
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 22 July 2022
Sec. Cardiovascular Therapeutics
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.894888
Purpose: Dabigatran concentrations monitoring are gaining importance of special situations, but limited data are available for the expected peak and trough levels. The hemoclot thrombin inhibitor (HTI) is dabigatran-calibrated quantitative determination of dabigatran concentration. This study aims to validate HTI assay as the quantification choice of dabigatran, and providing the expected peak and trough levels.
Materials and Methods: This is a multi-center methodology validate study, including seven hospitals from Beijing, Shanghai, Henan, Hunan, Chongqing, and Fujian. We retrospectively analyzed plasma samples taken from 118 healthy subjects and 183 patients receiving dabigatran. Dabigatran concentrations were measured with HTI assay and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Linear regression, Spearman correlation and Bland-Altman analysis were used in this study.
Results: The mean concentration ratio of HPLC-MS/MS and HTI assays was 1.03 and 0.98 at 2 and 12 h, and the acceptance ranges for both the ratio limit as well as the limit of agreement were met, suggesting good agreement between the HTI-derived plasma concentrations and HPLC-MS/MS. The reference detection range of single dose dabigatran 150 mg in healthy subjects was 33–159 ng/ml. About 500 blood samples were taken from 183 patients suggested that the expected peak and trough levels range of dabigatran 110 mg was about 95–196 and 36–92 ng/ml.
Conclusion: Hemoclot thrombin inhibitor assay can be a good quantitative detection method of dabigatran. Expected peak and trough levels provide a basis for the rational use of dabigatran, and provide important Asian population data for the update of the international clinical guidelines for hematological testing.
Clinical Trial Registration: [https://clinicaltrials.gov], identifier [NCT03161496].
– Hemoclot thrombin inhibitor (HTI) assay can be a good quantitative detection method of dabigatran in Asian population.
– The expected peak and trough levels (ng/mL) range derived from HTI assay of dabigatran 110 mg were 95–196 and 36–92.
– A multi-center quantitative assay of dabigatran was conducted for the first time in the Asian population.
Dabigatran etexilate (Pradaxa®) is a prodrug of dabigatran, a direct inhibitor of thrombin. It is approved for ischemic stroke and systemic embolism in patients with non-valvular atrial fibrillation (NVAF) prevention, and prevention/treatment of venous thrombosis and thromboembolism (1–3). Studies indicated that all oral anticoagulants seem to have similar pharmacokinetic variability and a narrow therapeutic range (4). Thus, oral anticoagulants, such as dabigatran, will require monitoring of anticoagulation to determine the dose that best meets individualized therapy. Anticoagulation reduces stroke risk in atrial fibrillation, yet a retrospective cohort study found that in patients with incident atrial fibrillation, race/ethnicity was independently associated with initiating any anticoagulant therapy and direct-acting oral anticoagulant use among anticoagulant initiators (5). Compared with White patients, the adjusted odds ratio (OR) of initiating any anticoagulant therapy was significantly lower for Asian [OR, 0.82; 95% confidence interval (CI), 0.72–0.94] and Black (OR, 0.90; 95% CI 0.85–0.95) patients. Moreover, more adverse events have emerged in recent years among patients of taking dabigatran, including gastrointestinal discomfort, bleeding, etc. (6). Thus, thrombolysis in ischemic stroke, major bleeding events or prior to urgent invasive treatments, a possibility of monitoring dabigatran is deemed to optimize its dosage regimens, so as to perioperative care in time and change urgently demanded thrombolytic therapy (7). Our team found that 30 new potential SNPs of 13 reported candidate genes (ABCB1, ABCC2, ABCG2, CYP2B6, CYP1A2, CYP2C19, CYP3A5, CES1, SLCO1B1, SLC22A1, UGT1A1, UGT1A9, and UGT2B7) were associated with dabigatran metabolism. Testing of coagulation in patients treated with non-vitamin K antagonist oral anticoagulants was described in non-Chinese populations (8, 9), but limited data are available for Chinese population. Therefore, more data of dabigatran detection in Chinese or Asian population are needed to guide clinical use of personal therapy of dabigatran.
Activated partial thromboplastin (APTT) or prothrombin time (PT) were low sensitivity, reagent-specific or coagulometer-specific variations render standard coagulation assays (10, 11). So, they are less suitable for dabigatran detection and just can be used for qualitative determination (12, 13). Drug concentration is usually determined by high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) method. However, only a small number of specialized laboratories carried out HPLC-MS/MS project of dabigatran (14, 15). The present studies demonstrate that dabigatran can be determined under real-world conditions using clot-based ecarin clotting time assay (ECT), hemoclot thrombin inhibitor (HTI), and chromogenic assays from patients on treatment (16–18). However, ECT is limited for the low availability and insufficient standardization (19, 20). The HTI is specifically calibrated for dabigatran, so as to be a quantitative determination of dabigatran concentration. In the guidelines of 2019 “International Council for Standardization in Haematology Recommendations for Hemostasis Critical Values, Tests, and Reporting (ICSH),” the plasma concentration ranges data of dabigatran are mainly from a few kinds of literature. Moreover, limited information exists on their performance and expected peak/trough levels, especially in their ability to accurately measure concentrations in the Chinese population.
Thus, we aimed to validate HTI assay as a quantification choice of dabigatran in the Chinese population and provided the expected peak and trough levels. The study analyzed consistency of plasma dabigatran levels between HIT assay and HPLC-MS/MS in healthy subjects, and expected peak-trough levels were recommended.
This study was based on a bioequivalence (BE) trial conducted in China, which was mainly performed to assess the BE of domestic generic dabigatran etexilate capsules, with reference to the original product (brand name: Pradaxa®) in healthy subjects. Based on the BE trial, we added PD parameter tests that were conducted during any one of the two reference periods. We investigated the consistency of HIT assay with HPLC in the Chinese healthy population, and also tested it in a patient population to provide a possible reference range. Seven hospitals from Beijing, Shanghai, Henan, Hunan, Chongqing, and Fujian participated in this study. The data presented in this study were from Pradaxa. The study protocol was approved by the ethics committee and the Institutional Review Board of Peking University First Hospital and all sub-central hospitals. Registration number in Clinical Trial system is NCT03161496.
Healthy volunteers were required to be 18–45 years old, with a Body Mass Index ranging from 18–26 kg/m2. Health status was confirmed via medical history interview, physical examination, vital signs and laboratory examination. Patients meeting the following criteria were included: (1) patients with NVAF using dabigatran for prevention of ischemic stroke and systemic embolism; (2) Age older than 18; (3) Baseline data were available.
The venous blood samples were collected 0 h and post-dose at 16–19 time points in 3.0 ml EDTA-K2 tubes and centrifuged at 4°C for 10 min at 3,000 g. The plasma samples were stored at –80°C until analysis.
The total anti-thrombin effect from dabigatran etexilate is a result of dabigatran (alone) and dabigatran acylglucuronides. An assay for total dabigatran (the sum of free dabigatran and the contribution from dabigatran acylglucuronides) in plasma is developed, validated and applied (21, 22). Thus, dabigatran concentrations were determined by validated HPLC-MS/MS assays at Shimadzu 30 series, API 6500 (Applied Biosystems, Inc., American). The standard calibration curves with good linearity were built for dabigatran within the concentration range of 1.0–300.0 ng/mL, and the lower limit of quantitation (LOQ) was 1.0 ng/mL. The precision (% CV), intra-batch accuracy and inter-batch accuracy were within 6.2–9.2, 90.3–104.3, and 94.6–103.8%, respectively. PK parameters were analyzed by non-compartmental methods using Phoenix Win-Non-lin 7.0 (A Certara™ Company, Princeton, NJ, United States). Cmax (Maximum concentration) and Tmax (the time to Cmax) were obtained from the observed data, while terminal half-life (t1/2), plasma concentration vs. time from 0 to the last measurable time point (AUC0–t) were calculated.
For detecting pharmacodynamics (PD) parameters, the sampling times were 0, 2, 8, 12 h after dabigatran administration respectively. In patients’ population, plasma sample obtained longer than 10 h after the previous dose was considered for trough concentration; average 2.0 h (range: 1.0–3.0 h) post dose was considered for peak concentration. The reference detection range refers to plasma dabigatran concentrations between the 2.5th and 97.5th percentiles of all values determined by HPLC-MS/MS.
Blood samples were collected via 2.7 ml sodium citrate tubes, then centrifuged for 15 min at 2,500 g at room-temperature. The plasma samples were stored at –70°C within 6 months until analysis.
Pharmacodynamic assessment was performed at a centralized facility in Peking University First Hospital. The HTI, PT, and APTT were measured on Sysmex CS-2100i (Sysmex, Kobe, Japan). PT and APTT were tested using Coagulation Method Assay Kits (Thromborel-S® and Actin®, Marburg, Germany). The normal reference range for the PT and APTT assays used in this study are 10.1–12.6 s for PT and 26.9–37.6 s for APTT. Concentration detection is divided into two standard curves, one is suitable for normal and slightly high concentration range, another one is suitable for low concentration range. When the concentration is below 120 ng/ml, the low concentration standard curve is switched to obtain a more accurate concentration value. PK assessment was done in each sub-center, without significant matrix effect found in any sub-center. PD assessment was all performed in our hospital.
Statistical analyses were conducted through Statistical Package for Social Sciences (SPSS version 22.0, SPSS Inc., Chicago, IL, United States). Correlation of coagulation assays and HPLC-MS/MS was performed using linear regression, Spearman correlation analysis (p < 0.05 means significant correlation). Difference between HPLC-MS/MS and specific dabigatran assay results was calculated by Bland-Altman analysis. Mean ± standard deviation (SD), frequencies and percentages were presented for continuous and categorical variables respectively.
Total 118 healthy subjects taking single dose dabigatran 150 mg were enrolled. Demographics characteristics are provided in Table 1. No significant difference was found in the baseline.
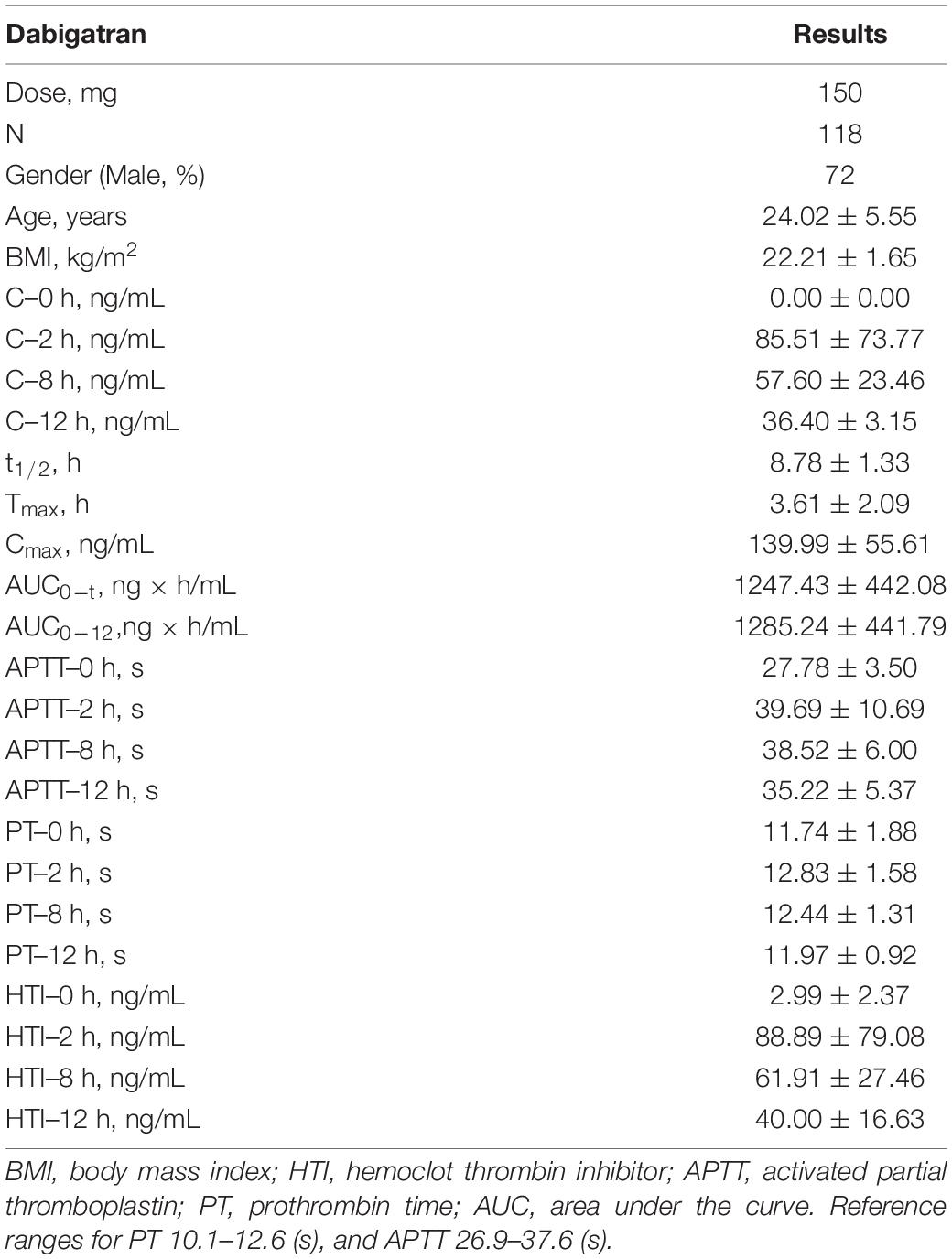
Table 1. The baseline characteristics, pharmacokinetics (PK) and pharmacodynamics (PD) parameters result of enrolled healthy subjects.
About 205 patients taking dabigatran 110 mg bid were included in the study, but, 22 people were lost to follow-up or blood samples were not collected. Thus, 183 patients were finally included in our analysis. The average age was 72.11 ± 9.86 (mean ± SD) years old, and 59.6% was male. The baseline of fibrinogen, D-dimer, TT, creatinine, glomerular filtration rate, etc., were provided in Table 2.
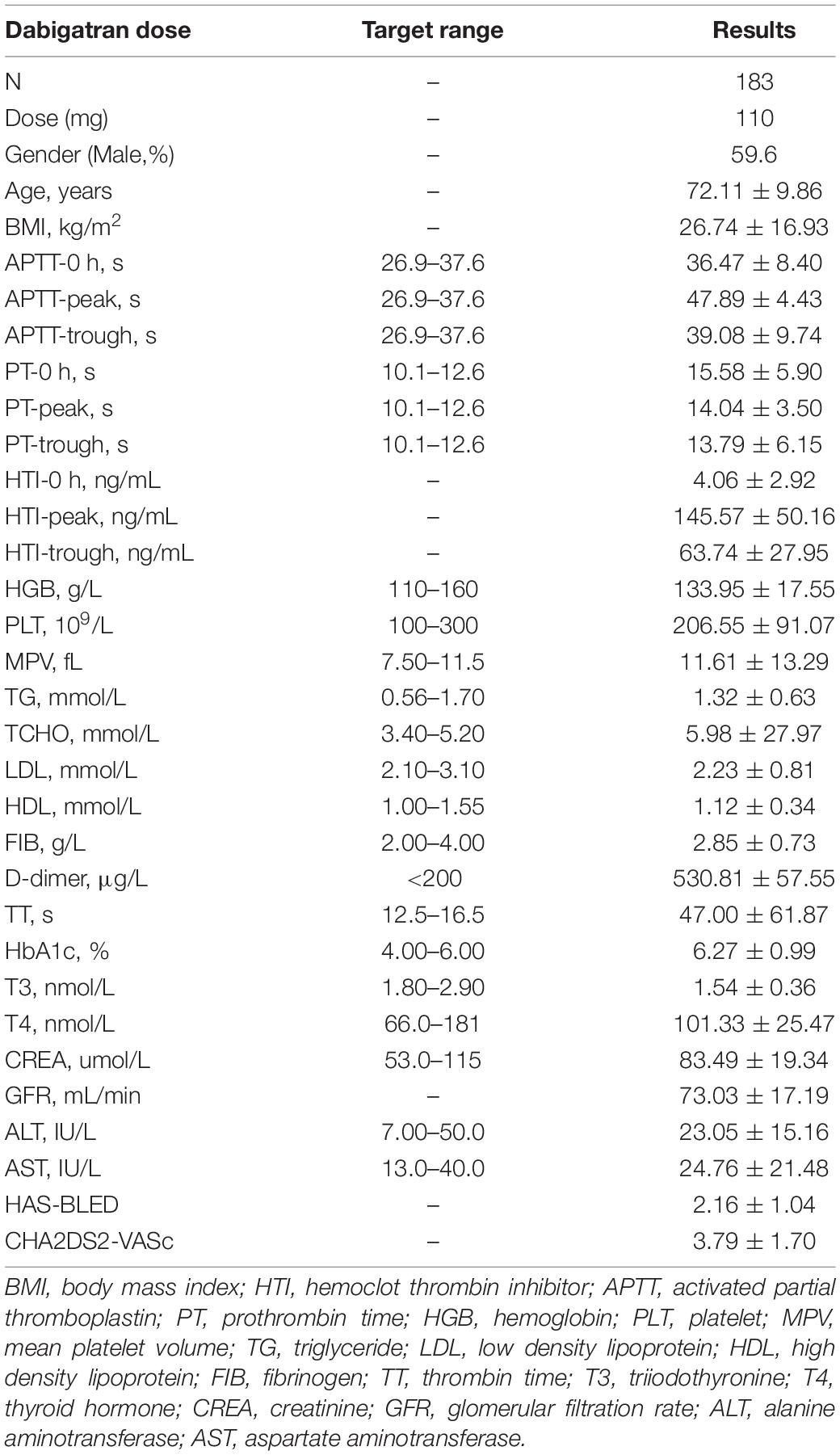
Table 2. Baseline characteristics and pharmacodynamics (PD) parameters result of the patients with dabigatran.
In healthy subjects, mean concentration (ng/mL) of 2, 8, and 12 h after taking dabigatran 150 mg (single dose) via HPLC-MS/MS were 85.51, 57.60, and 36.40. The mean t1/2, Tmax and Cmax were 8.78 h, 3.61 h, and 139.99 ng/ml. The HTI assay of mean dabigatran concentration (ng/ml) of 2, 8, and 12 h were 88.89, 61.91, and 40.00 (Table 1). Thus, in our study, the reference detection range of single dose dabigatran 150 mg in healthy subjects was 33–159 ng/ml.
Bland-Altman difference plot analysis was used to assess agreement between the HIT test method and the reference method LC-MS/MS. The mean concentration ratio of LC-MS/MS and HTI assays was 1.03 and 0.98 at 2 and 12 h, thus fulfilling the suggested acceptance criterion for bioanalytical sample analysis (23); ratio limit range was 0.98–1.08, 0.86–1.12 for 2 and 12 h (acceptance range, 0.83–1.20); and limit of agreement was 0.89–1.18, 0.80–1.19 for 2 and 12 h (acceptance range, 0.83–1.20). The acceptance ranges for both the ratio limit as well as the limit of agreement were met, suggesting good agreement between the HTI-derived plasma concentrations and LC-MS/MS (Figure 1). In addition, correlation and linear regression analysis of PT, APTT, HTI with drug concentration measured by HPLC-MS/MS in healthy subjects were done in the study. APTT showed significant correlation with concentration of dabigatran at 2, 8 and 12 h (p = m 5.41 × 10–28, 0.90 × 10–4, 7.90 × 10–5); PT had correlation with concentration of dabigatran only at 2 h (p = 1.90 × 10–5); HTI showed significant correlation with concentration of dabigatran at 2, 8, and 12 h (p = 5.97 × 10–55, 3.97 × 10–32, 1.48 × 10–27). In the linear regression analysis, APTT consistent with concentration of dabigatran only at 2 h (p = 0.02); PT had no consistency with concentration of dabigatran (p > 0.05); HTI had significant consistency with concentration of dabigatran at 2, 8, and 12 h (p = 0.20 × 10–3, 7.64 × 10–12, 9.13 × 10–17, r = 0.94, 0.84, 0.80, Table 3). HIT had good correlation with HPLC-MS/MS at all collection time (p < 0.001), suggesting that it was good quantitative detection method of dabigatran in Asian population.
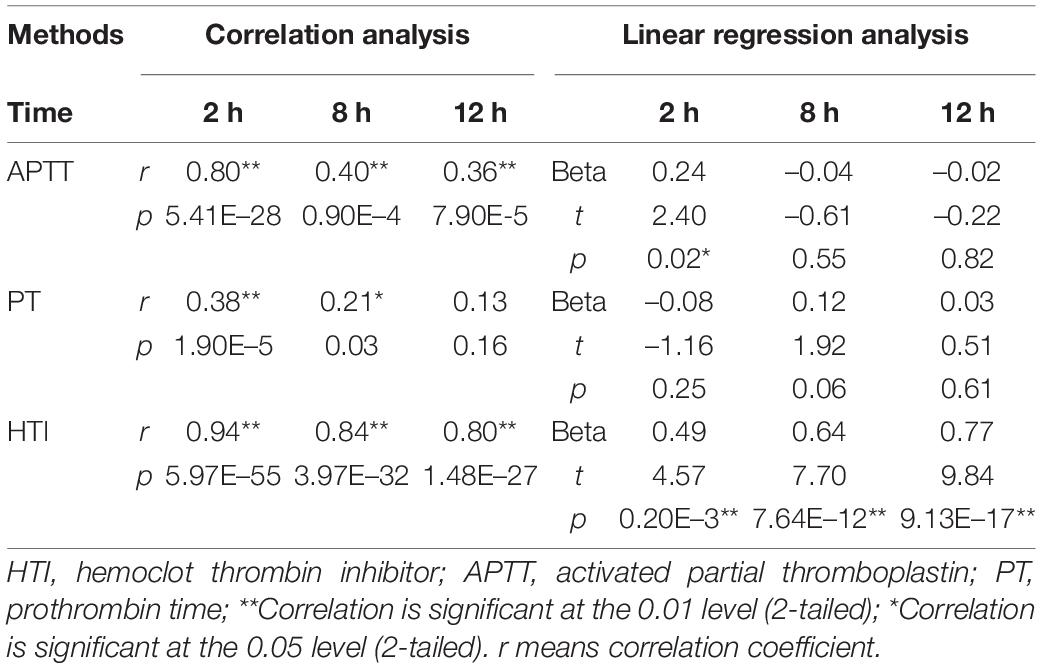
Table 3. Correlation and linear regression analysis of prothrombin time (PT), activated partial thromboplastin (APTT), hemoclot thrombin inhibitor (HTI) with drug concentration measured by HPLC-MS in healthy subjects.
Scatter plot of HTI and blood concentration of HPLC-MS/MS is showed in Figure 2. Difference between HPLC-MS/MS and HTI was calculated using Bland-Altman plot analysis at 2 and 12 h (Figure 2).
After blood collection, HTI, APTT, and PT were detected in the enrolled 183 patients (Table 2). The mean concentration of dabigatran 110 mg bid at pre-dose, peak and trough were 4.06, 145.57, and 63.74. APTT (s) was 36.47, 47.89, and 39.08; PT (s) was 15.58, 14.04, and 13.79, respectively. PD parameters at different collection points of dabigatran 110 mg is shown in Figure 3. Compared with healthy subjects’ HTI results, patients taking lower dose had higher values, especially in peak concentration. The above indicated that dabigatran concentration should be monitored in specific clinical practice. HTI assay of about 500 blood samples from 183 patients suggested that the expected peak and trough levels range of dabigatran 110 mg bid was about 95–196 and 36–92 ng/ml.
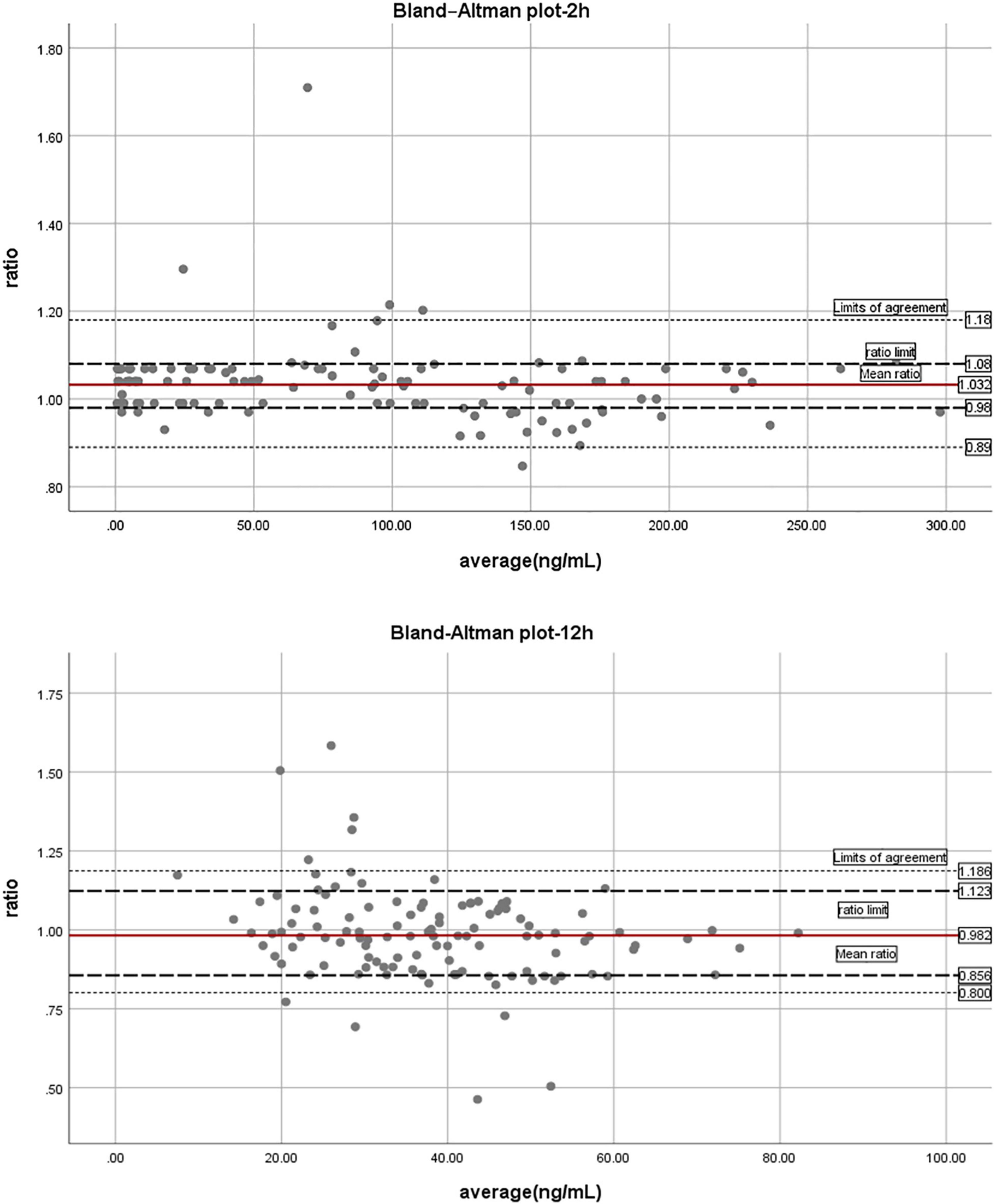
Figure 3. The trend chart of pharmacodynamic parameters at different blood collection points of dabigatran 110 mg.
In patients with incident atrial fibrillation, race/ethnicity was reported to be independently associated with initiating any anticoagulant therapy and direct-acting oral anticoagulant use among anticoagulant initiators. Moreover, limited data is available for testing of coagulation in patients treated with non-vitamin K antagonist oral anticoagulants in Chinese populations. We present a multi-center study of more than 1,000 blood samples from 301 subjects, assessing pharmacokinetics and PD of dabigatran. The more novel data is the expected peak and trough levels range for Asian patients taking dabigatran.
In the study, HTI had good correlation with HPLC-MS/MS at all collection time (p < 0.001), and we used Sysmex reagent to ensure the accuracy of detection results at low concentrations. In addition, the HTI method only takes 5 min, providing a reliable technical method for rapid and accurate clinical evaluation of dabigatran concentration. Thus, HIT assay can be a good quantitative detection method for dabigatran in Asian population. The expected peak and trough levels (ng/ml) range derived from HTI assay of dabigatran 110 mg were 95–196 and 36–92.
Clinical physicians prescribed the 110 mg dose instead of the 150 mg dose in Asian patient group, several reasons were taken in consideration for this. Firstly, Asians are smaller in body size and have lower body mass index than non-Asians: in the subgroup analysis of the RE-LY trial, the Asians have substantially lower body weight than the non-Asians (66 vs. 86 kg) (24). Therefore, the clinical physicians in China/Asian tend to prescribe a lower dose of dabigatran. Secondly, Asians have a high risk for warfarin-related intracranial hemorrhage when compared with whites (HR, 4.06) (25), this make physicians more conservative in prescribing other new anticoagulants as well.
Quantitative measurements of dabigatran are gaining importance in emergency and special situations, especially in the low concentration range. APTT or PT are influenced by biological factors or method-specific components (26). The HTI and ECT assays are specific modified thrombin clotting time assays, thus they are less likely to be influenced by the above factors. Dabigatran concentration was reported to be determined using the thrombin-based Hemoclot coagulation assay, similar to our patients (27).
Apart from HPLC-MS/MS, studies measured dabigatran concentrations via HTI and ECA (28). They suggested that HTI showed good agreement with HPLC-MS, in line with results observed in our study. In addition, HTI had good sensitivity and specificity in determination of dabigatran at the 50 ng/ml, but with impaired sensitivity (73%) at the 30 ng/ml. In our study, dabigatran concentrations lower than the 30 ng/ml can also be determined by a low range HTI calibrator. In another study (16), the dilute thrombin time and chromogenic and ECA were proved to accurately identify therapeutic and supratherapeutic dabigatran levels. And, the study demonstrated that the reference detection range of dabigatran 150 mg was 27–411 ng/ml in American population. Inconsistent with their findings, in our study, the reference detection range of single dose dabigatran 150 mg in healthy subjects was 23–159 ng/ml; The expected peak and trough levels (ng/ml) range derived from HTI assay of dabigatran 110 mg were 95–196 and 36–92. The results of this study can provide a quantitative method for reference and the expected trough and peak concentrations for Asian patients taking dabigatran.
In the guidelines of 2019 “International Council for Standardization in Hematology Recommendations for Hemostasis Critical Values, Tests, and Reporting (ICSH),” the plasma concentration ranges of dabigatran are mainly from a few literatures. A multiple logistic regression model showed that ischemic events was inversely related to trough dabigatran concentrations (c-statistic 0.66, 95% CI 0.61–0.71, p = 0.045), with age and previous stroke (p < 0.0001) as significant covariates (29). Multiple logistic regression showed major bleeding risk increased with dabigatran exposure (c-statistic 0.72, 95% CI 0.69–0.74, p < 0.0001), while age, ASA use, and diabetes as significant covariates (p < 0.0001, <0.0003, 0.018). Moreover, limited information exists on their performance and expected peak/trough levels, especially in the ability to measure low/high concentrations of Asian population accurately. In this study, a multi-center quantitative assay of dabigatran was conducted for the first time in the Chinese population, providing a basis for the rational use of dabigatran in the Asian population, and also providing important Asian population data for the update of the international clinical guidelines for hematological testing.
The range for peak and trough levels of dabigatran is wide according to the guideline and our study. Some potential reasons and measures might affect the range and accuracy of the drug concentration. Previous study reported that bodyweight and serum creatinine were key factors in predicting trough concentration (30). Collectively, present study demonstrated that large variability in dabigatran concentration could be predicted by age, BMI and history of heart failure based on the data from 86 Chinese patients with NVAF receiving 110 mg dabigatran bid (31). Moreover, dabigatran monitoring was also Influenced by TT reagent with different thrombin concentrations: Sysmex-TT was very sensitive at low concentrations of dabigatran (0–100 ng/mL), while Instrument Laboratory (TT-5 ml) and Stago-TT were sensitive at medium concentrations of dabigatran (0–300 ng/ml), and Instrument Laboratory (TT-2 ml) was less sensitive for a wide concentration of dabigatran (0–500 ng/ml; p = 0.007) (32).
In our study, we used Sysmex reagent to ensure the accuracy of detection results at low concentrations, and the HTI assay only takes 5 min, providing a reliable technical method for rapid and accurate clinical evaluation of dabigatran concentration.
This study has some limitations. Reagents from different manufacturers might affect the results, such as Stago, Werfen, and Siemens. Second, we monitored trough and peak levels of dabigatran, but the interindividual coefficient of variability was not examined in the study.
In the study, we present a multi-center study of more than 1,000 blood samples from 301 subjects on assessment pharmacokinetics of dabigatran, and PD with different assays. The more novel data are the expected peak and trough levels range for Asian patients taking dabigatran, and provided important Asian population data for the update of the international clinical guidelines for hematological testing.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by an independent Ethics Committee and the Institutional Review Board of Peking University First Hospital and all participating research sub-central hospitals. The patients/participants provided their written informed consent to participate in this study.
YC, QX, and JJ: conception and design. ZL, QfX, QX, HZ, and GM: provision of study materials or patients and collection and assembly of data. ZL, QfX, HZ, and QX: data analysis and interpretation. All authors: manuscript writing and Final approval of manuscript.
This study was supported by grants from the National High Level Hospital Clinical Research Funding (Scientific and Technological Achievements Transformation Incubation Guidance Fund Project of Peking University First Hospital, 2022CX04), Capital’s Funds for Health Improvement and Research (2022-2Z-40712), the National Key R&D Program of China (2016YFC0904900), and National Natural Science Foundation of China (81872940, 81973395, and 82073935).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all the authors for their contributions in data analysis, collation, and manuscript writing. We also thank all the sub-center staff who participated in this clinical study.
1. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. (2009) 361:1139–51.
2. Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. (2009) 361:2342–52.
3. Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. (2013) 368:709–18. doi: 10.1056/NEJMoa1113697
4. Wright DF, Al-Sallami HS, Duffull SB. Is the dose of dabigatran really more predictable than warfarin? Br J Clin Pharmacol. (2013) 76:997–8. doi: 10.1111/bcp.12144
5. Essien UR, Kim N, Hausmann LRM, Mor MK, Good CB, Magnani JW, et al. Disparities in anticoagulant therapy initiation for incident atrial fibrillation by race/ethnicity among patients in the Veterans health administration system. JAMA Netw Open. (2021) 4:e2114234. doi: 10.1001/jamanetworkopen.2021.14234
6. Lin S, Wang Y, Zhang L, Guan W. Dabigatran must be used carefully: literature review and recommendations for management of adverse events. Drug Des Devel Ther. (2019) 13:1527–33. doi: 10.2147/DDDT.S203112
7. Levy JH. Discontinuation and management of direct-acting anticoagulants for emergency procedures. Am J Med. (2016) 129:S47–53.
8. Wright C, Brown R, Cuker A. Laboratory measurement of the direct oral anticoagulants: indications and impact on management in clinical practice. Int J Lab Hematol. (2017) 39:31–6. doi: 10.1111/ijlh.12654
9. Martin K, Moll S. Direct oral anticoagulant drug level testing in clinical practice: a single institution experience. Thromb Res. (2016) 143:40–4. doi: 10.1016/j.thromres.2016.04.019
10. Ebner M, Peter A, Spencer C, Härtig F, Birschmann I, Kuhn J, et al. Point-of-care testing of coagulation in patients treated with non-vitamin K antagonist oral anticoagulants. Stroke. (2015) 46:2741–7. doi: 10.1161/STROKEAHA.115.010148
11. Ebner M, Birschmann I, Peter A, Härtig F, Spencer C, Kuhn J, et al. Emergency coagulation assessment during treatment with direct oral anticoagulants: limitations and solutions. Stroke. (2017) 48:2457–63. doi: 10.1161/STROKEAHA.117.017981
12. Douxfils J, Lessire S, Dincq AS, Hjemdahl P, Rönquist-Nii Y, Pohanka A, et al. Estimation of dabigatran plasma concentrations in the perioperative setting. An ex vivo study using dedicated coagulation assays. Thromb Haemost. (2015) 113:862–9.
13. Gosselin RC, Adcock DM, Bates SM, Douxfils J, Favaloro EJ, Gouin-Thibault I, et al. International council for standardization in haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. (2018) 118:437–50. doi: 10.1055/s-0038-1627480
14. Antovic JP, Skeppholm M, Eintrei J, Boija EE, Söderblom L, Norberg EM, et al. Evaluation of coagulation assays versus LC-MS/MS for determinations of dabigatran concentrations in plasma. Eur J Clin Pharmacol. (2013) 69:1875–81. doi: 10.1007/s00228-013-1550-4
15. Douxfils J, Dogné JM, Mullier F, Chatelain B, Rönquist-Nii Y, Malmström RE, et al. Comparison of calibrated dilute thrombin time and aPTT tests with LC-MS/MS for the therapeutic monitoring of patients treated with dabigatran etexilate. Thromb Haemost. (2013) 110:543–9. doi: 10.1160/TH13-03-0202
16. Hawes EM, Deal AM, Funk-Adcock D, Gosselin R, Jeanneret C, Cook AM, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost. (2013) 11:1493–502. doi: 10.1111/jth.12308
17. Du S, Weiss C, Christina G, Krämer S, Wehling M, Krämer R, et al. Determination of dabigatran in plasma, serum, and urine samples: comparison of six methods. Clin Chem Lab Med. (2015) 53:1237–47. doi: 10.1515/cclm-2014-0991
18. Solbeck S, Jensen AS, Maschmann C, Stensballe J, Ostrowski SR, Johansson PI. The anticoagulant effect of therapeutic levels of dabigatran in atrial fibrillation evaluated by thrombelastography (TEG§), hemoclot thrombin inhibitor (HTI) assay and Ecarin clotting time (ECT). Scand J Clin Lab Invest. (2018) 78:25–30. doi: 10.1080/00365513.2017.1408138
19. Douxfils J, Mullier F, Robert S, Chatelain C, Chatelain B, Dogne JM. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost. (2012) 107:985–97. doi: 10.1160/TH11-11-0804
20. Matsuno K, Usami T, Hatuse M, Shimizu C. Monitoring of oral thrombin inhibitor. Rinsho Byori. (2014) 62:958–64.
21. Saffian SM, Zhang M, Leong Chin PK, Jensen BP. Quantification of dabigatran and indirect quantification of dabigatran acylglucuronides in human plasma by LC-MS/MS. Bioanalysis. (2015) 7:957–66. doi: 10.4155/bio.15.32
22. Zhang M, Moore GA, Chin PKL. Simultaneous determination of dabigatran, rivaroxaban, and apixaban in human plasma by liquid chromatography/tandem mass spectrometry. Ther Drug Monit. (2020) 42:473–80. doi: 10.1097/FTD.0000000000000744
23. Rocci ML Jr, Devanarayan V, Haughey DB, Jardieu P. Confirmatory reanalysis of incurred bioanalytical samples. AAPS J. (2007) 9:E336–43. doi: 10.1208/aapsj0903040
24. Hori M, Connolly SJ, Zhu J, Liu LS, Lau CP, Pais P, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. (2013) 44:1891–6.
25. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. (2007) 50:309–15. doi: 10.1016/j.jacc.2007.01.098
26. Douxfils J, Chatelain C, Chatelain B, Dogne JM, Mullier F. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost. (2013) 110:283–94. doi: 10.1160/TH12-12-0898
27. Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. (2013) 369:1206–14.
28. Jaffer IH, Chan N, Roberts R, Fredenburgh JC, Eikelboom JW, Weitz JI. Comparison of the ecarin chromogenic assay and diluted thrombin time for quantification of dabigatran concentrations. J Thromb Haemost. (2017) 15:2377–87.
29. Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY trial (Randomized evaluation of long-term anticoagulation therapy). J Am Coll Cardiol. (2014) 63:321–8. doi: 10.1016/j.jacc.2013.07.104
30. Lin SY, Tang SC, Kuo CH, Tsai LK, Yeh SJ, Shen LJ, et al. Factors affecting serum concentration of dabigatran in Asian patients with non-valvular atrial fibrillation. J Formos Med Assoc. (2019) 118:1154–60. doi: 10.1016/j.jfma.2018.11.013
31. Zhu Z, Shen Z, Shi A, Su C, Mao J, Tao H, et al. Dabigatran plasma concentration indicated the risk of patients with non-valvular atrial fibrillation. Heart Vessels. (2022) 37:821–7. doi: 10.1007/s00380-021-01974-0
Keywords: dabigatran, hemoclot thrombin inhibitor, quantitative detection, drug safety, cardiovascular medicine
Citation: Liu Z, Mu G, Xie Q, Zhang H, Jiang J, Xiang Q and Cui Y (2022) Hemoclot Thrombin Inhibitor Assay and Expected Peak-Trough Levels of Dabigatran: A Multicenter Study. Front. Cardiovasc. Med. 9:894888. doi: 10.3389/fcvm.2022.894888
Received: 12 March 2022; Accepted: 13 May 2022;
Published: 22 July 2022.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Paul Ken Leong Chin, University of Otago, New ZealandCopyright © 2022 Liu, Mu, Xie, Zhang, Jiang, Xiang and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yimin Cui, Y3VpLnBoYXJtQHBrdWZoLmNvbQ==; Qian Xiang, eGlhbmdxekBwa3VmaC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.