- 1Department of Cardiology, Guangzhou Panyu Central Hospital, Guangzhou, China
- 2Department of Cardiac Surgery, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 3Forensic and Pathology Laboratory, Department of Pathology, Institute of Forensic Science, Jiaxing University, Jiaxing, China
- 4Department of Pathology and Municipal Key-Innovative Discipline of Molecular Diagnostics, Jiaxing Hospital of Traditional Chinese Medicine, Jiaxing University, Jiaxing, China
Aim: The aim of this study was to examine the utility of liver function tests (LFTs) in predicting the prognosis of critically ill patients with primary pulmonary hypertension (PPH) with/without liver disease.
Methods: We retrieved the Medical Information Mart for Intensive Care III (MIMIC-III) database to acquire clinical data. From the database, we recruited adult patients that were equal to or older than 18 years with primary pulmonary hypertension (PPH) discharge from intensive care unit (ICU). Then, the relationship between LFTs and duration of hospitalization and ICU stays was examined based on the Spearman correlation. The chi-square assessment was conducted to examine the correlation between LFTs and death rates. Survival curves were plotted with the aid of the Kaplan-Meier technique, and the curves were subsequently compared utilizing the log-rank test. The LFTs were identified as independent predictive variables of death according to the results of multivariable logistic regression. The specificity and sensitivity for mortality were calculated utilizing receiver operating characteristic (ROC) curves and the area under the curve (AUC).
Results: In total, 198 patients satisfying the inclusion criteria were recruited, among which there were 23 patients with liver disease. Only ALB was correlated with the length of ICU stay in the total PPH group. ALB independently served as a risk variable for hospital mortality and 90-day mortality and was significantly associated with 90-day and 4-year survival rates in both total PPH and PPH without liver disease. AST was correlated with hospital mortality and 90-day survival curves in both total PPH and PPH without liver disease and independently served as a risk factor for hospital and 90-day mortality only in the total PPH group. ALT independently acted as a risk variable for hospital mortality and total bilirubin was correlated with hospital mortality in the total group. The diagnostic performance of the predictive model combining the LFTs was moderately good for the hospital, 90-day, and 4-year mortality. Both Modelı End-Stage ıLiverı Disease (MELD) score and albumin-bilirubin (ALBI) score were independent risk factors for short- and long-term prognosis. And they were also significantly associated with short- and long-term prognosis.
Conclusion: Among critically ill patients with PPH and with or without liver illness, aberrant LFT was linked to short- and long-term prognoses.
Introduction
Primary pulmonary hypertension (PPH), one of the pulmonary arterial hypertension (PAH), is an uncommon chronic illness hallmarked by right ventricle (RV) failure and poor prognosis (1). In recent years, PAH has shown to be a multiorgan systemic disorder hallmarked by anomalies in the immune system, skeletal muscle, kidneys, peripheral and central nervous system, as well as systemic circulation (2, 3). Due to the liver’s close physiological and anatomical link to the RV, it is among the vulnerable organs to be affected by RV failure from PAH, which can lead to a variety of liver abnormalities (4).
The WHO functional class, average right atrial pressure, 6-min walking distance, and brain natriuretic peptide (BNP) plasma levels served as prognostic indicators for PAH in this research (5). Recently, abnormal liver function tests (LFTs) were indicated to significantly affect the prognosis of PAH. Decreased ALB was associated with decreased PAH survival (6, 7). Increased bilirubin level was correlated with an elevated mortality risk of PAH (8, 9). Another research report showed that it was not associated with PAH survival (10). The inconsistency of the current studies suggested the mechanistic complexity of LFTs in PAH. However, not only ischemic hepatitis and congestive hepatopathy caused by heart failure due to PAH but also preexisting liver disease could cause abnormal LFTs. Although the correlation between LFT abnormalities and PAH was recently reported, few investigations compared the abnormal liver markers caused by PAH with and without liver disease.
The objective of this research was to examine if there was a correlation between LFTs and duration of hospitalization and ICU stays, inpatient mortality, 90-day mortality, and 4-year mortality among critically ill PPH patients with or without liver illness.
Materials and Methods
Data Source
A retrospective cohort approach was adopted in this research. The ICU database, which is a public critical care repository of Medical Information Mart for Intensive Care III (MIMIC-III), was retrieved to obtain data. Beginning from 2001 to 2012, the clinical data from patients from ICU admissions at Beth Israel Deaconess Medical Center (BIDMC) were gathered (11). The access approval to the database was granted by the institutional review boards of both the BIDMC and the Massachusetts Institute of Technology Affiliates. Since the data had been de-identified, there was no need for informed consent.
Patient Selection
The MIMIC-III database was utilized for the purpose of identifying clinical data from patients who met the criteria for inclusion in this research. The aforementioned criteria were (1) patients who have undergone a definite diagnosis of PPH. Diagnoses of PPH were made in accordance with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. For PPH, the ICD-90-CM code was 4160. (2) Patients older than 18 years, which was reported previously (12). (3) Patients who underwent routine blood tests 24 h upon admission, and the tested indicators included white blood cell (WBC), serum sodium, serum potassium, platelets, blood urea nitrogen (BUN), creatinine, total bilirubin, blood alanine transaminase (ALT), blood aspartate transaminase (AST), and blood albumin (ALB). The criteria for exclusion used in this research were (1) patients who did not develop PPH; (2) patients under the age of 18; (3) patients whose blood test results were not complete; and (4) patients with PPH suffering from systemic immune system diseases, active infectious disease; other important functional organs severely damaged, malignant tumors, etc.
Data Acquisition
The data were acquired and collected with the aid of the Structured Query Language (SQL), and the administration platform used was pgAdmin4 for PostgreSQL. The acquired data predominantly consist of demographics (gender and age), vital signs [temperature, percutaneous oxygen saturation (SpO2), respiratory rate (RR), systolic blood pressure (SBP), heart rate (HR), and diastolic blood pressure (DBP)], comorbid conditions (renal failure, liver disease, complicated diabetes, uncomplicated diabetes, chronic pulmonary, hypertension, peripheral vascular, pulmonary circulation disorder, congestive heart failure, valvular illness, and cardiac arrhythmias), laboratory tests (serum potassium, platelets, BUN, WBC, creatinine, ALB, total bilirubin, AST, ALT, and serum sodium), Model End-Stage Liver Disease (MELD), the Sequential Organ Failure Assessment (SOFA) score, and the Simplified Acute Physiology Score (SAPS) II. ALBI score was calculated using albumin and total bilirubin, and FIB-4 index was calculated using age, ALT, AST, and platelet by formula as described before (13, 14). The formulae for calculating the scores are MELD score = 3.78 × ln [(bilirubin (mg/dl)] + 11.2 × ln (INR) + 9.57 × ln [creatinine (mg/dl) + 6.43 × (for cholestatic or alcoholic liver disease and 1 for others)]; ALBI score = [log10 bilirubin (μmol/L) × 0.66] + [albumin (g/L) × −0.085]; FIB-4 index = [age (years) × AST (U/L)]/[Platelet count (109/L) × ALT (U/L)1/2]. ALBI scores were divided into three levels, namely, grade 1, ALBI ≤ −2.60; grade 2, −2.60 < ALBI ≤ −1.39; grade 3: ALBI > −1.39. MELD scores were divided into three levels: grade 1: MELD ≤ 14; grade 2: 14 < MELD ≤ 18; and grade 3: MELD > 18. As the percentage of incomplete information for each variable was less than 1.5%, it is reasonable to ignore these variables in subsequent analyses.
Outcome Variables
The outcome variables that were obtained in this research are hospital mortality, duration of ICU stay, duration of hospitalization stay, 90-day mortality (post-ICU admission), as well as 4-year mortality. As a patient might undergo ICU admissions several times throughout a single hospitalization, the time spent in the ICU was exclusively determined by the first ICU hospitalization. Only individuals in the CareVue system who had been monitored for no less than 4 years were included in the analysis for 4-year mortality.
Statistical Analysis
Categorical data are expressed as numerals with percentages and were examined by performing the χ2 test. Continuous variables are reported as mean ± standard deviation or median (interquartile range) and were subjected to a comparison by performing the Mann–Whitney U-test and t-test. The non-parametric Spearman’s rank correlation test was conducted for the purpose of assessing the correlation between the duration of ICU and hospital stays as well as the lab measurements. We included all patients with PPH, containing survivors and non-survivors, in the prognostic analysis. Survival curves were plotted with the help of the Kaplan–Meier technique and subjected to a subsequent comparison utilizing the log-rank test. We performed multivariate and univariate logistic regression analyses to screen for independent predictive indicators for PPH-related mortality (including hospital, 90-day, and 4-year mortality). To account for possible confounding factors, two varied models were developed. For total patients with PPH, model 1 was corrected for BUN, valvular disease, SOFA scores, liver disease, SpO2, and RR, whereas model 2 was adjusted for SAPS II score and valvular disease. For PAH patients without liver-related disorders, model 1 was corrected for BUN, SOFA score, SpO2, valvular disorder, and MELD score, whereas model 2 was corrected for SAPS II score and valvular disease. Statistical significance was set as a p-value < 0.05. Additionally, the specificity and sensitivity were assessed with the aid of receiver operating characteristic (ROC) curves and the area under the curve (AUC) values. STATA, version 14.0 (StataCorp, College Station, TX, United States) was employed to perform all the analyses of statistical data.
Results
The Study Population’s Baseline Parameters
In total, 198 patients who satisfied the inclusion requirements were included in this research, with 26 patients (13.13%) dying in the hospital. Table 1 summarizes the baseline features of the included patients, including demographics, lab tests, vital signs, comorbid conditions, and scores.
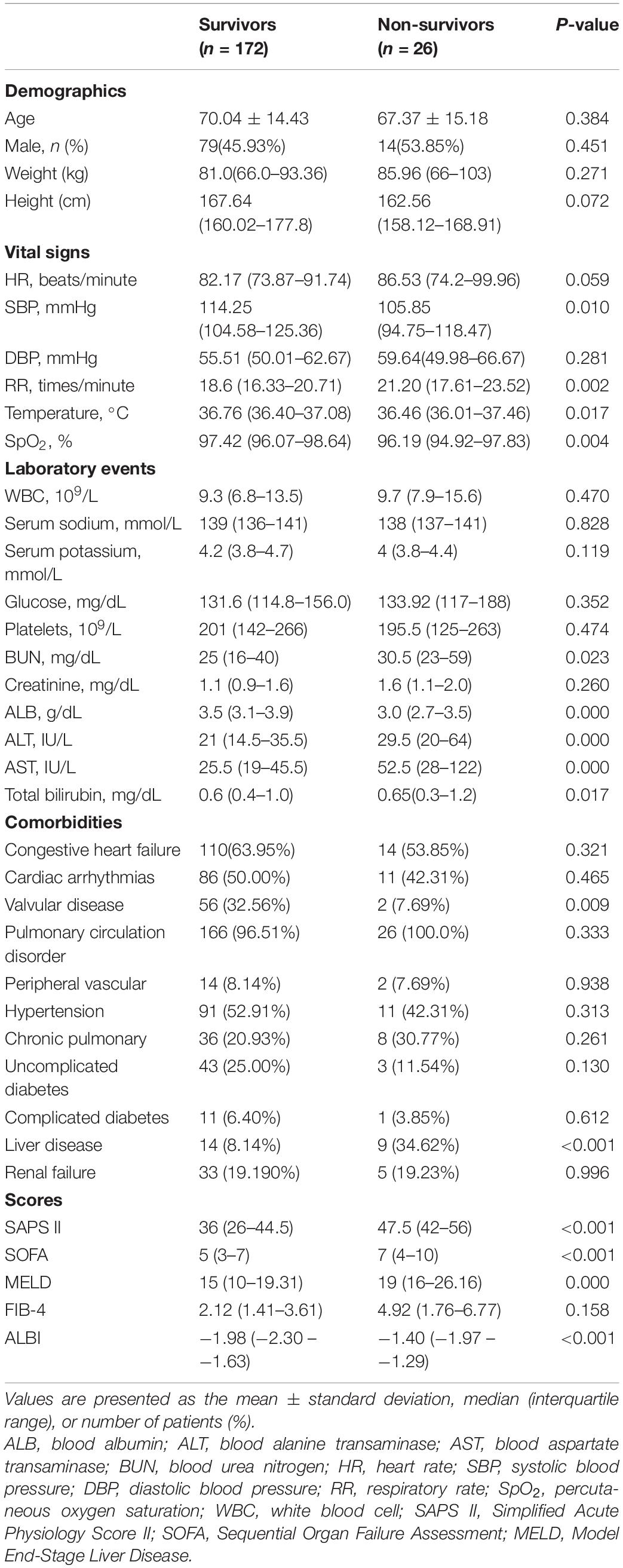
Table 1. Baseline characteristics of the study population with different survival status in hospital.
Table 1 displays the demographic data from the research sample. Among the survivors and non-survivors, no remarkable differences were found in age, gender, weight, or height. Non-survivors exhibited remarkably lowered ALB levels (3.5 vs. 3.0, p < 0.001) and elevated levels of total bilirubin (0.6 vs. 0.65, p < 0.001), AST (25.5 vs. 52.5, p < 0.001), ALT (21 vs. 29.5, p < 0.001), MELD score (15 vs. 19, p < 0.001), and ALBI score (−1.98 vs. −1.40, p < 0.001) (Table 1). Non-survivors exhibited decreased SpO2, temperature, SBP levels and incidence of valvular disorder, as well as elevated BUN, RR, SOFA, SAPS II, and MELD scores, and also experienced a history of liver (Table 1).
The Liver Function Index and Its Prognostic Value in Primary Pulmonary Hypertension
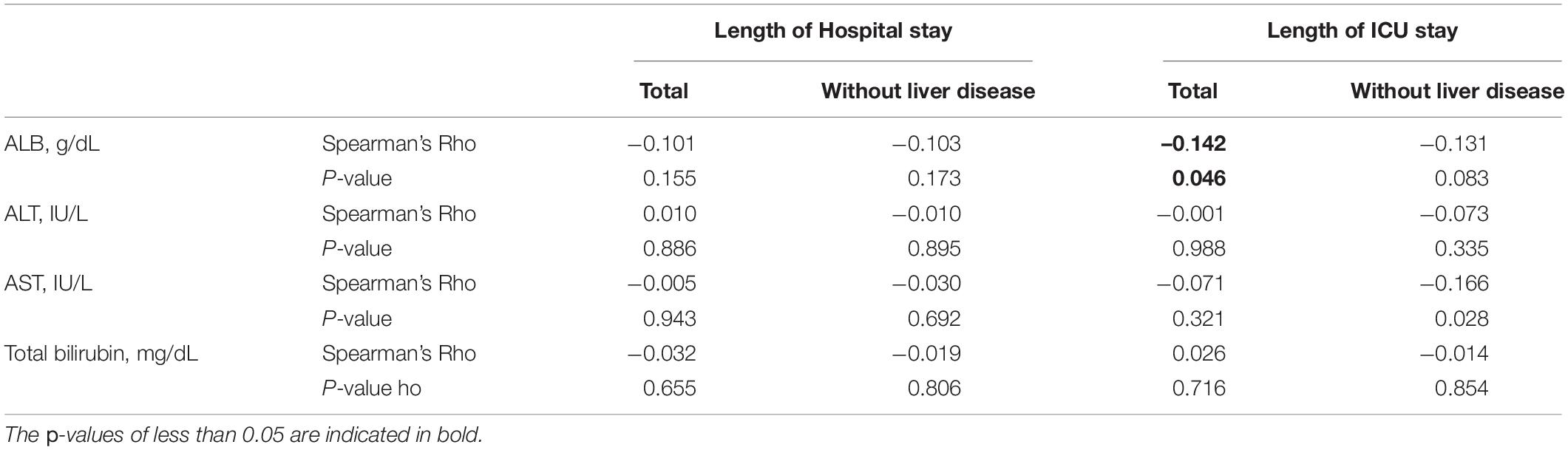
Table 2 illustrates a correlation between the liver function index and the duration of hospitalization and ICU stays among patients with PPH (total patients with PPH or patients with PPH who do not have liver illness), which was investigated by performing Spearman’s rank correlation analysis. Only ALB exhibited a remarkable negative correlation with the duration of ICU stay for total patients with PPH (Spearman’s rho = −0.142, p = 0.046).
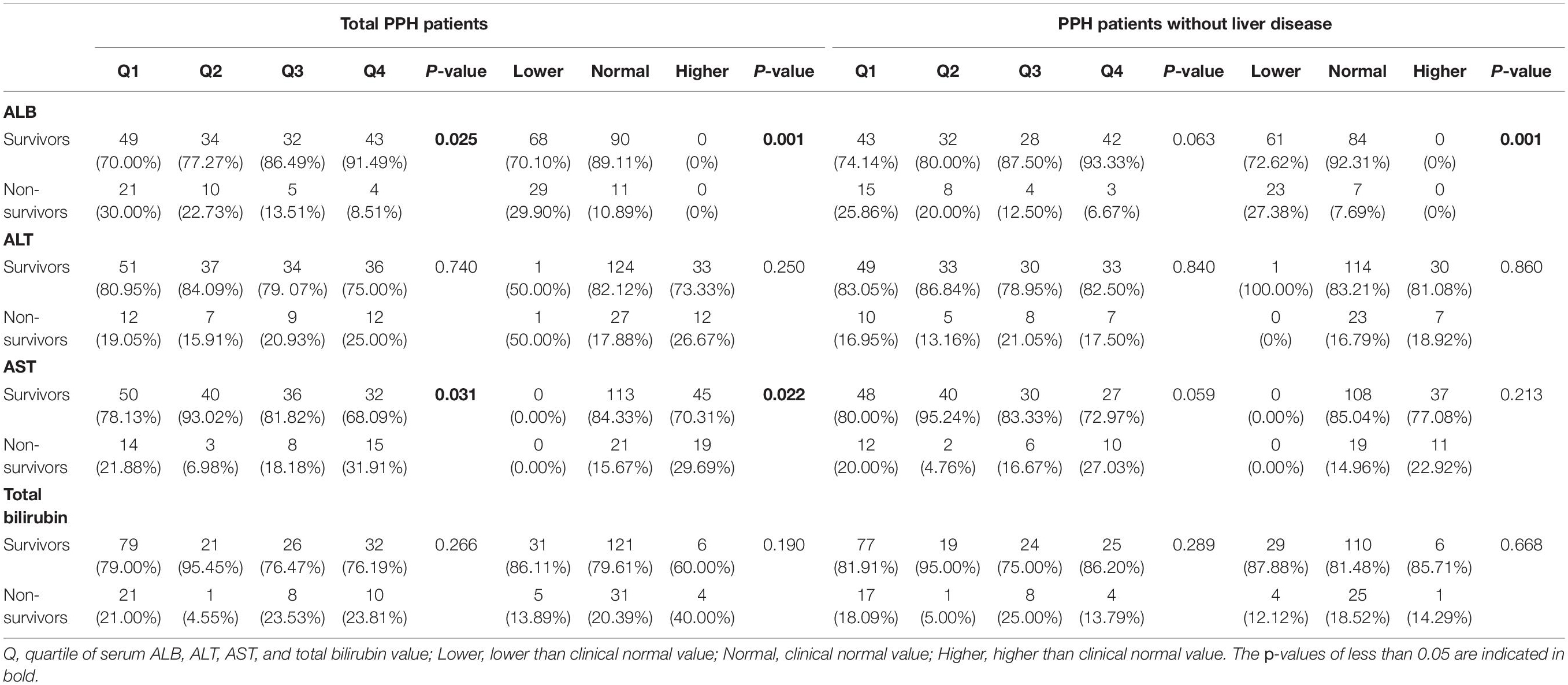
Then, we probed into the relationship between liver function index and hospital mortality among patients with PPH (total patients with PPH or patients with PPH who do not have liver illness). A remarkable correlation was discovered between quartiles of ALB and AST and hospital mortality in total patients with PPH and patients with PPH and without liver disease (for total patients with PPH, ALB: p = 0.006; AST: p = 0.012; for patients with PPH and without liver disease, ALB: p = 0.023; AST: p = 0.042) (Table 3). In both total PPH and PPH without liver disease groups, a greater incidence of hospital mortality was reported during the 1st quartile for ALB, whereas a greater proportion of hospital mortality was observed within the 4th quartile for AST. In the case where clinical normal values were grouped together, similar tendencies emerged. In both total PPH and PPH without liver disease groups, a greater incidence of hospital mortality was found among the group exhibiting a lesser value compared to normal for ALB, whereas in the total PPH group, a greater proportion of patients experiencing hospital mortality was reported among the group with a greater level in contrast with normal for AST (for total patients with PPH, ALB: p = 0.008; AST: p = 0.003; for patients with PPH and without liver disease, ALB: p = 0.013) (Table 3). Within the total PPH group, patients with a greater value in total bilirubin exhibited an elevated incidence of hospital mortality (p = 0.035).
The relationship between the liver function index and PPH mortality over 90 days was also studied, with the findings reported in Table 4. For ALB, a higher incidence rate of 90-day mortality was found in the 1st quartile. In addition, for AST, a higher incidence rate of 90-day mortality was found in the 4th quartile in the total PPH group but not PPH without liver disease (ALB: p = 0.025; AST: p = 0.031). In the case where clinical normal values were grouped together, comparable tendencies emerged. For ALB, in both total PPH and PPH without liver disease groups, the group exhibiting a lesser value compared to the normal had a greater risk of 90-day mortality. In addition, for AST, a higher incidence rate of hospital mortality was found in patients belonging to the group with a higher value than normal only in the total PPH group (for total patients with PPH, ALB: p = 0.001; AST: p = 0.022; for patients with PPH and without liver disease, ALB: p = 0.001) (Table 4).
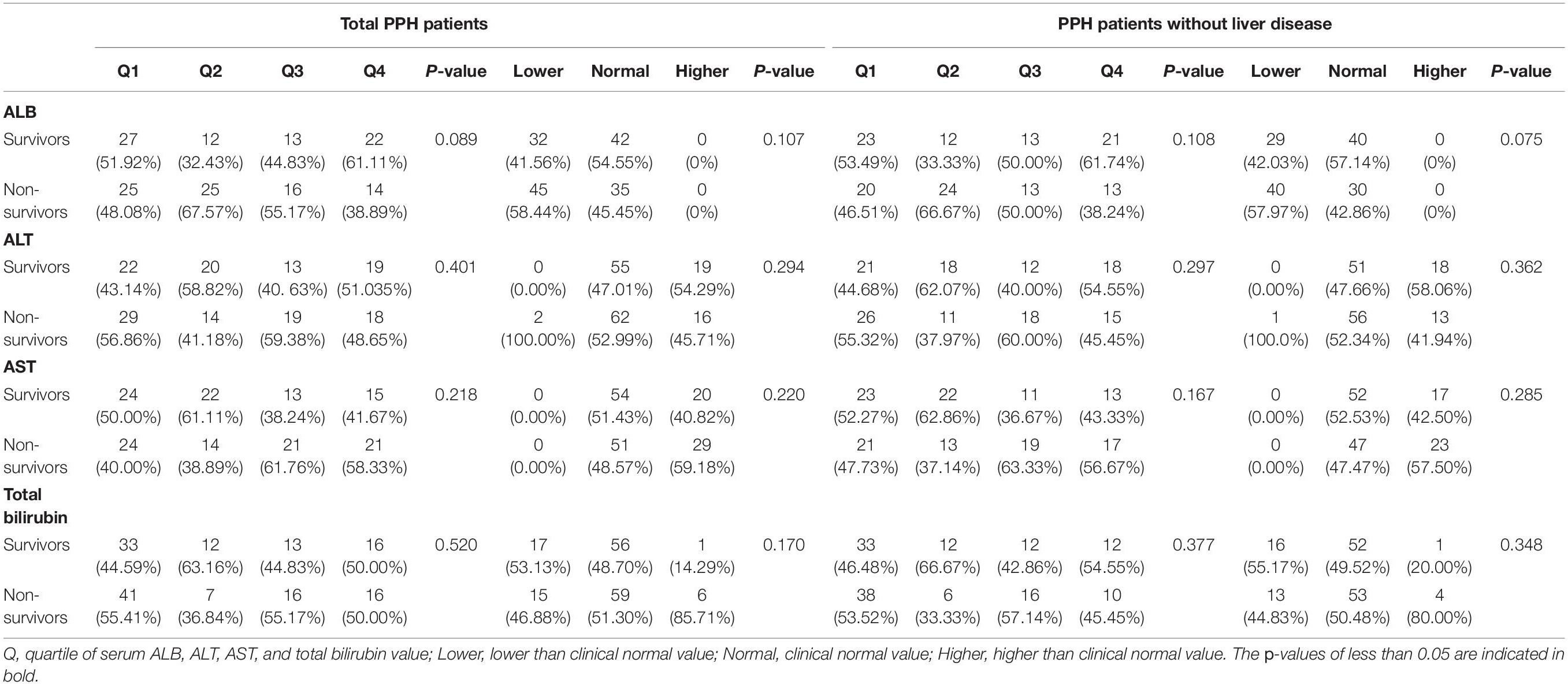
Only participants in CareVue who underwent a follow-up for no less than 4 years were examined for 4-year mortality. According to the results, no considerable association was discovered between the liver function index and PPH-related 4-year mortality, as indicated in Table 5.
Figures 1–4 display Kaplan–Meier survival curves comparing patients based on their liver function indices and liver function score levels. As depicted by Figure 1, patients within the first and fourth quartile of ALB and AST, respectively, exhibited the worst 90-day survival in both total PPH and PPH without liver disease groups, while patients with lower ALB than normal in both total PPH and PPH without liver disease groups, and higher AST than normal in total PPH group exhibited a reduced 90-day survival rate (all p < 0.05) (Figures 1A–F). As depicted in Figure 2, patients within grade 3 of MELD score exhibited the worst 90-day survival in both total PPH groups, while patients within grade 3 of ALBI score, respectively, exhibited the worst 90-day survival in both total PPH and PPH without liver disease groups (all p < 0.05) (Figures 2A–D). As displayed in Figure 3, patients within the fourth quartile of ALB had the highest 4-year survival in PPH without liver disease group, while patients with lower ALB than normal in both total PPH and PPH without liver disease groups had reduced 4-year survival rate (all p < 0.05) (Figures 3A–F). As displayed in Figure 4, patients within grade 3 of MELD score had the worst 4-year survival in both total PPH and PPH without liver disease groups (all p < 0.01); however, there are no differences among the 4-year survival of the three grades of ALBI score (Figures 4A–D).
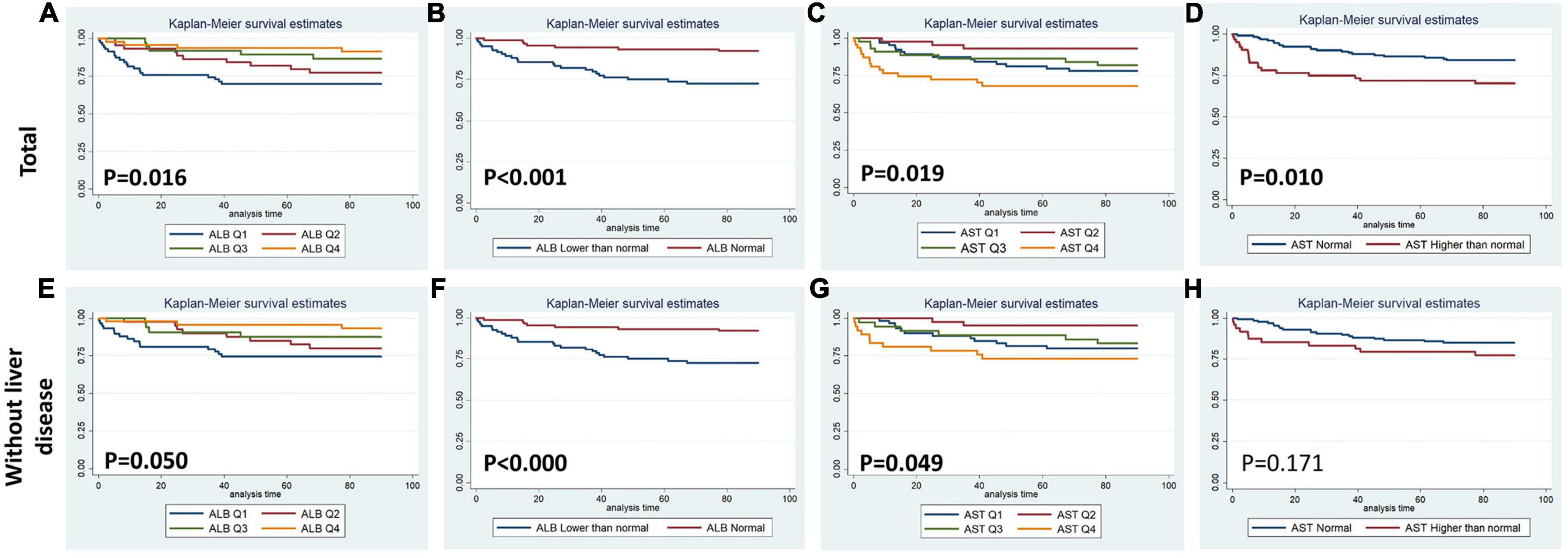
Figure 1. Kaplan–Meier 90-day survival curves comparing patients by liver function index levels. Q, quartile of serum ALB, ALT, AST, and total bilirubin value; normal, clinical normal value; ALB, blood albumin; ALT, blood alanine transaminase; AST, blood aspartate transaminase. (A–D) Total patients with PPH. (E–H) Patients with PPH and without liver disease.
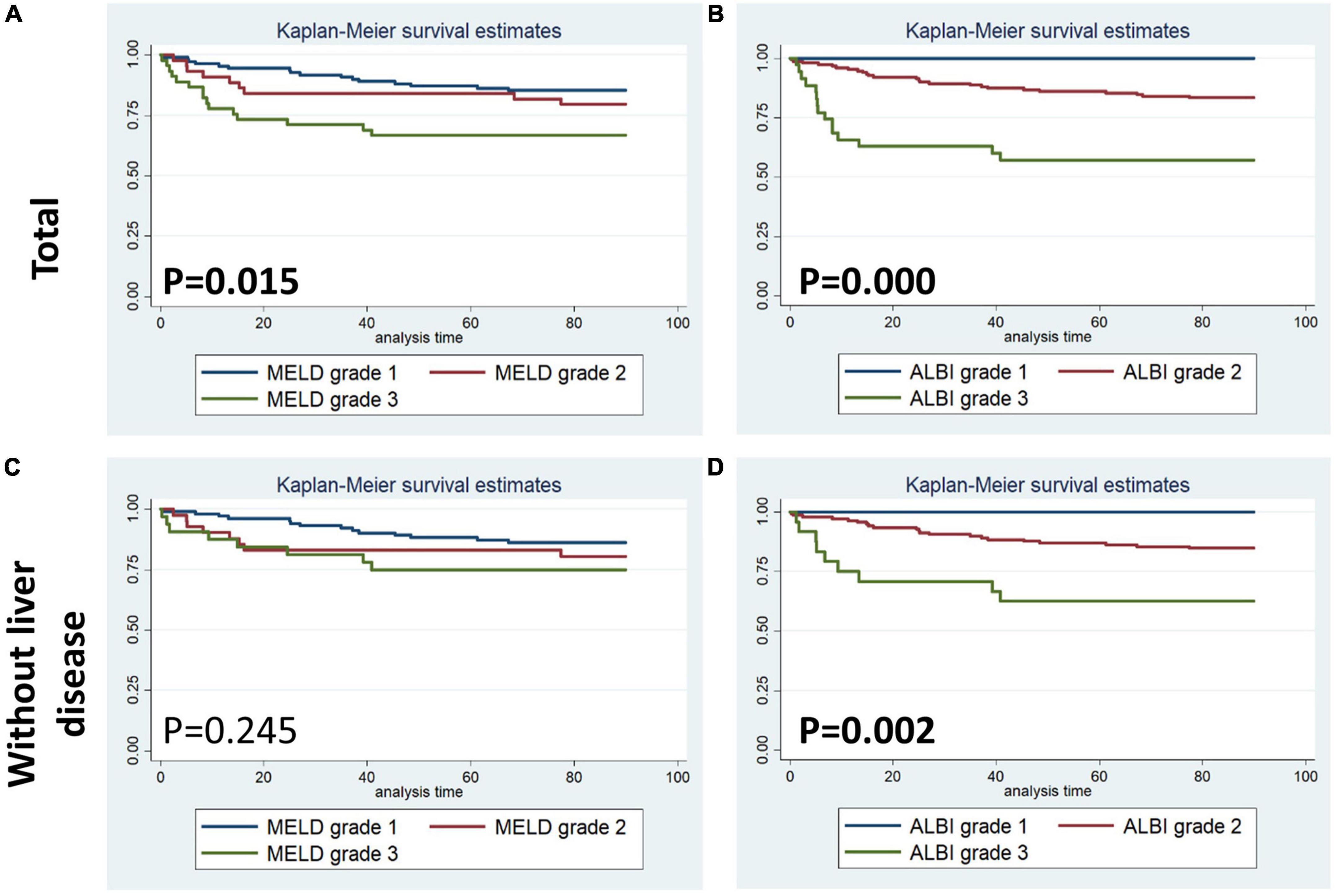
Figure 2. Kaplan–Meier 90-day survival curves comparing patients by liver function score levels (MELD and ALBI score). (A,B) Total patients with PPH. (C,D) Patients with PPH and without liver disease.
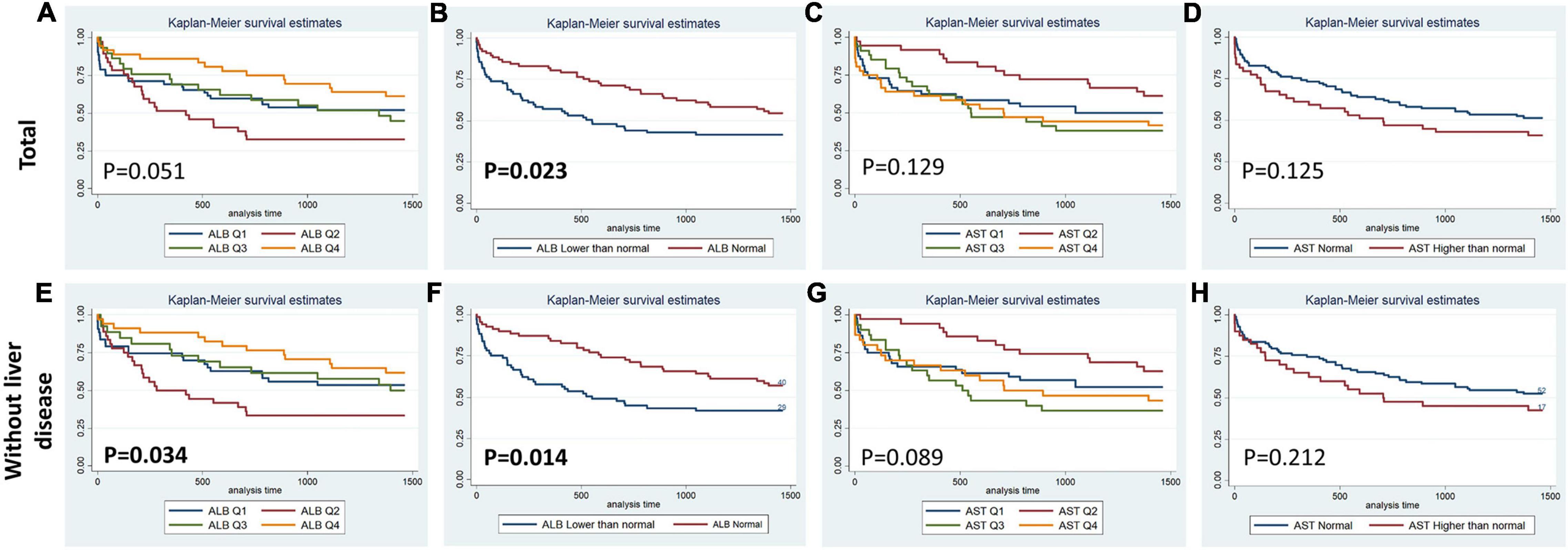
Figure 3. Kaplan–Meier 4-year survival curves comparing patients by liver function index levels. Q, quartile of serum ALB, ALT, AST, and total bilirubin value; Normal, clinical normal value; ALB, blood albumin; ALT, blood alanine transaminase; AST, blood aspartate transaminase. (A–D) Total patients with PPH. (E–H) Patients with PPH and without liver disease.
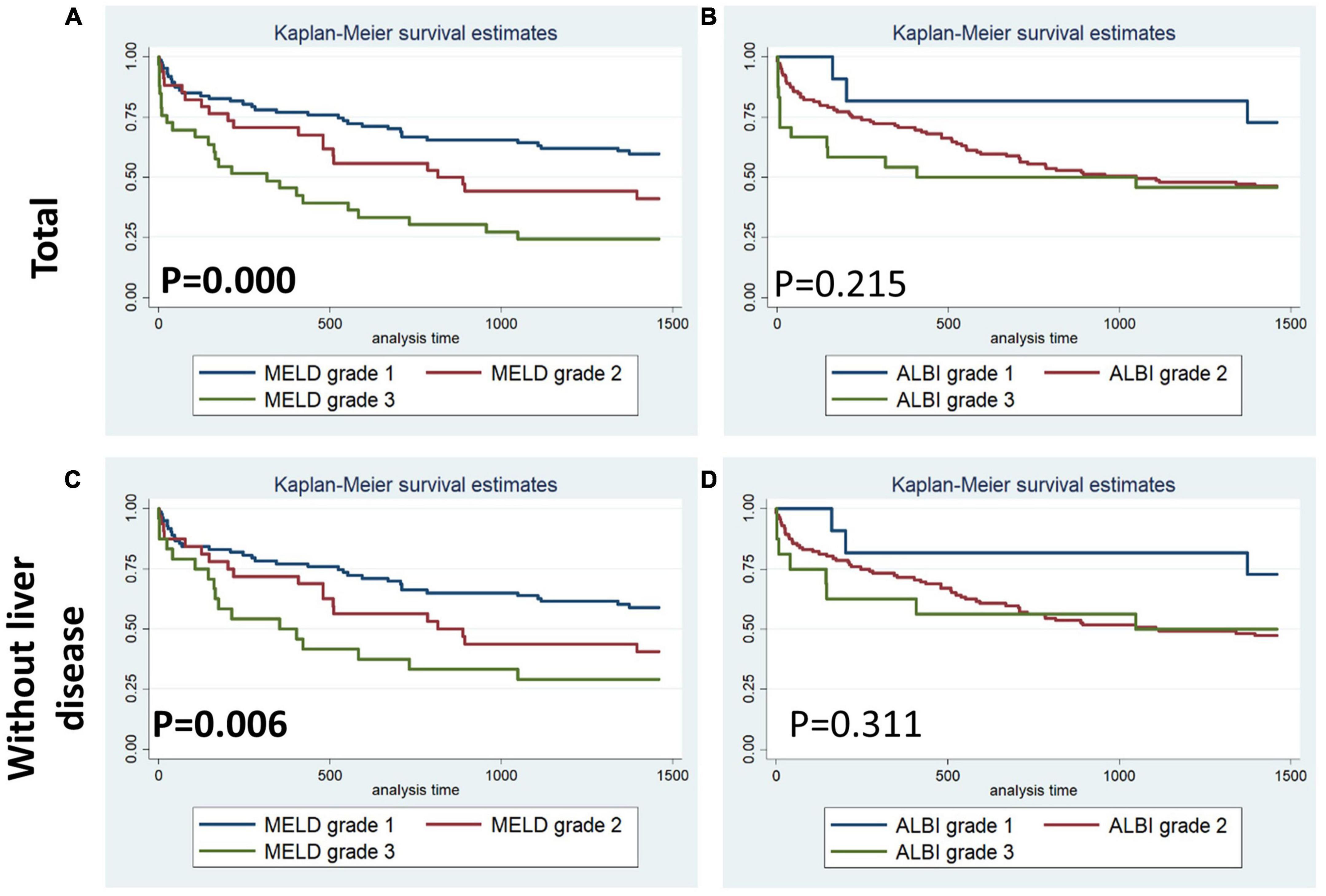
Figure 4. Kaplan–Meier 4-year survival curves comparing patients by liver function score levels (MELD and ALBI score). (A,B) Total patients with PPH. (C,D) Patients with PPH and without liver disease.
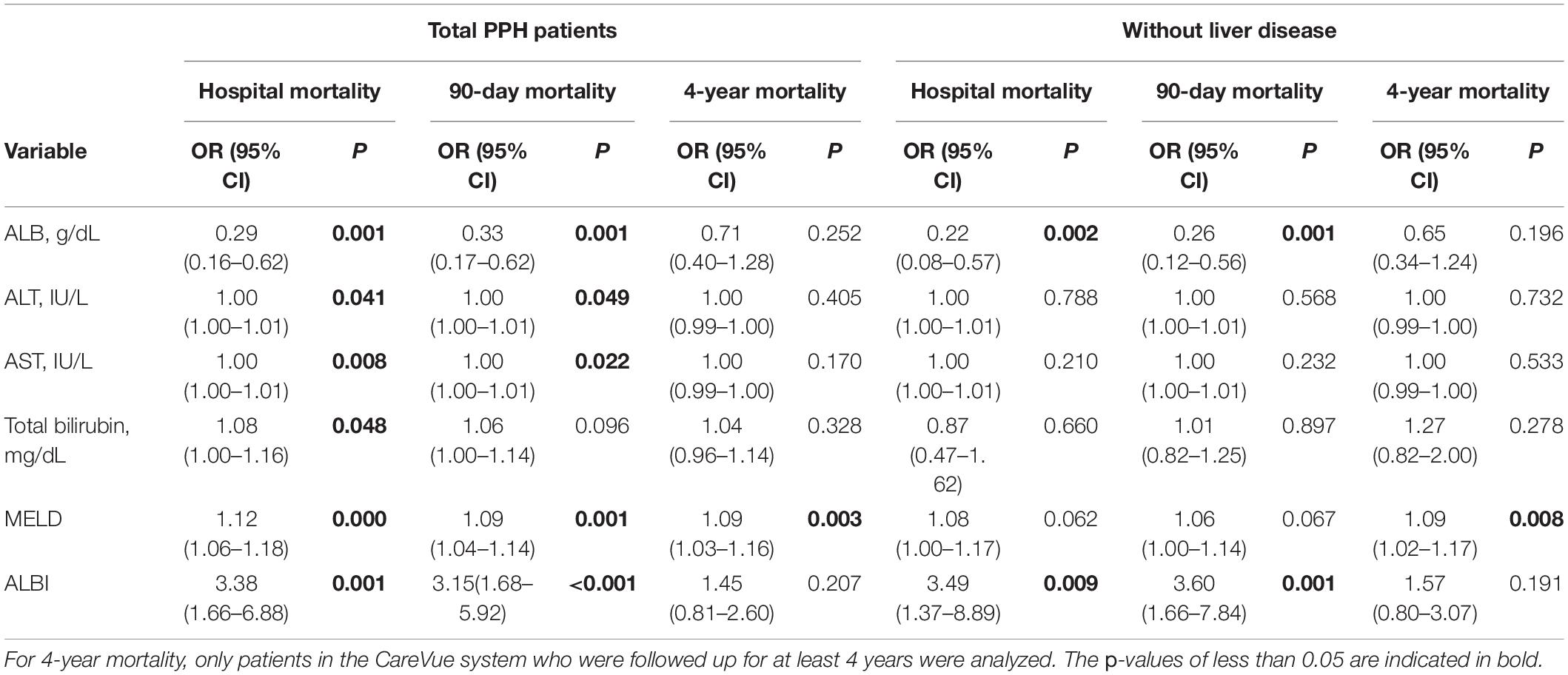
Table 6 presents the findings of the univariate logistic regression analysis. Accordingly, ALB was correlated with hospital mortality (total: OR = 0.29, 95% CI = 0.16–0.62, p = 0.001; without liver disease: OR = 0.22, 95% CI = 0.08–0.57, p = 0.002) and 90-day mortality (total: OR = 0.33, 95% CI = 0.17–0.62, p = 0.001; without liver disease: OR = 0.26, 95% CI = 0.12–0.56, p = 0.001) in both total PPH and PPH without liver disease groups. ALT and AST were correlated with hospital mortality and 90-day mortality in total patients with PPH (For ALT, hospital mortality: OR = 1.00, 95% CI = 1.00–1.01, p = 0.041, 90-day mortality: OR = 1.00, 95% CI = 1.00–1.01, p = 0.049; for AST, hospital mortality: OR = 1.00, 95% CI = 1.00–1.01, p = 0.008, 90-day mortality: OR = 1.00, 95% CI = 1.00–1.01, p = 0.022). In the total PPH group, total bilirubin was correlated with hospital mortality (OR = 1.08, 95% CI = 1.00–1.16, p = 0.048). MELD score was correlated with hospital mortality (total: OR = 1.12, 95% CI = 1.06–1.18, p < 0.001), 90-day mortality (total: OR = 1.09, 95% CI = 1.04–1.14, p = 0.001) and 4-year mortality (total: OR = 1.09, 95% CI = 1.03–1.16, p = 0.003) in total PPH, and 4-year mortality (total: OR = 1.09, 95% CI = 1.02–1.17, p = 0.008) in PPH without liver disease groups. ALBI score was correlated with hospital mortality (total: OR = 3.38, 95% CI = 1.66–6.88, p = 0.001; without liver disease: OR = 3.49, 95% CI = 1.37–8.89, p = 0.009) and 90-day mortality (total: OR = 3.15, 95% CI = 1.68–5.92, p < 0.001; without liver disease: OR = 3.60, 95% CI = 1.66–7.84, p = 0.001) in both total PPH and PPH without liver disease groups.
Table 7 presents the outcomes of the multivariate analysis. For performing multivariate analyses, model 1 was corrected for SOFA scores, BUN, valvular illness, liver illness, SpO2, and RR, whereas model 2 was corrected for SAPS II score and valvular disease for total patients with PPH. For patients with PPH and without liver disease, model 1 was corrected for BUN, SpO2, SOFA scores, valvular disorder, and MELD score, whereas model 2 was corrected for SAPS II score and valvular disease. ALB was correlated with hospital stay and 90-day mortality in both total PPH and PPH without liver disease groups (hospital mortality for total PPH group, model 1: OR = 0.38, 95% CI = 0.16–0.92, p = 0.027, model 2: OR = 0.39, 95% CI = 0.17–0.91, p = 0.028; hospital mortality for PPH without liver disease group, model 1: OR = 0.27, 95% CI = 0.10–0.76, p = 0.015, model 2: OR = 0.32, 95% CI = 0.12–0.88, p = 0.027; 90-day mortality for total PPH group, model 1: OR = 0.40, 95% CI = 0.20–0.79, p = 0.008, model 2: OR = 0.40, 95% CI = 0.20–0.79, p = 0.009; 90-day mortality for PPH without liver disease group, model 1: OR = 0.28, 95% CI = 0.12–0.62, p = 0.002, model 2: OR = 0.33, 95% CI = 0.15–0.73, p = 0.006). ALT and AST were correlated with hospital mortality within the total PPH group (ALT: model 2, OR = 1.00, 95% CI = 1.00–1.01, p = 0.036; AST, model 1: OR = 1.00, 95% CI = 1.00–1.01, p = 0.029, model 2: OR = 1.00, 95% CI = 1.00–1.01, p = 0.003). AST was correlated with 90-day mortality in the total PPH group (model 2, OR = 1.00, 95% CI = 1.00–1.01, p = 0.037). MELD score was correlated with hospital stay, 90-day mortality, and 4-year mortality in total PPH, and was associated with hospital stay and 4-year mortality in PPH without liver disease groups (hospital mortality for total PPH group, model 2: OR = 1.08, 95% CI = 1.02–1.15, p = 0.007; hospital mortality for PPH without liver disease group, model 1: OR = 1.07, 95% CI = 1.00–1.14, p = 0.045; 90-day mortality for total PPH group, model 2: OR = 1.06, 95% CI = 1.01–1.12, p = 0.017; 4-year mortality for total PPH group, model 1: OR = 1.06, 95% CI = 1.00–1.13, p = 0.048, model 2: OR = 1.08, 95% CI = 1.03–1.14, p = 0.003; 4-year mortality for PPH without liver disease group, model 2: OR = 1.08, 95% CI = 1.02–1.15, p = 0.011). ALBI score was correlated with hospital stay and 90-day mortality in total PPH, and was associated with 90-day mortality in PPH without liver disease groups (hospital mortality for total PPH group, model 2: OR = 2.54, 95% CI = 1.16–5.53, p = 0.019; 90-day mortality for total PPH group, model 1: OR = 2.45, 95% CI = 1.19–5.01, p = 0.014, model 2: OR = 2.69, 95% CI = 1.38–5.27, p = 0.004; 90-day mortality for PPH without liver disease group, model 1: OR = 2.31, 95% CI = 1.16–4.61, p = 0.017, model 2: OR = 2.97, 95% CI = 1.33–6.67, p = 0.008).
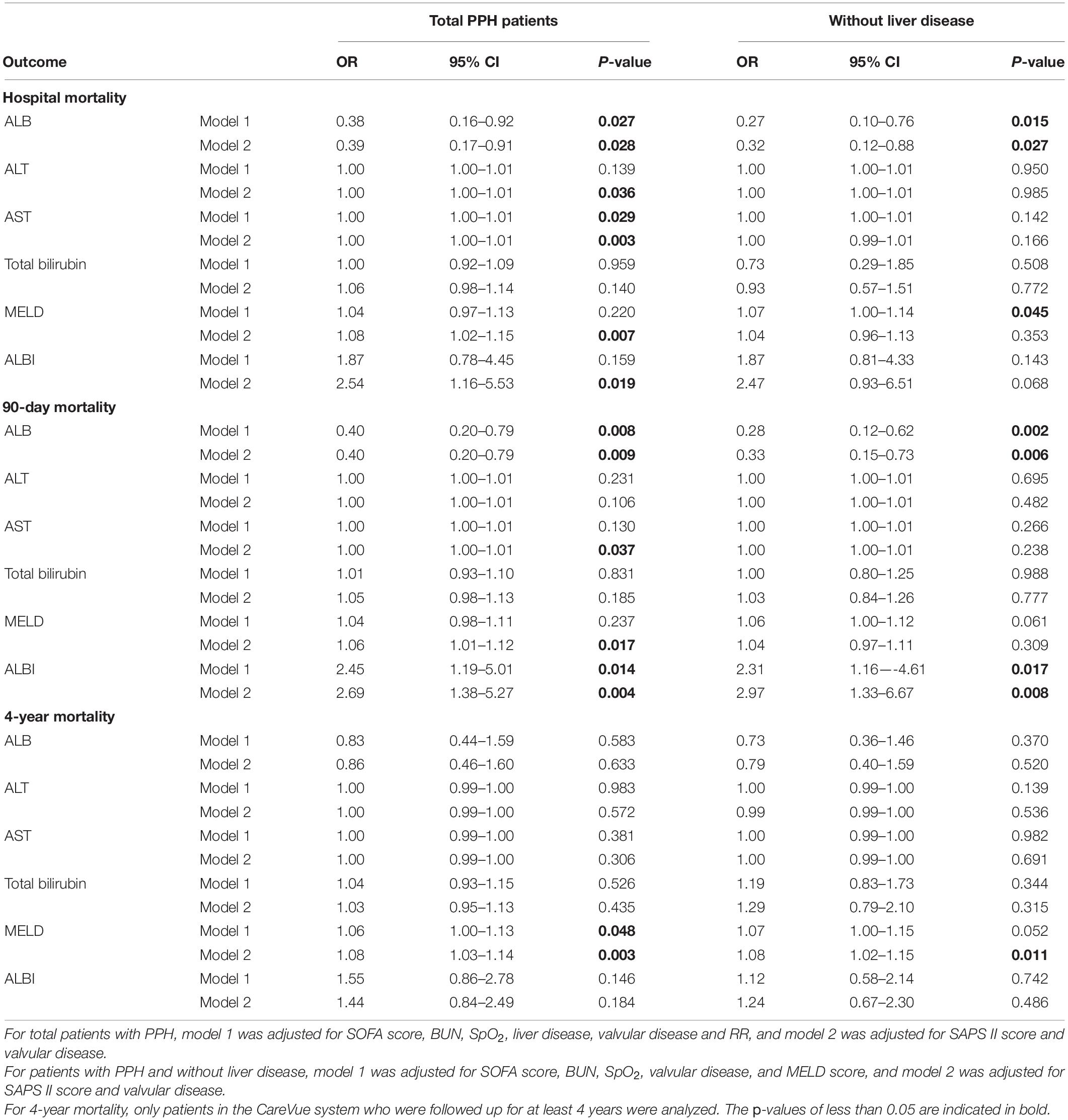
Table 7. Association between ALB, ALT, AST, and total bilirubin with prognosis of patients with PPH.
Predictive Ability of the Liver Function Index for the Prognosis of Patients With Primary Pulmonary Hypertension
The prediction model incorporating the total bilirubin, AST, ALB, and ALT was evaluated utilizing ROC curves for the purpose of determining the diagnostic significance. For hospital, 90-day and 4-year mortality, the diagnostic performance of liver function index was satisfactory (hospital mortality: AUC for total PPH group = 0.755, AUC for PPH without liver disease = 0.760; 90-day mortality: AUC for total PPH group = 0.717, AUC for PPH without liver disease = 0.710; 4-year mortality: AUC for total PPH group = 0.608, AUC for PPH without liver disease = 0.636, all p < 0.05) (Figure 5).
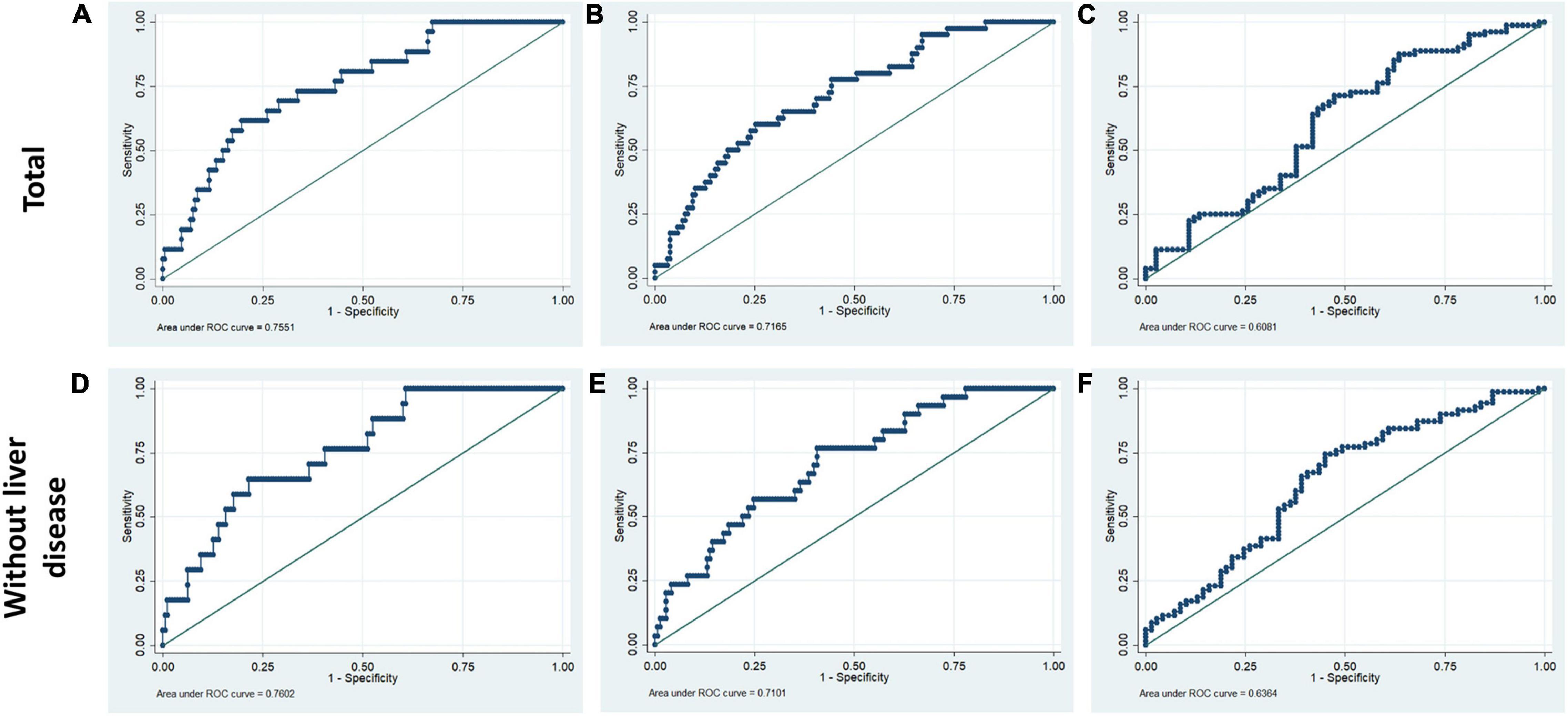
Figure 5. Receiver operating characteristic curves for liver function tests in hospital mortality, 90-day mortality, and 4-year mortality of patients with PPH. The ROC included ALB, ALT, AST, and total bilirubin. (A,D) Hospital mortality; (B,E) 90-day mortality; (C,F) 4-year mortality. (A–C) Total patients with PPH. (D–F) Patients with PPH and without liver disease.
Discussion
Within the human adult body, the liver is identified as the biggest solid organ, and it plays a crucial role in a variety of biological activities. The liver, lungs, and heart have a bidirectional and complicated interaction. Liver cirrhosis was linked to pulmonary arterial hypertension (portal hypertension) or aberrant pulmonary blood vessels dilation in the presence of severe hypoxia (hepatopulmonary syndrome) (15). In contrast, liver disease may be caused by heart and lung problems. Ischemic hepatitis and congestive liver disease are linked to cardiogenic shock and congestive heart failure, correspondingly. However, venous congestion caused by right ventricular malfunction could result in a significant elevation in hepatic blood volume, affecting liver function (16, 17). Unfortunately, there are no available outcome data for PPH patients without a history of hepatic failure or primary severe liver disease. Only ALB was correlated with the duration of ICU stay in the total PPH group, according to our findings. Abnormal ALB independently served as a risk variable for hospital mortality and 90-day mortality but not for 4-year mortality; however, it was significantly associated with 90-day and 4-year survival curves in both total PPH and PPH without liver disorders (Tables 2–7 and Figures 1, 3). Aberrant AST was linked to hospital mortality and 90-day survival curve in both total PPH and PPH without liver disease and independently served as risk variable for hospital and 90-day mortality only in total PPH group (Tables 2–7 and Figures 1, 3). ALT independently acted as a risk indicator for hospital mortality, whereas total bilirubin was only linked to hospital mortality only in the total group (Tables 6, 7). MELD score was an independent risk factor for hospital stay, 90-day mortality, and 4-year mortality in total PPH, and for hospital stay and 4-year mortality in PPH without liver disease groups. It was also significantly associated with 90-day and 4-year survival rates in total PPH and 4-year survival rates in PPH without liver disease (Tables 6, 7 and Figures 2, 4). ALBI score was an independent risk factor for hospital stay and 90-day mortality in total PPH, and for 90-day mortality in PPH without liver disease groups. It was also significantly associated with 90-day survival rates in both total PPH and PPH without liver disease (Tables 6, 7 and Figures 2, 4). The diagnostic performance of the predictive model combining the total bilirubin, AST, ALT, and ALB was moderately good for the hospital, 90-day, and 4-year mortality (Figure 5). In patients with PPH and with or without liver disorders, aberrant LFT was linked to short- and long-term patient prognosis, according to our findings.
Hypoalbuminemia may be a non-specific risk indicator for later-stage PAH since serum ALB plays a role in multiple physiological and pathological processes related to PAH progression, including hepatic dysfunction, starvation, and systemic inflammatory processes (18–21). Kawut et al. found that greater expression levels of ALB, as well as warfarin usage, acute vasoreactivity, and cardiac index, were independently linked to elevated transplant-free survival rates among patients with PAH (6). Snipelisky et al. showed that reduced serum albumin levels among patients with PAH are linked to elevated mortality rate and might function as an indicator of disease severity (7). Haddad et al. also showed a low level of serum ALB and elevated hospital mortality among patients with PAH representing a particularly high-risk cohort (22). Our research subject emphasized the analysis of patients with PPH and with and without liver disease, and our results were consistent with previous studies. Abnormal ALB independently functioned as a risk variable for hospital mortality and 90-day mortality and was significantly associated with 90-day and 4-year survival curves in both total PPH and PPH without liver disorders (Tables 2–7 and Figures 1, 3), suggesting that ALB may serve as an independent prognostic marker for PPH, irrespective of the presence or absence of liver disease in PPH patients. For patients with PPH and without liver disease, ALB was the main predictor, which may be a better surrogate for patient frailty and overall nutritional status and stage of RV dysfunction.
Hepatocellular injury pattern shaped by acute heart failure with reduced cardiac shock and output may cause a rapid increase in serum aminotransferase (AST/ALT) (23, 24). Meanwhile, patients with hepatic venous congestion caused by chronic heart failure are predominantly manifested by elevated cholestatic liver enzymes [hypoalbuminemia, gamma-glutamyltransferase (GGT), alkaline phosphatase (ALP), and bilirubin] rises (25, 26). Acute decompensated heart failure with reduced cardiac output causes a combined liver biochemical pattern with signals of hepatocyte damage (ALT and AST elevations) and cholestatic liver enzymes (ALP and bilirubin) elevations (23, 24). In the majority of research reports, bilirubin increase at baseline was linked to a worse outcome among patients with PAH. For PAH, increased total bilirubin levels were observed in 15–20% of patients (27). One further research discovered an increase in direct bilirubin level among 37% of patients and independently functioned as a risk indicator for mortality in individuals with idiopathic PAH (28). However, Hu et al. illustrated that total bilirubin was not correlated with the idiopathic PAH survival (10). This research illustrated that increased total bilirubin was correlated with hospital mortality for patients with PPH (Tables 3, 6). The inconsistency of the results may be due to the fact that this study included severe patients with pulmonary hypertension and right heart failure discharged from intensive care unit, while previous studies included mild and severe patients. In addition, the complexity of the disease may have resulted in inconsistent results across the included populations. Moreover, several smaller studies showed mildly elevated total bilirubin, ALP, and GGT for patients with PAH, while elevation in AST/ALT was less common (6, 8, 22, 29, 30). However, our results showed that aberrant AST was linked to hospital mortality and 90-day survival curve in both total PPH and PPH without liver disease, while served as a risk variable for hospital and 90-day mortality in an independent manner only in the total PPH group (Tables 2–7 and Figures 1, 3). ALT independently functioned as a risk variable for hospital mortality was associated with hospital mortality only in the total group (Tables 6, 7). These results suggested AST but not ALT was more sensitive for predicting mortality in patients with PPH and without liver disorders, and it could be involved in the PPH process. However, when we considered these four indicators in combination, rather than analyzing them individually, we found that the model composed of these four indicators had an outstanding prognostic predictive ability for mortality in patients with PPH irrespective of the presence or absence of liver disease (Figure 5). Taken together, LFTs might be a viable prognostic indicator for PPH.
Limitations
This study has some limitations. First, this was a single-center retrospective non-randomized study. Second, the therapy of pulmonary hypertension has drastically changed during the last decade. More recent data should be analyzed for more precise conclusion. Moreover, some PH drugs affect liver function; however, drug information for primary pulmonary arterial hypertension in the database was seriously missing. Therefore, we had to abandon the analysis in this study. Furthermore, the sample size of the study was small, especially the low number of non-survivors. More robust studies with larger sample size and meta-analyses should be done to further illustrate the relationship between LTFs and pulmonary hypertension. Then, the disease definition of the MIMIC III database is based on the ICD-9-CM code. Therefore, some important information was lacking because of the limitations of the database itself, such as echocardiography results, electrocardiogram results, pulmonary artery pressure, and risk scores. These were the limitations of the database itself.
Conclusion
In this research, we discovered that aberrant ALB was associated with longer ICU stay among critically ill patients with PPH. Among patients with PPH and with or without liver illness, aberrant LFTs were linked to increased hospital mortality, 90-day mortality, and 4-year mortality. The model composed of LFTs had a good prognostic predictive ability for mortality in critically ill patients with PPH irrespective of the presence or absence of liver disease.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional review boards of the MIT (Cambridge, MA, United States) and BIDMC (Boston, MA, United States). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JH and BH conceived and designed the study. DW, SH, GX, SW, ZL, and LX provided, selected, assembled, analyzed, and interpreted the data. All authors contributed to data analysis, drafting and critically revising the article, and agreed to be accountable for all aspects of the work. All authors have read and confirmed that they met ICMJE criteria for authorship.
Funding
Financial support was provided by the National Natural Science Foundation of China (81900294), the Major Transverse Research Projects of Jiaxing University, China (00619006), and the Sci-Tech Planning Project of Jiaxing, China (2019AY32008).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
References
1. Higenbottam T, Wheeldon D, Wells F, Wallwork J. Long-term treatment of primary pulmonary hypertension with continuous intravenous epoprostenol (prostacyclin). Lancet. (1984) 1:1046–7. doi: 10.1016/s0140-6736(84)91452-1
2. Nickel NP, Yuan K, Dorfmuller P, Provencher S, Lai YC, Bonnet S, et al. Beyond the lungs: systemic manifestations of pulmonary arterial hypertension. Am J Respir Crit Care Med. (2020) 201:148–57. doi: 10.1164/rccm.201903-0656CI
3. Rosenkranz S, Howard LS, Gomberg-Maitland M, Hoeper MM. Systemic consequences of pulmonary hypertension and right-sided heart failure. Circulation. (2020) 141:678–93. doi: 10.1161/CIRCULATIONAHA.116.022362
4. Nickel NP, Galura GM, Zuckerman MJ, Hakim MN, Alkhateeb H, Mukherjee D, et al. Liver abnormalities in pulmonary arterial hypertension. Pulm Circ. (2021) 11:20458940211054304.
5. Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. (2000) 102:865–70.
6. Kawut SM, Horn EM, Berekashvili KK, Garofano RP, Goldsmith RL, Widlitz AC, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol. (2005) 95:199–203. doi: 10.1016/j.amjcard.2004.09.006
7. Snipelisky D, Jentzer J, Batal O, Dardari Z, Mathier M. Serum albumin concentration as an independent prognostic indicator in patients with pulmonary arterial hypertension. Clin Cardiol. (2018) 41:782–7. doi: 10.1002/clc.22954
8. Stepnowska E, Lewicka E, Dabrowska-Kugacka A, Danilowicz-Szymanowicz L, Zagozdzon P, Kaminski R, et al. Predictors of poor outcome in patients with pulmonary arterial hypertension: a single center study. PLoS One. (2018) 13:e0193245. doi: 10.1371/journal.pone.0193245
9. Benza RL, Gomberg-Maitland M, Naeije R, Arneson CP, Lang IM. Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trials. J Heart Lung Transplant. (2011) 30:982–9. doi: 10.1016/j.healun.2011.03.011
10. Hu EC, He JG, Liu ZH, Ni XH, Zheng YG, Gu Q, et al. High levels of serum lactate dehydrogenase correlate with the severity and mortality of idiopathic pulmonary arterial hypertension. Exp Ther Med. (2015) 9:2109–13. doi: 10.3892/etm.2015.2376
11. Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. (2016) 3:160035. doi: 10.1038/sdata.2016.35
12. Hu B, Xu G, Jin X, Chen D, Qian X, Li W, et al. Novel prognostic predictor for primary pulmonary hypertension: focus on blood urea nitrogen. Front Cardiovasc Med. (2021) 8:724179. doi: 10.3389/fcvm.2021.724179
13. Pinato DJ, Sharma R, Allara E, Yen C, Arizumi T, Kubota K, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol. (2017) 66:338–46. doi: 10.1016/j.jhep.2016.09.008
14. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. (2006) 43:1317–25. doi: 10.1002/hep.21178
15. Mandell MS. Hepatopulmonary syndrome and portopulmonary hypertension in the model for end-stage liver disease (MELD) era. Liver Transpl. (2004) 10:S54–8. doi: 10.1002/lt.20260
16. Lightsey JM, Rockey DC. Current concepts in ischemic hepatitis. Curr Opin Gastroenterol. (2017) 33:158–63. doi: 10.1097/MOG.0000000000000355
18. Ha CE, Bhagavan NV. Novel insights into the pleiotropic effects of human serum albumin in health and disease. Biochim Biophys Acta. (2013) 1830:5486–93. doi: 10.1016/j.bbagen.2013.04.012
19. Arques S, Ambrosi P. Human serum albumin in the clinical syndrome of heart failure. J Card Fail. (2011) 17:451–8. doi: 10.1016/j.cardfail.2011.02.010
20. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. (2004) 17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x
21. Ballmer PE. Causes and mechanisms of hypoalbuminaemia. Clin Nutr. (2001) 20:271–3. doi: 10.1054/clnu.2001.0439
22. Haddad F, Peterson T, Fuh E, Kudelko KT, de Jesus Perez V, Skhiri M, et al. Characteristics and outcome after hospitalization for acute right heart failure in patients with pulmonary arterial hypertension. Circ Heart Fail. (2011) 4:692–9. doi: 10.1161/CIRCHEARTFAILURE.110.949933
23. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. (2013) 34:835–43. doi: 10.1093/eurheartj/ehs444
24. van Deursen VM, Damman K, Hillege HL, van Beek AP, van Veldhuisen DJ, Voors AA. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail. (2010) 16:84–90. doi: 10.1016/j.cardfail.2009.08.002
25. Samsky MD, Patel CB, DeWald TA, Smith AD, Felker GM, Rogers JG, et al. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol. (2013) 61:2397–405. doi: 10.1016/j.jacc.2013.03.042
26. Arques S, Roux E, Sbragia P, Gelisse R, Pieri B, Ambrosi P. Usefulness of serum albumin concentration for in-hospital risk stratification in frail, elderly patients with acute heart failure. Insights from a prospective, monocenter study. Int J Cardiol. (2008) 125:265–7. doi: 10.1016/j.ijcard.2007.07.094
27. Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL). Circulation. (2010) 122:164–72. doi: 10.1161/CIRCULATIONAHA.109.898122
28. Xu XQ, Lv ZC, Liu QQ, Zhao QH, Wu Y, Sun K, et al. Direct bilirubin: a new risk factor of adverse outcome in idiopathic pulmonary arterial hypertension. Int J Cardiol. (2017) 228:895–9. doi: 10.1016/j.ijcard.2016.11.036
29. Hao YJ, Jiang X, Zhou W, Wang Y, Gao L, Wang Y, et al. Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur Respir J. (2014) 44:963–72. doi: 10.1183/09031936.00182813
Keywords: primary pulmonary hypertension, 90-day mortality, long-and short-term prognoses, hospital mortality, 4-year mortality, liver function tests, intensive care unit
Citation: Wang D, Huang S, Xu G, Wu S, Liu Z, Xu L, Hu B and Hou J (2022) Abnormal Liver Function Tests Were Related to Short- and Long-Term Prognosis in Critically Ill Patients With Primary Pulmonary Hypertension. Front. Cardiovasc. Med. 9:897040. doi: 10.3389/fcvm.2022.897040
Received: 15 March 2022; Accepted: 09 May 2022;
Published: 02 June 2022.
Edited by:
Robert Jeenchen Chen, Stanford University, United StatesReviewed by:
Yosuke Nabeshima, University of Occupational and Environmental Health Japan, JapanEmmanouil Tampakakis, Johns Hopkins University, United States
Copyright © 2022 Wang, Huang, Xu, Wu, Liu, Xu, Hu and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dayu Wang, d2FuZ2RheXVAcHlob3NwaXRhbC5jb20uY24=; Bo Hu, aGJvamxAMTI2LmNvbQ==; Jian Hou, Y2huaG91akAxNjMuY29t
†These authors have contributed equally to this work
 Dayu Wang1*†
Dayu Wang1*† Suiqing Huang
Suiqing Huang Guangtao Xu
Guangtao Xu Long Xu
Long Xu Bo Hu
Bo Hu Jian Hou
Jian Hou