- 1Department of Endocrinology and Metabolism, The First Affiliated Hospital of Jinan University, Guangzhou, China
- 2Department of Functional Examination, Gansu Provincial Maternal and Child Health Hospital, Lanzhou, China
- 3Clinical Experimental Center, The First Affiliated Hospital of Jinan University, Guangzhou, China
Background and Objective: Relevant data of PARADIGM-HF reveals sacubitril/valsartan (SV) therapy led to a greater reduction in the risks of arrhythmia, and sudden cardiac death than angiotensin converting enzyme inhibitor (ACEI)/angiotensin receptor inhibitor (ARB) therapy in HFrEF, however, inconsistent results were reported in subsequent studies. Here, we conduct a meta-analysis of related randomized controlled trials (RCTs) to evaluate the protective effect of SV on reducing the risk of arrhythmias.
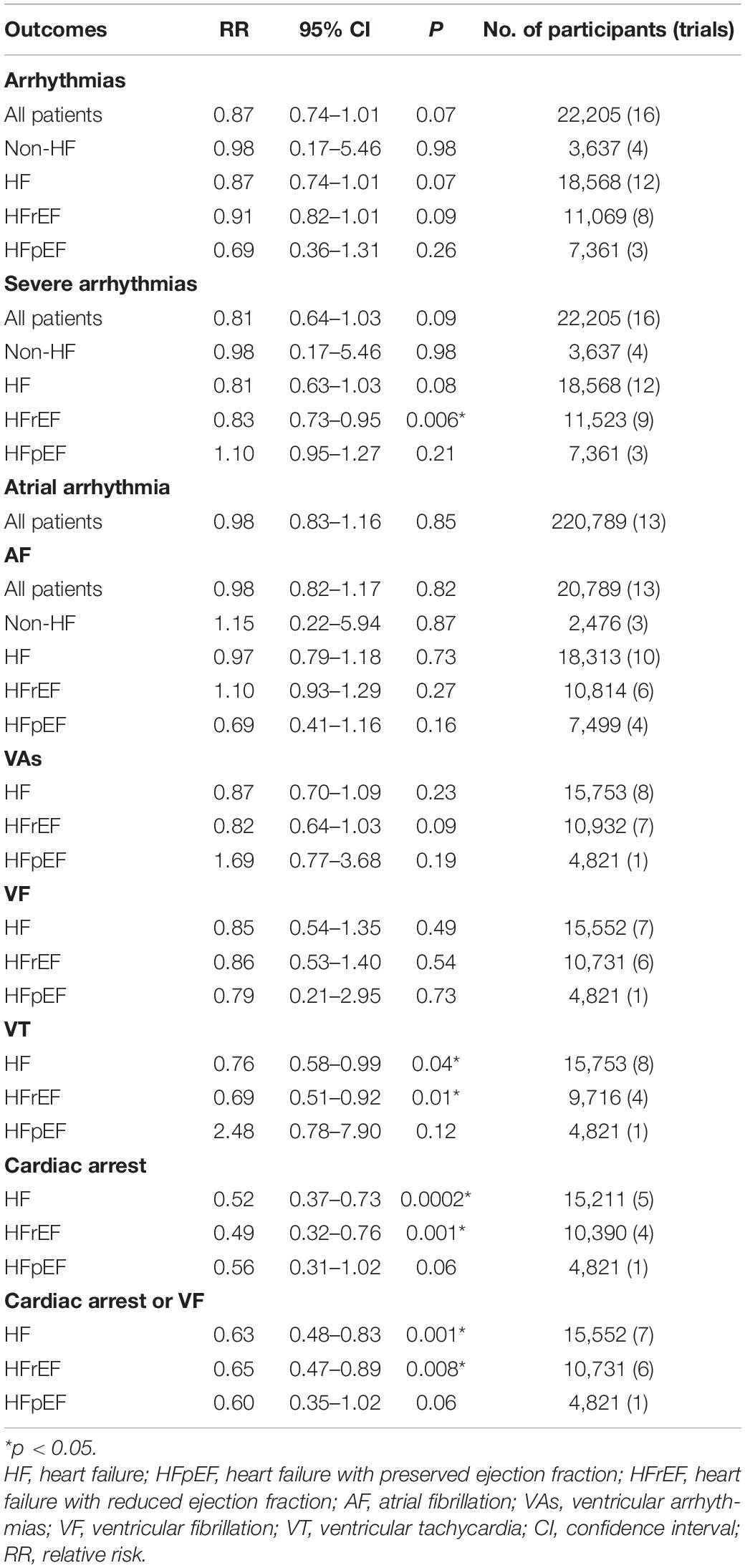
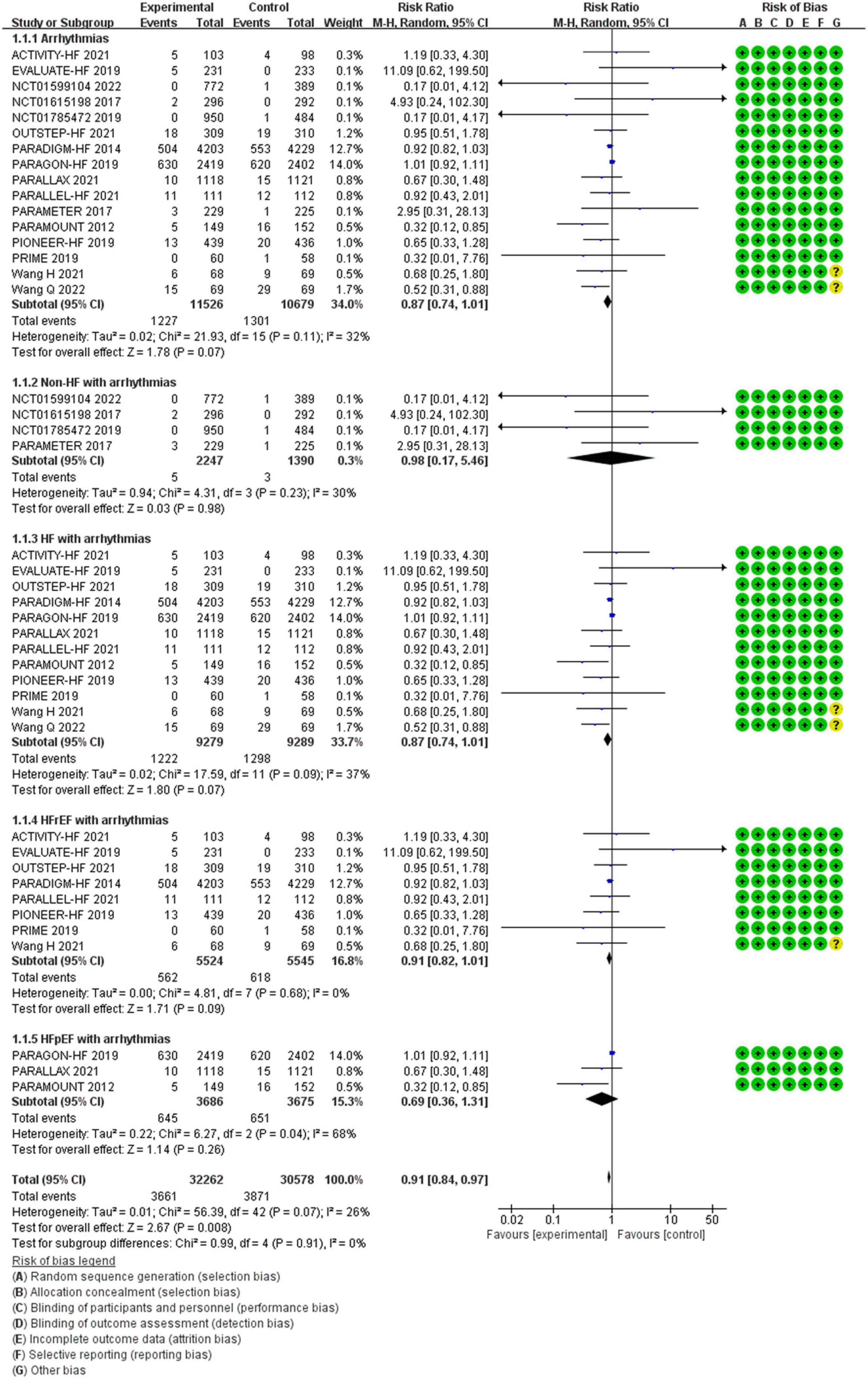
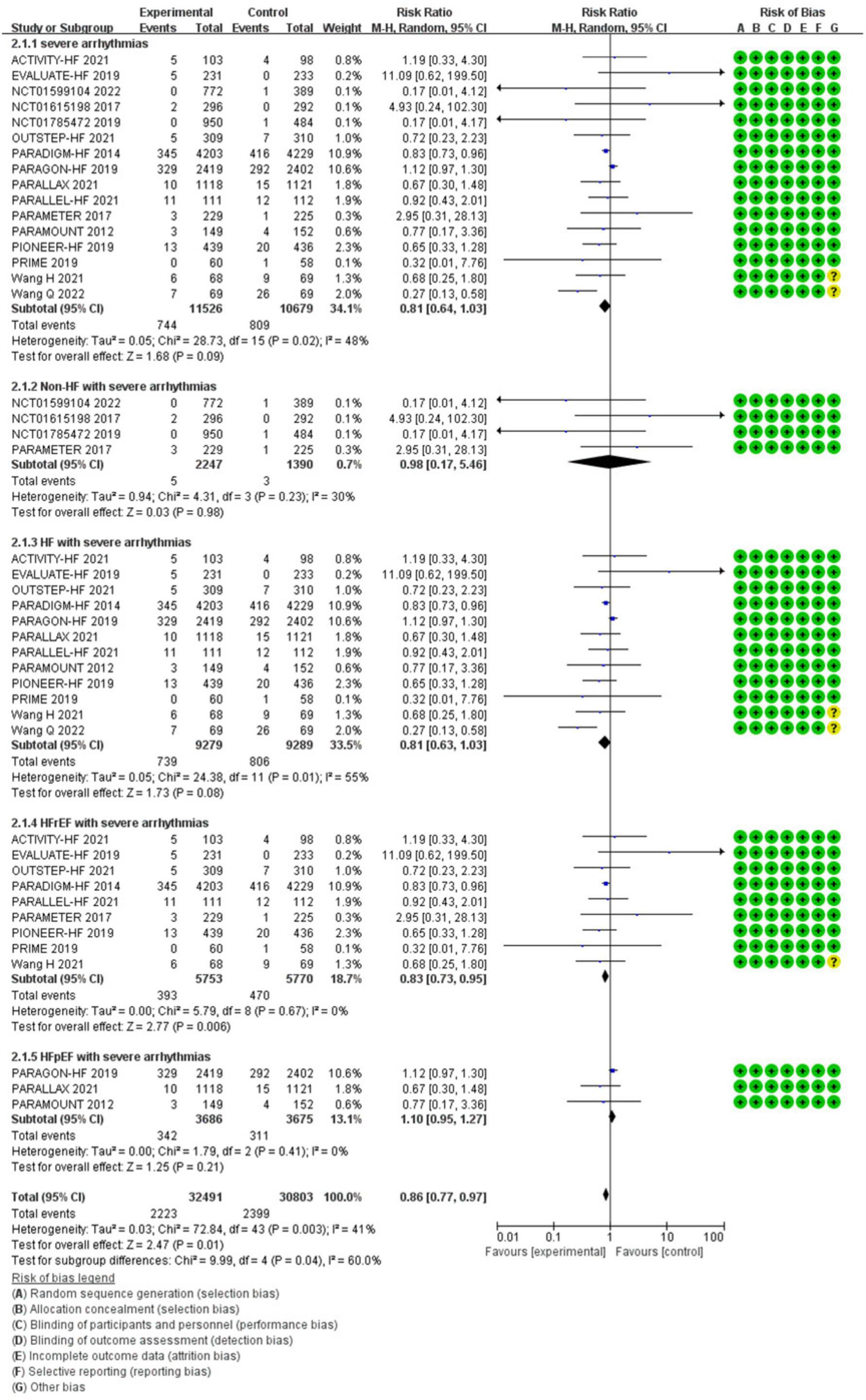
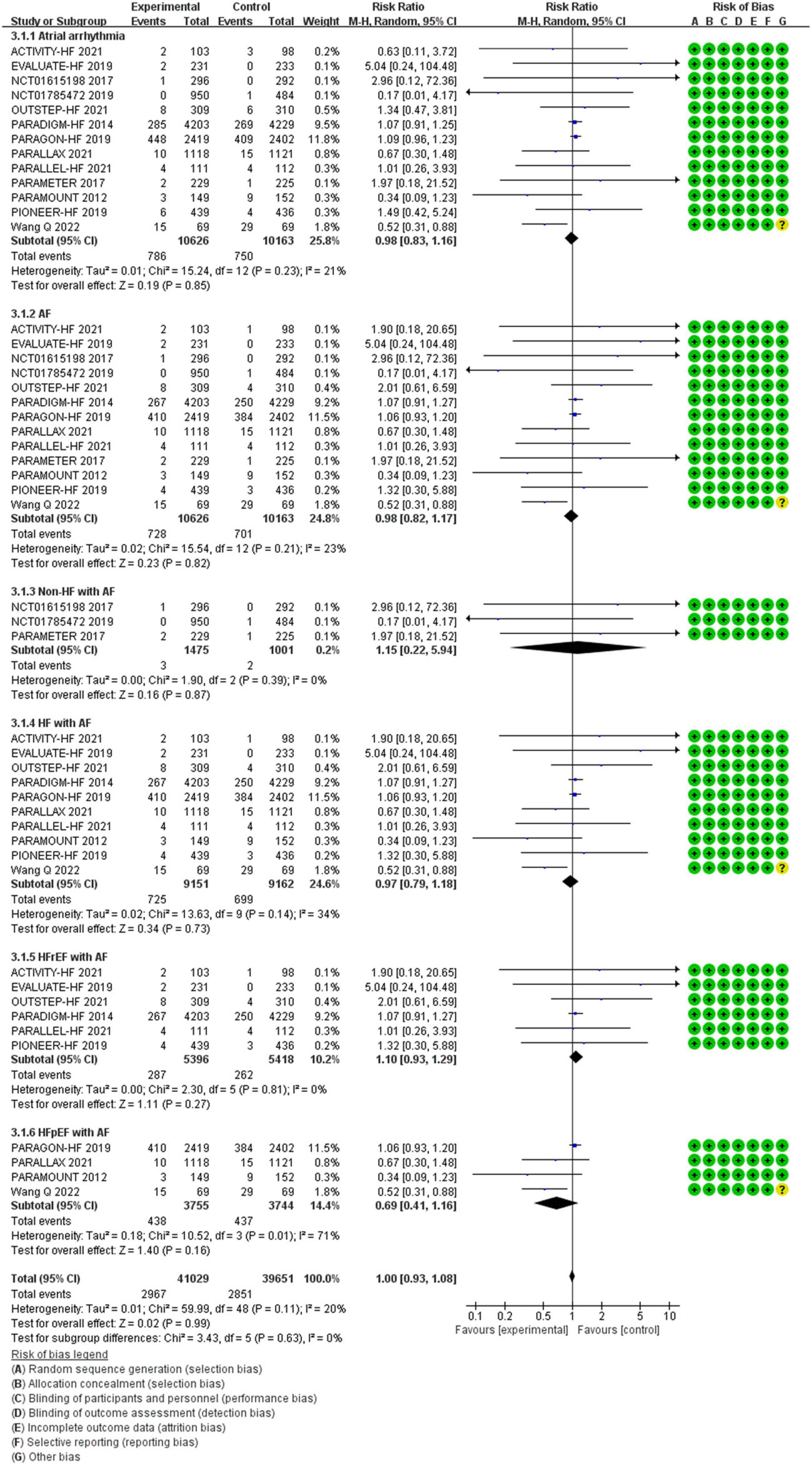
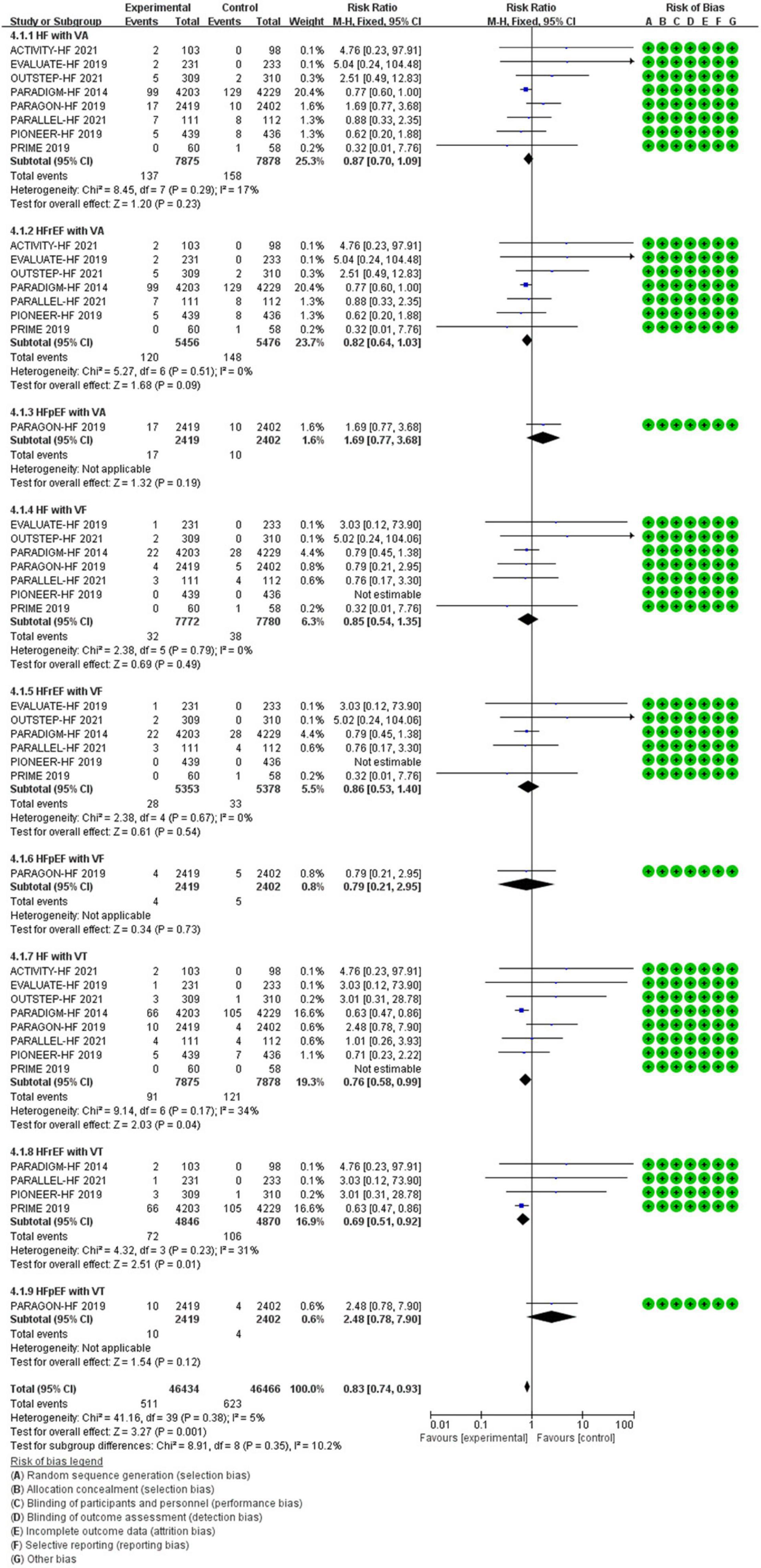
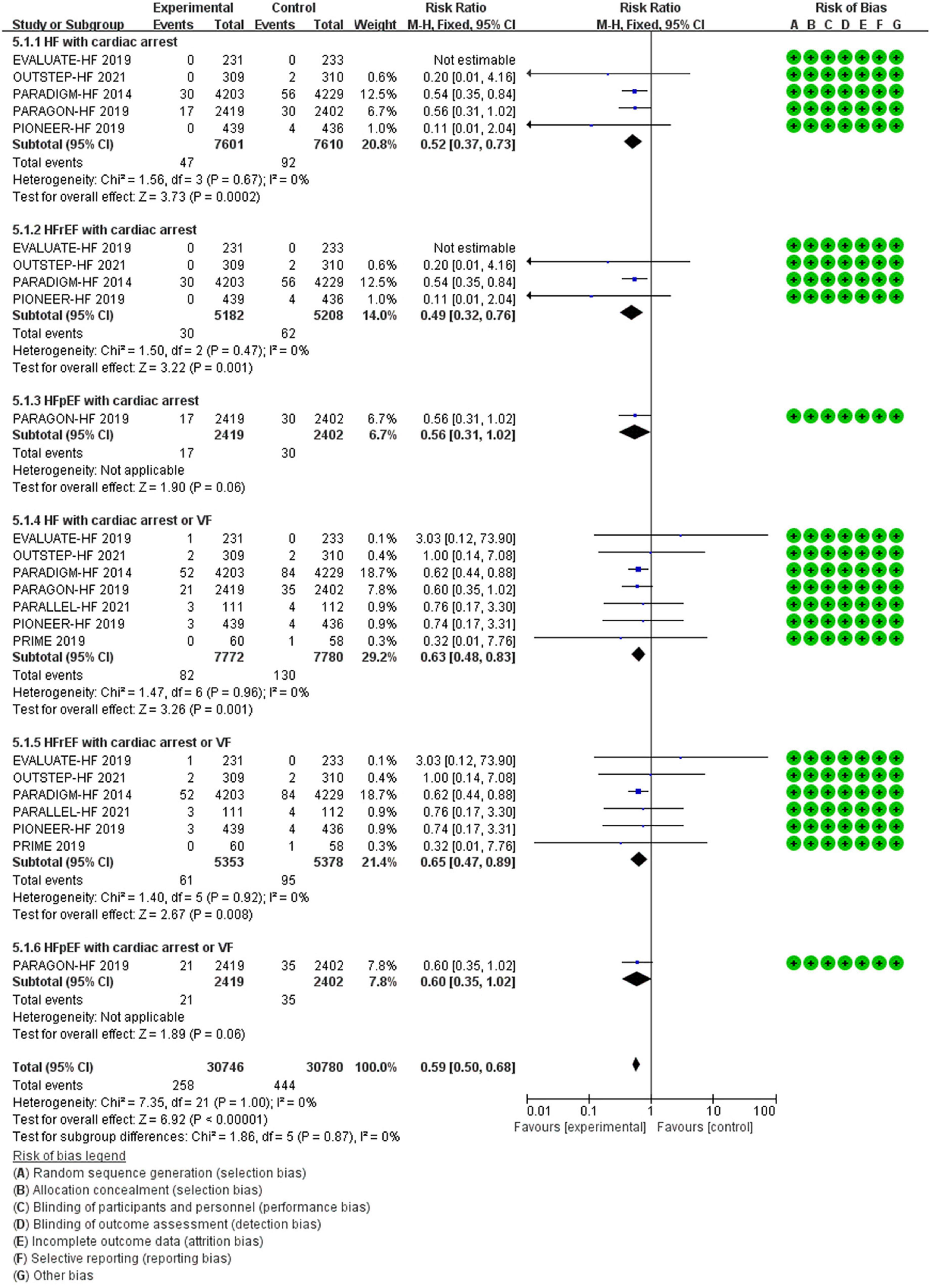
Methods and Results: RCTs focused on the difference in therapeutic outcomes between SV and ACEI/ARB were searched from PUBMED, EMBASE, ClinicalTrials.gov, and Cochrane Library. The results were extracted from each individual study, expressed as binary risk, 95% confidence interval (CI) and relative risk (RR). Sixteen RCTs including 22, 563 patients met the study criteria. Compared with ACEI/ARB therapy, SV therapy did significantly reduce in the risks of severe arrhythmias among patients with heart failure with reduced ejection fraction (HFrEF) (RR 0.83, 95% CI 0.73–0.95, p = 0.006), ventricular tachycardia (VT) among patients with HFrEF (RR 0.69, 95% CI 0.51–0.92, p = 0.01), cardiac arrest among patients with heart failure (HF) (RR 0.52, 95% CI 0.37–0.73, p = 0.0002), cardiac arrest among patients with HFrEF (RR 0.49, 95% CI 0.32–0.76, p = 0.001), cardiac arrest or ventricular fibrillation (VF) among patients with HF (RR 0.63, 95% CI 0.48–0.83, p = 0.001), and cardiac arrest or VF among patients with HFrEF (RR 0.65, 95% CI 0.47–0.89, p = 0.008), but reduced the risks of arrhythmias (RR 0.87, 95% CI 0.74–1.01, p = 0.07), atrial arrhythmias (RR 0.98, 95% CI 0.83–1.16, p = 0.85), and atrial fibrillation (RR 0.98, 95% CI 0.82–1.17, p = 0.82) among all patients with no significant between-group difference. The merged result was robust after sensitivity analysis, and there was no publication bias.
Conclusion: Our meta-analysis provides evidence that, compared with ACEI/ARB, SV can additionally reduce the risks of most arrhythmias, just the significant differences are revealed in reducing the risks of VT, severe arrhythmias, and cardiac arrest in patients with HFrEF. Besides, the positive effect of SV on VF according to statistical result of combining VF with cardiac arrest in patients with HFrEF is credibility.
Introduction
Arrhythmia is one of the most common diseases in cardiovascular field, which has complex relationships with multiple diseases (1), especially heart failure (HF). Destruction of normal periodicity and regularity of the electrical activity in heart has been recognized as the mechanism of arrhythmia. Atrial fibrillation (AF) and ventricular arrhythmia (VA) are common and serious in patients with HF, which can cause various complications and ultimately lead to disability or death, such as thrombus caused by AF, sudden cardiac death (SCD) caused by sustained ventricular tachycardia (VT) or ventricular fibrillation (VF), etc. (2). The risk of AF has increased 3-fold in the world over the past 50 years based on the results of Framingham Heart Study (3), and the global prevalence of AF was about 46.3 million in 2016 according to the estimate of WHO (4). AF is the most common persistent arrhythmia with an average prevalence of 25% in HF (5), which can increase the risks of stroke and death (6). Sustained VA is a serious complication of HF, as it accounts for 75–80% of SCD, while about 30–50% of cardiovascular death in patients with HF was attributed to SCD (7). Arrhythmia is a challenging problem especially under the circumstance of HF, as their mutual interaction could further aggravating the state of an illness. Therefore, received widespread attention in terms of prevention, diagnosis, and treatment of arrhythmia. At present, a part of arrhythmias can be cured by new technologies with the wider application of interventional and surgical therapy (8), nevertheless, drug therapy is still currently the most important mean of the prevention and treatment of arrhythmias due to economic, scope of application of new technologies, etc.
The data of PARADIGM-HF prompted that sacubitril/valsartan (SV) therapy led to a greater reduction in the risk of SCD and significant survival benefit of SCD observed from the Kaplan-Meier curves than enalapril therapy among patients with heart failure with reduced ejection fraction (HFrEF), indicating SV may directly reduce the risk of SCD (9). Since then, the effect of SV on arrhythmia has attracted widespread concern. The effect of SV in arrhythmia is uncertain although it has been fully affirmed in HF and hypertension (10, 11). The researches of Russo et al. (12), Tsai et al. (13), and Curtain et al. (14) revealed that SV could improve ventricular remodeling while reducing the risk of VA compared with angiotensin converting enzyme inhibitor (ACEI)/angiotensin receptor inhibitor (ARB), meanwhile, it could improve atrial remodeling and atrial arrhythmia by the researches of Suo et al. (15) and Li et al. (16). Nevertheless, different results on arrhythmias were reported by El-Battrawy et al. (17), Martens et al. (18), Solomon et al. (19), Velazquez et al. (20), and McMurray et al. (10), etc.
Thus far, few specifically studies about the effect of SV on arrhythmia have been investigated. The purpose of this meta-analysis is to provide some new approaches for the treatment of arrhythmia by analyzing the risks of arrhythmias in randomized controlled trials (RCTs) for SV.
Materials and Methods
Search Strategy
We searched the Cochrane Library, Embase, PubMed and ClinicalTrials.gov, the current four major medical databases, which contain the vast majority of medical research literatures, as of February 21, 2022, and it was re-run twice on March 3, 2022. The search conditions include: sacubitril valsartan, sacubitril/valsartan, sacubitril, entresto, LCZ696, AHU377, angiotensin receptor neprilysin inhibitor, neprilysin inhibitor, sacubitril valsartan sodium hydrate, sacubitril valsartan drug combination. There was no protocol for expected registration, but the search terms, inclusion criteria, and data collection forms were pre-specified in the analysis plan and remain unchanged during data collection and analysis. The publication date and language restrictions were not applied, and reference lists of related articles were also used to supplement search terms. The study included only RCT.
Study Selection
The trials included in this study need to meet the following conditions: (1) The trial is an RCT. (2) The control group is intervened with ACEI/ARB, the experimental group is intervened with SV. (3) All studies must have data on adverse events endpoint or adverse reaction of arrhythmias.
Data Extraction
The two researchers independently extracted data from RCTs that met the criteria and Cochrane reviewer’s handbook. In the event of discrepancies, all authors discussed the results. The research data was retrieved from the original published manuscript or the results in ClinicalTrials.gov. Extracted the following data from each trial: 1. Name of the trial, author, registration number; 2. year of publication; 3. number of people enrolled; 4. characteristics of participants at baseline, including arrhythmia, age, gender, etc.; 5. drug in control group; 6. study duration; 7. main outcome.
Quality Assessment
Two researchers separately assessed the risk of bias for each qualified trial by the Cochrane Collaboration’s tool and compiled a bias risk table as described in Cochrane Handbook (21). The quality items used to evaluate each study were as follows: sequence generation of allocation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other biases.
Outcome Measures
The number of arrhythmias listed in results of trials, including atrial arrhythmia, AF, VA, etc.
Statistical Analyses
Data of arrhythmias was analyzed by Review Manager 5.4, sensitivity analysis and publication bias detection by Stata 17.0, and I2 was used to assess heterogeneity. I2 ≥ 50% or the corresponding P-value (p < 0.05) was considered to have obvious heterogeneity results and then we used a random model. I2 < 50% and the corresponding P-value (p > 0.05) was considered to have no obvious heterogeneity results and then we used a fixed model (22). The results were extracted from each trial, expressed as binary risk, 95% confidence interval (CI), and relative risk (RR). The Mantel-Haenszel method and Z test were used to determine the overall results to determine the significance of RR. The heterogeneity was assessed with the I2 test, P < 0.05 was considered statistically significant. All results are in line with the declarations of PRISMA and Meta-analysis (23).
Publication Bias and Sensitivity Analysis
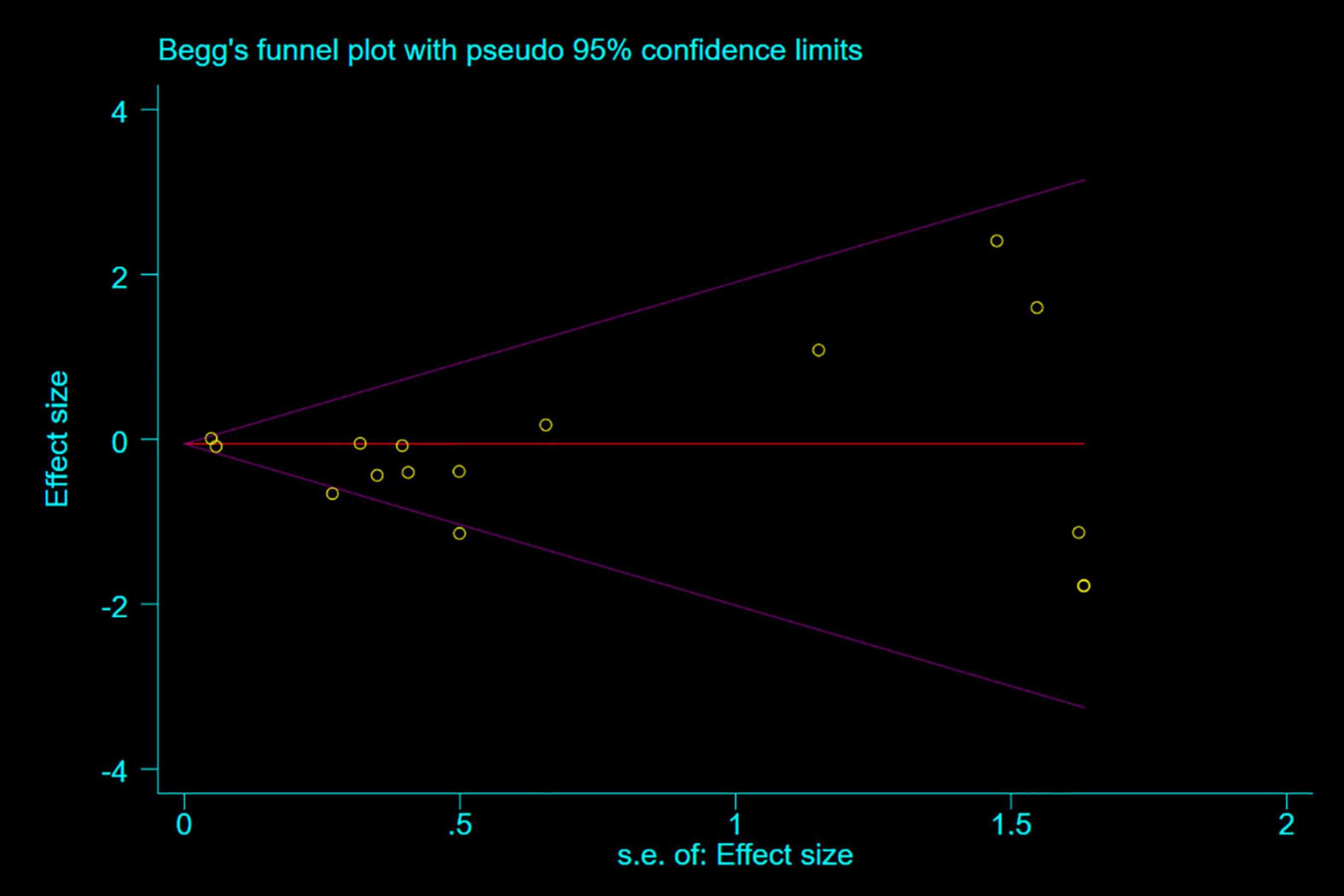
Publication bias was judged using funnel plot, Begg and Berlin (24), and Egger’s test (25), P < 0.05 was considered statistically significant.
Results
Description of Selected Studies
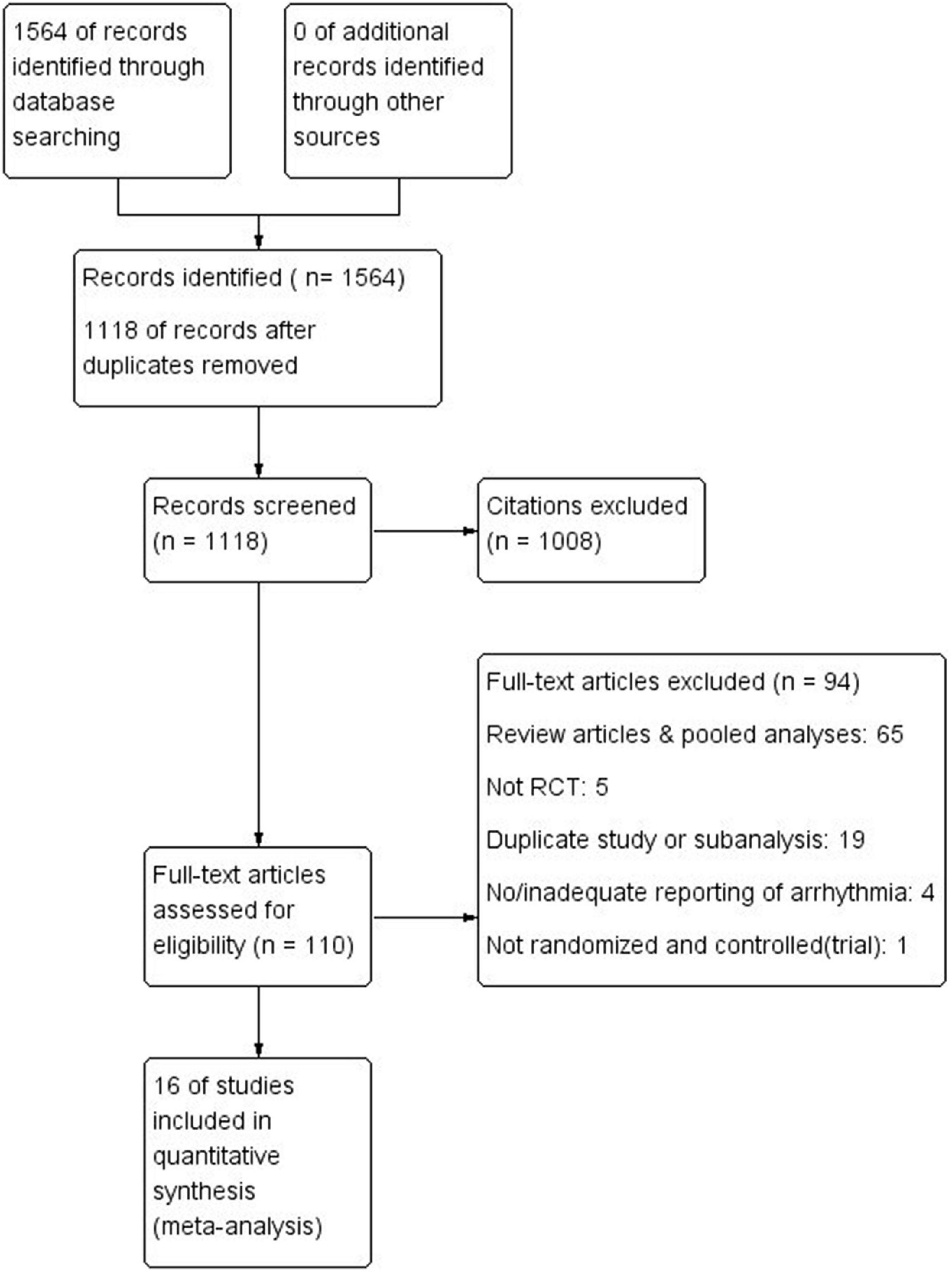
A total of 1, 564 possible articles or studies were initially identified, and 446 possible articles were left after filtered repeated research by endnote. The remaining articles were judged by the two researchers according to criteria, final 16 RCTs were included for analysis. The flow chart is shown in Figure 1.
Study Characteristics
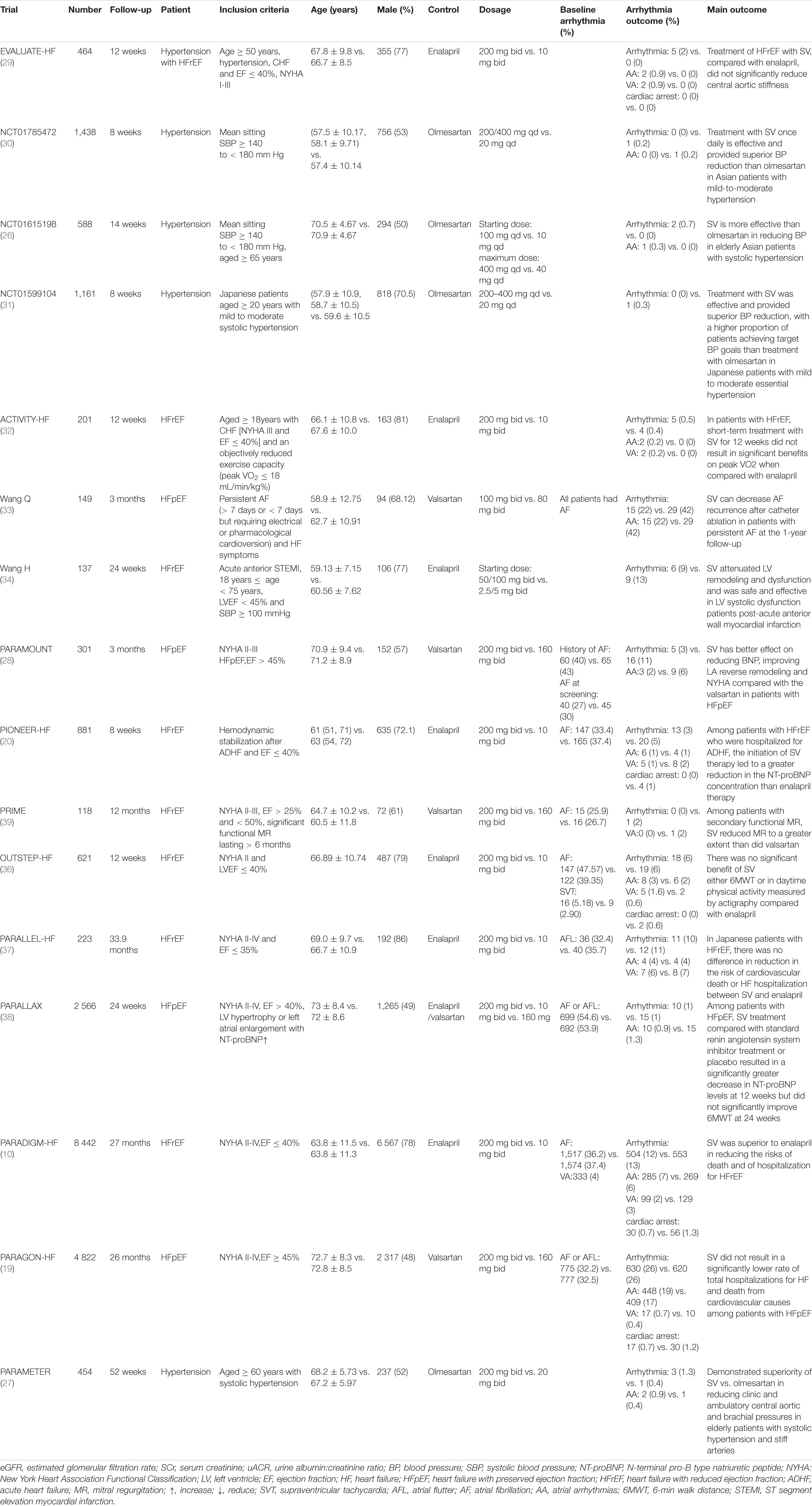
The detailed characteristics of 16 studies (10, 19, 20, 26–38) are shown in Table 1. Most of the data are come from ClinicalTrials.gov, 14 trials are multi-center and 12 trials are aimed at patients with HF. The total number of subjects is 22, 563 and the follow-up ranged from 8 weeks to 33.9 months. All are RCTs.
Quality Assessment
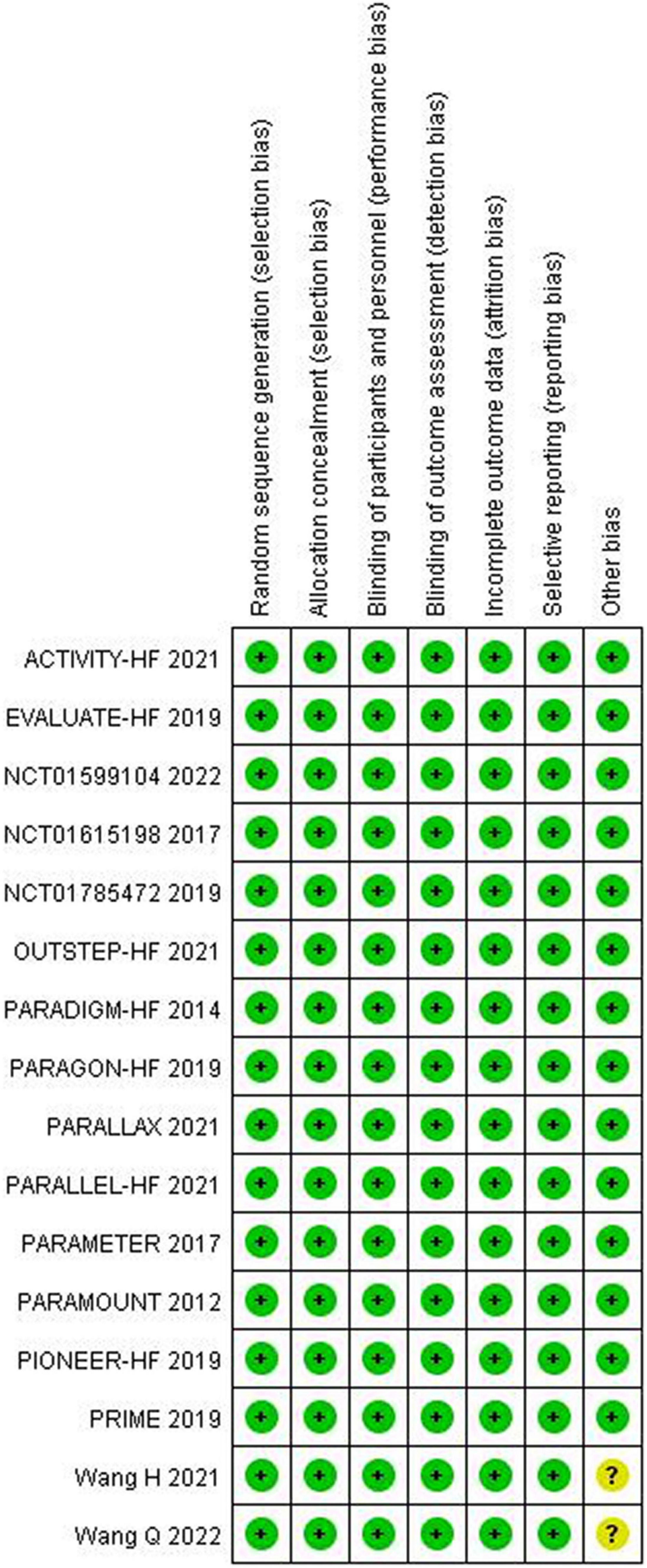
The quality assessment for the included studies is presented in Figures 2, 3. Randomization assignment was conducted using computer-generated random numbers in a majority of the trials and prespecified outcomes were reported by all trials. Individual studies did not specifically describe the methods used to hide and allocate sequences. Overall, the included studies are of high quality.
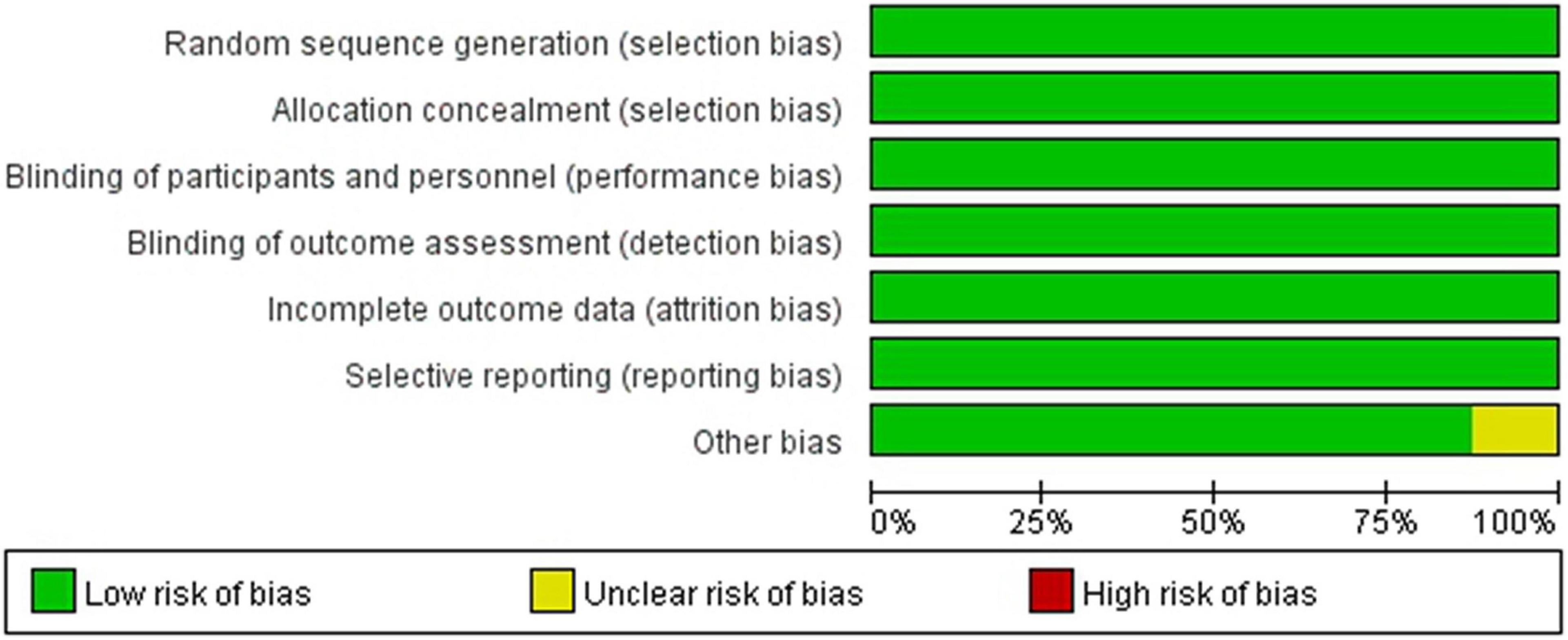
Figure 2. Methodological quality graph: author’s judgments about each methodological quality item presented as a percentage across all included studies.
Clinical Outcomes Evaluation
The analysis results are summarized in Table 2.
The Efficacy of Sacubitril/Valsartan Compared to Angiotensin Converting Enzyme Inhibitor/Angiotensin Receptor Inhibitor on Arrhythmias
Between the two groups, the results revealed that there was no difference in reduction in the risks of arrhythmias among all patients (RR 0.87, 95% CI 0.74–1.01, p = 0.07), arrhythmias among patients with non-HF (RR 0.98, 95% CI 0.17–5.46, p = 0.98), HF (RR 0.87, 95% CI 0.74–1.01, p = 0.07), HFrEF (RR 0.91, 95% CI 0.82–1.01, p = 0.09), and HF with preserved ejection fraction (HFpEF) (RR 0.69, 95% CI 0.36–1.31, p = 0.26) (Figure 4).
The Efficacy of Sacubitril/Valsartan Compared to Angiotensin Converting Enzyme Inhibitor/Angiotensin Receptor Inhibitor on Severe Arrhythmias
Compared with ACEI/ARB therapy, SV therapy did significantly reduce in the risks of severe arrhythmias among patients with HFrEF (RR 0.83, 95% CI 0.73–0.95, p = 0.006), but the reductions in the risks of severe arrhythmias among all patients (RR 0.81, 95% CI 0.64–1.03, p = 0.09), severe arrhythmias among patients with non-HF (RR 0.98, 95% CI 0.17–5.46, p = 0.98), HF (RR 0.81, 95% CI 0.63–1.03, p = 0.08), and HFpEF (RR 1.10, 95% CI 0.95–1.27, p = 0.21) were no significant between-group difference (Figure 5).
The Efficacy of Sacubitril/Valsartan Compared to Angiotensin Converting Enzyme Inhibitor/Angiotensin Receptor Inhibitor on Atrial Arrhythmias
Between the two groups, the results revealed that there was no difference in reduction in the risks of atrial arrhythmias among all patients (RR 0.98, 95% CI 0.83–1.16, p = 0.85), AF among all patients (RR 0.98, 95% CI 0.82–1.17, p = 0.82), AF among patients with non-HF (RR 1.15, 95% CI 0.22–5.94, p = 0.87), HF (RR 0.97, 95% CI 0.79–1.18, p = 0.73), HFrEF (RR 1.10, 95% CI 0.93–1.29, p = 0.27), and HFpEF (RR 0.69, 95% CI 0.41–1.16, p = 0.16) (Figure 6).
The Efficacy of Sacubitril/Valsartan Compared to Angiotensin Converting Enzyme Inhibitor/Angiotensin Receptor Inhibitor on Ventricular Arrhythmias
All data of VAs were came from patients with HF. Compared with ACEI/ARB therapy, SV therapy did significantly reduce in the risks of VT among patients with HFrEF (RR 0.69, 95% CI 0.51–0.92, p = 0.01), but the reductions in the risks of VA among patients with HF (RR 0.87, 95% CI 0.70–1.09, p = 0.23), VA among patients with HFrEF (RR 0.82, 95% CI 0.64–1.03, p = 0.09), VA among patients with HFpEF (RR 1.69, 95% CI 0.77–3.68, p = 0.19), VF among patients with HF (RR 0.85, 95% CI 0.54–1.35, p = 0.49), VF among patients with HFrEF (RR 0.86, 95% CI 0.53–1.40, p = 0.54), VF among patients with HFpEF (RR 0.79, 95% CI 0.21–2.95, p = 0.73), and VT among patients with HFpEF (RR 2.48, 95% CI 0.78–7.90, p = 0.12) were no significant between-group difference (Figure 7).
The Efficacy of Sacubitril/Valsartan Compared to Angiotensin Converting Enzyme Inhibitor/Angiotensin Receptor Inhibitor on Cardiac Arrest or Cardiac Arrest Combined With Ventricular Fibrillation
Compared with ACEI/ARB therapy, SV therapy did significantly reduce in the risks of cardiac arrest among patients with HF (RR 0.52, 95% CI 0.37–0.73, p = 0.0002), cardiac arrest among patients with HFrEF (RR 0.49, 95% CI 0.32–0.76, p = 0.001), cardiac arrest or VF among patients with HF (RR 0.63, 95% CI 0.48–0.83, p = 0.001), and cardiac arrest or VF among patients with HFrEF (RR 0.65, 95% CI 0.47–0.89, p = 0.008), but the reductions in the risks of cardiac arrest among patients with HFpEF (RR 0.56, 95% CI 0.31–1.02, p = 0.06) and cardiac arrest or VF among patients with HFpEF (RR 0.60, 95% CI 0.35–1.02, p = 0.06) were no significant between-group difference (Figure 8).
Sensitivity Analysis and Publication Bias
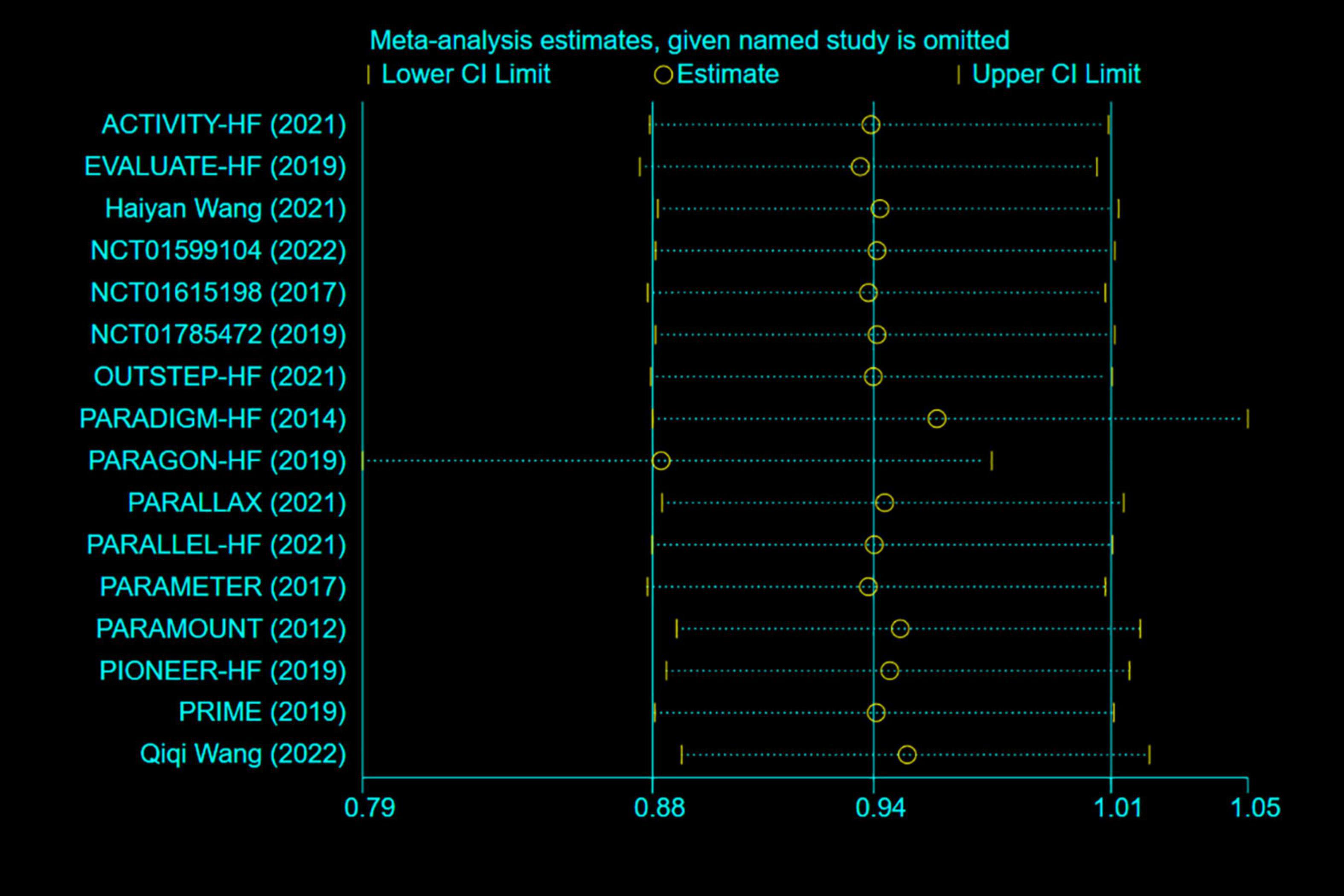
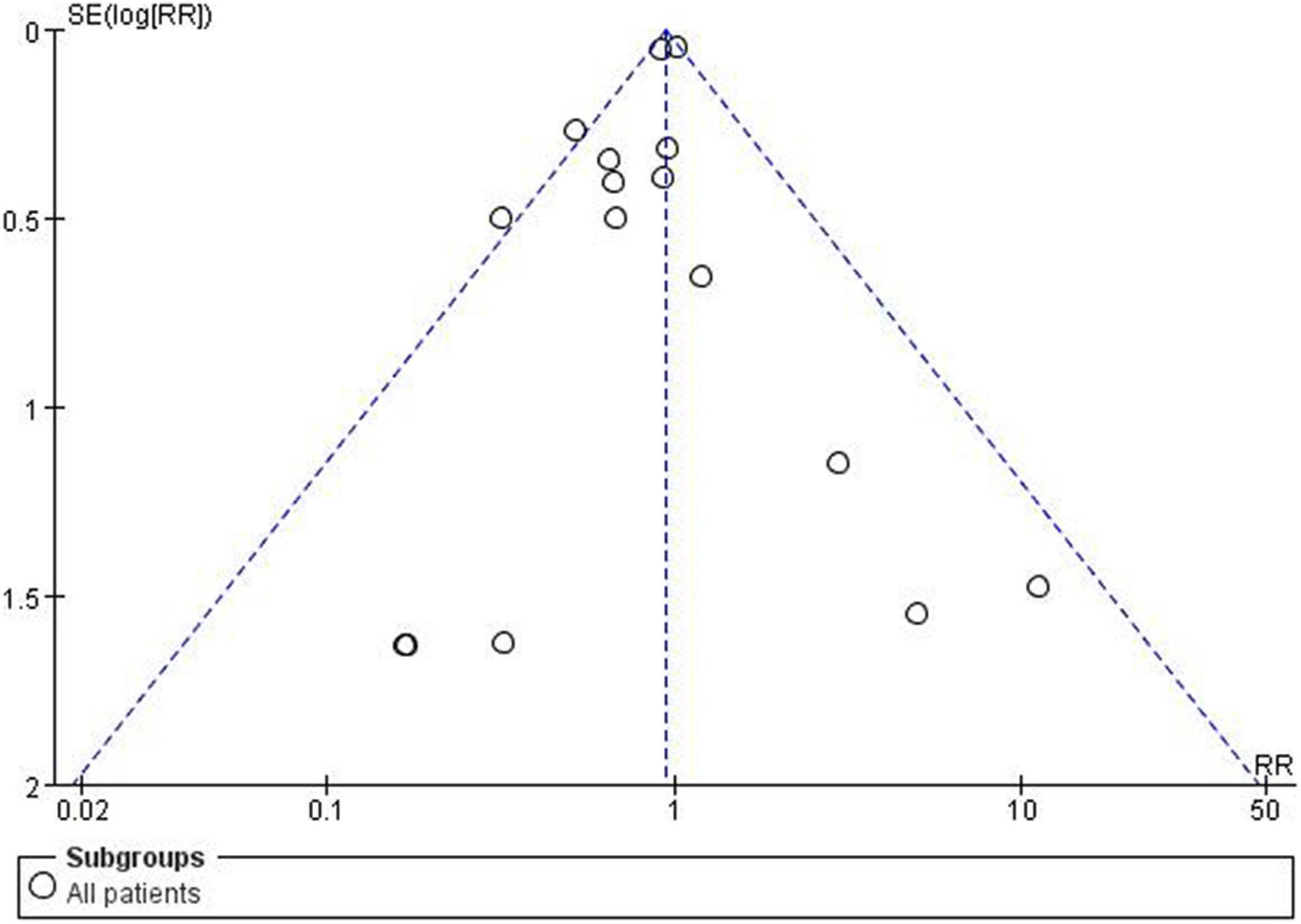
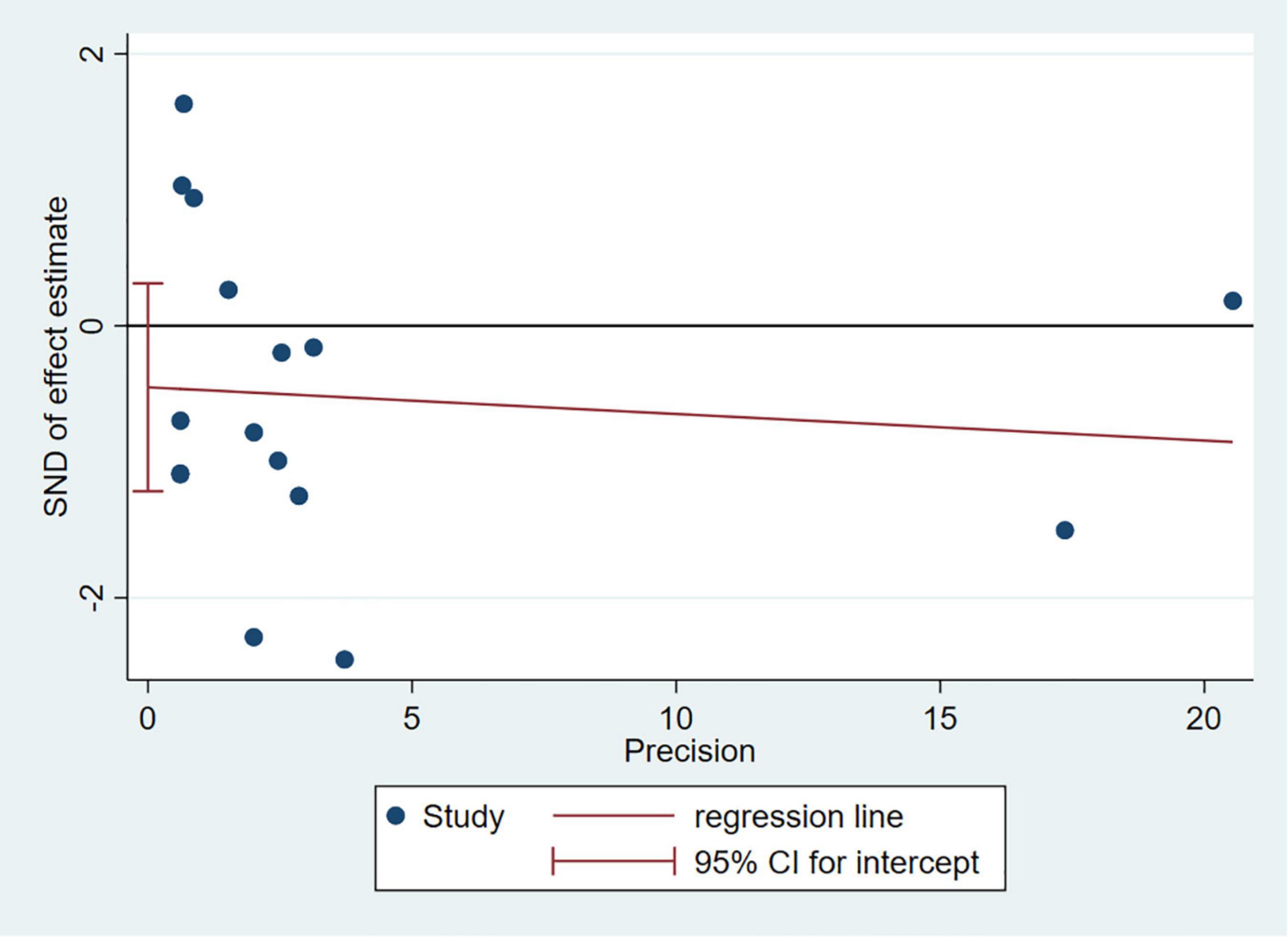
Any single study excluded would not affected the significance of our combined effect size for either outcome by sensitivity analysis (Figure 9). No obvious publication bias was found in the visual inspection of funnel plots (Figure 10), Egger’s test (Figure 11) for OS with a p-value of 0.225 and Begg’s Test (Figure 12) for OS with a p-value of 0.822 also proved it. Therefore, we could conclude that all the included studies have no obvious publication bias and the result is stable.
Discussion
Major Findings
Compared with ACEI/ARB therapy, SV therapy did significantly reduce in the risks of severe arrhythmias, VT and cardiac arrest among patients with HFrEF, and cardiac arrest among patients with HF by 17, 31, 51, and 48%, respectively, and the reductions in the risks of arrhythmias, severe arrhythmias, atrial arrhythmias, AF and VAs among patients with HF were 13, 19, 22, and 13%, respectively with no significant between-group difference. Although the specific relationship between the data of cardiac arrest and VF in this meta-analysis cannot be determined, considering that the main cause of cardiac arrest is VF, we combined the data for statistical analysis, and the results revealed that SV therapy did significantly reduce in reducing the risks of cardiac arrest or VF among patients with HF, HFrEF by 37 and 35%, respectively compared with ACEI/ARB therapy. Therefore, we consider that SV therapy did significantly reduce in the risks of cardiac arrest in patients with HF, mainly HFrEF, compared with ACEI/ARB therapy. Similarly, we can speculate about the superior role of SV therapy in reducing the use of implantable cardioverter-defibrillator from SV therapy led to a reduction in the risks of VT, VF, and cardiac arrest or VF compared with ACEI/ARB therapy in HFrEF. Further research is needed to confirm these speculations. Overall, the results of our meta-analysis revealed that SV therapy in specific groups are effective and provided superior arrhythmias reduction than ACEI/ARB therapy.
Mechanisms
Cardiovascular disease mainly leads to the activation of the neuroendocrine system, including sympathetic nervous system (SNS), renin-angiotensin-aldosterone system (RAAS), and vasoactive peptides (mainly natriuretic peptides) (40). Despite the initial functional response, chronic SNS and RAAS activation increases cardiac afterload, which increasing myocardial oxygen consumption and leading to deleterious proliferative remodeling effects (41). Natriuretic peptide system (NPs) mediates the activation of cyclic guanosine monophosphate-dependent signaling pathways through corresponding receptors, resulting in vasodilation, natriuretic diuresis, and lowering of blood pressure (40). In addition, cardiac filling, preload, and ventricular remodeling are reduced by NPs and corresponding receptors through inhibiting endothelin secretion, and activation of RAAS and SNS. The effect of SV is mainly considered to enhance NPs and inhibit RAAS by inhibiting neprilysin, moreover, it has more effective dual inhibitory effect on the neuroendocrine system by combining with ARB. Furthermore, enhancing the NPs has favorable cardiovascular effects in HF and is an ideal complementary therapeutic target for RAAS and SNS blockade. Enkephalinase inhibitor (NEPI) in SV exerts natriuretic diuresis, dilates blood vessels, and reduces blood pressure by upregulating the levels of NPs, bradykinin, and adrenomedullin. Also, inhibition of sympathetic tone and RAAS can reduce aldosterone, myocardial fibrosis and hypertrophy, and ventricular remodeling. In addition to counteracting the vasoconstriction problem caused by the increased concentration of angiotensin II caused by NEPI, the combined use of valsartan can enhance the inhibition of vasoconstriction, cardiomyocyte proliferation and fibrosis, and myocardial remodeling by further inhibit SNS and decreases aldosterone levels by inhibiting sustained activation of RAAS (42, 43).
The mechanism of SV improving arrhythmia is not clear, which may be related to the treatment with SV provided superior cardiac remodeling (including structure and electrophysiology) and fibrosis (44) reduction than angiotensin inhibition, as cardiac remodeling can lead to increased susceptibility to arrhythmia and fibrosis, a well-recognized factor for malignant VA, is an important basis of cardiac remodelling (45, 46). Besides, NPs regulation has affect on myocardial electrophysiological properties and anatomical substrate, which are major factors for the development of sustained VA. The inhibition of SV on SNS and RAAS may exert a direct effect on reducing the risk of arrhythmia in HF. SNS and RAAS are activated and the secretion of neurohormone is increased in HF, lead to arrhythmia by increasing myocardial automaticity, altering conductivity and refractory periods, constricting of blood vessels to alter cardiac load, and promoting ventricular remodeling together (47, 48). In pre-clinical studies, SV improved the risk of persistent VA by reducing wall stress (49) and the activity of membrane ion channels, such as sodium channel NaV1.5 protein and potassium channel proteins, associated with VT/VF, which decrease trigger factors and perpetuating/maintaining the event of Vas (50). Moreover, demonstrated superiority of SV vs. ACEI/ARB in reducing VAs though improving potential systolic and diastolic function, calcium homeostasis and conduction delay, increased pacing threshold to induce arrhythmia, a decrease of action potential duration (APD) and the maximum slope of APD restitution by inhibiting the CaMK II pathway, and down-regulation of small-conductance Ca2+-activated potassium channel type 2 (44, 51, 52).
Findings Relevant to Other Studies
The post hoc study of PARADIGM-HF revealed that, compared with enalapril therapy, SV therapy did significantly reduce in reducing the risk of ventricular arrhythmia and the composite arrhythmia outcome in HFrEF (14). A retrospective analysis of 1-year telemonitoring in 151 SV-treated HFrEF patients with implantable cardioverter-defibrillator/cardiac-resynchronization-therapy (18) revealed that SV treatment resulted in a reduction in mean VT/VF burden, non-sustained VT and treatments, which were associated with improved biventricular pacing and a higher degree of reverse remodeling. These results are consistent with our findings that SV therapy led to a greater reduction in the risks of VT/VF compared with ACEI/ARB therapy in HFrEF. The results of the PARADIGM-HF (9) revealed that SV had a direct effect on reducing the risk of SCD compared with enalapril, which is consistent with the findings of our study that SV therapy reduced the risk of cardiac arrest in HFrEF by 51% compared with ACEI/ARB therapy. SV therapy did not affect AF burden in the study of Martens (18), and the same is true of our study. Other than these, SV therapy could reduce the risk of severe arrhythmias by 17% in HFrEF compared with ACEI/ARB therapy. Above results are consistent with consistent with our findings, confirming the reliability of our results.
Thoughts on Difference of Therapy Effect
Our study finds that SV therapy is superior in reducing the risk of certain arrhythmias merely in HFrEF compared with ACEI/ARB therapy, which may be related to the different therapeutic effects of SV in different EF spectra (53, 54). Although has been recommended for the treatment of HFpEF by guidelines, SV merely has a significant therapeutic advantage in morbidity among patients with HFrEF, which could be found in other large clinical trials, due to multi-factors. The pathophysiological heterogeneity within the broader clinical spectrum of HFpEF, which may represent a different progression or disease, lead to the effect of neurohormone antagonists on HFpEF is relatively weak compared with HFrEF (55). Moreover, there is a significant correlation between the degree of systolic dysfunction and frequency of arrhythmias, mainly VAs, in HFrEF, and improvement in LVEF was associated with a significant reduction in VAs and mortality (56–58). However, as normal EF of HF, HFpEF may not have a similar phenomenon. Interestingly, arrhythmias mainly existed among patients with HF in trials included, of which atrial arrhythmias are more common than VAs, and VAs mainly came from patients with HFrEF, while atrial arrhythmias mainly came from patients with HFpEF.
From the phenomena, we could speculate that HFrEF is mainly related to ventricular remodeling, while HFpEF related to atrial remodeling, and SV therapy is more advantageous in improving ventricular remodeling. Besides, differences in atrial fibrillation prevalence at baseline may have contributed to this result. Above may be the major reasons of inconsistent results in our study. Besides, the different levels of indexes of echocardiography at baseline also lead to different results of arrhythmias, such as left atrial strain and atrial volume are associated with AF (59), global longitudinal strain and mechanical dispersion are associated with VAs, etc. It is difficult to analyze the relation between indexes of echocardiography at baseline and results of arrhythmias in our study, because only EVALUATE-HF, PRIME, and Wang H provide the data of echocardiography, while few participants and inconsistent indexes are included in the trials.
Strength and Limitations
We conducted a reasonable search of the literature and carefully screened it using strict standards, and the study included a large sample size. This is a more comprehensive analysis of the effect of SV in reducing the risk of arrhythmia, which only includes RCTs. Most of the studies in this analysis are large multicenter clinical trials, so the quality of our meta-analysis is very high. Our study confirms the advantage of SV in reducing VT, VF and cardiac arrest. However, several possible deficiencies should be noted: arrhythmias were not the main objective of these trials, the observation period of individual studies were short, most studies did not mention the occurrence of arrhythmias at baseline, only 4 trials on patients with non-HF, and the types of diseases targeted are limited. In addition, another large clinical study PARADISE-MI (ClinicalTrials.gov ID: NCT02924727) cannot be included for its data on arrhythmia has not published.
Conclusion
This meta-analysis reveals that compared with ACEI/ARB therapy, SV therapy can reduce the risks of most arrhythmias, just the significant differences were revealed in reducing the risks of VT, severe arrhythmias, and cardiac arrest among patients with HFrEF. Besides, the positive effect of SV on VF according to statistical result of combining VF with cardiac arrest in patients with HFrEF is credibility. The result of our study provides more useful information for strengthening the clinical application of SV, especially among patients with high-risk factors for VT, VF, cardiac arrest, etc. By comparing with ACEI/ARB, we can infer that the additional antiarrhythmic effect of SV may originate from the increase of endogenous vasoactive peptides through inhibiting neprilysin. Of course, the exact mechanism and beneficiary population of SV therapy on arrhythmia need to be further clarified by further studies.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
All procedures strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). All included studies were published without ethical and informed consent dispute.
Author Contributions
RW, HY, JW, and LM contributed to the literature database search, data collection, data extraction, data analysis, and writing of the manuscript. RW, HY, JW, and YW performed data analysis of the results. XZ and LW were conceptualized the topic. XZ and LW reviewed and revised this article. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Talent introduction funding project of the First Affiliated Hospital of Jinan University (no. 808026).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.890481/full#supplementary-material
References
1. Vukmir RB. Cardiac arrhythmia diagnosis. Am J Emerg Med. (1995) 13:204–10. doi: 10.1016/0735-6757(95)90095-0
2. Huizar JF, Ellenbogen KA, Tan AY, Kaszala K. Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:2328–44. doi: 10.1016/j.jacc.2019.02.045
3. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham heart study: a cohort study. Lancet. (2015) 386:154–62. doi: 10.1016/S0140-6736(14)61774-8
4. Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association[J]. Circulation, (2019) 139:e56–e528. doi: 10.1161/CIR.0000000000000659
5. Lardizabal JA, Deedwania PC. Atrial fibrillation in heart failure. Med Clin North Am. (2012) 96:987–1000. doi: 10.1016/j.mcna.2012.07.007
6. Gillis AM. Atrial fibrillation and ventricular arrhythmias: sex differences in electrophysiology, epidemiology, clinical presentation, and clinical outcomes. Circulation. (2017) 135:593–608. doi: 10.1161/circulationaha.116.025312
7. Saltzman HE. Arrhythmias and heart failure. Cardiol Clin. (2014) 32:125–33. doi: 10.1016/j.ccl.2013.09.005
8. Anselmino M, De Ferrari GM. Future perspectives and new “Frontiers” in cardiac rhythmology. Front Cardiovasc Med. (2020) 7:126. doi: 10.3389/fcvm.2020.00126
9. Desai AS, Mcmurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. (2015) 36:1990–7. doi: 10.1093/eurheartj/ehv186
10. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. (2014) 371:993–1004. doi: 10.1056/nejmoa1409077
11. Crea F. The ESC guidelines on heart failure, sacubitril-valsartan in resistant hypertension, and new therapeutic targets in myocardial hypertrophy. Eur Heart J. (2021) 42:3581–5. doi: 10.1093/eurheartj/ehab627
12. Russo V, Bottino R, Rago A, Papa AA, Liccardo B, Proietti R, et al. The effect of sacubitril/valsartan on device detected arrhythmias and electrical parameters among dilated cardiomyopathy patients with reduced ejection fraction and implantable cardioverter defibrillator. J Clin Med. (2020) 9:1111. doi: 10.3390/jcm9041111
13. Tsai YN, Cheng WH, Chang YT, Hsiao YW, Chang TY, Hsieh YC, et al. Mechanism of angiotensin receptor-neprilysin inhibitor in suppression of ventricular arrhythmia. J Cardiol. (2021) 78:275–84. doi: 10.1016/j.jjcc.2021.04.011
14. Curtain JP, Jackson AM, Shen L, Jhund PS, Docherty KF, Petrie MC, et al. Effect of sacubitril/valsartan on investigator-reported ventricular arrhythmias in PARADIGM-HF. Eur J Heart Fail. (2022) 24:551–61. doi: 10.1002/ejhf.2419
15. Suo Y, Yuan M, Li H, Zhang Y, Li Y, Fu H, et al. Sacubitril/valsartan improves left atrial and left atrial appendage function in patients with atrial fibrillation and in pressure overload-induced mice. Front Pharmacol. (2019) 10:1285. doi: 10.3389/fphar.2019.01285
16. Li SN, Zhang JR, Zhou L, Xi H, Li CY, Zhao L. Sacubitril/valsartan decreases atrial fibrillation susceptibility by inhibiting angiotensin II-induced atrial fibrosis through p-Smad2/3, p-JNK, and p-p38 signaling pathways. J Cardiovasc Transl Res. (2022) 15:131–42. doi: 10.1007/s12265-021-10137-5
17. El-Battrawy I, Pilsinger C, Liebe V, Lang S, Kuschyk J, Zhou X, et al. Impact of sacubitril/valsartan on the long-term incidence of ventricular arrhythmias in chronic heart failure patients. J Clin Med. (2019) 8:1582. doi: 10.3390/jcm8101582
18. Martens P, Nuyens D, Rivero-Ayerza M, Van Herendael H, Vercammen J, Ceyssens W, et al. Sacubitril/valsartan reduces ventricular arrhythmias in parallel with left ventricular reverse remodeling in heart failure with reduced ejection fraction. Clin Res Cardiol. (2019) 108:1074–82. doi: 10.1007/s00392-019-01440-y
19. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. (2019) 381:1609–20. doi: 10.1056/NEJMoa1908655
20. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. (2019) 380:1090. doi: 10.1056/NEJMx190009
21. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
22. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
23. Page MJ, Mckenzie JE, Kirkham J, Dwan K, Forbes A. Bias due to selective inclusion and reporting of outcomes and analyses in systematic reviews of randomised trials of healthcare interventions. Cochrane Database Syst Rev. (2014) 32:45–7. doi: 10.1016/j.enfi.2020.12.001
24. Begg CB, Berlin JA. Publication bias: a problem in interpreting medical data. J R Stat Soc. (1988) 181:419–45. doi: 10.2307/2982993
25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
26. Supasyndh O, Wang J, Hafeez K, Zhang Y, Zhang J, Rakugi H. Efficacy and safety of sacubitril/valsartan (LCZ696) compared with olmesartan in elderly Asian patients (=65 Years) with systolic hypertension. Am J Hypertens. (2017) 30:1163–9. doi: 10.1093/ajh/hpx111
27. Williams B, Cockcroft JR, Kario K, Zappe DH, Brunel PC, Wang Q, et al. Effects of sacubitril/valsartan versus olmesartan on central hemodynamics in the elderly with systolic hypertension: the PARAMETER study. Hypertension. (2017) 69:411–20. doi: 10.1161/hypertensionAHA.116.08556
28. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. (2012) 380:1387–95. doi: 10.1016/S0140-6736(12)61227-6
29. Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, et al. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. (2019) 322:1077–84. doi: 10.1001/jama.2019.12843
30. Huo Y, Li W, Webb R, Zhao L, Wang Q, Guo W. Efficacy and safety of sacubitril/valsartan compared with olmesartan in Asian patients with essential hypertension: a randomized, double-blind, 8-week study. J Clin Hypertens (Greenwich). (2019) 21:67–76. doi: 10.1111/jch.13437
31. Rakugi H, Kario K, Yamaguchi M, Sasajima T, Gotou H, Zhang J. Efficacy of sacubitril/valsartan versus olmesartan in Japanese patients with essential hypertension: a randomized, double-blind, multicenter study. Hypertens Res. (2022) 45:824–33. doi: 10.1038/s41440-021-00819-7
32. Halle M, Schöbel C, Winzer EB, Bernhardt P, Mueller S, Sieder C, et al. A randomized clinical trial on the short-term effects of 12-week sacubitril/valsartan vs. enalapril on peak oxygen consumption in patients with heart failure with reduced ejection fraction: results from the ACTIVITY-HF study. Eur J Heart Fail. (2021) 23:2073–82. doi: 10.1002/ejhf.2355
33. Wang Q, Zhuo C, Xia Q, Jiang J, Wu B, Zhou D, et al. Sacubitril/valsartan can reduce atrial fibrillation recurrence after catheter ablation in patients with persistent atrial fibrillation. Cardiovasc Drugs Ther. (2022). doi: 10.1007/s10557-022-07315-1
34. Wang H, Fu X. Effects of sacubitril/valsartan on ventricular remodeling in patents with left ventricular systolic dysfunction following acute anterior wall myocardial infarction. Coron Artery Dis. (2021) 32:418–26. doi: 10.1097/MCA.0000000000000932
35. Kang DH, Park SJ, Shin SH, Hong GR, Lee S, Kim MS, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. (2019) 139:1354–65. doi: 10.1161/circulationAHA.118.037077
36. Piepoli MF, Hussain RI, Comin-Colet J, Dosantos R, Ferber P, Jaarsma T, et al. OUTSTEP-HF: randomised controlled trial comparing short-term effects of sacubitril/valsartan versus enalapril on daily physical activity in patients with chronic heart failure with reduced ejection fraction. Eur J Heart Fail. (2021) 23:127–35. doi: 10.1002/ejhf.207
37. Tsutsui H, Momomura SI, Saito Y, Ito H, Yamamoto K, Sakata Y, et al. Efficacy and safety of sacubitril/valsartan in Japanese patients with chronic heart failure and reduced ejection fraction- results from the PARALLEL-HF study. Circ J. (2021) 85:584–94. doi: 10.1253/circj.CJ-20-0854
38. Pieske B, Wachter R, Shah SJ, Baldridge A, Szeczoedy P, Ibram G, et al. Effect of sacubitril/valsartan vs standard medical therapies on plasma NT-proBNP concentration and submaximal exercise capacity in patients with heart failure and preserved ejection fraction: the PARALLAX randomized clinical trial. JAMA. (2021) 326:1919–29. doi: 10.1001/jama.2021.18463
39. Kang DH, Park SJ, Shin SH, Hong GR, Lee S, Kim MS, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation: the PRIME study. Circulation. (2018) 139:1354–65. doi: 10.1161/circulationAHA.118.037077
40. Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol. (2015) 6:187–214. doi: 10.1002/cphy.c140055
41. Hanna P, Shivkumar K, Ardell JL. Calming the nervous heart: autonomic therapies in heart failure. Card Fail Rev. (2018) 4:92–8. doi: 10.15420/cfr.2018.20.2
42. Chen CH. Critical questions about PARADIGM-HF and the future. Acta Cardiol Sin. (2016) 32:387–96. doi: 10.6515/acs20151120a
43. Diez J. Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: implications for therapy. Eur J Heart Fail. (2017) 19:167–76. doi: 10.1002/ejhf.656
44. Chang PC, Lin SF, Chu Y, Wo HT, Lee HL, Huang YC, et al. LCZ696 therapy reduces ventricular tachyarrhythmia inducibility in a myocardial infarction-induced heart failure rat model. Cardiovasc Ther. (2019) 2019:6032631. doi: 10.1155/2019/6032631
45. von Lueder TG, Wang BH, Kompa AR, Huang L, Webb R, Jordaan P, et al. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail. (2015) 8:71–8. doi: 10.1161/circheartfailure.114.001785
46. Iborra-Egea O, Gálvez-Montón C, Roura S, Perea-Gil I, Prat-Vidal C, Soler-Botija C, et al. Mechanisms of action of sacubitril/valsartan on cardiac remodeling: a systems biology approach. NPJ Syst Biol Appl. (2017) 3:12. doi: 10.1038/s41540-017-0013-4
47. Yeh YH, Wakili R, Qi XY, Chartier D, Boknik P, Kääb S, et al. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol. (2008) 1:93–102. doi: 10.1161/CIRCEP.107.754788
48. Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. (2007) 87:425–56. doi: 10.1152/physrev.00014.2006
49. Levine YC, Rosenberg MA, Mittleman M, Samuel M, Methachittiphan N, Link M, et al. B-type natriuretic peptide is a major predictor of ventricular tachyarrhythmias. Heart Rhythm. (2014) 11:1109–16. doi: 10.1016/j.hrthm.2014.04.024
50. Landstrom AP, Dobrev D, Wehrens XHT. Calcium signaling and cardiac arrhythmias. Circ Res. (2017) 120:1969–93. doi: 10.1161/circresaha.117.310083
51. Sung YL, Lin TT, Syu JY, Hsu HJ, Lin KY, Liu YB, et al. Reverse electromechanical modelling of diastolic dysfunction in spontaneous hypertensive rat after sacubitril/valsartan therapy. ESC Heart Fail. (2020) 7:4040–50. doi: 10.1002/ehf2.13013
52. Chang PC, Wo HT, Lee HL, Lin SF, Chu Y, Wen MS, et al. Sacubitril/valsartan therapy ameliorates ventricular tachyarrhythmias inducibility in a rabbit myocardial infarction model. J Card Fail. (2020) 26:527–37. doi: 10.1016/j.cardfail.2020.03.007
53. Gallo G, Volpe M, Battistoni A, Russo D, Tocci G, Musumeci MB. Sacubitril/valsartan as a therapeutic tool across the range of heart failure phenotypes and ejection fraction spectrum. Front Physiol. (2021) 12:652163. doi: 10.3389/fphys.2021.652163
54. Solomon SD, Vaduganathan MBLC, Packer M, Zile M, Swedberg K, Rouleau J, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. (2020) 141:352–61. doi: 10.1161/circulationAHA.119.044586
55. Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2020) 17:559–73. doi: 10.1038/s41569-020-0363-2
56. Krebs C. Frequency and significance of ventricular arrhythmia in patients with reduced ejection fraction (less than or equal to 30%). J Am Coll Cardiol. (1987) 10:717–8. doi: 10.1016/s0735-1097(87)80220-6
57. Linton NW. Arrhythmia and left ventricular ejection fraction: once broken, always broken? Heart Rhythm. (2013) 10:847–8. doi: 10.1016/j.hrthm.2013.03.018
58. Hallstrom A, Pratt CM, Greene HL, Huther M, Gottlieb S, DeMaria A, et al. Relations between heart failure, ejection fraction, arrhythmia suppression and mortality: analysis of the cardiac arrhythmia suppression trial. J Am Coll Cardiol. (1995) 25:1250–7. doi: 10.1016/0735-1097(94)00553-3
Keywords: sacubitril/valsartan, arrhythmia, atrial arrhythmia, ventricular arrhythmia, cardiac arrest, ACEI, ARB
Citation: Wang R, Ye H, Ma L, Wei J, Wang Y, Zhang X and Wang L (2022) Effect of Sacubitril/Valsartan on Reducing the Risk of Arrhythmia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 9:890481. doi: 10.3389/fcvm.2022.890481
Received: 07 March 2022; Accepted: 25 April 2022;
Published: 01 July 2022.
Edited by:
Leon Ferder, Vice Chancellor Maimonides University Argentina, ArgentinaReviewed by:
Massimo Volpe, Sapienza University of Rome, ItalyEmilia D’Elia, Papa Giovanni XXIII Hospital, Italy
Copyright © 2022 Wang, Ye, Ma, Wei, Wang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofang Zhang, ODU3MTU0NTg2QHFxLmNvbQ==; Lihong Wang, bmQ2Njg4QDE2My5jb20=
†These authors have contributed equally to this work
 Ruxin Wang
Ruxin Wang Haowen Ye
Haowen Ye Li Ma2
Li Ma2 Jinjing Wei
Jinjing Wei