- 1Division of Cardiovascular Diseases, Department of Internal Medicine and Medical Specialties, University of Genoa, Genoa, Italy
- 2Cardiology Unit, DICATOV - Cardiothoracic and Vascular Department, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ospedale Policlinico San Martino, Genoa, Italy
Myocardial infarction with non-obstructive coronary arteries (MINOCA), despite a lower burden of coronary atherosclerosis, has a non-negligible prognostic impact. The label of MINOCA includes an array of different aetiologies and pathologic conditions, thus the identification of the underlying disease is crucial to patient management. Myocardial infarction with obstructive coronary artery disease and MINOCA share only some risk factors and comorbid conditions. While traditional cardiovascular risk factors have a lower prevalence in MINOCA patients, atypical ones—e.g., anxiety, depression, and autoimmune diseases—are much more frequent in this population. Other conditions—e.g., pregnancy, cancer, and anti-cancer therapy—can predispose to or even induce MINOCA through various mechanisms. The evidence of such risk factors for MINOCA is still scarce and contradicting, as no randomised controlled trials exist in this field. In our work, we performed a review of registries, clinical studies, and case reports of MINOCA, in order to summarise the available data and analyse its possibile pathogenic mechanisms.
Introduction
Myocardial infarction with non-obstructive coronary arteries (MINOCA) is defined as myocardial infarction (MI)—according to the universal definition criteria—in the absence of obstructive coronary arteries on angiography—i.e., no coronary artery stenosis ≥50% in any potential infarct-related artery—, provided that no overt specific cause for the presentation has been found (1, 2). Non-obstructive coronary arteries include normal or near-normal coronary arteries—i.e., with no ≥30% stenosis—and mild coronary atherosclerosis—i.e., stenosis >30% but <50%. The 50% cutoff is partly controversial, owing to inter- and intra-observer variability in stenosis estimation on traditional coronary angiography as well as possible changes in vessel diameter resulting from a fluctuating vasomotor tone and an unstable plaque (3). Notably, MINOCA accounts for about 6–9% of patients with MI (4, 5). However, since the label of MINOCA includes an array of different etiologies and underlying pathologic conditions, it should be considered a working diagnosis, which highlights the importance of identifying its specific mechanisms in order to inform patient management.
There are currently few data on predisposing factors and comorbidities associated with MINOCA. In this work, we performed an extensive search of the literature on PubMed and Google Scholar using the algorithm “MINOCA” AND (“typical” OR “atypical” OR “unusual”) AND (“risk factors” OR “comorbidities”). We considered studies, case reports/series, registries, and meta-analyses in English language providing data about MINOCA, particularly its association with typical and unusual risk factors as well as comorbid conditions. Finally, among the retrieved results, we identified and selected the most recent articles—including 11 observational studies, three case reports/series, one registry, and one meta-analysis—and reported their most significant findings.
Classification
Based on the underlying mechanism, MINOCA may be classified into two main subgroups, i.e., epicardial and microvascular (5). Furthermore, other forms of type 2 MI should be considered in the differential diagnosis. Despite myocardial ischaemia likely playing a role in its pathogenesis, the inclusion of takotsubo syndrome (TTS) in MINOCA is still debated, as it shows unique features (1). A summary of MINOCA classification is reported in Figure 1.
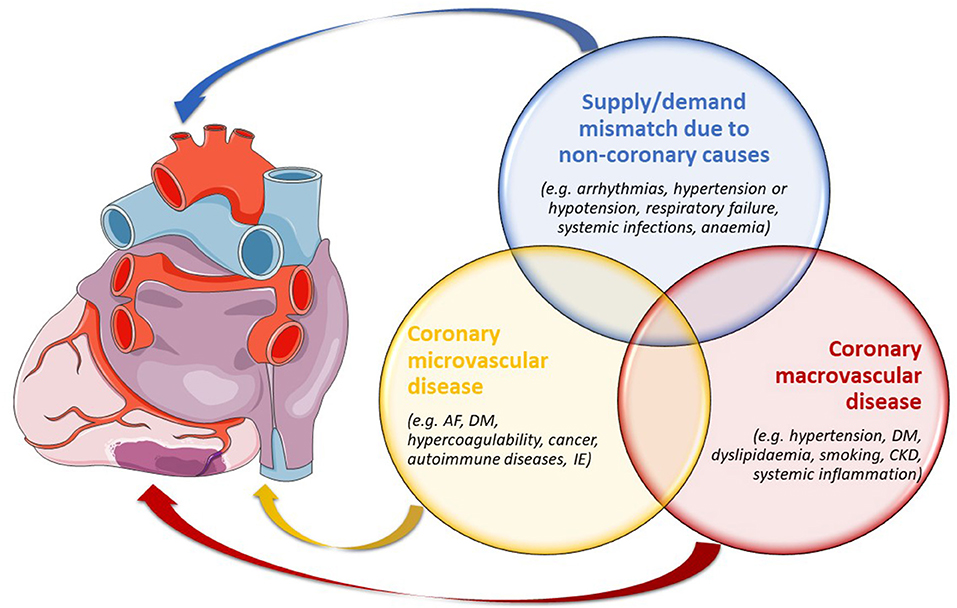
Figure 1. The principal types of MINOCA as per aetiologies and pathogeneses, potentially overlapping between one another. AF, atrial fibrillation; CKD, chronic kidney disease; DM, diabetes mellitus; IE, infective endocarditis.
Epicardial Causes
Coronary plaque disease may explain almost half of cases of MINOCA, as documented by intracoronary imaging (6). Despite the absence of critical coronary stenoses, the two main pathologic findings are plaque rupture and plaque erosion, just like in type 1 MI. MINOCA may depend on thrombosis, thromboembolism, superimposed vasospasm, or a combination of these processes, and spontaneous thrombolysis has been suggested to explain patency of the coronary arteries (5). Thrombosis complicating a calcified nodule may constitute another possible cause of MINOCA on intracoronary imaging (7).
Coronary artery spasm reflects a vascular smooth muscle hyper-reactivity to specific triggers, which may be endogenous or exogenous (8). Provocative tests with intracoronary ergonovine or acetylcholine were reported positive—i.e., ST-segment changes and angina with ≥90% coronary artery diameter reduction—in up to 46% of patients presenting with MINOCA and suspected coronary vasomotor abnormalities, also defining a subpopulation with worse outcomes (9, 10).
Spontaneous coronary artery dissection (SCAD) may present as either a proper dissection or an intramural haematoma without intimal tear (11). SCAD is responsible for about 1–4% of acute coronary syndromes (ACSs) overall and 25–35% of MIs in women ≤ 50 years of age (12, 13). It predominantly affects young white females, most often occurring in patients with a low prevalence of traditional cardiovascular risk factors (14). The left anterior descending artery is more frequently involved, probably owing to haemodynamic peculiarities (15).
Microvascular Causes
Coronary microvascular spasm, indicated by ST-segment changes and angina with no or <90% coronary artery diameter reduction, is reported in about 25% of patients with MINOCA. Acetylcholine provocation testing can portend the diagnosis (10). Whereas long-term mortality rate seems very low, angina often persists despite medical therapy, having a negative impact on the quality of life (16).
Coronary thromboembolism usually involves the microcirculation, although large thrombi or emboli may abruptly interrupt coronary flow at epicardial level. Coronary thromboembolism may result from coagulation disorders—i.e., thrombophilia—as well as various embolic sources and predisposing conditions, including atrial fibrillation, mechanical valve prostheses, and right-to-left interatrial shunt. Moreover, MINOCA may rarely result from septic coronary embolism in patients with infective endocarditis (5).
Takotsubo Syndrome
Takotsubo syndrome (TTS) often presents as an acute, reversible heart failure in postmenopausal women, in the absence of obstructive coronary artery disease (CAD) (17). Since TTS may mimic both myocarditis and type 1 MI, the differential diagnosis is extremely challenging and is usually ascertained after transthoracic echocardiography, coronary angiography, and ventriculography. Contrast-enhanced cardiac magnetic resonance shows a typical pattern and may be useful in unclear cases (18). Pathophysiology of TTS is multifaceted and may significantly vary between patients. Among possible mechanisms, multi-vessel epicardial spasm, catecholamine-induced myocardial stunning, spontaneous lysis of coronary thrombus, and acute microvascular spasm appear the most plausible. Particularly, diffuse coronary microvascular dysfunction is a common determinant of TTS (19). Long-term prognosis is generally good, although in-hospital mortality reaches 8%, mainly due to complications such as heart failure, ventricular arrhythmias, and left ventricular free wall rupture (20).
Epidemiology and Prognosis
Owing to a significantly distinct pathophysiology, MI with concomitant obstructive CAD and MINOCA share only some risk factors and comorbid conditions. Moreover, each MINOCA subtype does have its own peculiarities.
Compared with patients with obstructive CAD, those with MINOCA are younger, less often smokers and diabetic, and have lower values of total and low-density lipoprotein cholesterol. They more frequently present with non-ST-segment elevation MI, a greater left ventricular ejection fraction, and lower Killip classes, showing reduced rates of in-hospital death. Notably, MINOCA is significantly associated with pulmonary disease, which marks its different epidemiological profile (21). Despite a lower burden of coronary atherosclerosis, MINOCA has a non-negligible prognosis since, according to emerging data, long-term mortality and rates of major adverse cardiovascular events (MACE) are as high as in MI with obstructive CAD (9, 22). Nonetheless, these results have been refuted by other studies and warrant further corroboration (4, 23). Contradictory findings may result from the extreme variability of the populations enrolled in the studies. However, it seems evident that the prognosis of MINOCA mainly depends on the underlying mechanism, also considering high-risk subsets of patients—e.g., those with coronary plaque rupture or epicardial vasospasm (24).
Risk Factors and Comorbidities
The main links between MINOCA and its risk factors and comorbidities, as discussed in detail below, are presented in Figure 2.
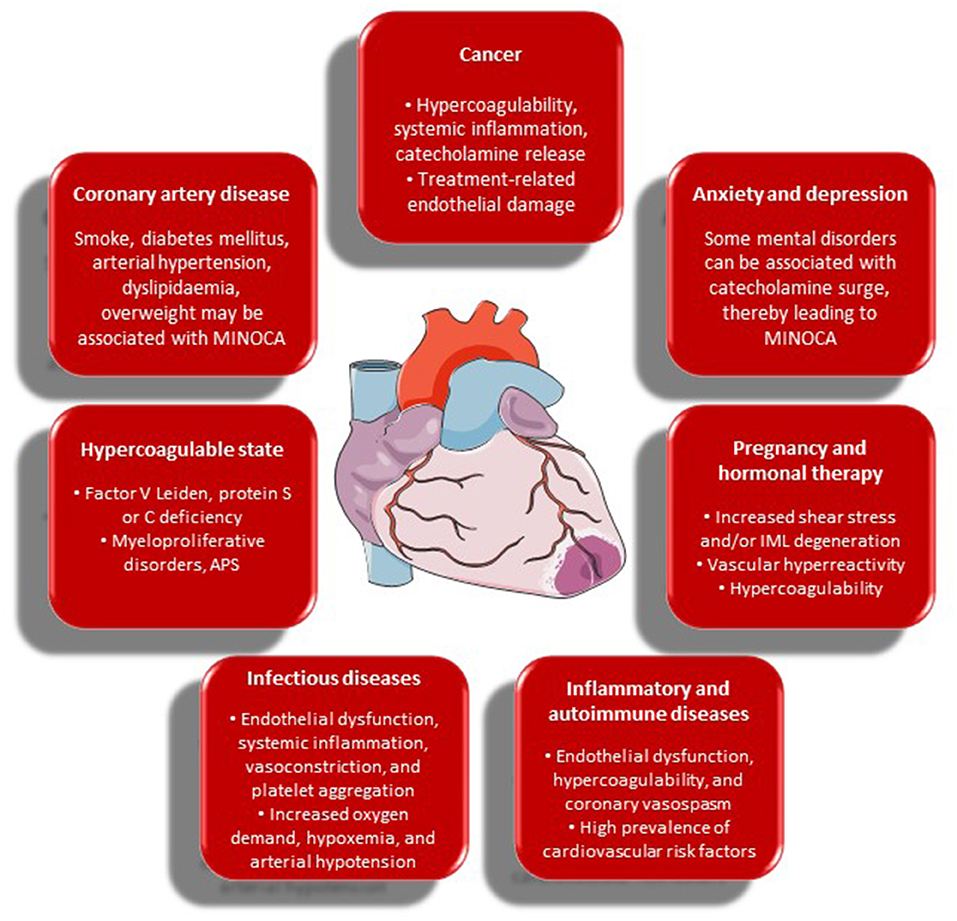
Figure 2. Risk factors and comorbidities of MINOCA and their possible contributory role in its occurrence. APS, antiphospholipid syndrome; IML, intima-media layer; MINOCA, myocardial infarction with non-obstructive coronary arteries.
- Cardiovascular risk factors: compared to MI with obstructive CAD, the prevalence of conventional cardiovascular risk factors is significantly lower in MINOCA patients (25). According to the VIRGO study, smoking, arterial hypertension, diabetes mellitus, dyslipidaemia, obesity, family history of CAD, and prior MI or peripheral artery disease showed a decreased frequency within the MINOCA population. Conversely, unconventional risk factors—e.g., history of illicit drug use, known renal dysfunction, autoimmune diseases, and venous thromboembolism—did not differ between the groups (22).
- Hypercoagulable state: coronary thrombosis may arise from hereditary or acquired thrombophilia, while coronary emboli may occur from coronary or systemic arterial thrombi (5). Paradoxical embolism can be related to patent foramen ovale, atrial septal defect, or coronary arteriovenous fistula (26). Hereditary thrombophilia includes factor V Leiden and protein S or C deficiency. In patients with MINOCA, the incidence of hypercoagulability is higher than in the general population (27). Several studies have demonstrated the presence of factor V Leiden in about 10% of MINOCA cases, with higher rates of genetically determined hypercoagulable state in younger patients (28–30). Acquired thrombophilia should also be considered—e.g., in antiphospholipid syndrome and myeloproliferative disorders—, and hematology profile together with transoesophageal and bubble-contrast echocardiography may be necessary to identify a specific thromboembolic cause and to inform therapy (5).
- Anxiety and depression: although anxiety and depression are rather frequent both in MINOCA and in MI with obstructive CAD, psychosocial disorders are more typical of MINOCA (31, 32). In a Taiwanese nationwide population-based study including more than 10,000 patients with non-obstructive CAD, over 38% had a prior anxiety disorder (33). In another study, one in five patients had a history of psychiatric disorders, and more than half reported physical and/or emotional distress within one week prior to admission, especially when diagnosed with TTS (34). Anxiety and depression may play a role in the pathogenesis of MINOCA due to their potential to trigger vasospasm and endothelial dysfunction through catecholamine surge in predisposed patients—e.g., post-menopausal women. Finally, psychiatric disorders—mainly depression—are associated with adverse clinical outcomes, MACE, and all-cause mortality in MINOCA patients as much as in patients with obstructive CAD (35).
- Cancer: cancer and MINOCA have a non-negligible relationship. A recent meta-analysis by Pelliccia et al. has shown that a previous diagnosis of cancer is present in about 2.5% of patients with MINOCA, which outperforms the current prevalence of malignancy in the general population (36). This observation is consistent with the results of a registry-based cohort study of patients admitted to Swedish coronary care units (37). Multiple pathophysiologic mechanisms probably play a role, including hypercoagulability, systemic inflammation, and treatment-related endothelial damage. Tumor cells can trigger coagulation through procoagulant, antifibrinolytic, and aggregating activities; release of inflammatory and angiogenic cytokines; and interaction with vascular and blood cells through adhesion molecules. Thrombophilia may also facilitate systemic embolism in atrial fibrillation (38). Enhanced inflammation and/or catecholamine release might play a role in the pathophysiology of MINOCA—i.e., epicardial or microvascular spasm (36). Finally, MINOCA may present as a complication of either chemotherapy or radiotherapy, likely due to drug-related endothelial damage. Specifically, 5-fluorouracile may induce coronary vasospasm and endothelial dysfunction. Vascular endothelial growth factor and aromatase inhibitors may favor thromboembolism. Cisplatin may induce arterial thrombosis with subsequent myocardial or cerebral ischaemia in about 2% of patients, owing to direct toxicity on the endothelium, whereas immune checkpoint inhibitors may play a role through multiple immune-related mechanisms (38, 39). Finally, several types of cancer treatment—e.g., anthracycline-, taxane-, and platinum-based chemotherapy—, increase the risk of venous thromboembolism. Thus, anti-cancer therapy may also determine MINOCA-mimicking conditions—e.g., pulmonary embolism—, making differential diagnosis very challenging in this setting.
- Inflammatory and autoimmune diseases: there is ample evidence that patients with chronic inflammatory and autoimmune disorders—e.g., rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, ankylosing spondylitis, inflammatory bowel diseases, psoriasis, and periodontitis—have an increased risk of cardiovascular diseases (40). Accordingly, a meta-analysis of 41,490 patients showed that cardiovascular risk was 48% higher in patients with rheumatoid arthritis as compared to healthy individuals (41). This is partly mediated by traditional cardiovascular risk factors, but mainly depends on systemic inflammation as a promoter of endothelial dysfunction (42). Systemic lupus erythematosus and antiphospholipid syndrome have been studied most in this specific setting, as an association between them and both obstructive CAD and MINOCA was demonstrated (43). Among possible pathophysiological mechanisms, hypercoagulability can enhance thromboembolism, but microvascular dysfunction and coronary vasospasm have been described as well (41, 44). The majority of studies point to the fundamental role of systemic endothelial dysfunction in rheumatic diseases, which seems to coincide with or precede both macrovascular and microvascular involvement. Moreover, a recent observational study reported that the number of pro-inflammatory conditions—i.e., autoimmune disorders, connective tissue diseases, active cancer, and infections—was significantly higher in the MINOCA group. Nonetheless, neither all-cause mortality nor hospitalisations for cardiovascular causes differed between the groups at follow-up (31). At present, the role of inflammatory and autoimmune diseases in the development of SCAD remains controversial.
- Infectious diseases: acute myocardial injury is common during systemic infections—particularly pneumonia and sepsis—, and a significant association between respiratory infections—especially influenza—and acute MI was largely demonstrated (45). Coronary instability in course of an infectious illness may result from acute inflammation, biomechanical stress, and vasoconstriction. Moreover, infections may promote platelet activation and endothelial dysfunction, increase metabolic demand, and induce hypoxaemia and hypotension (46). During the coronavirus disease 2019 (COVID-19) pandemic, new challenges arose in the diagnosis and treatment of patients with acute myocardial injury or ACSs (47). Whereas severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection primarily affects the lungs, a heightened risk of thromboembolism has widely been reported (48). Accordingly, MINOCA has been increasingly described in COVID-19 patients, due to various mechanisms. First, increased sympathetic activation associated with viral infection may promote ACSs in patients with pre-existing CAD (47). Similarly, cytokine storm may lead to coronary plaque instability as well as initiation and perpetuation of a pro-thrombotic milieu. Furthermore, direct myocardial injury due to viral cardiomyocyte infection might play a role. Vasoconstriction and platelet activation may also occur, contributing to endothelial damage. Finally, significant hypoxia in course of viral pneumonia may not only trigger coronary plaque rupture and thrombosis, but also cause type 2 MI because of reduced oxygen supply. This deleterious pathogenic ensemble seems to be responsible for ACSs in COVID-19 (47). In addition, myocarditis, TTS, and acute heart failure are important aetiologies of myocardial injury in COVID-19, albeit non-cardiac conditions should also be considered. Sepsis—primarily originating from the lower respiratory tract—may also determine myocardial injury or type 2 MI (49). Overall, in SARS-CoV-2 infection, patients with myocardial injury have a worse prognosis and are more likely to be admitted to an intensive care unit (50). Another peculiar cause of MINOCA is coronary embolisation of an endocardial vegetation fragment. Despite its rarity—observational studies have shown that the incidence of coronary embolism during infective endocarditis approximates 0.5%—, it constitutes a potentially lethal complication (51). Oftentimes, a septic embolus is not visible on coronary angiography owing to its microvascular localisation, but should be suspected in this specific clinical setting, especially in case of multiple embolic events.
- Pregnancy and hormonal therapy: physiological changes associated with pregnancy and labor can facilitate SCAD, particularly in the peripartum period and in the presence of fibromuscular dysplasia or other predisposing conditions—e.g., twin gestation and pre-existing or gestational arterial hypertension—that further increase endothelial haemodynamic shear stress (52–54). Moreover, high plasma levels of both estrogen and progesterone associated not only with pregnancy, but also with hormonal contraception or hormone replacement therapy, can determine degeneration of the arterial intima-media layer in predisposed individuals, mainly through a combination of decreased release of extracellular matrix and production of matrix metalloproteinases (14, 55, 56). MINOCA due to coronary thrombosis without CAD is a rather frequent cause of pregnancy-related MI, mainly depending on the hypercoagulable state related to gestation (57, 58). Moreover, pre-eclampsia heightens the risk of coronary spasm, since it provokes endothelial dysfunction due to an imbalance in the release of endothelin and thromboxane (59). Pregnancy-related coronary spasm may also result from enhanced vascular reactivity to some hormones, such as angiotensin II and noradrenaline (60).
Conclusions
The definition of MINOCA groups several conditions that share the absence of obstructive coronary arteries. As such, under the MINOCA umbrella, each pathologic process shows its own peculiarities. Unfortunately, all the available data are currently retrospective and have limited quality, often showing evident contradictions. There is utter need for further clarification of possible mechanisms and prognostic implications in specific subsets of patients. Accordingly, as MINOCA still remains somewhat indefinite and nebulous, the identification of the underlying disease and associated comorbidities is paramount for an in-depth understanding of pathophysiology, prevention, and specific treatment targets.
Author Contributions
AM, VG, and IP contributed to conception and design of the study. AM wrote the first draft of the manuscript. AM, AT, EP, and VG wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. (2017) 38:143–53. doi: 10.1093/eurheartj/ehw149
2. Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: A Scientific Statement From the American Heart Association. Circulation. (2019) 139:e891–908. doi: 10.1161/CIR.0000000000000670
3. Toth GG, Toth B, Johnson NP, De Vroey F, Di Serafino L, Pyxaras S, et al. Revascularization decisions in patients with stable angina and intermediate lesions: results of the international survey on interventional strategy. Circ Cardiovasc Interv. (2014) 7:751–9. doi: 10.1161/CIRCINTERVENTIONS.114.001608
4. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. (2015) 131:861–70. doi: 10.1161/CIRCULATIONAHA.114.011201
5. Scalone G, Niccoli G, Crea F. Editor's choice- pathophysiology, diagnosis and management of MINOCA: an update. Eur Heart J Acute Cardiovasc Care. (2019) 8:54–62. doi: 10.1177/2048872618782414
6. Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. (2011) 124:1414–25. doi: 10.1161/CIRCULATIONAHA.111.026542
7. Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. (2013) 62:1748–58. doi: 10.1016/j.jacc.2013.05.071
8. Kaski JC, Crea F, Meran D, Rodriguez L, Araujo L, Chierchia S, et al. Local coronary supersensitivity to diverse vasoconstrictive stimuli in patients with variant angina. Circulation. (1986) 74:1255–65. doi: 10.1161/01.CIR.74.6.1255
9. Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Camma G, et al. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J. (2018) 39:91–8. doi: 10.1093/eurheartj/ehx667
10. Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas A, et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. (2020) 41:3504–20. doi: 10.1093/eurheartj/ehaa503
11. Antonsen L, Thayssen P, Jensen LO. Large coronary intramural hematomas: a case series and focused literature review. Cardiovasc Revasc Med. (2015) 16:116–23. doi: 10.1016/j.carrev.2014.10.009
12. Nishiguchi T, Tanaka A, Ozaki Y, Taruya A, Fukuda S, Taguchi H, et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. (2016) 5:263–70. doi: 10.1177/2048872613504310
13. Saw J, Aymong E, Mancini GB, Sedlak T, Starovoytov A, Ricci D. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol. (2014) 30:814–9. doi: 10.1016/j.cjca.2014.01.011
14. Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. (2012) 126:579–88. doi: 10.1161/CIRCULATIONAHA.112.105718
15. Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, et al. Spontaneous coronary artery dissection: current state of the science: A Scientific Statement From the American Heart Association. Circulation. (2018) 137:e523–57. doi: 10.1161/CIR.0000000000000564
16. Masumoto A, Mohri M, Takeshita A. Three-year follow-up of the Japanese patients with microvascular angina attributable to coronary microvascular spasm. Int J Cardiol. (2001) 81:151–6. doi: 10.1016/S0167-5273(01)00540-X
17. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. (2008) 155:408–17. doi: 10.1016/j.ahj.2007.11.008
18. Collste O, Sorensson P, Frick M, Agewall S, Daniel M, Henareh L, et al. Myocardial infarction with normal coronary arteries is common and associated with normal findings on cardiovascular magnetic resonance imaging: results from the Stockholm Myocardial Infarction with Normal Coronaries study. J Intern Med. (2013) 273:189–96. doi: 10.1111/j.1365-2796.2012.02567.x
19. Galiuto L, De Caterina AR, Porfidia A, Paraggio L, Barchetta S, Locorotondo G, et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J. (2010) 31:1319–27. doi: 10.1093/eurheartj/ehq039
20. Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. (2007) 50:448–52. doi: 10.1016/j.jacc.2007.03.050
21. Williams MJA, Barr PR, Lee M, Poppe KK, Kerr AJ. Outcome after myocardial infarction without obstructive coronary artery disease. Heart. (2019) 105:524–30. doi: 10.1136/heartjnl-2018-313665
22. Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, et al. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO Study. J Am Heart Assoc. (2018) 7:e009174. doi: 10.1161/JAHA.118.009174
23. Pustjens TFS, Appelman Y, Damman P, Ten Berg JM, Jukema JW, de Winter RJ, et al. Guidelines for the management of myocardial infarction/injury with non-obstructive coronary arteries (MINOCA): a position paper from the Dutch ACS working group. Neth Heart J. (2020) 28:116–30. doi: 10.1007/s12471-019-01344-6
24. Niccoli G, Camici PG. Myocardial infarction with non-obstructive coronary arteries: what is the prognosis? Eur Heart J Suppl. (2020) 22(Suppl. E):E40–5. doi: 10.1093/eurheartj/suaa057
25. Kilic S, Aydin G, Coner A, Dogan Y, Arican Ozluk O, Celik Y, et al. Prevalence and clinical profile of patients with myocardial infarction with non-obstructive coronary arteries in Turkey (MINOCA-TR): a national multi-center, observational study. Anatol J Cardiol. (2020) 23:176–82. doi: 10.14744/AnatolJCardiol.2019.46805
26. Crump R, Shandling AH, Van Natta B, Ellestad M. Prevalence of patent foramen ovale in patients with acute myocardial infarction and angiographically normal coronary arteries. Am J Cardiol. (2000) 85:1368–70. doi: 10.1016/S0002-9149(00)00772-4
27. Stepien K, Nowak K, Wypasek E, Zalewski J, Undas A. High prevalence of inherited thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries: comparison with cryptogenic stroke. Int J Cardiol. (2019) 290:1–6. doi: 10.1016/j.ijcard.2019.05.037
28. Da Costa A, Tardy B, Haouchette K, Mismetti P, Cerisier A, Lamaud M, et al. Long term prognosis of patients with myocardial infarction and normal coronary angiography: impact of inherited coagulation disorders. Thromb Haemost. (2004) 91:388–93. doi: 10.1160/TH03-07-0442
29. Mansourati J, Da Costa A, Munier S, Mercier B, Tardy B, Ferec C, et al. Prevalence of factor V Leiden in patients with myocardial infarction and normal coronary angiography. Thromb Haemost. (2000) 83:822–5. doi: 10.1055/s-0037-1613927
30. Van de Water NS, French JK, Lund M, Hyde TA, White HD, Browett PJ. Prevalence of factor V Leiden and prothrombin variant G20210A in patients age <50 years with no significant stenoses at angiography three to four weeks after myocardial infarction. J Am Coll Cardiol. (2000) 36:717–22. doi: 10.1016/S0735-1097(00)00772-5
31. Lopez-Pais J, Izquierdo Coronel B, Galan Gil D, Espinosa Pascual MJ, Alcon Duran B, Martinez Peredo CG, et al. Clinical characteristics and prognosis of myocardial infarction with non-obstructive coronary arteries (MINOCA): a prospective single-center study. Cardiol J. (2020). doi: 10.5603/CJ.a2020.0146. [Epub ahead of print].
32. Asbury EA, Creed F, Collins P. Distinct psychosocial differences between women with coronary heart disease and cardiac syndrome X. Eur Heart J. (2004) 25:1695–701. doi: 10.1016/j.ehj.2004.07.035
33. Hung MY, Mao CT, Hung MJ, Wang JK, Lee HC, Yeh CT, et al. Coronary artery spasm as related to anxiety and depression: a nationwide population-based study. Psychosom Med. (2019) 81:237–45. doi: 10.1097/PSY.0000000000000666
34. Daniel M, Agewall S, Berglund F, Caidahl K, Collste O, Ekenback C, et al. Prevalence of anxiety and depression symptoms in patients with myocardial infarction with non-obstructive coronary arteries. Am J Med. (2018) 131:1118–24. doi: 10.1016/j.amjmed.2018.04.040
35. Watkins LL, Koch GG, Sherwood A, Blumenthal JA, Davidson JR, O'Connor C, et al. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc. (2013) 2:e000068. doi: 10.1161/JAHA.112.000068
36. Pelliccia F, Pasceri V, Tanzilli G, Speciale G, Camici PG, Gaudio C. Malignancy in patients with myocardial infarction and non-obstructive coronary arteries: a systematic review and meta-regression. Eur J Intern Med. (2020) 81:38–43. doi: 10.1016/j.ejim.2020.06.018
37. Eggers KM, Hjort M, Baron T, Jernberg T, Nordenskjold AM, Tornvall P, et al. Morbidity and cause-specific mortality in first-time myocardial infarction with nonobstructive coronary arteries. J Intern Med. (2019) 285:419–28. doi: 10.1111/joim.12857
38. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. (2016) 37:2768–801. doi: 10.1093/eurheartj/ehw211
39. Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. (2020) 9:e013757. doi: 10.1161/JAHA.119.013757
40. Vancheri F, Longo G, Vancheri S, Henein M. Coronary microvascular dysfunction. J Clin Med. (2020) 9:2880. doi: 10.3390/jcm9092880
41. Konst RE, Guzik TJ, Kaski JC, Maas A, Elias-Smale SE. The pathogenic role of coronary microvascular dysfunction in the setting of other cardiac or systemic conditions. Cardiovasc Res. (2020) 116:817–28. doi: 10.1093/cvr/cvaa009
42. Arida A, Protogerou AD, Kitas GD, Sfikakis PP. Systemic inflammatory response and atherosclerosis: the paradigm of chronic inflammatory rheumatic diseases. Int J Mol Sci. (2018) 19:1890. doi: 10.3390/ijms19071890
43. Ramjas V, Jain A, Lee RDM, Fioni F, Tawfik N, Sandhu O, et al. Unraveling the association between myocardial infarction of nonobstructive coronary arteries and antiphospholipid syndrome. Cureus. (2021) 13:e17002. doi: 10.7759/cureus.17002
44. Gandhi H, Ahmed N, Spevack DM. Prevalence of myocardial infarction with non-obstructive coronary arteries (MINOCA) amongst acute coronary syndrome in patients with antiphospholipid syndrome. Int J Cardiol Heart Vasc. (2019) 22:148–49. doi: 10.1016/j.ijcha.2018.12.015
45. Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. (2018) 378:345–53. doi: 10.1056/NEJMoa1702090
46. Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. (2010) 10:83–92. doi: 10.1016/S1473-3099(09)70331-7
47. Manolis AS, Manolis AA, Manolis TA, Melita H. COVID-19 and acute myocardial injury and infarction: related mechanisms and emerging challenges. J Cardiovasc Pharmacol Ther. (2021) 26:399–414. doi: 10.1177/10742484211011026
48. Burkert FR, Niederreiter L, Dichtl W, Mayr A, Virgolini I, Klauser A, et al. Case report of a COVID-19-associated myocardial infarction with no obstructive coronary arteries: the mystery of the phantom embolus or local endothelitis. Eur Heart J Case Rep. (2021) 5:ytaa521. doi: 10.1093/ehjcr/ytaa521
49. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Glob Heart. (2018) 13:305–38. doi: 10.1016/j.gheart.2018.08.004
50. Anupama BK, Chaudhuri D. A review of acute myocardial injury in coronavirus disease 2019. Cureus. (2020) 12:e8426. doi: 10.7759/cureus.8426
51. Zhao J, Yang J, Chen W, Yang X, Liu Y, Cong X, et al. Acute myocardial infarction as the first sign of infective endocarditis: a case report. J Int Med Res. (2020) 48:300060520980598. doi: 10.1177/0300060520980598
52. Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott NS, et al. Long-term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol. (2019) 74:2743–54. doi: 10.1016/j.jacc.2019.09.052
53. Rajagopalan S, Nwazota N, Chandrasekhar S. Outcomes in pregnant women with acute aortic dissections: a review of the literature from 2003 to 2013. Int J Obstet Anesth. (2014) 23:348–56. doi: 10.1016/j.ijoa.2014.05.001
54. Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation. (2014) 130:1915–20. doi: 10.1161/CIRCULATIONAHA.114.011422
55. Madu EC, Kosinski DJ, Wilson WR, Burket MW, Fraker TD Jr, Ansel GM. Two-vessel coronary artery dissection in the peripartum period. Case report and literature review. Angiology. (1994) 45:809–16. doi: 10.1177/000331979404500909
56. Wingrove CS, Garr E, Godsland IF, Stevenson JC. 17beta-oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim Biophys Acta. (1998) 1406:169–74. doi: 10.1016/S0925-4439(97)00097-5
57. Goland S, Elkayam U. Anticoagulation in pregnancy. Cardiol Clin. (2012) 30:395–405. doi: 10.1016/j.ccl.2012.05.003
58. Raphael CE, Heit JA, Reeder GS, Bois MC, Maleszewski JJ, Tilbury RT, et al. Coronary embolus: an underappreciated cause of acute coronary syndromes. JACC Cardiovasc Interv. (2018) 11:172–80. doi: 10.1016/j.jcin.2017.08.057
59. Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. (2014) 63:1815–22. doi: 10.1016/j.jacc.2014.02.529
Keywords: myocardial infarction, MINOCA, coronary artery disease, risk factor, comorbidities
Citation: Merlo AC, Troccolo A, Piredda E, Porto I and Gil Ad V (2022) Myocardial Infarction With Non-obstructive Coronary Arteries: Risk Factors and Associated Comorbidities. Front. Cardiovasc. Med. 9:895053. doi: 10.3389/fcvm.2022.895053
Received: 12 March 2022; Accepted: 14 April 2022;
Published: 02 May 2022.
Edited by:
Josip A. Borovac, University of Split, CroatiaReviewed by:
Konstantin Schwarz, University Hospital Sankt Pölten, AustriaAleksandra Ga̧secka, Medical University of Warsaw, Poland
Copyright © 2022 Merlo, Troccolo, Piredda, Porto and Gil Ad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vered Gil Ad, dmVyZWRnaWxhZEBnbWFpbC5jb20=
†ORCID: Andrea Carlo Merlo orcid.org/0000-0002-7219-9624
Italo Porto orcid.org/0000-0002-9854-5046
Vered Gil Ad orcid.org/0000-0003-0631-162X
 Andrea Carlo Merlo
Andrea Carlo Merlo Alessandro Troccolo
Alessandro Troccolo Elisa Piredda
Elisa Piredda Italo Porto
Italo Porto Vered Gil Ad
Vered Gil Ad