- 1The First School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, China
- 2School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
Background: Previous observational studies have suggested that the causal role of systemic lupus erythematosus (SLE) in the risk of cardiovascular diseases (CVDs) remained inconsistent. In this study, we aimed to investigate the causal relationship between SLE and CVDs by two-sample Mendelian randomization (MR) analysis.
Methods: Genetic instruments for SLE were obtained from a public genome-wide association study (GWAS) with 4,036 patients with SLE and 6,959 controls. Summary statistical data for CVDs, including coronary artery disease (CAD), myocardial infarction (MI), atrial fibrillation (AF), ischemic stroke (IS), and its subtypes, were identified from other available GWAS meta-analyses. The inverse-variance weighted (IVW) method was used as the primary method to estimate the causal effect. The simple- and weighted-median method, MR-Egger method, and MR pleiotropy residual sum and outlier (MR-PRESSO) were provided as a supplement to the IVW method. Besides, we performed sensitivity analyses, including Cochran's Q test, MR-Egger intercept test, and leave-one-out analysis, to evaluate the robustness of the results.
Results: A total of 15 single-nucleotide polymorphisms (SNPs) were identified after excluding linkage disequilibrium (LD) and potential confounding factors. According to the IVW results, our MR study indicated that genetically predicted SLE was not causally connected with the risk of CVDs [CAD: odds ratio (OR) = 1.005, 95% confidence interval (CI) = 0.986–1.024, p-value = 0.619; MI: OR = 1.002, 95% CI = 0.982–1.023, p-value = 0.854; AF: OR = 0.998, 95% CI = 0.982–1.014, p-value = 0.795; IS: OR = 1.006, 95% CI = 0.984–1.028, p-value = 0.621; cardioembolic stroke (CES): OR = 0.992, 95% CI = 0.949–1.036, p-value = 0.707; small vessel stroke (SVS): OR = 1.014, 95% CI = 0.964–1.067, p-value = 0.589; large artery stroke (LAS): OR = 1.030, 95% CI = 0.968–1.096, p-value = 0.352]. Analogical findings could be observed in supplementary MR methods. Sensitivity analyses suggested that the causal estimates were robust.
Conclusion: Our two-sample MR analysis provided no evidence that genetically determined SLE was causally associated with the risk of CVDs.
Introduction
Systemic lupus erythematosus (SLE) is one of the archetypal autoimmune diseases that can involve numerous organs or systems, including the skin, kidney, hematologic and nervous system, among others (1). The global prevalence of SLE ranged from 13 to 7,713.5 in every 100,000 individuals (2). In recent decades, with the improved awareness of SLE, comprehensive therapy, and prevention of complications, the overall survival rate has enhanced dramatically, with 5-, 10-, and 15-year survival rates of 96, 93, and 76%, respectively (3). However, the all-cause standardized mortality ratio for patients with SLE was still 2.6 times higher than that for the general population (4).
Several studies suggested that patients with SLE were more susceptible to cardiovascular diseases (CVDs), including coronary artery disease (CAD), myocardial infarction (MI), atrial fibrillation (AF), and stroke. A recent meta-analysis proclaimed that, compared with the unexposed cohort, the relative risk of patients with SLE suffering from CVDs was 1.98, especially lupus nephritis (5). Another retrospective cohort indicated that SLE could increase the incidence of CAD (OR 1.42, 95% CI 1.40–1.44) by enrolling 252,676 patients with SLE and 758,034 matched patients without SLE (6). In Korea, similar results for MI, AF, and stroke could be observed after an 8-year follow-up (7, 8). On the contrary, another meta-analysis and cohort study suggested that the incidence of CVDs did not differ significantly between patients with SLE and the control population (9, 10). Since the observational study was more likely prone to confounders, such as smoking, type 2 diabetes (T2D), and low-density lipoprotein (LDL), different studies were more likely to reach divergent conclusions. Therefore, it is necessary to further explore the causal association between SLE and CVDs.
Mendelian randomization (MR) is an epidemiological method that assesses causality between exposure and outcome depending on genetic variants (11). As genotypes are assigned randomly during meiosis and preceded phenotypes, it can address the influence of confounding factors and reverse causation (12). In recent years, MR study was widely applied to explore the causal association between multiple exposures and CVDs, such as sleep duration (13), alcohol intake (14), obesity (15), blood pressure (16), and major depressive disorder (17).
In this study, we aimed to estimate the potential causal relationship for SLE with the risk of CVDs that encompassed CAD, AF, MI, ischemic stroke (IS), and its subtypes by a two-sample MR study.
Methods
Study design
The persuasive conclusion is obtained only when the following assumptions for the MR study are satisfied. First, the genetic instrumental variables (IVs) should be powerfully related to exposure (SLE). Second, the genetic IVs have nothing to do with the potential confounders, including body mass index (BMI), LDL, T2D, total cholesterol (TC), triglyceride (TG), hypertension, C-reactive protein (CRP), and others. Third, the genetic IVs only affect the outcome (CVDs) through SLE. Figure 1 shows three key assumptions of this MR study. Ethical approval of participants was not necessary as this research was based on the publicly available database.
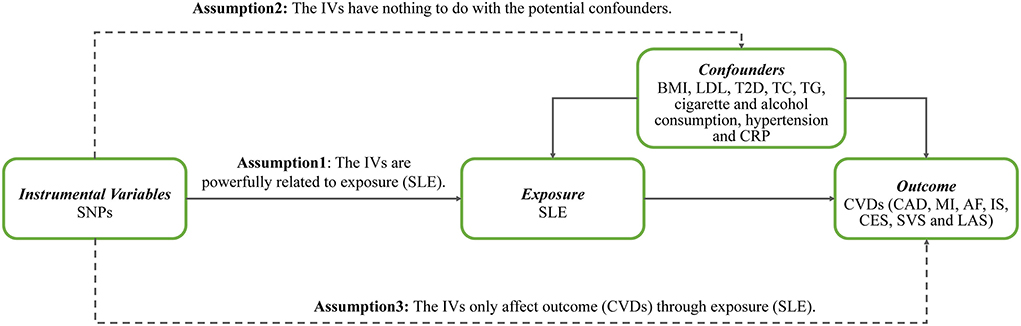
Figure 1. Three key assumptions of Mendelian randomization study. SNPs, single-nucleotide polymorphisms; IVs, instrumental variables; SLE, systemic lupus erythematosus; BMI, body mass index; LDL, low-density lipoprotein; T2D, type 2 diabetes; TC, total cholesterol; TG, triglyceride; CRP, C-reactive protein; CVDs, cardiovascular diseases; CAD, coronary artery disease; MI, myocardial infarction; AF, atrial fibrillation; IS, ischemic stroke; CES, cardioembolic stroke; SVS, small vessel stroke; LAS, large artery stroke.
Data sources
Summary data on SLE were obtained from a meta-analysis of genome-wide association studies (GWASs) including 10,995 European participants with 4,036 cases and 6,959 controls (18). This study totally covered 644,674 single-nucleotide polymorphisms (SNPs), and all cases fulfilled the SLE diagnostic criteria of the American College of Rheumatology.
The GWAS meta-analysis with 48 studies was applied to extract the genetic variants associated with CAD and MI (19). This study assembled 60,801 cases (~70% cases had a reported history of MI) and 123,504 controls, nearly 77% individuals with European ancestry. All cases satisfied the CAD diagnosis, including MI, acute coronary syndrome, chronic stable angina, or coronary stenosis >50%. Atrial Fibrillation Genetics Consortium conducted a large-scale GWAS meta-analysis with 65,446 cases (84.2% European individuals) and 522,744 controls, reporting 12,149,979 SNPs (20). All cases suffered from paroxysmal, permanent AF, or atrial flutter. Genetic variants related to IS and its subtypes were based on another GWAS study from the MEGASTROKE consortium, covering ~8 million SNPs (21). According to the Trial of Org 10172 in Acute Stroke Treatment criteria, IS was further classified as cardioembolic stroke (CES) with 7,193 cases, small vessel stroke (SVS) with 5,386 cases, and large artery stroke (LAS) with 4,373 cases. Supplementary Table 1 describes the basic information of the above GWAS studies.
SNPs selection
In the original study, Bentham et al. (18) identified 25 SNPs of genome-wide significance (p-value < 5 × 10−8). To ensure the independence of these 25 SNPs, we used LD-pruned (pairwise r2 < 0.001, window size = 10,000 kb) by the clump_data command using the R software (22). In addition, these SNPs were searched at the GWAS threshold (p-value < 5 × 10−8) by the PhenoScanner V2 database (http://www.phenoscanner.medschl.cam.ac.uk/) to rule out the influence of potential confounders (BMI, LDL, T2D, TC, TG, cigarette and alcohol consumption, hypertension, and CRP) (23). Furthermore, we calculated the F-statistic to assess the extent of weak instrument bias (24). The proportion of variance (R2), which was explained by these selected SNPs, was evaluated by the formula of 2 × MAF × (1 – MAF) × β2 (25). The smallest effect detected by the sample size of the outcome to provide 80% statistical power at an α level of 5% was calculated by using the online mRnd power tool [https://shiny.cnsgenomics.com/mRnd/ (26)].
Statistical analysis
In this two-sample MR analysis, we used five methods [inverse-variance weighted (IVW), simple- and weighted-median, MR-Egger, and MR pleiotropy residual sum and outlier test (MR-PRESSO)] to derive the causal estimates between SLE and CVDs. The IVW analysis is the primary method in our MR study because it provides the most persuasive estimates when the directional pleiotropy of the IVs is absent (27, 28). The simple median yields causal effects, where <50% of information comes from valid IVs; the weighted median requires more than 50% of valid IVs (29). The MR-Egger method provides the causal estimates based on the slope from the weighted regression of the IVs-outcome relationship on the IVs-exposure relationship (27). The MR-PRESSO method detects horizontal pleiotropy and reappraises the causal effect after eliminating the pleiotropic IVs (30).
To further explain the potential pleiotropy, we conducted a series of sensitivity analyses. Cochran's Q-test quantifies the heterogeneity of IVs, with a value of p < 0.05 indicating heterogeneity (31). The deviation of the MR-Egger intercept from zero determines whether there exists a directional horizontal pleiotropy. Furthermore, we also used the leave-one-out analysis to assess whether the causal effect was disturbed by a single SNP (27). Figure 2 displays the flowchart of IVs selection and MR analyses.

Figure 2. The flowchart of this Mendelian randomization study. IVs, instrumental variables; SLE, systemic lupus erythematosus; GWAS, genome-wide association study; CAD, coronary artery disease; MI, myocardial infarction; AF, atrial fibrillation; IS, ischemic stroke; IVW, inverse-variance weighted method; MR-PRESSO, Mendelian randomization pleiotropy residual sum and outlier test.
All statistical analyses were performed by MR-PRESSO (1.0) (30) and TwoSampleMR (0.5.5) (22) packages using the R software (3.6.1), and a value of p < 0.05 was considered statistically significant if not otherwise stated.
Result
On account of linkage disequilibrium (LD) and confounding factors (BMI, LDL, T2D, TC, TG, cigarette and alcohol consumption, hypertension, and CRP), 10 SNPs were excluded. Finally, a total of 15 SNPs were taken as effective IVs. The F-statistic of all selected SNPs was over 10, which ranged from 137.29 to 1,817.47, suggesting that there was no weak instrument bias. Supplementary Table 2 describes the detailed information about these SNPs.
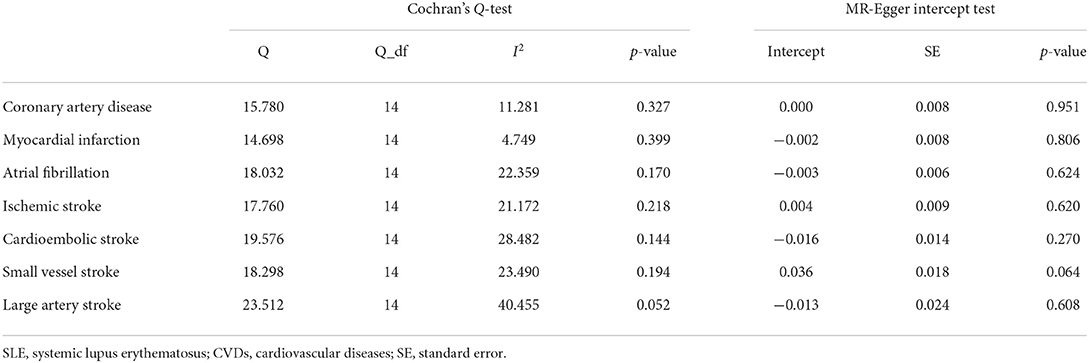
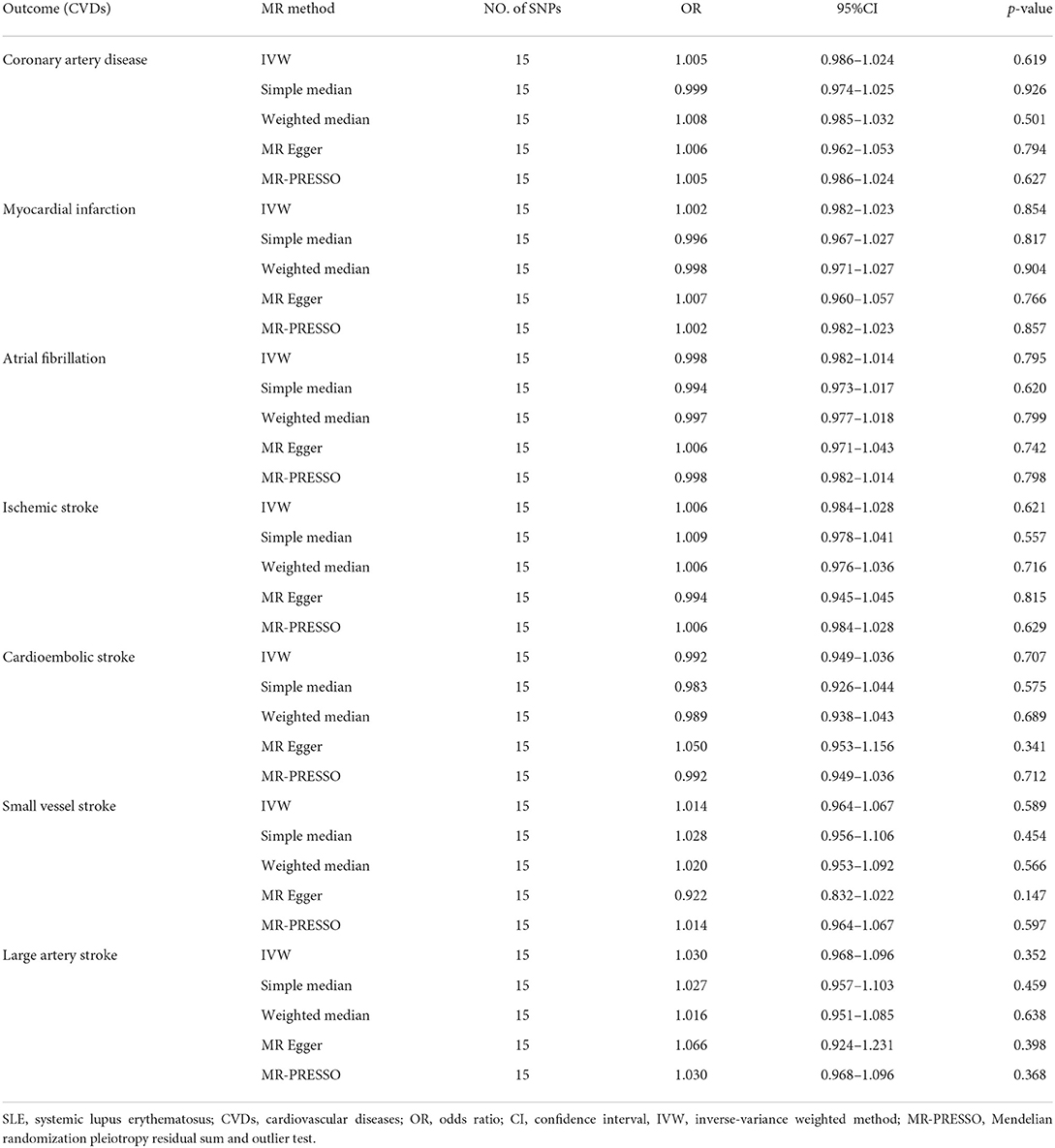
Cochran's Q-test revealed no significant heterogeneity among 15 SNPs (CAD: p-value = 0.327, MI: p-value = 0.399; AF: p-value = 0.170; IS: p-value = 0.218; CES: p-value = 0.144; SVS: p-value = 0.194; LAS: p-value = 0.052) (Table 1). Therefore, we chose the fixed-effects IVW method, indicating that there was no evidence to support the causal association between SLE and the risk of CVDs (CAD: OR 1.005, 95% CI 0.986–1.024, p-value = 0.619; MI: OR 1.002, 95% CI 0.982–1.023, p-value = 0.854; AF: OR 0.998, 95% CI 0.982–1.014, p-value = 0.795; IS: OR 1.006, 95% CI 0.984–1.028, p-value = 0.621; CES: OR 0.992, 95% CI 0.949–1.036, p-value = 0.707; SVS: OR 1.014, 95% CI 0.964–1.067, p-value = 0.589; LAS: OR 1.030, 95% CI 0.968–1.096, p-value = 0.352) (Table 2; Figure 3; Supplementary Figure 1). The effect estimates of the simple- and weighted-median method, MR-Egger, and MR-PRESSO were parallel to that of the IVW method (Table 2).
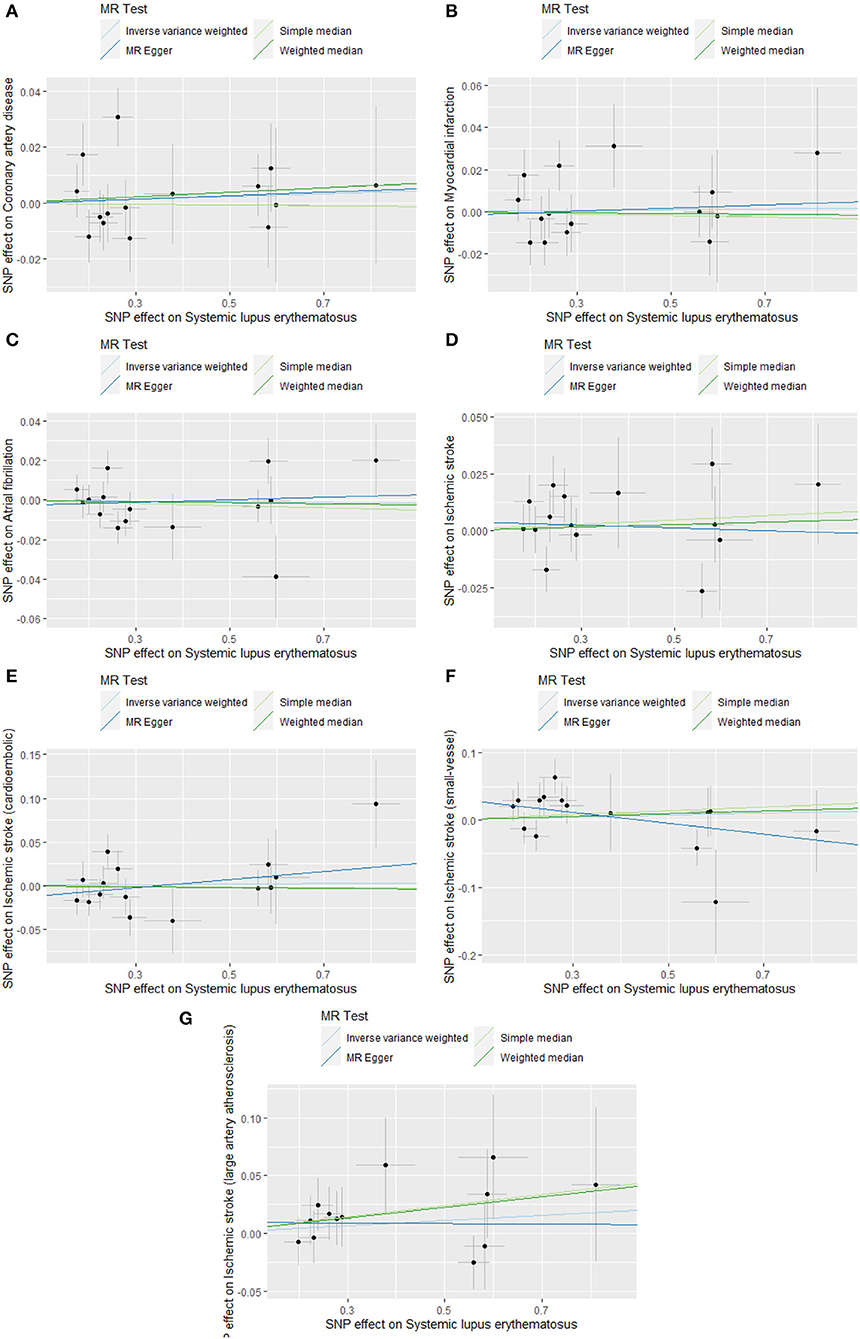
Figure 3. Scatter plots of causal associations of SLE on CVDs. SLE, systemic lupus erythematosus; CVDs, cardiovascular diseases. (A) coronary artery disease; (B) myocardial infarction; (C) atrial fibrillation; (D) ischemic stroke; (E) cardioembolic stroke; (F) small vessel stroke; (G) large artery stroke.
To reduce the bias resulting from horizontal pleiotropy, we performed a MR-Egger intercept test and leave-one-out analysis. The result of MR-Egger intercept test did not reveal directional pleiotropy (CAD: intercept = 0.000, p-value = 0.951; MI: intercept = −0.002, p-value = 0.806; AF: intercept = −0.003, p-value = 0.624; IS: intercept = 0.004, p-value = 0.620; CES: intercept = −0.016, p-value= 0.270; SVS: intercept = 0.036, p-value = 0.064; LAS: intercept = −0.013, p-value = 0.608) (Table 1). Meanwhile, the leave-one-out analysis also demonstrated the robustness of the MR effect estimates (Supplementary Figure 2). Based on the sample size of the CVDs GWAS meta-analysis, there was >80% power to detect the associations of SLE with the risk of CAD, MI, AF, IS, and its subtypes for effect size (OR) of ~0.9 (Supplementary Table 3).
Discussion
In the study, we systematically evaluated the causal association between SLE and the risk of CVDs by a two-sample MR analysis. We did not provide the causal evidence that genetically predicted SLE could increase the risk of CVDs (CAD, MI, AF, IS, CES, SVS, and LAS). The sensitivity analyses also displayed that horizontal pleiotropy was absent, revealing the robustness of effect estimates.
Previous multiple observational studies have proclaimed that SLE was positively associated with the incidence of CVDs. A 10-year follow-up cohort study performed by Yafasova et al. (32) found that patients with SLE had a higher risk of AF, IS, and MI. In line with the above study, another meta-analysis also suggested that the incidence of cardiovascular events was 25.4% among patients with SLE, including acute MI (4.1%) and stroke (7.3%) (33).
However, other studies revealed that the prevalence rate of coronary heart disease (CHD) and IS did not appear differently between patients with SLE and age- and gender-matched controls (34, 35). Our MR analysis also did not provide sufficient evidence that genetically predicted SLE was in connection with the increased risk of CVDs. Given the random allocation of genetic variants, MR analysis could provide more reliable results, compared with the observational study. Previous positive results might be triggered by several common confounders shared by SLE and CVDs. Obesity is a well-acknowledged risk factor for CVDs (36, 37), and it is also related to worse disease activity and higher levels of inflammation markers for SLE (37). Therefore, obesity is an extremely important common risk factor for SLE and CVDs. Apart from obesity, cigarette consumption also tended to act as another common risk (38, 39). In addition, long-term use of glucocorticoids, the fundamental drug for SLE, could lead to glucose-lipid metabolism disturbance to further induce the development of CHD, IS, and MI (40, 41).
The main strength of this study was that we estimated the causal relationship of SLE with CAD, MI, AF, IS, and its subtypes by two-sample MR analysis for the first time. In addition, we largely overcame the interference of potential confounders. However, there were still several limitations to this study. First, studies indicated disease activity was positively associated with CVDs among patients with SLE (42, 43). However, disease activity was not reported in the original SLE GWAS, which could have contributed to the definition of SLE status (active or remission), so we could not explore whether higher disease activity of SLE increased the rate of cardiovascular events. Second, the incidence of CVDs was different in different racial patients with SLE (44). As ~15.8% of AF and 23% of CAD were participants without European ancestry, our MR study might be slightly influenced by population effects. In the end, based on our MR results, although the ORs of AF and CES were <1 and others were more than 1, all the p-values were considerably >0.05. This inconsistency may be due to the insufficient sample size. Therefore, it is necessary to conduct SLE GWAS with a larger sample size to more demonstrate whether there is a causality between SLE and CVDs.
Conclusion
Overall, our research provided no evidence that genetically predicted SLE was causally associated with the risk of CVDs by a two-sample MR analysis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JB and YF provided the idea, designed this study protocol, and revised the manuscript. SH and FH collected and analyzed data. CM and FT were responsible for the drawing. SH, FH, and CM contributed to the manuscript writing. All authors read and consented to the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81803980) and the Research Project of Zhejiang Chinese Medical University (Grant Nos. 2019ZG22 and 2021JKZKTS012B).
Acknowledgments
We sincerely appreciated all researchers who provided summary statistical data on GWAS or GWAS meta-analysis for SLE, CAD, MI, AF, IS, and its subtypes. On account of providing public data, we could smoothly carry out our MR research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.896499/full#supplementary-material
References
1. Zhang L, Qing P, Yang H, Wu Y, Liu Y, Luo Y. Gut microbiome and metabolites in systemic lupus erythematosus: link, mechanisms and intervention. Front Immunol. (2021) 12:686501. doi: 10.3389/fimmu.2021.686501
2. Barber MRW, Drenkard C, Falasinnu T, Hoi A, Mak A, Kow NY, et al. Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol. (2021) 17:515–32. doi: 10.1038/s41584-021-00668-1
3. Fors Nieves CE, Izmirly PM. Mortality in systemic lupus erythematosus: an updated review. Curr Rheumatol Rep. (2016) 18:21. doi: 10.1007/s11926-016-0571-2
4. Lee YH, Choi SJ Ji JD, Song GG. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. (2016) 25:727–34. doi: 10.1177/0961203315627202
5. Restivo V, Candiloro S, Daidone M, Norrito R, Cataldi M, Minutolo G, et al. Systematic review and meta-analysis of cardiovascular risk in rheumatological disease: symptomatic and non-symptomatic events in rheumatoid arthritis and systemic lupus erythematosus. Autoimmun Rev. (2022) 21:102925. doi: 10.1016/j.autrev.2021.102925
6. Katz G, Smilowitz NR, Blazer A, Clancy R, Buyon JP, Berger JS. Systemic lupus erythematosus and increased prevalence of atherosclerotic cardiovascular disease in hospitalized patients. Mayo Clin Proc. (2019) 94:1436–43. doi: 10.1016/j.mayocp.2019.01.044
7. Lim SY, Bae EH, Han KD, Jung JH, Choi HS, Kim HY, et al. Systemic lupus erythematosus is a risk factor for cardiovascular disease: a Nationwide, Population-Based Study in Korea. Lupus. (2018) 27:2050–6. doi: 10.1177/0961203318804883
8. Lim SY, Bae EH, Han KD, Jung JH, Choi HS, Kim CS, et al. Systemic lupus erythematosus is a risk factor for atrial fibrillation: a Nationwide, Population-Based Study. Clin Exp Rheumatol. (2019) 37:1019–25.
9. Lu X, Wang Y, Zhang J, Pu D, Hu N, Luo J, et al. Patients with systemic lupus erythematosus face a high risk of cardiovascular disease: a systematic review and meta-analysis. Int Immunopharmacol. (2021) 94:107466. doi: 10.1016/j.intimp.2021.107466
10. Chuang YW Yu MC, Lin CL Yu TM, Shu KH, Kao CH. Risk of peripheral arterial occlusive disease in patients with systemic lupus erythematosus: a Nationwide Population-Based Cohort Study. Medicine. (2015) 94:e2121. doi: 10.1097/MD.0000000000002121
11. Burgess S, Foley CN, Allara E, Staley JR, Howson JMM. A Robust and efficient method for mendelian randomization with hundreds of genetic variants. Nat Commun. (2020) 11:376. doi: 10.1038/s41467-019-14156-4
12. Aikens RC, Zhao W, Saleheen D, Reilly MP, Epstein SE, Tikkanen E, et al. Systolic blood pressure and risk of type 2 diabetes: a mendelian randomization study. Diabetes. (2017) 66:543–50. doi: 10.2337/db16-0868
13. Ai S, Zhang J, Zhao G, Wang N, Li G, So HC, et al. Causal associations of short and long sleep durations with 12 cardiovascular diseases: linear and nonlinear mendelian randomization analyses in Uk biobank. Eur Heart J. (2021) 42:3349–57. doi: 10.1093/eurheartj/ehab170
14. Larsson SC, Burgess S, Mason AM, Michaelsson K. Alcohol consumption and cardiovascular disease: a mendelian randomization study. Circ Genom Precis Med. (2020) 13:e002814. doi: 10.1161/CIRCGEN.119.002814
15. Riaz H, Khan MS, Siddiqi TJ, Usman MS, Shah N, Goyal A, et al. Association between obesity and cardiovascular outcomes: a systematic review and meta-analysis of mendelian randomization studies. JAMA Netw Open. (2018) 1:e183788. doi: 10.1001/jamanetworkopen.2018.3788
16. Wan EYF, Fung WT, Schooling CM, Au Yeung SL, Kwok MK Yu EYT, et al. Blood pressure and risk of cardiovascular disease in Uk biobank: a mendelian randomization study. Hypertension. (2021) 77:367–75. doi: 10.1161/HYPERTENSIONAHA.120.16138
17. Zhang F, Cao H, Baranova A. Shared genetic liability and causal associations between major depressive disorder and cardiovascular diseases. Front Cardiovasc Med. (2021) 8:735136. doi: 10.3389/fcvm.2021.735136
18. Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. (2015) 47:1457–64. doi: 10.1038/ng.3434
19. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. (2015) 47:1121–30. doi: 10.1038/ng.3396
20. Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. (2018) 50:1225–33. doi: 10.1038/s41588-018-0133-9
21. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. (2018) 50:524–37. doi: 10.1038/s41588-018-0058-3
22. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The Mr-base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
23. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. Phenoscanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. (2019) 35:4851–3. doi: 10.1093/bioinformatics/btz469
24. Burgess S, Thompson SG, CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036
25. Park JH, Wacholder S, Gail MH, Peters U, Jacobs KB, Chanock SJ, et al. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet. (2010) 42:570–5. doi: 10.1038/ng.610
26. Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in mendelian randomization studies. Int J Epidemiol. (2013) 42:1497–501. doi: 10.1093/ije/dyt179
27. Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing mendelian randomization investigations. Wellcome Open Res. (2019) 4:186. doi: 10.12688/wellcomeopenres.15555.1
28. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
29. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
30. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
31. Del Greco MF, Minelli C, Sheehanc NA, Thompsonc JR. Detecting pleiotropy in mendelian randomisation studies with summary data and a continuous outcome. Stat Med. (2015) 34:2926–40. doi: 10.1002/sim.6522
32. Yafasova A, Fosbol EL, Schou M, Baslund B, Faurschou M, Docherty KF, et al. Long-term cardiovascular outcomes in systemic lupus erythematosus. J Am Coll Cardiol. (2021) 77:1717–27. doi: 10.1016/j.jacc.2021.02.029
33. Ballocca F, D'Ascenzo F, Moretti C, Omede P, Cerrato E, Barbero U, et al. Predictors of cardiovascular events in patients with systemic lupus erythematosus (sle): a systematic review and meta-analysis. Eur J Prev Cardiol. (2015) 22:1435–41. doi: 10.1177/2047487314546826
34. Tziomalos K, Gkougkourelas I, Sarantopoulos A, Bekiari E, Makri E, Raptis N, et al. Arterial stiffness and peripheral arterial disease in patients with systemic lupus erythematosus. Rheumatol Int. (2017) 37:293–8. doi: 10.1007/s00296-016-3610-4
35. Hammad SM, Hardin JR, Wilson DA, Twal WO, Nietert PJ, Oates JC. Race disparity in blood sphingolipidomics associated with lupus cardiovascular comorbidity. PLoS ONE. (2019) 14:e0224496. doi: 10.1371/journal.pone.0224496
36. Kim MS, Kim WJ, Khera AV, Kim JY, Yon DK, Lee SW, et al. Association between adiposity and cardiovascular outcomes: an umbrella review and meta-analysis of observational and mendelian randomization studies. Eur Heart J. (2021) 42:3388–403. doi: 10.1093/eurheartj/ehab454
37. Correa-Rodriguez M, Pocovi-Gerardino G, Callejas Rubio JL, Rios Fernandez R, Martin Amada M, Cruz Caparros M, et al. The impact of obesity on disease activity, damage accrual, inflammation markers and cardiovascular risk factors in systemic lupus erythematosus. Panminerva Med. (2020) 62:75–82. doi: 10.23736/S0031-0808.19.03748-0
38. Colpani V, Baena CP, Jaspers L, van Dijk GM, Farajzadegan Z, Dhana K, et al. Lifestyle factors, cardiovascular disease and all-cause mortality in middle-aged and elderly women: a systematic review and meta-analysis. Eur J Epidemiol. (2018) 33:831–45. doi: 10.1007/s10654-018-0374-z
39. Chua MH, Ng IA, Mike WC, Mak A. Association between cigarette smoking and systemic lupus erythematosus: an updated multivariate bayesian metaanalysis. J Rheumatol. (2020) 47:1514–21. doi: 10.3899/jrheum.190733
40. Wilson JC, Sarsour K, Gale S, Petho-Schramm A, Jick SS, Meier CR. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res. (2019) 71:498–511. doi: 10.1002/acr.23611
41. Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. (2016) 15:457–65. doi: 10.1517/14740338.2016.1140743
42. Romero-Diaz J, Vargas-Vorackova F, Kimura-Hayama E, Cortazar-Benitez LF, Gijon-Mitre R, Criales S, et al. Systemic lupus erythematosus risk factors for coronary artery calcifications. Rheumatology. (2012) 51:110–9. doi: 10.1093/rheumatology/ker307
43. Wang XY, Tang XQ, Huang YJ, Chen WY Yu XQ. Frequency of established cardiovascular disease and its risk factors in Chinese patients with systemic lupus erythematosus. Clin Rheumatol. (2012) 31:669–75. doi: 10.1007/s10067-011-1910-3
Keywords: systemic lupus erythematosus, cardiovascular disease, coronary artery disease, myocardial infarction, atrial fibrillation, ischemic stroke, Mendelian randomization, single nucleotide polymorphism
Citation: Huang S, Huang F, Mei C, Tian F, Fan Y and Bao J (2022) Systemic lupus erythematosus and the risk of cardiovascular diseases: A two-sample Mendelian randomization study. Front. Cardiovasc. Med. 9:896499. doi: 10.3389/fcvm.2022.896499
Received: 15 March 2022; Accepted: 08 August 2022;
Published: 02 September 2022.
Edited by:
Edoardo Sciatti, Local Social Health Agency Garda, ItalyReviewed by:
Fabio Fimiani, Azienda Ospedaliera dei Colli, ItalyMichael C. Mahaney, The University of Texas Rio Grande Valley, United States
Copyright © 2022 Huang, Huang, Mei, Tian, Fan and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Bao, c2lua3liakAxMjYuY29t; Yongsheng Fan, Znlzemp0Y21AMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Shuo Huang
Shuo Huang Fugang Huang1†
Fugang Huang1† Fengyuan Tian
Fengyuan Tian Jie Bao
Jie Bao