
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 03 June 2022
Sec. General Cardiovascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.890228
This article is part of the Research Topic Abdominal Aortic Aneurysms: Advancements in diagnosis, biomarkers, drug therapeutics, surgical and endovascular treatment View all 21 articles
Background: Etiology and risk factors of peripheral artery disease (PAD) include age, smoking, and hypertension, etc. , which are shared by an abdominal aortic aneurysm (AAA). Concomitance with AAA in patients with PAD is not rare but is easily overlooked in the clinical situation, though management strategies are altered totally. This study aims to investigate diagnostic biomarkers for the prediction of AAA in patients with PAD.
Methods: A total of 684 patients diagnosed with AAA and/or PAD were enrolled and analyzed retrospectively. Each patient with PAD and AAA was gender and age-matched. Demographic data, medical history, and serum laboratory test profiles were obtained. Statistical analysis was performed to determine diagnostic biomarkers of AAA in patients with PAD.
Results: Firstly, 320 patients with PAD-only and 320 patients with AAA-only were compared. Levels of bilirubin and D-Dimer were decreased, while the incidence of diabetes mellitus, levels of fibrinogen, and platelet count were increased significantly in patients with PAD-only compared with those in patients with AAA-only (P < 0.001). Next, 364 patients with PAD (44 patients with AAA) and 364 patients with AAA (44 patients with PAD) were compared. Multivariate logistic regression analysis confirmed the differential distribution of bilirubin, D-dimer, fibrinogen, and platelet count between patients with AAA and patients with PAD (P < 0.05). Receiver operator curves (ROC) showed that the area under the curve (AUC) of total bilirubin, direct bilirubin, D-dimer, fibrinogen, and platelet count was 0.6113, 0.5849, 0.7034, 0.6473, and 0.6785, respectively. Finally, to further validate the predictive efficacy of mentioned markers, a multivariable logistics regression analysis was performed between the PAD only group and the PAD with AAA group. The results suggested increased levels of D-dimer in the PAD with AAA group compared to the PAD only group (OR: 2.630, 95% CI:1.639–4.221; P < 0.001). In particular, the Youden index suggested that the cut-off value of D-dimer for predicting AAA in patients with PAD was 0.675 mg/L with a sensitivity of 76.9% and a specificity of 84.9% (AUC = 0.8673; 95% CI, 0.8106–0.9240, P < 0.001). In all 364 patients with PAD, 41.46% patients were diagnosed AAA when D-dimer is >0.675 mg/L, while only 3.55% patients were diagnosed AAA when D-dimer ≤ 0.675 mg/L.
Conclusions: PAD and AAA exert different clinical and serum profiles; D-dimer (>0.675 mg/L) is a reliable biomarker for the prediction of AAA in patients with PAD.
Abdominal aortic aneurysm (AAA) is defined as the dilation of the abdominal aortic artery (≥30 mm, ≥50% normal size) (1, 2). With the aging of the population, the incidence of AAA has been rising significantly, which has become a serious problem threatening health conditions (3, 4). In particular, mortality of ruptured AAA ~90% if untreated and 40% if treated (5, 6). Early diagnosis and careful monitoring are key to better managing the issue.
Peripheral arterial disease (PAD) is a chronic atherosclerotic disease. PAD leads to chronic ischemia of the lower extremity, manifesting as intermittent claudication or critical limb ischemia. PAD occurs mainly in elderly male patients (7). The prevalence of PAD was up to 10% around the world and is associated with increased mortality and morbidity (8).
Risk factors of PAD include age, gender (male), smoking, hypertension, abnormal blood lipid (8–14), and others, which are shared by AAA. The etiology of these two diseases is also atherosclerosis (15, 16), meaning it is clinically possible that patients with the mentioned risk factors can have PAD and AAA simultaneously (12.1% in the present study), which is easily overlooked by clinicians given that AAA is mostly asymptomatic. However, therapeutic options may vary among those patients. For example, patients with PAD may need first to receive surgical repair of AAA if the diameter is ≥55 mm or careful monitoring of the aorta if the diameter is ≤ 55 mm.
Therefore, markers to predict AAA in patients with PAD are needed to facilitate health care providers to better screen out AAA in patients with PAD. To date, relatively few studies have specifically investigated the potential value of a blood marker-based approach.
The aim of this study is to: (1) investigate the similarity and differentia of risk factors of the two diseases, and (2) to explore serum-based diagnostic biomarkers to predict AAA in patients with PAD.
Six hundred and eighty-four patients who were diagnosed with AAA and/or PAD at the department of vascular surgery, Xiangya Hospital, Central South University from January 2010 to May 2019 were enrolled in this study. Patients can be divided into different groups based on diagnosis: including the AAA only group (n = 320), in which patients were diagnosed with AAA, but not with PAD; the PAD only group (n = 320); in which patients were diagnosed with PAD but not with AAA; and the PAD + AAA group (n = 44), in which patients were diagnosed with both AAA and PAD (Figure 1). All procedures were approved by the Institute Review Board of Xiangya Hospital, Central South University (IRB No. 201905267). Patients were informed, and consent forms were obtained for research purposes.
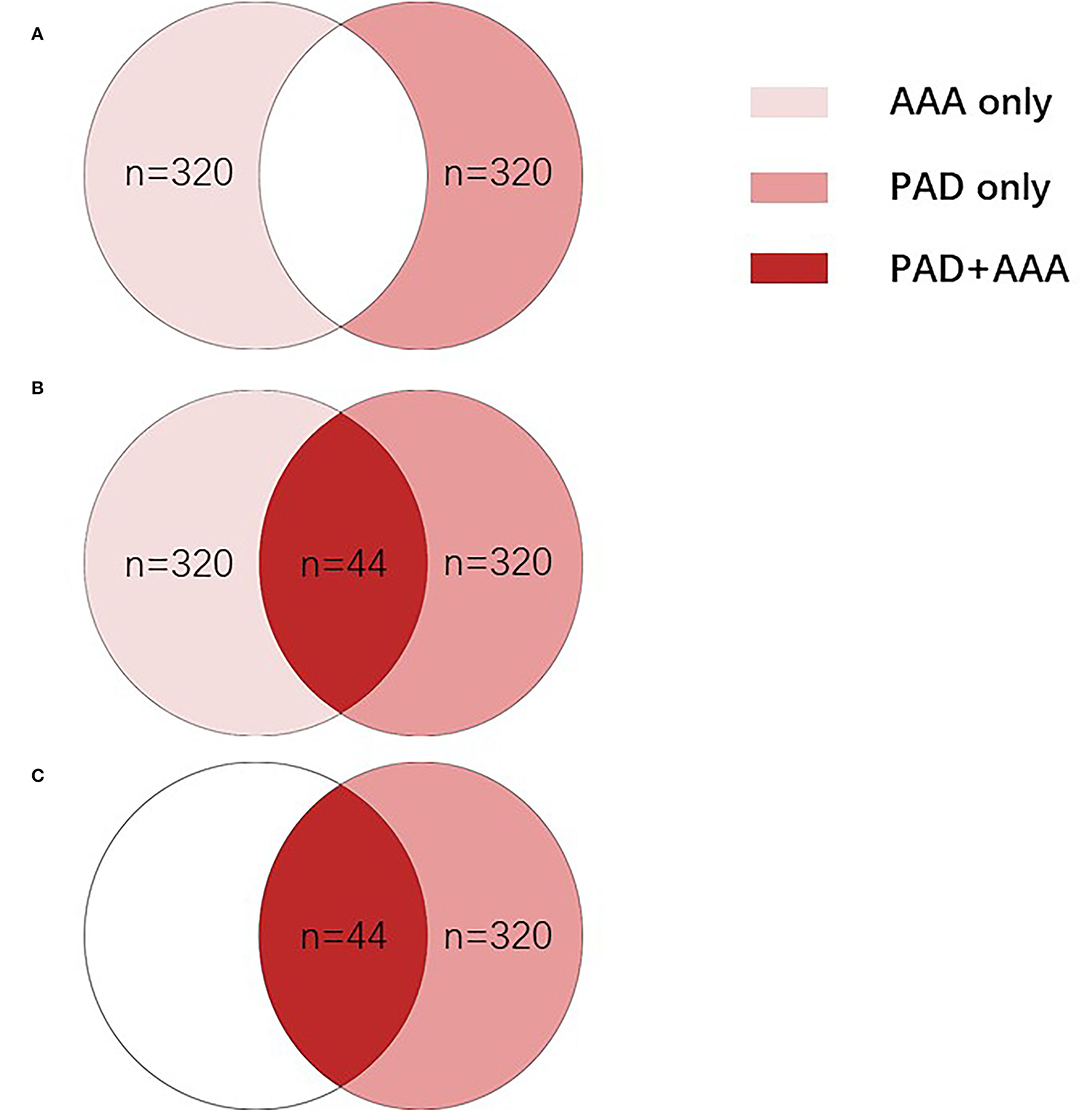
Figure 1. All patients were divided into different groups based on diagnosis. (A) To compare the difference between the PAD group and AAA group, AAA only group (n = 320), in which patients were diagnosed with AAA without PAD, and the PAD only group (n = 320), in which patients were diagnosed with PAD without AAA were enrolled. (B) To evaluate whether different indicators can be used to distinguish patients with PAD and patients with AAA, all 684 patients were divided into two groups: the AAA group (n = 364), in which patients were diagnosed with AAA only (n = 320) or both AAA and PAD (n = 44); and the PAD group (n = 364), in which patients were diagnosed with PAD only (n = 320) or both PAD and AAA (n = 44). (C) To evaluate whether d-dimer can clinically distinguish patients with PAD with and without AAA, 364 patients were enrolled, including the PAD only group (n = 320), in which patients were diagnosed with AAA without PAD and the PAD + AAA group (n = 44), in which patients were diagnosed with both AAA and PAD. AAA, abdominal aortic aneurysm; PAD, peripheral arterial disease.
Including criteria for AAA: (1) Age >40 years old; (2) AAA was confirmed by CTA or MRA.
The exclusion criteria are as follows: (1) infectious AAA; (2) secondary AAA with certain causes (such as injury); (3) severe mental illness leading to incapability of cooperation; (4) severe infectious or autoimmune diseases; (5) pregnancy or malignant tumor; and (6) thrombotic diseases such as venous thromboembolism.
The inclusion criteria for PAD were: (1) Age > 40 years; (2) High-risk factors such as smoking, diabetes, hypertension, hyperlipidemia, and others; (3) Symptoms of the ischemic lower extremity; (4) The pulse of the distal arteries of the ischemic limb is weakened or disappeared; (5) Ankle-brachial index (ABI) of ≤ 0.9; (6) Artery stenosis or occlusion were confirmed by CTA or ultrasound. The clinical diagnosis of PAD can be made if four of the above diagnostic criteria were met.
The exclusion criteria of PAD are as follows: (1) severe infectious or connective tissue diseases; (2) pregnancy or malignant tumor; (3) severe mental illness leading to the incapability of cooperation; and (4) thrombotic diseases such as venous or arterial thromboembolism.
Demographic characteristics, history, and personal history were recorded. Peripheral blood was collected at the time of administration. Specifically, levels of D-dimer were obtained at the time of admission by enzyme-linked immunosorbent assay. Laboratory tests were performed and data were collected.
Kolmogorov-Smirnov test was used to determine whether quantitative data were normally distributed. Quantitative data are shown as a mean ± standard deviation (SD) for normally distributed variables or median (interquartile range) for non-normally distributed variables. Qualitative data are presented as relative frequencies. Chi-square test or Fisher exact test was performed among qualitative data, and Student's t-test or Mann Whitney U-test was conducted among quantitative data. Multivariate logistic regression analysis was utilized to determine the differential distribution of biomarkers. Receiver operating characteristics (ROC) curves and the area under the curve (AUC) were performed to further assess the diagnostic efficacy of biomarkers. All statistical analyses were performed using the Statistical Product and Service Solutions (SPSS) 22.0 statistical software package (IBM Analytics, Armonk, NY, USA).
Demographic and medical history were recorded. To screen the potential differentially distributed parameters in patients with AAA and patients with PAD, statistical analysis was performed in AAA only group (n = 320) and PAD only group (n = 320). As shown in Table 1, the incidence of diabetes mellitus decreased significantly in AAA only group compared to PAD only group (11.25 vs. 31.56%, P < 0.01). No obvious difference in age, gender, hypertension, dyslipidemia, coronary heart disease, cerebral infarction, smoking, or drinking was founded between AAA only group and PAD only group (P > 0.05).
To screen the potential differentially distributed serum markers among patients with PAD and patients with AAA, peripheral blood samples were collected at the time of admission, and laboratory tests were performed (liver function, renal function, lipids, coagulation, and platelet count). As shown in Table 2, levels of total bilirubin, direct bilirubin, and D-dimer were increased, while levels of fibrinogen and platelet were decreased significantly in AAA only group when compared with those in PAD only group (P < 0.001). No obvious difference in TBA, BUN, SCR, uric acid, fasting glucose, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, total cholesterol, PT, or hemoglobin were found between AAA only group and PAD only group (P > 0.05). This suggests that patients with AAA may experience relatively high levels of bilirubin and D-dimer, while patients with PAD may show relatively high levels of fibrinogen and platelet.
The absolute value of circulating parameters in Table 2 cannot fully reflect the health status of patients since whether those markers are in the normal range is more clinically relevant. Thus, patients in the AAA only group and PAD only group are sub-grouped into a normal or abnormal group (Table 3). Statistical analysis was carried out to validate the association of variables with AAA and PAD. As shown in Table 3, patients with AAA-only manifested a higher possibility of having an abnormal status of D-dimer, fibrinogen, and platelet count, and a lower possibility of diabetes mellitus when compared with patients with PAD-only (P < 0.001). No obvious difference in the abnormal status of total bilirubin and direct bilirubin was identified between AAA only group and PAD only group (P > 0.05), which is reasonable due to the median (Q1/Q3) value of total bilirubin and direct bilirubin in Table 2 are in the normal range (normal range for total bilirubin and is ≤ 17.1 and ≤ 6.8, respectively).
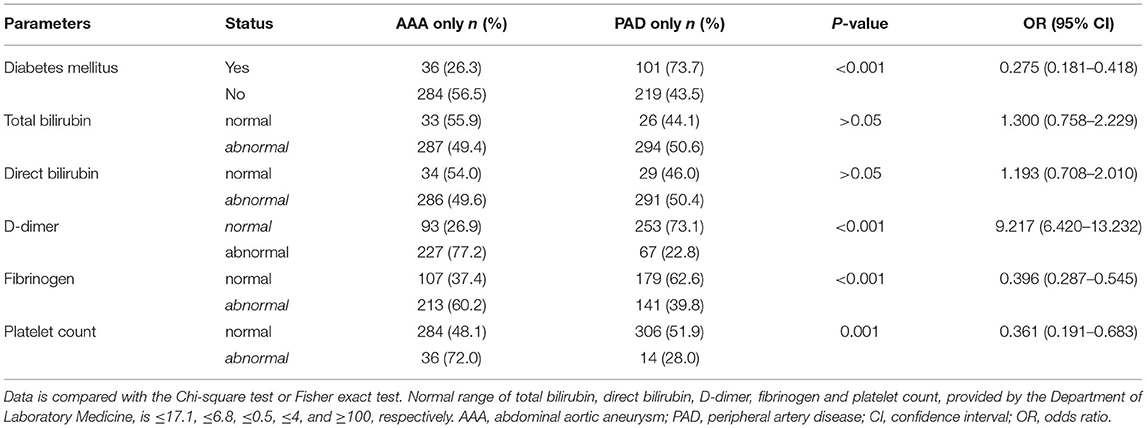
Table 3. Differential distribution of clinical and blood laboratory test parameters in AAA only and PAD only group.
Together, the above results suggested that diabetes mellitus, D-dimer, fibrinogen, and platelet count were differentially distributed in patients with AAA when compared to patients with PAD.
The AAA and PAD share common risk factors including atherosclerosis, age, gender (male), smoking and hypertension, and others. This makes it clinically realistic that a patient with PAD is concomitant with AAA, which is easily overlooked by clinicians, although management strategies are altered totally. To find out variants to distinguish between patients with AAA and patients with PAD, multivariable logistics regression analysis was performed in the AAA group (n = 364, Figure 1B) and PAD group (n = 364, Figure 1B). Results in Table 4 indicated that total bilirubin, direct bilirubin, D-dimer, fibrinogen, and platelet count could be used to distinguish between patients with AAA and patients with PAD (P < 0.05).
To further evaluate the diagnostic efficacy of the mentioned markers, the receiver operating characteristic (ROC) curve of the five risk factors mentioned above was performed in the AAA group (n = 364, Figure 1B) and PAD group (n = 364, Figures 1B, 2). Figure 2F summarizes detailed information on ROC curves in Figure 2. As indicated in Figure 2F, D-dimer may be the most optimal biomarker for the distinction between patients with AAA and patients with PAD with the area under the curve (AUC) of 0.7034.
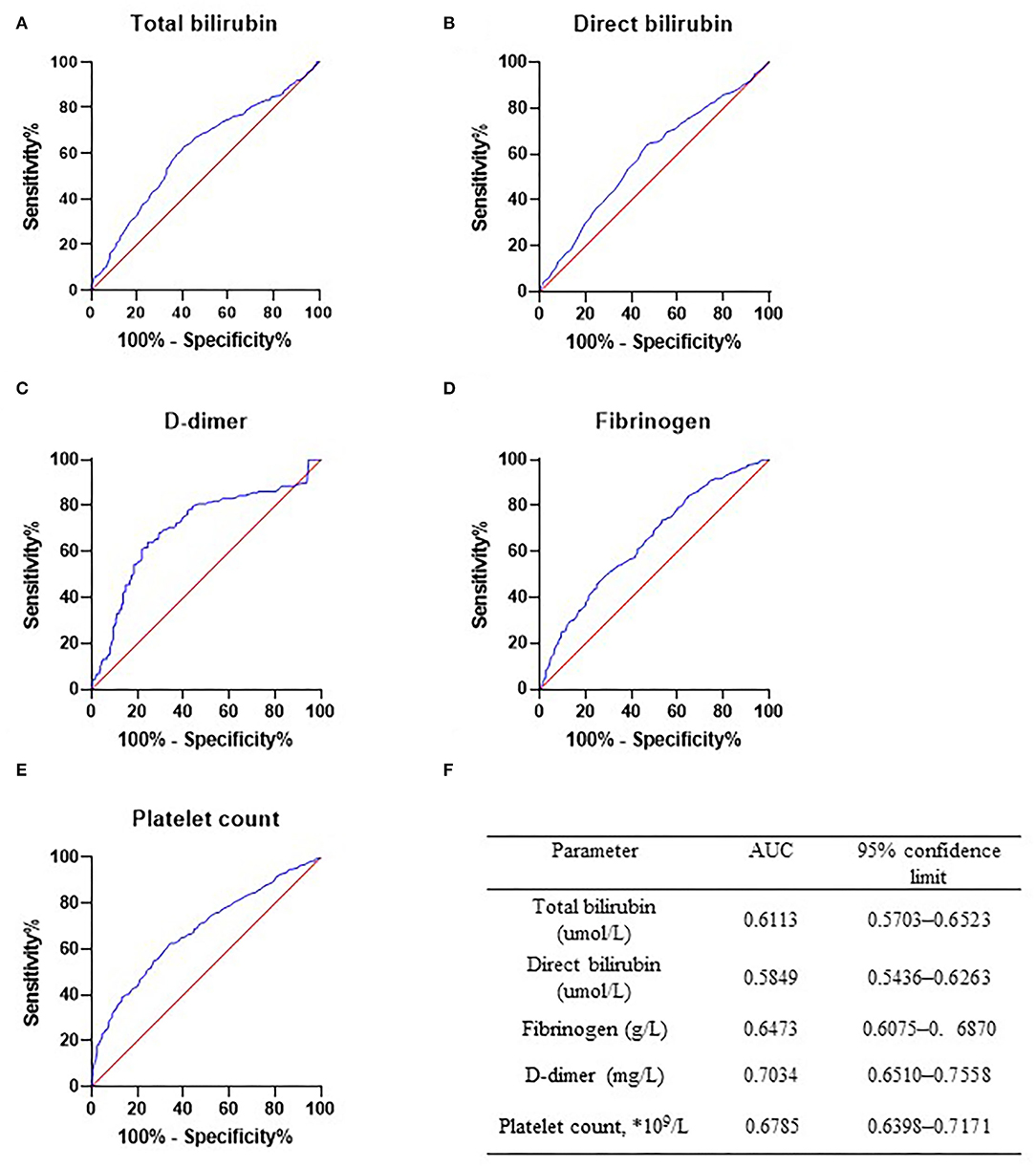
Figure 2. Receiver operator characteristic curve in abdominal aortic aneurysm (AAA) group and peripheral arterial disease (PAD) group. Receiver operator characteristic curve of total bilirubin (A), direct bilirubin (B), D-dimer (C), fibrinogen (D), and platelet count (E) was drawn in PAD only and AAA only patients. Diagnostic efficacy of these markers was determined. (F) Detailed information of ROC curves. AAA, abdominal aortic aneurysm; PAD, peripheral arterial disease.
As mentioned above, the main objective of this study was to find out ideal biomarkers for the prediction of AAA in patients with PAD. Total bilirubin, direct bilirubin, D-dimer, fibrinogen, and platelet may potentially be predictive biomarkers of AAA, though OR value or AUC was not that promising between patients with AAA (n = 364) and patients with PAD (n = 364). To further validate the efficacy of these biomarkers in a more clinically realistic condition, statistical analysis was performed again between the AAA + PAD group (n = 44) and the PAD group (n = 364).
As shown in Table 5, multivariate logistic regression analysis showed that the serum levels of D-dimer increased in AAA + PAD group compared with the PAD group (OR: 2.630, 95% CI:1.639–4.221; P < 0.001). No significant difference in fibrinogen and platelet count was found between the AAA + PAD group and the PAD group (P > 0.05).
Furthermore, the ROC curve of the D-dimer was carried out (Figure 3), and AUC was 0.867. The Youden index suggested that the best cutoff value of D-dimer was 0.675 mg/L (Table 6 with partial data provided).
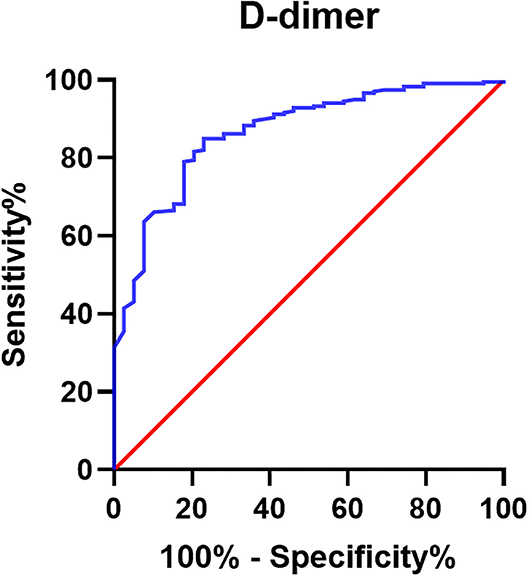
Figure 3. Receiver operator characteristic curve of D-dimer for prediction of AAA in PAD group. Receiver operator characteristic curve of D-dimer was drawn for the prediction of AAA in patients with PAD (n = 364). The AUC of D-dimer is 0.867.
To better visualize the predictive efficacy of D-dimer for AAA, the 364 patients with PAD were divided into two groups based on D-dimer: D-dimer > 0.65 mg/L, and D-dimer ≤ 0.65 mg/L. As shown in Figure 4, 41.46% of patients with PAD were concomitant with AAA when D-dimer was > 0.65 mg/L, while only 3.55% of patients with PAD were concomitant with AAA when D-dimer was ≤ 0.65 mg/L.
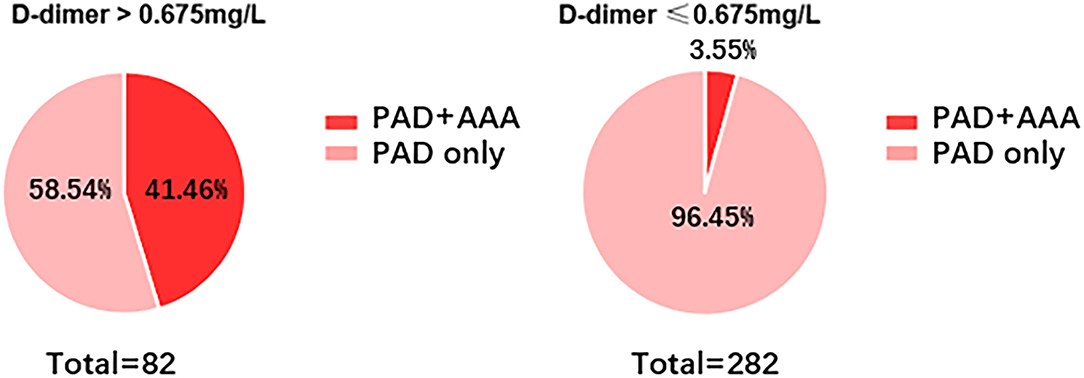
Figure 4. Incidence of AAA in patients with PAD with different levels of D-dimer. Incidence of AAA was determined in patients with PAD when D-dimer is ≤ 0.675 mg/L or ≥0.675 mg/L.
In this study, we demonstrated that AAA and PAD share a group of risk factors, such as hypertension, dyslipidemia, smoking, and drinking. However, patients with AAA experienced a decreased incidence of diabetes mellitus, lowered levels of fibrinogen and platelet count, and upregulated levels of D-dimer and bilirubin. Our study further validates the diagnostic efficacy of these markers for the prediction of AAA and suggests that D-dimer is a promising biomarker with satisfying sensitivity and specificity. Patients with PAD with D-dimer of ≥0.675 indicated a highly possible presence of AAA.
Studies have revealed that hypertension, smoking, cardiovascular diseases, dyslipidemia, cerebrovascular disease, and renal insufficiency are well-defined risk factors for AAA (17–22). Researchers have also suggested that hypertensive, smoking, and dyslipidemia individuals have a higher risk of PAD (14, 23, 24). The dominant etiology of PAD and AAA is arterial atherosclerosis. Thus, it is clinically realistic that patients with PAD are concomitant with AAA (Figure 1). To date, relatively few studies have specifically focused on the potential value of a blood marker-based approach for clinicians to screen whether patients have PAD combined with AAA. Our results show that D-dimer helps predict AAA in patients with PAD in a time- and cost-effective manner over ultrasound-based screening of AAA. Coagulation status test, including D-dimer is routine, at least in China, prior to surgical treatment of PAD, thus, clinicians are less likely to ignore the dramatic increase of D-dimer in patients with PAD. The likelihood of neglecting AAA in patients with PAD is decreased significantly without the extra costs of time and money.
As is well-demonstrated, a large volume of intraluminal aortic thrombus is common in patients with AAA (25, 26), resulting from activated coagulation status inside an aneurysm. D-dimer is the degradative product of fibrin, and increased levels of D-dimer indicate the presence of coagulation procedure and secondary hyperfibrinolysis. Thus, circulating D-dimer in patients with AAA originates from fibrinolysis of intraluminal thrombus, which explains increased levels of D-dimer in patients with AAA (Table 2). Our finding is consistent with previous studies, which have confirmed the diagnostic value of D-dimer for AAA (27–30). However, control group in those studies is non-atherosclerotic population even healthy subjects. The conclusions obtained from these studies may not be applicable among athero-thrombosis diseases, for example, PAD, since AAA and PAD share common risk factors and etiologies. Our research has filled gaps in knowledge and the inclusion of patients with PAD in this study is important in distinguishing patients with AAA from PAD not only in the general population, but serum D-dimer also facilitates the diagnosis of AAA in symptomatic PAD individuals.
Currently, D-dimer is widely used as an exclusion of venous thrombo-embolism and is regarded as cost- and time-effective. As shown in Figure 4, only 3.55% of patients with PAD were diagnosed with AAA. Therefore, we can similarly take advantage of D-dimer ≤ 0.675 mg/L as an exclusion biomarker of AAA in patients with PAD.
As we know, platelet and fibrinogen are fundamental materials for coagulation, and conversion of fibrinogen to fibrin and platelet aggression are key steps in the coagulation procedure. Thus, constant coagulation will lead to the consumption of platelet and fibrinogen. This may explain the findings of the present study, that patients with AAA exerted decreased levels of platelet and fibrinogen compared with patients with PAD (Tables 2–4). These observations are consistent with previous publications (31–33).
In accordance with others, the prevalence of diabetes is decreased in patients with AAA and increased significantly in patients with PAD (Tables 1, 3). Some epidemiological studies indicate that diabetes is a protective factor for AAA and can inhibit the occurrence and progression of AAA (34, 35). The underlying mechanism may be that metformin prescription is associated with decreased AAA enlargement (36). Diabetes is also a well-recognized risk factor for PAD.
Our study has several limitations. First, the study was performed in a single-center with a limited sample size. A large scale and multicenter study is a necessity to further validate the conclusions. Second, diagnostic accuracy can be compromised when patients with PAD are experiencing atherosclerotic thrombosis due to active coagulation and fibrinolysis processes in the lower extremities.
Our study has revealed differentially distributed serum markers (bilirubin, D-dimer, fibrinogen, and platelet) and the prevalence of diabetes among patients with AAA and patients with PAD. Particularly, the serum level of D-dimer is a promising biomarker for the presence of AAA in patients with PAD. A patient with PAD with a D-dimer of ≥0.675 mg/L requires further evaluation for the presence of AAA.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of Xiangya Hospital, Central South University. The patients/participants provided their written informed consent to participate in this study.
WW and JH conceived and designed the study. HC, LW, SL, KW, and PY collected the data. HC, BP, JX, LW, SL, and WW analyzed the data. HC and BP wrote the manuscript. BP, KW, PY, JH, and WW provided a substantial revision of the manuscript. WW obtained funding. All authors have read and approved the final manuscript.
This study was funded by the National Natural Science Foundation of China (No. 81873525), the Clinical Research Project of Xiangya hospital, CSU, and the project was sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor ZL is currently organizing a Research Topic with the WW.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. LeFevre ML. Screening for abdominal aortic aneurysm: U.S. preventive services task force recommendation statement annals of internal medicine. Ann Intern Med. (2014) 161:281–90. doi: 10.7326/M14-1204
2. Alcorn HG, Wolfson SK Jr, Sutton-Tyrrell K, Kuller LH, O'Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in the cardiovascular health study. Arterioscler Thromb Vasc Biol. (1996) 16:963–70. doi: 10.1161/01.ATV.16.8.963
3. Huang T, Liu S, Huang J, Xu B, Bai Y, Wang W. Meta-analysis of the growth rates of abdominal aortic aneurysm in the Chinese population. BMC Cardiovasc Disord. (2019) 19:204. doi: 10.1186/s12872-019-1160-x
4. Yu J, Liu S, Huang J, Wang W. Current theories and clinical trial evidence for limiting human abdominal aortic aneurysm growth. Curr Drug Targets. (2018) 19:1302–8. doi: 10.2174/1389450118666171113114310
5. Sampson UK, Norman PE, Fowkes FG, Aboyans V, Song Y, Harrell FE Jr, et al. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob Heart. (2014) 9:159–70. doi: 10.1016/j.gheart.2013.12.009
6. Huang J, Li G, Wang W, Wu K, Le T. 3D printing guiding stent graft fenestration: a novel technique for fenestration in endovascular aneurysm repair. Vascular. (2017) 25:442–6. doi: 10.1177/1708538116682913
7. Hamur H, Onk OA, Vuruskan E, Duman H, Bakirci EM, Kucuksu Z, et al. Determinants of chronic total occlusion in patients with peripheral arterial occlusive disease. Angiology. (2017) 68:151–8. doi: 10.1177/0003319716641827
8. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-society consensus for the management of peripheral arterial disease (Tasc II). J Vasc Surg. (2007) 45:S5–67. doi: 10.1016/j.jvs.2006.12.037
9. Folsom AR, Yao L, Alonso A, Lutsey PL, Missov E, Lederle FA, et al. Circulating biomarkers and abdominal aortic aneurysm incidence: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. (2015) 132:578–85. doi: 10.1161/CIRCULATIONAHA.115.016537
10. Guirguis-Blake JM, Beil TL, Senger CA. Whitlock EP. Ultrasonography screening for abdominal aortic aneurysms: a systematic evidence review for the U.S. preventive services task force. Ann Intern Med. (2014) 160:321–9. doi: 10.7326/M13-1844
11. Barba A, Vega de. Ceniga M, Estallo L, de la Fuente N, Viviens B, Izagirre M. Prevalence of abdominal aortic aneurysm is still high in certain areas of Southern Europe. Ann Vasc Surg. (2013) 27:1068–73. doi: 10.1016/j.avsg.2013.01.017
12. Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. (2010) 52:539–48. doi: 10.1016/j.jvs.2010.05.090
13. Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the tromso study, 1994–2001. Circulation. (2009) 119:2202–8. doi: 10.1161/CIRCULATIONAHA.108.817619
14. Mascarenhas JV, Albayati MA, Shearman CP, Jude EB. Peripheral arterial disease. Endocrinol Metab Clin North Am. (2014) 43:149–66. doi: 10.1016/j.ecl.2013.09.003
15. Wang W, Xu B, Xuan H, Ge Y, Wang Y, Wang L, et al. Hypoxia-inducible factor 1 in clinical and experimental aortic aneurysm disease. J Vasc Surg. (2018) 68:1538–50 e2. doi: 10.1016/j.jvs.2017.09.030
16. Yu J, Liu R, Huang J, Wang L, Wang W. Inhibition of phosphatidylinositol 3-kinease suppresses formation and progression of experimental abdominal aortic aneurysms. Sci Rep. (2017) 7:15208. doi: 10.1038/s41598-017-15207-w
17. Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol. (2019) 16:225–42. doi: 10.1038/s41569-018-0114-9
18. Li X, Zhao G, Zhang J, Duan Z, Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population—a meta-analysis. PLoS ONE. (2013) 8:e81260. doi: 10.1371/journal.pone.0081260
19. Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. (2006) 26:2605–13. doi: 10.1161/01.ATV.0000245819.32762.cb
20. Singh K, Bonaa KH, Jacobsen BK, Bjork L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the tromso study. Am J Epidemiol. (2001) 154:236–44. doi: 10.1093/aje/154.3.236
21. Jamrozik K, Norman PE, Spencer CA, Parsons RW, Tuohy R, Lawrence-Brown MM, et al. Screening for abdominal aortic aneurysm: lessons from a population-based study. Med J Aust. (2000) 173:345–50. doi: 10.5694/j.1326-5377.2000.tb125684.x
22. Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) veterans affairs cooperative study group. Ann Internal Med. (1997) 126:441–9. doi: 10.7326/0003-4819-126-6-199703150-00004
23. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the national health and nutrition examination survey, 1999–2000. Circulation. (2004) 110:738–43. doi: 10.1161/01.CIR.0000137913.26087.F0
24. Murabito JM, D'Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from the Framingham heart study. Circulation. (1997) 96:44–9. doi: 10.1161/01.CIR.96.1.44
25. Golledge J, Wolanski P, Parr A, Buttner P. Measurement and determinants of infrarenal aortic thrombus volume. Eur Radiol. (2008) 18:1987–94. doi: 10.1007/s00330-008-0956-3
26. Fan YN, Ke X, Yi ZL, Lin YQ, Deng BQ, Shu XR, et al. Plasma D-dimer as a predictor of intraluminal thrombus burden and progression of abdominal aortic aneurysm. Life Sci. (2020) 240:117069. doi: 10.1016/j.lfs.2019.117069
27. Sidloff DA, Stather PW, Choke E, Bown MJ, Sayers RD. A systematic review and meta-analysis of the association between markers of hemostasis and abdominal aortic aneurysm presence and size. J Vasc Surg. (2014) 59:528–35.e4. doi: 10.1016/j.jvs.2013.10.088
28. Golledge J, Muller R, Clancy P, McCann M, Norman PE. Evaluation of the diagnostic and prognostic value of plasma D-dimer for abdominal aortic aneurysm. Eur Heart J. (2011) 32:354–64. doi: 10.1093/eurheartj/ehq171
29. Takagi H, Manabe H, Kawai N, Goto S, Umemoto T. Plasma fibrinogen and D-dimer concentrations are associated with the presence of abdominal aortic aneurysm: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. (2009) 38:273–7. doi: 10.1016/j.ejvs.2009.05.013
30. Yamazumi K, Ojiro M, Okumura H, Aikou T. An activated state of blood coagulation and fibrinolysis in patients with abdominal aortic aneurysm. Am J Surg. (1998) 175:297–301. doi: 10.1016/S0002-9610(98)00014-2
31. Monaco M, Di Tommaso L, Stassano P, Smimmo R, De Amicis V, Pantaleo A, et al. Impact of blood coagulation and fibrinolytic system changes on early and mid term clinical outcome in patients undergoing stent endografting surgery. Interact Cardiovasc Thorac Surg. (2006) 5:724–8. doi: 10.1510/icvts.2006.136507
32. Milne AA, Adam DJ, Murphy WG, Ruckley CV. Effects of asymptomatic abdominal aortic aneurysm on the soluble coagulation system, platelet count and platelet activation. Eur J Vasc Endovasc Surg. (1999) 17:434–7. doi: 10.1053/ejvs.1998.0790
33. Bradbury A, Adam D, Garrioch M, Brittenden J, Gillies T, Ruckley CV. Changes in platelet count, coagulation and fibrinogen associated with elective repair of asymptomatic abdominal aortic aneurysm and aortic reconstruction for occlusive disease. Eur J Vasc Endovasc Surg. (1997) 13:375–80. doi: 10.1016/S1078-5884(97)80079-2
34. De Rango P, Farchioni L, Fiorucci B, Lenti M. Diabetes and abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. (2014) 47:243–61. doi: 10.1016/j.ejvs.2013.12.007
35. Lederle FA. The strange relationship between diabetes and abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. (2012) 43:254–6. doi: 10.1016/j.ejvs.2011.12.026
Keywords: abdominal aortic aneurysm, peripheral artery disease, D-dimer, fibrinogen, platelet count
Citation: Cai H, Pan B, Xu J, Liu S, Wang L, Wu K, Yang P, Huang J and Wang W (2022) D-Dimer Is a Diagnostic Biomarker of Abdominal Aortic Aneurysm in Patients With Peripheral Artery Disease. Front. Cardiovasc. Med. 9:890228. doi: 10.3389/fcvm.2022.890228
Received: 05 March 2022; Accepted: 22 April 2022;
Published: 03 June 2022.
Edited by:
Zhenjie Liu, The Second Affiliated Hospital of Zhejiang University School of Medicine, ChinaReviewed by:
Jinwei Xie, Sichuan University, ChinaCopyright © 2022 Cai, Pan, Xu, Liu, Wang, Wu, Yang, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang, d2FuZ3dlaWNzdUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.