
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Cardiovasc. Med., 13 May 2022
Sec. General Cardiovascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.887236
Heart failure (HF) development is a common complication of myocardial infarction (MI), which warrants a search for novel therapies able to prevent left ventricular remodeling after an MI. In a recent article, Batkai et al. evaluated CDR132L, a synthetic antisense inhibitor of miR-132, in a pig model of reperfused MI (1). The authors report that monthly intravenous administration of CDR132L is safe and effective in preventing HF development. They expect CDR132L to have an additive, and possibly synergistic, effect to standard-of-care therapies [beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEi/ARB), and mineralocorticoid receptor antagonists (MRA)] because of their distinct first targets. Nonetheless, a degree of overlap in the final effects of CDR132L and current therapies might exist, given that ACEi/ARB and MRA ultimately modulate myocardial inflammation and fibrosis, as CDR132L do (1).
We assessed this point by searching for similar changes in protein expression between MI therapies and CDR132L.
We retrieved the 14 mRNAs significantly altered in the myocardium of pigs receiving CDR132L compared with control pigs: BMPR2, ADRA1D, GCLC, CD44, PRDX1, ECM1, LEP, GATA3, GPX1, EIF4G1, ACE2, HMOX1, RTN4, and LIFR. Except for RTN4, all these mRNAs were downregulated by CDR132L (1). We assumed a close correlation between changes in mRNA levels and the expression of the corresponding proteins, as previously demonstrated (2). By using massive public databases, such as Drugbank (3), the Open Targets Platform (4) and the Human Protein Atlas (5), we identified all approved, investigational and experimental drugs reported to modulate the expression of at least one of these 14 proteins in any setting (Table 1).
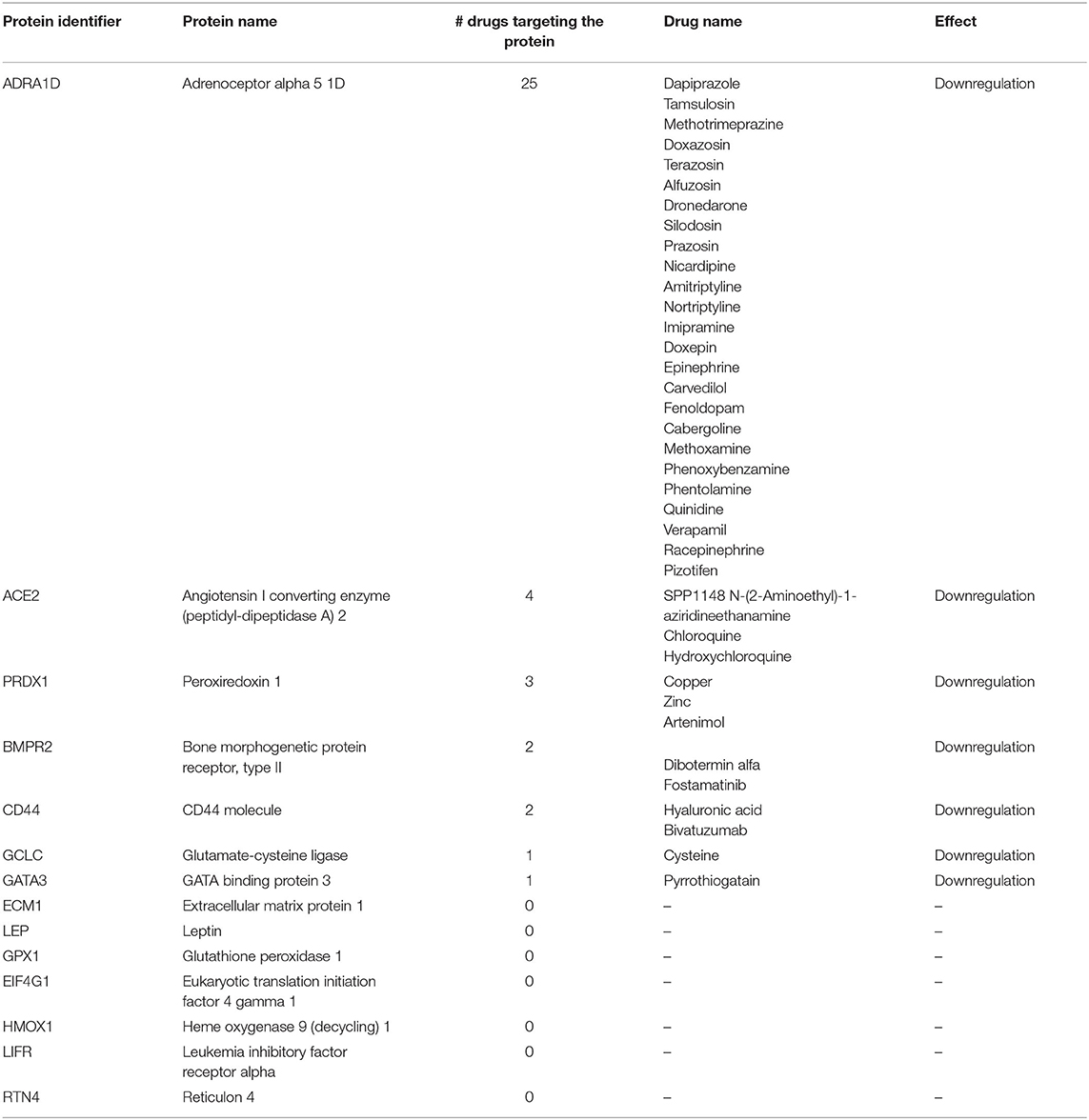
Table 1. All drugs/compounds that target one or more of the proteins encoded by the 14 mRNAs candidates.
We did not find any drug modulating more than one of the 14 proteins at the same time. Therefore, no drug, including ACEi/ARB or MRA, proved able to mimic the effects of CDR132L on protein expression. This finding corroborates the conclusion that CDR132L might have an additive or synergistic action to standard drugs, given the different effects on the profiles of protein expression.
Next, we performed a protein-protein interaction analysis to know if the candidates were biologically related to each other. Then, by using unsupervised algorithms (K-means clustering, elbow method K = 4) we wanted to assess if these interactions corresponded to proteins grouped in the same cluster (and thus share similar biological properties or pathways) or are among proteins from distinct clusters that could indicate more complex biological mechanisms at play. Here we found that HMOX1, GPX1, GCLC, and PRDX1 work to tightly regulate endothelial cell proliferation [false discovery rate (FDR) = 0.002] and hydrogen peroxide catabolic processes (FDR = 0.001). Although we could not find any report on novel drugs or compounds acting to modulate this specific cluster (or the individual proteins), this analysis indicates that a drug targeting them could be highly specific and a possible novel treatment in HF.
OI-E and AA contributed to conception and design of the study. OI-E performed the in silico analysis. AA wrote the first draft of the manuscript. OI-E, AA, and AB-G wrote sections of the manuscript. AB-G supervised the study. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was supported in part by grants from MICINN (PID2019-110137RB-I00 and PLEC2021-008194), Instituto de Salud Carlos III (PIC18/00014, ICI19/00039, ICI20/00135, PI21/01700, and PI21/01703), Red RICORS (PI21/01703), CIBERCV (CB16/11/00403) as a part of the Plan Nacional de I + D + I, and it was co-funded by ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER) and AGAUR (2017-SGR-483 and 2019PROD00122).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Batkai S, Genschel C, Viereck J, Rump S, Bär C, Borchert T, et al. CDR132L improves systolic and diastolic function in a large animal model of chronic heart failure. Eur Heart J. (2020) 42:192–201. doi: 10.1093/eurheartj/ehaa791
2. Koussounadis A, Langdon SP, Um IH, Harrison DJ, Smith VA. Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci Rep. (2015) 5:10775. doi: 10.1038/srep10775
3. Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. (2018) 46:D1074–82. doi: 10.1093/nar/gkx1037
4. Ochoa D, Hercules A, Carmona M, Suveges D, Gonzalez-Uriarte A, Malangone C, et al. Open Targets platform: supporting systematic drug–target identification and prioritization. Nucleic Acids Res. (2021) 49:D1302–10. doi: 10.1093/nar/gkaa1027
Keywords: heart failure, CDR132L, therapy, myocardial infarction, models
Citation: Iborra-Egea O, Aimo A and Bayes-Genis A (2022) Different Effects on Protein Expression of CDR132L, an Antisense Inhibitor of miR-132, and Standard Therapies for Myocardial Infarction. Front. Cardiovasc. Med. 9:887236. doi: 10.3389/fcvm.2022.887236
Received: 01 March 2022; Accepted: 28 April 2022;
Published: 13 May 2022.
Edited by:
Emma Louise Robinson, University of Colorado, United StatesReviewed by:
Amela Jusic, Luxembourg Institute of Health, LuxembourgCopyright © 2022 Iborra-Egea, Aimo and Bayes-Genis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antoni Bayes-Genis, YWJheWVzZ2VuaXNAZ21haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.