
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 19 October 2022
Sec. General Cardiovascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.896173
Objective: To analyze the predictive values of D-dimer in Chinese patients with non-ST-segment elevation myocardial infarction (NSTEMI).
Methods: We retrospectively retrieved consecutive patients hospitalized due to acute NSTEMI from January 2015 to December 2018 from the Electronic Medical Record (EMR) library. Clinical and follow-up data were collected. The primary endpoint was major adverse composite cardiovascular events (MACEs), such as all-cause death, non-fatal myocardial infarction, and non-fatal stroke. The secondary endpoints included all-cause death, non-fatal myocardial infarction, non-fatal stroke, heart failure, and severe arrhythmias. The Cox regression model was used to evaluate the association between risk factors and clinical outcomes in Chinese patients with NSTEMI.
Results: A total of 673 patients were included; the median age was 64.0 (53.0–75.0) years old and 76.2% were men. Patients with higher D-dimer levels were more often women, older, had a higher Charlson Comorbidity Index, and had a higher incidence of MACEs (59.9 vs. control 9.0%; p < 0.001) and all-cause death (49.1 vs. control 2.2%; p < 0.001). The multivariate Cox analysis suggested that the D-dimer level was an independent predictor of MACEs (hazard ratio [HR]: 1.069, 95% CI: 1.010–1.132, p = 0.021). The receiver operating characteristic (ROC) analysis suggested that D-dimer levels were better than the Charlson Comorbidity Index in all-cause death.
Conclusion: In Chinese patients with acute NSTEMI, higher D-dimer levels on admission suggest a poor long-term prognosis.
Acute myocardial infarction is a common critical illness based on atherosclerotic thrombosis and is a leading cause of morbidity and mortality (1). Patients exhibit increased coagulation system activity during the acute phase of unstable angina and myocardial infarction and manifest a persistent hypercoagulable state with minimal generation of fibrin over the next 6 months (2). Plasma D-dimer, a soluble degradation product of cross-linked fibrin during fibrinolysis (3), is a specific marker of thrombosis and indicates a hypercoagulable state. Previous studies reported that D-dimer correlates with vascular endothelial dysfunction and is a risk factor for the onset of major adverse composite cardiovascular events (MACEs) and all-cause death (4, 5). Several studies have suggested that increased D-dimer levels are associated with the presence and severity of coronary disease (6–8) and indicate an unfavorable prognosis for stable coronary disease (9). Therefore, D-dimer may be a valuable marker for predicting ischemic events (10). However, to our knowledge, the role of D-dimer in predicting the incidence of adverse events in Chinese patients with non-ST-segment elevation myocardial infarction (NSTEMI) has not been thoroughly studied. Therefore, we explored the predictive value of D-dimer on the prognosis of Chinese patients with NSTEMI based on our previous clinical data.
This was a retrospective study designed to investigate associated risk factors and clinical outcomes among Chinese patients with NSTEMI. We retrospectively retrieved and anonymously obtained the consecutive hospitalized patients due to acute NSTEMI between January 2015 and December 2018 from the Electronic Medical Record (EMR) library of Dongguan People's Hospital. The inclusion criteria were as follows: (1) age >18 years and (2) a diagnosis of NSTEMI based on the International Classification of Diseases 11th Revision (ICD-11) (such as, BA41, BA41.1, and BA41); (3) meets the fourth universal definition of myocardial infarction (11); and (4) the onset of myocardial infarction was less than 48 h. Exclusion criteria were as follows: (1) an electrocardiogram (ECG) with persistent ST-segment elevation in at least two consecutive leads; (2) duplicate cases; and (3) patients who were lost during the first 1-year follow-up period. This study is exempt from the need for informed consent according to China's “Ethical Review Approaches for Biomedical Research Involving Humans” 2016, Article 39 (1) (12). The study was approved by the institute ethics committee and was conducted following the Declaration of Helsinki (Ethics Approval Number: KYKT2022-012).
The baseline clinical characteristics included the patient's gender, age, smoking history, systolic blood pressure (SBP), diastolic blood pressure (DBP), blood glucose (BG), body mass index (BMI = weight (kg)/ [height (m)]2), and past medical histories. The age-adjusted Charlson Comorbidity Index (ACCI) (13, 14) was introduced to weigh the burden of comorbidities. Blood samples were drawn from fasting patients by venipuncture within 24 h after admission and stored in 3.2% citrated tubes or plain tubes according to the clinical laboratory requirements. Plasma D-dimer and fibrinogen (Fib) were detected by latex agglutination turbidimetry using a STARMAX automatic coagulation apparatus (STAGO, France). The normal range of plasma D-dimer levels was < 0.5 μg/ml. Low-density lipoprotein cholesterol (LDL-C) was measured using clinical laboratory methods (Beckman CX9, USA). C-reactive protein (CRP) was measured based on an immunofluorescence dry quantitative method using an automatic dry immunoassay analyzer (A5000, Boditech Biotechnology, China). B-Type natriuretic peptide (BNP) was measured by UniCel Dxi800 Access (Beckman Coulter, China). Troponin T (TNT) was measured using a Roche Cobas h 232 immunoassay analyzer (Roche Diagnostics, Mannheim, Germany). The left ventricular ejection fraction (LVEF) was estimated using the Simpson method.
A clinical follow-up visit was performed every 3 months by the treating physicians. An independent research team evaluated patients' status and end-point events according to the follow-up records. Any inconsistencies were resolved by the researcher through telephone interviews with the treating physician and/or patients. The follow-up period ended at death or November 2021. The primary endpoint event was defined as a major adverse cardiovascular event (MACE), which included non-fatal myocardial infarction, non-fatal cerebral infarction, and all-cause death. The secondary endpoint events were single events, such as all-cause death, non-fatal myocardial infarction, non-fatal stroke, heart failure, and severe arrhythmias.
Patients were grouped according to their quartiles of plasma D-dimer level at admission. The normal distribution of continuous variables was verified by the Kolmogorov–Smirnov test. Continuous variables are presented as the mean ± standard deviation (SD) or median and interquartile range (IQR) and were tested by one-way analysis of variance (ANOVA) or the Mann–Whitney U-test, as appropriate. The count data are presented as frequencies (n) and percentages (%), and the comparisons of the groups were conducted using the χ2 test. Patient survival in different groups was described by Kaplan–Meier analyses and log-rank tests. Risk factors for endpoint events were assessed using a univariate and/or multivariate Cox regression analysis. A p-value < 0.05 was considered statistically significant (a two-tailed test). Analyses were performed using the SPSS 22.0 software package for Windows (IBM, USA).
According to the inclusion and exclusion criteria, a total of 673 patients (Figure 1) were finally included (median age, 64.0 (53.0–75.0) years; men, 76.2%; and BMI, 24.62 ± 3.40 kg/m2). The baseline median plasma D-dimer level was 0.46 (0.30–0.96) mg/dl. A total of 60.2% (405/673) of patients had a history of smoking, 62.6% (421/673) of patients had a history of hypertension, and 33.4% (225/673) of patients had a history of diabetes.
The median DBP was 72.00 (83.00–96.00) mmHg. The median LVEF was 62.00 (55.00–68.00)%. The median fibrinogen was 3.75 (3.16–4.74) g/L. The median CRP level was 145 (62.50–427.90). The median ACCI was 4 (3–6). The baseline characteristics of the patients are listed in Table 1. Table 2 shows the medication status of the four groups. Except for antihyperlipidemic agents, there were significant differences in the use of the other 5 drugs among the groups.
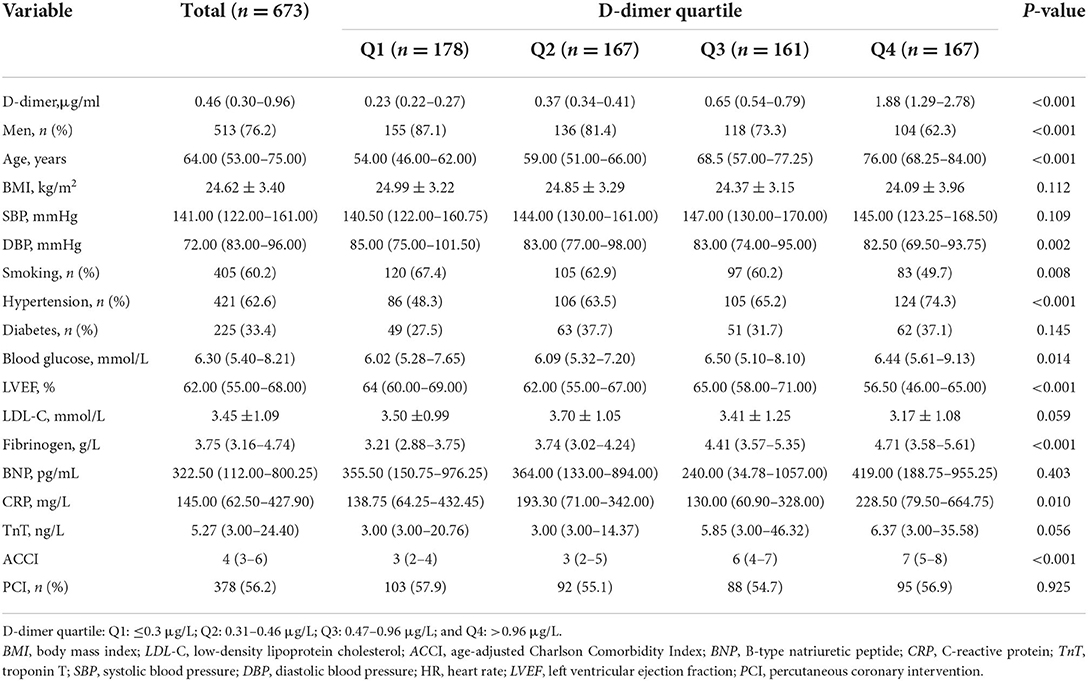
Table 1. Baseline characteristics of patients with non-ST-segment elevation myocardial infarction (NSTEMI).
Patients were followed up for a total of 1,936.4 person·years, and the average follow-up time was 1050.19 ± 548.26 days. During the follow-up period, 191 (28.4%) patients experienced MACEs, 1,119 (17.7%) patients died, 49 (7.3%) patients developed non-fatal myocardial infarction, 20 (3.0%) patients developed non-fatal cerebral infarction, 40 (5.9%) patients suffered heart failure, and 15 (2.2%) patients experienced severe arrhythmias. The incidence of clinical outcomes is shown in Table 3. As shown in the Kaplan–Meier analysis (Figure 2), plasma D-dimer levels were significantly correlated with MACEs (log-rank χ2 = 142.9, p < 0.001) and all-cause death (log-rank χ2 = 181.1, p < 0.001).
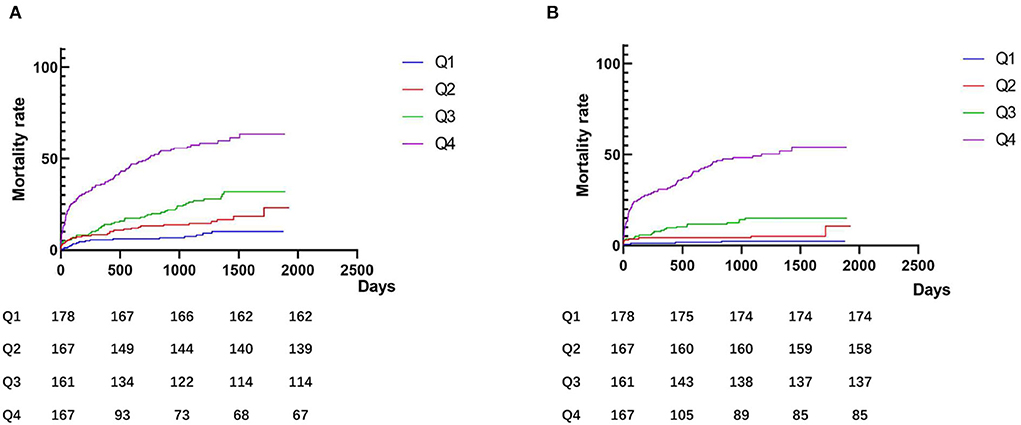
Figure 2. Kaplan–Meier curves according to groups of admission D-dimer levels. (A) Major adverse composite cardiovascular events (MACEs); and (B) All-cause death. Q1: ≤ 0.3 μg/L; Q2: 0.31–0.46 μg/L; Q3: 0.47–0.96 μg/L; and Q4: >0.96 μg/L.
The results of the univariate and multivariate Cox regression analyses of MACEs are listed in Table 4.
A univariate Cox regression analysis showed that the occurrence of MACEs were significantly correlated with gender [hazard ratio [HR]: 1.487, 95% CI: 1.090–2.027, p = 0.012], age [HR: 1.058, 95% CI: 1.046–1.070, p < 0.001], D-dimer [HR:1.152, 95% CI: 1.120–1.185, p < 0.001], fibrinogen [HR: 1.272, 95% CI: 1.154–1.402, p < 0.001], ACCI [HR: 1.419, 95% CI: 1.339–1.503, p < 0.001], BNP [HR:1.176, 95% CI: 1.064–1.299; p < 0.001], CRP [HR: 1.241, 95% CI: 1.104–1.395; p < 0.001], LVEF [HR: 1.010, 95% CI: 1.026–1.116, p < 0.001], aspirin [HR: 0.450, 95% CI: 0.303–0.668, p < 0.001], clopidogrel [HR: 0.402, 95% CI: 0.250–0.646, p < 0.001], angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) [HR: 0.384, 95% CI: 0.289–0.511, p < 0.001], β-blocker [HR: 0.382, 95% CI: 0.287–0.509, p < 0.001], calcium channel blockers (CCB) [HR: 1.825, 95% CI: 1.346–2.474, p < 0.001], and hypertension [HR: 1.894, 95% CI: 1.369–2.621, p < 0.001], but not with smoking, fasting blood-glucose, aspirin, diabetes, and percutaneous coronary intervention (PCI).
A multivariate Cox regression analysis based on age, sex, ACCI, fibrinogen, CRP, LVEF, aspirin, clopidogrel, ACEI/ARB, β-blocker, hypertension, and D-dimer indicated that plasma D-dimer [HR: 1.071, 95% CI: 1.013–1.133, p < 0.017], ACCI [HR: 1.264, 95% CI: 1.141–1.400, p < 0.001], LVEF [HR: 1.069, 95% CI: 1.026–1.114, p = 0.001], aspirin [HR: 0.604, 95% CI: 0.369–0.990, p = 0.046], ACEI/ARB [HR: 0.661, 95% CI: 0.457–0.956, p = 0.028], and CCB [HR: 1.484, 95% CI: 1.018–2.164, p = 0.040] were independent risk factors for MACEs.
When using D-dimer as a categorical variable, the multivariate Cox regression analysis indicated that the hazard ratios pertaining to MACEs in patients with the Q2, Q3, and Q4 groups compared with the Q1 group were 1.348 [HR: 1.348, 95% CI: 0.649–2.799, p = 0.423], 1.551 [HR: 1.551, 95% CI: 0.770–3.124, p = 0.220], and 2.336 [HR: 2.336, 95% CI: 1.144–4.768, p = 0.020], respectively. Therefore, the Q4 group of patients was at the highest risk of developing MACEs, as shown in Table 4.
A univariate regression analysis assessed predictors of all-cause death (Table 5). Furthermore, Table 5 shows the final multivariate Cox proportional models of predictors for all-cause death. After adjustment for multiple relevant covariables, ACCI [HR: 1.292, 95% CI: 1.131–1.476, p < 0.001] and D-dimer [HR: 1.082, 95% CI: 1.021–1.147, p = 0.008] were still independent predictors for all-cause death in the study population. When using D-dimer as a categorical variable, the multivariate Cox regression analysis indicated that the hazard ratios pertaining to all-cause death of patients in the Q2, Q3, and Q4 groups compared with patients in the Q1 group were 1.258 [HR: 1.258, 95% CI: 0.609–2.599, p = 0.535], 1.463 [HR: 1.463, 95% CI: 0.726–2.947, p = 0.287], and 2.338 [HR: 2.338, 95% CI: 1.139–4.801, p = 0.021], respectively. Following the above analysis, we found that the Q4 group of patients was at the highest risk of developing all-cause death, as shown in Table 5.
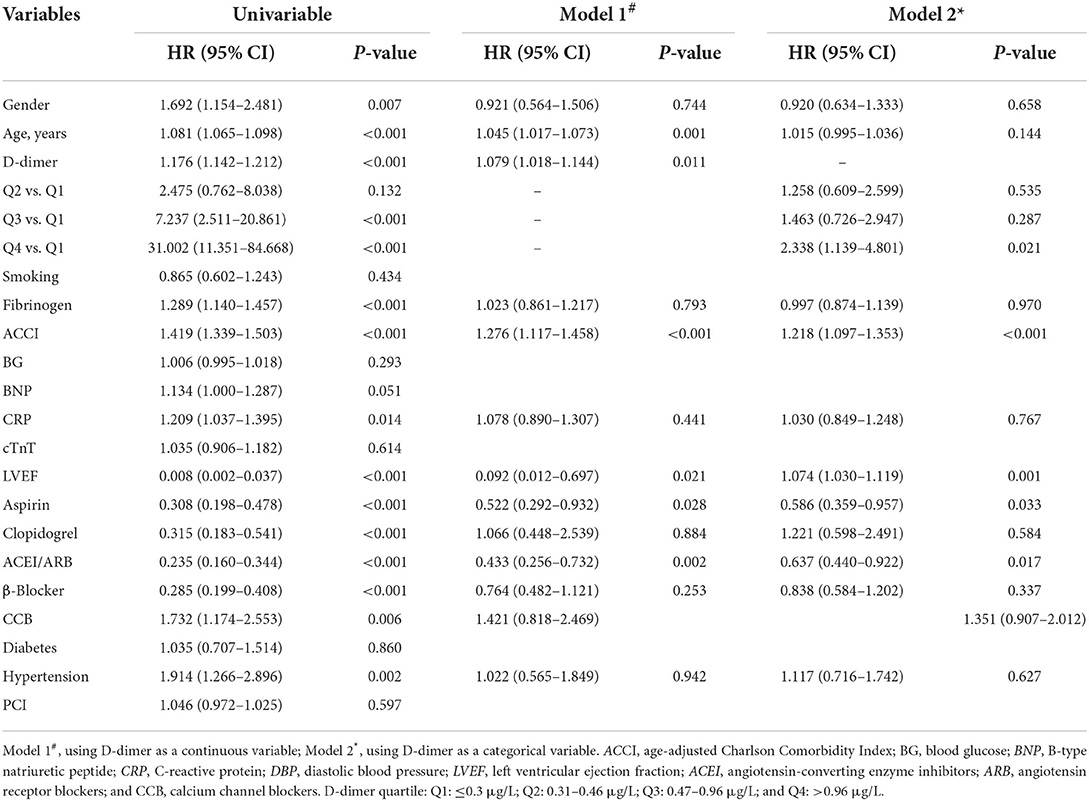
Table 5. The univariate and multivariate Cox regression analyses of all-cause death in patients with NSTEMI.
The receiver operating characteristic (ROC) curves are shown in Figure 3. An ROC analysis revealed that the area under the curve (AUC) of plasma D-dimer for MACEs [0.763 (95% CI: 0.722–0.804, p < 0.001)] was similar to that of ACCI [0.768 (95% CI: 0.729–0.808, p < 0.001)]. The AUC of plasma D-dimer was greater than that of CRP [0.580 (95% CI: 0.532–0.628, p = 0.001)] and LVEF [0.607 (95% CI: 0.554–0.661, p < 0.001)]. In addition, we used a combination of indicators to predict MACEs. We found that the AUC of the combination of D-dimer, LVEF, CRP, and ACCI [0.802 (95% CI: 0.763–0.840, p < 0.001)] to predict MACEs was greater than the combination of AUC of LVEF, CRP, and ACCI [0.789 (95% CI: 0.749–0.829, p < 0.001)] to predict MACEs.
For all-cause death, we found that the AUC of plasma D-dimer [0.836 (95% CI 0.796–0.876, P < 0.001)] was higher than the AUC of the ACCI [0.809 (95% CI 0.767–0.851, P < 0.001)], age [0.789 (95% CI 0.746–0.833, P < 0.001)] and LVEF [0.656 (95% CI 0.593–0.720, P < 0.001)]. Similarly, we used combined indicators to predict all-cause death. We found that the AUC of the combination of D-dimer, LVEF, age, and ACCI [0.875 (95% CI 0.841–0.910, P < 0.001)] was greater than the combination of AUC of Age, LVEF, and ACCI [0.860 (95% CI 0.824–0.896, P < 0.001)] to predict all-cause death.
Non-ST-segment elevation myocardial infarction is a common acute coronary syndrome with a high morbidity and mortality rate. The identification of methods to further improve the survival of patients remains a great challenge. In this study, we found that the ACCI and plasma D-dimer levels were independent risk factors for the occurrence of MACEs and all-cause death during long-term follow-up in Chinese patients with NSTEMI. Patients with plasma D-dimer >0.96 μg/L were at a high risk of developing MACEs and all-cause death.
The ACCI has provided a simple, readily applicable, and a valid method of estimating clinical outcomes from comorbid diseases for use in longitudinal studies (13). Our study demonstrated again that, after adjusting for age, sex, and other factors, the ACCI was still independently associated with the occurrence of MACEs and all-cause deaths. By comparing the areas under the ROC curve, D-dimer levels were superior to ACCI in predicting all-cause death. Compared with the complex calculation of ACCI based on medical history, D-dimer could be identified only by a blood test, which might be more suitable for patients with critical illnesses or speech disorders. In a real-world retrospective study, Li et al. (10) observed that patients with STEMI who had a higher plasma D-dimer might benefit from the use of thrombus aspiration during primary PCI. In addition, we used joint indicators to predict MACEs and all-cause deaths. We found that the AUC of the joint indicator was greater for MACEs and all-cause deaths. The addition of D-dimer to clinical predictors can significantly improve risk predictions for MACEs and all-cause deaths. This facilitates decision-making on treatment strategies (15).
However, there are still some disputes about the relationship between plasma D-dimer and clinical outcomes in patients with the coronary heart disease. Oldgren et al. (16) demonstrated that higher D-dimer levels are associated with worse clinical outcomes in patients with unstable coronary diseases. The greater the D-dimer level is, the greater the thrombosis load (17). Higher D-dimer levels were associated with the severity of myocardial infarction in patients with STEMI undergoing primary PCI (18). Zhao et al. (19) considered a potential benefit of lowering D-dimer levels among DM patients with de novo lesions. Our study included more diseases in addition to diabetes, and we used the ACCI to score common diseases. Our study reached similar conclusions in Chinese patients with NSTEMI and more strongly demonstrated the predictive value of D-dimer for clinical outcomes in patients with acute coronary syndromes.
Nevertheless, Tello-Montoliu et al. (20) did not observe any advantage of plasma D-dimer when troponin T, CRP, and NT-ProBNP were considered in patients with NSTEMI during 6 months of follow-up. This difference in studies might be due to variations in blood sampling time points and the administration of anticoagulants, such as heparin and other antithrombotic agents, used in the first 36 h, which might influence plasma D-dimer levels. In addition, a positive correlation was noted between D-dimer and other biomarkers, such as troponin T, CRP, and NT-proBNP, in patients with non-ST-elevation acute coronary syndrome (20). The GRACE score has been recognized as a powerful tool for risk assessment in patients with NSTEMI (21). Satilmisoglu et al. (22) found that D-dimer was not an independent predictor of mortality risk, but a higher D-dimer level was associated with a higher GRACE score and mortality risk in the univariate analysis. Lu et al. (23) suggested that NT-proBNP, D-dimer, and fibrinogen combined with the GRACE score exhibited a higher predictive power for the risk of MACE events in patients with NSTEMI than the GRACE score alone. The disparate conclusions of these studies might be attributed to their limited sample size and event records during the follow-up period, which precludes researchers from reaching more definitive conclusions about the predictive values of the D-dimer assay for adverse outcomes in patients with NSTEMI.
It is worth noting that only patients with a clear diagnosis of myocardial infarction were included in the present study, and those without increased troponin levels were excluded. This population selection might weaken the predictive value of troponin and BNP, so there was no overall difference in the plasma cardiac troponin-T and BNP levels among the groups in our study. In addition, we found that the patients with plasma D-dimer >0.96 μg/L had significantly higher MACEs and all-cause mortality than the other groups based on the multivariate Cox regression analysis. Similarly, Gong et al. (24) found that higher baseline D-dimer levels were related to a higher risk of MACEs in patients not taking anticoagulant drugs. In the ATLAS ACS TIMI-46 trial substudy (25), increased baseline D-dimer was associated with an increased risk of MACEs within 6 months of the ACS event, and administration of the Factor Xa inhibitor contributed to lower D-dimer levels at day 30 and 180. Therefore, we should perform a more rigorous follow-up and standardized treatment for patients at high risk (D-dimer >0.96 μg/L) to explore whether antithrombotic therapy aimed to lower D-dimer levels can improve prognosis in patients with NSTEMI.
There are several inherent limitations to the current study. First, this study was a single-center retrospective study with a limited sample, and patients who were lost to follow-up were excluded from this study, which may have lead to selection bias. Second, this study did not evaluate therapeutic strategies, such as optimizing medication and percutaneous coronary intervention, which have a substantial influence on clinical outcomes in patients with NSTEMI. In addition, we only observed the results of one D-dimer test after admission. It remains unclear whether monitoring and/or decreasing plasma D-dimer levels could improve clinical outcomes in patients with NSTEMI. Age can affect baseline plasma D-dimer levels (26). D-dimer levels were not age-normalized in this study. However, when age and other factors were included in the multivariate Cox regression analysis, the D-dimer level was still independently associated with MACEs and all-cause death risks.
In Chinese patients with acute NSTEMI, higher D-dimer levels at admission suggest a poor long-term prognosis. Physicians should take plasma D-dimer seriously, and treatment aimed at reducing plasma D-dimer might help to improve the prognosis of Chinese patients with NSTEMI.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of Dongguan People's Hospital. The Ethics Committee waived the requirement of written informed consent for participation.
XF and HW designed and performed data analysis. XF and TM wrote the manuscript. HW, SS, and BX reviewed the manuscript and supervised the work. All authors have read and approved the manuscript.
This study was supported by research grants from the Key Projects of Guangdong provincial basic and applied basic research fund (Provincial and Municipal Joint Fund, No. 2020B1515120003).
The authors would like to thank the staff of the Department of Cardiology, Dongguan People's Hospital, Southern Medical University for their cooperation. We would also like to thank the clinical laboratory of Dongguan People's Hospital for providing experimental technical support that was essential to the completion of this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mone P, Gambardella J, Pansini A, Rizzo M, Mauro C, Minicucci F, Santulli G. Impact of thrombus aspiration in frail STEMI patients. Aging Clin Exp Res. (2021) 33:3081-9. doi: 10.1007/s40520-021-01848-5
2. Merlini PA, Bauer KA, Oltrona L, Ardissino D, Cattaneo M, Belli C, Mannucci PM, Rosenberg RD. Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. CIrculation. (1994) 90:61–8. doi: 10.1161/01.CIR.90.1.61
3. Tripodi A. D-dimer testing in laboratory practice. Clin Chem. (2011) 57:1256–62. doi: 10.1373/clinchem.2011.166249
4. Hileman CO, Longenecker CT, Carman TL, Milne GL, Labbato DE, Storer NJ, White CA, mccomsey GA. Elevated D-dimer is independently associated with endothelial dysfunction: a cross-sectional study in HIV-infected adults on antiretroviral therapy. Antivir Ther. (2012) 17:1345–9. doi: 10.3851/IMP2297
5. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. CIrculation. (2007) 115:1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859
6. Tokita Y, Kusama Y, Kodani E, Tadera T, Nakagomi A, Atarashi H, Mizuno K. Utility of rapid D-dimer measurement for screening of acute cardiovascular disease in the emergency setting. J Cardiol. (2009) 53:334–40. doi: 10.1016/j.jjcc.2008.12.001
7. Smith A, Patterson C, Yarnell J, Rumley A, Ben-Shlomo Y, Lowe G. Which hemostatic markers add to the predictive value of conventional risk factors for coronary heart disease and ischemic stroke? The Caerphilly Study. CIrculation. (2005) 112: 3080-7. doi: 10.1161/CIRCULATIONAHA.105.557132
8. Eikelboom JW, Weitz JI, Budaj A, Zhao F, Copland I, Maciejewski P, Johnston M, Yusuf S. Clopidogrel does not suppress blood markers of coagulation activation in aspirin-treated patients with non-ST-elevation acute coronary syndromes. Eur Heart J. (2002) 23:1771–9. doi: 10.1053/euhj.2000.3234
9. Naruse H, Ishii J, Takahashi H, Kitagawa F, Okuyama R, Kawai H, Muramatsu T, Harada M, Yamada A, Motoyama S, Matsui S, Hayashi M, Sarai M, Watanabe E, Izawa H, Ozaki Y. prognostic value of combination of plasma D-Dimer concentration and estimated glomerular filtration rate in predicting long-term mortality of patients with stable coronary artery disease. CIrc J. (2017) 81:1506–13. doi: 10.1253/circj.CJ-16-1272
10. Li JF, Lin ZW, Chen CX, Liang SQ, Du LL, Qu X, Gao Z, Huang YH, Kong ST, Chen JX, Sun LY, Zhou H. Clinical impact of thrombus aspiration and interaction with D-dimer levels in patients with st-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Front Car diovasc Med. (2021) 8:706979. doi: 10.3389/fcvm.2021.706979
11. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. (2018) 72:2231–64. doi: 10.1016/j.jacc.2018.08.1038
12. Ethical Review Approaches for Biomedical Research Involving Humans. National Health Planning Commission of the People's Republic of China. (2017) 44–50. Available online at: http://www.gov.cn/gongbao/content/2017/content_5227817.htm (accessed March 14, 2022).
13. Charlson ME, Pompei P, Ales KL, mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J CHRonic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681 (87)90171-8
14. Asleh R, Enriquez-Sarano M, Jaffe AS, Manemann SM, Weston SA, Jiang R, Roger VL. Galectin-3 levels and outcomes after myocardial infarction: a population-based study. J Am Coll Cardiol. (2019) 73:2286–95. doi: 10.1016/j.jacc.2019.02.046
15. Chen R, Liu C, Zhou P, Tan Y, Sheng Z, Li J, Zhou J, Chen Y, Song L, Zhao H, Yan H. Prognostic value of D-dimer in patients with acute coronary syndrome treated by percutaneous coronary intervention: a retrospective cohort study. THRomb J. (2021) 19:30. doi: 10.1186/s12959-021-00281-y
16. Oldgren J, Linder R, Grip L, Siegbahn A, Wallentin L. Coagulation activity and clinical outcome in unstable coronary artery disease. Arterioscler THRomb Vasc Biol. (2001) 21:1059-64. doi: 10.1161/01.ATV.21.6.1059
17. Corban MT, Hung OY, Mekonnen G, Eshtehardi P, Eapen DJ, Rasoul-Arzrumly E, Al KH, Manocha P, Ko YA, Sperling LS, Quyyumi AA, Samady H. elevated levels of serum fibrin and fibrinogen degradation products are independent predictors of larger coronary plaques and greater plaque necrotic core. CIrc J. (2016) 80:931–7. doi: 10.1253/circj.CJ-15-0768
18. Choi S, Jang WJ, Song YB, Lima JA, Guallar E, Choe YH, Hwang JK, Kim EK, Yang JH, Hahn JY, Choi SH, Lee SC, Lee SH, Gwon HC. D-dimer levels predict myocardial injury in ST-segment elevation myocardial infarction: a cardiac magnetic resonance imaging STUDY. PLoS ONE. (2016) 11: e0160955. doi: 10.1371/journal.pone.0160955
19. Zhao X, Lan J, Yu X, Zhou J, Tan Y, Sheng Z, Li J, Wang Y, Chen R, Liu C, Zhou P, Chen Y, Song L, Zhao H, Yan H. Primary percutaneous coronary intervention in patients with type 2 diabetes with late/very late stent thrombosis and de novo lesions: a single-center observational cohort study of clinical outcomes and influencing factors. Front Cardiovasc Med. (2021) 8:653467. doi: 10.3389/fcvm.2021.653467
20. Tello-Montoliu A, Marín F, Roldán V, Mainar L, López MT, Sogorb F, Vicente V, Lip GY. A multimarker risk stratification approach to non-ST elevation acute coronary syndrome: implications of troponin T, CRP, NT pro-BNP and fibrin D-dimer levels. J Int Med. (2007) 262:651–8. doi: 10.1111/j.1365-2796.2007.01871.x
21. De Araújo GP, Ferreira J, Aguiar C, Seabra-Gomes R. TIMI, PURSUIT, and GRACE risk scores: sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur HEART J. (2005) 26:865–72. doi: 10.1093/eurheartj/ehi187
22. Satilmisoglu MH, Ozyilmaz SO, Gul M, Ak YH, Kayapinar O, Gokturk K, Aksu H, Erkanli K, Eksik A. Predictive values of D-dimer assay, GRACE scores and TIMI scores for adverse outcome in patients with non-ST-segment elevation myocardial infarction. Ther Clin risk Manag. (2017) 13:393–400. doi: 10.2147/TCRM.S124794
23. Lu PJ, Gong XW, Liu Y, Tian FS, Zhang WJ, Liu YW, Yao ZH, Wang JX, Han P, Yang YN, Cui Z, Gao J. Optimization of GRACE risk stratification by N-terminal Pro-B-type natriuretic peptide combined with d-dimer in patients with non-ST-elevation myocardial infarction. Am J Cardiol. (2021) 140:13–19. doi: 10.1016/j.amjcard.2020.10.050
24. Gong P, Yang SH, Li S, Luo SH, Zeng RX, Zhang Y, Guo YL, Zhu CG, Xu RX, Li JJ. Plasma d-dimer as a useful marker predicts severity of atherosclerotic lesion and short-term outcome in patients with coronary artery disease. Clin Appl THRomb Hemost. (2016) 22:633–40. doi: 10.1177/1076029616634885
25. Alkhalfan F, Kerneis M, Nafee T, Yee MK, Chi G, Plotnikov A, Braunwald E, Gibson CM. D-Dimer levels and effect of rivaroxaban on those levels and outcomes in patients with acute coronary syndrome (An ATLAS ACS-TIMI 46 Trial Substudy). Am J Cardiol. (2018) 122:1459–64. doi: 10.1016/j.amjcard.2018.07.032
Keywords: D-dimers, acute non-ST-segment myocardial infarction, coronary artery diseases, outcomes, retrospective study
Citation: Fan X, Min T, Su S, Xiong B and Wan H (2022) Validation of plasma D-dimer in Chinese patients with acute non-ST segment elevation myocardial infarction. Front. Cardiovasc. Med. 9:896173. doi: 10.3389/fcvm.2022.896173
Received: 14 March 2022; Accepted: 16 September 2022;
Published: 19 October 2022.
Edited by:
Shuyang Zhang, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Pasquale Mone, Albert Einstein College of Medicine, United StatesCopyright © 2022 Fan, Min, Su, Xiong and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huaibin Wan, ZG9jdmFuaGJAb3V0bG9vay5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.