharan, Gulf Medical University, United Arab Emirates
Copyright © 2022 Fu, Feng, Xu, Yang, Chen, He, Zhu, Yin, Xue and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengbiao Xue, NTc0Mzg0NDBAcXEuY29t; Bo Wei, d2JqeG1oQDE2My5jb20=
†These authors have contributed equally to this work
‡These authors share senior authorship
 Yonghui Fu1,2†
Yonghui Fu1,2† Shenghui Feng
Shenghui Feng Yuanjian Yang
Yuanjian Yang Wengen Zhu
Wengen Zhu