
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 07 April 2022
Sec. Thrombosis and Haemostasis
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.883986
This article is part of the Research TopicInsights in Thrombosis: 2022View all 9 articles
Background: Thrombophilia screening is widely done in clinical practice, and it is claimed that the extent of venous thromboembolism (VTE) recurrence risk in patients with common defects is still not fully understood.
Aim: We aimed to summarize data of all observational studies prospectively assessing the association of heterozygous factor V Leiden (FVL) mutation and recurrent VTE in patients with VTE, and to calculate pooled relative risks (RR), overall and in various subgroups.
Methods: We searched MEDLINE and EMBASE databases for cohort studies prospectively assessing VTE recurrence in patients with and without FVL mutation (PROSPERO: CRD42021182800). Data were extracted on cohort and study-level. The methodological quality was assessed using the Newcastle-Ottawa Scale (NOS). RR were calculated overall and in subgroups using a random-effects model.
Results: From 31 cohorts, 24 studies were finally included summarizing 13,571 patients. Heterozygous FVL mutation was identified in 2,840 individuals (21%). The methodological quality was estimated to be high in 20 studies (83%). The overall RR was 1.46 (95% CI: 1.31, 1.64), consistent across subgroups.
Conclusions: Pooling all high-quality epidemiological data, the risk of recurrent VTE was increased by 46% in patients with heterozygous FVL mutation. Against the background of established risk factors, the FVL mutation plays only a marginal role in the risk assessment for recurrent VTE.
Thrombophilia screening is still a popular tool in the workup of patients with venous thromboembolism (VTE) (1, 2). VTE is one of the most common cardiovascular diseases associated with high morbidity and mortality (3–7). More than 25% of unselected patients experience recurrent events, potentially resulting in a reduced quality of life or even death (8, 9). Thus, preventing recurrent VTE is an important goal of secondary prevention (4, 10–12). To accomplish this, high-risk patients must be identified (9, 13). Given the clustering of VTE in families or even in individuals, genetic factors are considered as promising targets (14–16). The most common inherited thrombophilia is heterozygous factor V Leiden (FVL) mutation, which is acknowledged as a relevant risk factor for first VTE (17, 18). Earlier investigations suggest a moderately increased risk only and current guidelines do not suggest thrombophilia testing in unselected patients (1, 19–26). However, the selection criteria are largely unclear and thrombophilia screening (including FVL mutation) is still frequently done in clinical practice (1, 2, 9, 20, 27–33). Besides, some authors claim that the knowledge is still limited, particularly within subgroups of patients, and that the presence of FVL mutation might sum up with other risk factors resulting in a modified treatment recommendation (14, 34, 35).
Various previous studies observed the association between the presence of FVL mutation and the risk of VTE recurrence and the results are conflicting. Some studies concluded that heterozygous FVL mutation increases the risk (10, 12, 36–41) and others do not (38, 42–48). In particular, some authors raise the question of whether FVL mutation increases the recurrence risk in specific subgroups such as men (36), young women without hormonal treatment (38), or cancer patients (49, 50). Indeed, FVL mutation was also detected in various genetic profiling studies (10, 23, 40, 41, 51–54), and it was included in one clinical prediction model (53). Thus, whether or not FVL mutation increases the risk of recurrent VTE to a relevant degree is not fully understood, and more data are needed to clarify this issue.
In a systematic review and meta-analysis, we aimed to summarize data of all observational studies prospectively assessing the association of heterozygous FVL mutation and recurrent VTE. We aimed to calculate relative risks (RR) overall and in various subgroups of patients. To set this into context, we observed the frequency of testing in Switzerland using a large claim-based dataset.
The study protocol was submitted to the PROSPERO international prospective register of systematic reviews (#CRD42021182800) and the manuscript was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (55).
A comprehensive search strategy for MEDLINE (1946 to February 03, 2022) and EMBASE (1974 to 2022 February 03) databases was developed, and the Ovid interface used (Supplementary List 1). The search strategy was based on three elements: heterozygous FVL mutation (patients); recurrent VTE (outcome); and prospective cohort study (study design). The search strategy was improved using keywords found in key publications and no limits were applied. The sensitivity was tested in eight key publications (100%). The literature search was completed by hand search using reference lists of articles retrieved. All included studies were checked for published errata. The last search run was done on the fourth of February 2022. All records were carefully assessed for eligibility by screening of title, abstract and full text by two reviewers in duplicate (D.E., M.N.).
The following inclusion criteria were applied: (a) prospective cohort studies, (b) patients tested for FVL mutation/ activated protein C resistance (APCR) at baseline, (c) objectively confirmed VTE, (d) recurrent VTE defined as primary outcome, and (e) numbers of recurrences or recurrence rates reported separately in patients with and without FVL mutation. Exclusion criteria were (1) retrospective studies, (2) case-control studies, case reports, and (3) studies conducted in close subgroups (e.g., children, perioperative VTE, upper extremity deep venous thrombosis, and homozygous FVL mutation). Articles based on the same cohort were compiled and the publication with the (a) highest number of patients, and (b) most complete clinical data were selected for meta-analysis.
Recurrent VTE was defined as objectively confirmed VTE. For deep venous thrombosis (DVT), one of the following imaging techniques must have been used: venography, duplex sonography, or compression ultrasonography. For pulmonary embolism (PE), ventilation-perfusion scan, spiral computed tomography, or pulmonary angiography should have been used (56–58).
First, several characteristics were retrieved to summarize each cohort: name of cohort, country, setting (type of health care institution), time period of patient recruitment, inclusion criteria and all publications. Secondly, detailed data were extracted out of the selected publication for meta-analysis: first author, year of publication, age of patients (mean or median), total number of patients, number of female patients, number of FVL mutation patients (at baseline), location of initial VTE (isolated distal DVT, proximal DVT/PE or mixed DVT/PE), triggering factor first VTE (unprovoked, provoked, mixed), duration of anticoagulation (months), type of anticoagulation [Vitamin-K antagonist (VKA), direct oral anticoagulants (DOAC)], absolute number of patients with unprovoked VTE, number of cancer patients, observation period (months), total number of patients with recurrence, number of FVL mutation patients with recurrence, number of non-FVL mutation patients with recurrence and recurrence rate of FVL mutation patients.
The methodological quality of the primary studies was assessed using the Newcastle-Ottawa Scale (NOS) for cohort studies (59). The following three domains were applied: (a) selection of patients, (b) comparability of study groups, and (c) outcome of interest. The questions were modified to fit the present research question (Supplementary List 2). The assessment was done in duplicate (D.E., M.N.) and discrepancies were resolved by discussion.
To set this analysis into context, we assessed the frequency and trends of testing for FVL mutation in the Swiss health care system. Health care claims data of Helsana, one of the largest Swiss health insurance companies were used. Approximately 15% of the Swiss population are insured with Helsana for obligatory basic insurance, and the population is considered representative (60, 61). All invoices submitted for reimbursement for FVL mutation (#6200.64) and APCR (#1086.00) of the list of analyses from the Federal Office of Public Health were retrieved between 2014 and 2020 (62).
Using the extracted data, the relative risks (RR) and their 95% confidence intervals (CI) were calculated for each primary study. The RRs were then calculated using a random-effects model based on the Mantel-Haenszel estimator, and the corresponding 95% CI were computed. Heterogeneity between studies was assessed using Higgins' I2. All analyses were performed using the “meta,” “etaphor,” and “dmetar” packages for R. As a first sensitivity analysis, a leave-one-out analysis was performed to check for outliers. Studentized residuals and Cook's distance were calculated, and studies with studentized residuals outside of −1 and 1, and Cook's distances >50% of the lower tail of a Chi-square distribution with p (p = number of model coefficients) degrees of freedom were flagged as potentially influential outliers. These studies were excluded from the overall analysis. Furthermore, subgroup analysis was performed for the following subgroups: Year of publication (<2000, 2000–2010, >2010), location of the initial VTE (mixed, proximal DVT/PE), presence of triggering risk factors for the initial VTE (unprovoked, provoked, and mixed), the anticoagulation drug used (VKA, DOAC), and the presence of cancer (no cancer, mixed). A funnel plot was additionally created to assess publication bias.
The literature search retrieved a total of 2,581 publications, 2,573 accessed in MEDLINE and EMBASE databases, and eight identified by manual review (Figure 1). After removing duplicates, the title and abstract of the 2,259 remaining publications were screened, giving 131 publications for full-text screening (including 100 journal articles and 21 conference abstracts). A total of 67 publications were excluded with reasons. Eventually, 31 different prospective cohort studies were identified (Table 1; Figure 1). Per cohort, the publication with the most complete clinical data was selected for further analysis. No publication with sufficient data were identified in seven cohorts (44, 45, 47, 54, 80, 101, 104). Twenty-four publications were finally considered for meta-analysis (Figure 1) (17, 36–41, 46, 48, 63–65, 67, 69–71, 73, 75, 78, 82–84, 86, 103).
Thirty-one prospective cohort studies conducted in Europe (n = 23), North America (n = 3), Europe and North America (n = 2), and other areas (n = 3) were identified. The number of publications per cohort ranged from 1 (17, 38–41, 45–48, 54, 63, 64, 69, 70, 73, 80, 82, 83, 103, 104) to 15 (86). Twenty-three cohorts included patients with a first VTE (36, 39–41, 44–46, 48, 54, 64, 65, 67, 69, 70, 75, 78, 80, 82, 84, 86, 101, 103, 104), and eight cohorts included patients with any VTE (17, 37, 38, 47, 63, 71, 73, 83). Detailed cohort characteristics are reported in Table 1.
Details of the primary studies included in the meta-analysis are reported in Table 2, summarizing data of 13,571 patients, including 2,840 patients with FVL mutation (21%). The number of patients varied between 72 (83) and 1,267 (37). The prevalence of FVL mutation ranged between 8.4% (36) and 28% (86). The mean or median age varied between 37 years (40) and 76 years (48). The observation periods varied from six (83) to 88 (63, 65) months. VKA were used in most studies (36, 37, 41, 46, 48, 64, 65, 67, 69, 71, 73, 75, 78, 82, 84, 86, 103), summarizing 8,654 patients (64%). DOAC were used as anticoagulant in one study (17), and the type of anticoagulant was not specified in six studies (38–40, 63, 70, 83). The inclusion criteria and the type and location of the primary event is reported in Table 1. Eight studies included patients with a first unprovoked VTE only (36, 41, 48, 69, 73, 84, 86, 103) and one study provided separate data (provoked/unprovoked) (67). Both provoked and unprovoked VTE were included in 15 primary studies (17, 37–40, 46, 63–65, 70, 71, 75, 78, 82, 83). Patients with cancer were excluded in 16 studies (36, 39–41, 46, 48, 64, 65, 67, 69, 73, 78, 82, 84, 86, 103) and not reported in two studies (63, 83). Overall, 341 cancer patients were reported in six studies (17, 37, 38, 70, 71, 75). A funnel plot is given in Supplementary Figure S1.
A summary of the methodological quality according to the NOS tool is given in Figure 2; detailed results for all studies are reported in the Supplementary Table S1. With at least six NOS criteria fulfilled in twenty studies, the overall methodological quality was high (17, 36–41, 48, 63–65, 67, 69, 71, 73, 75, 78, 82, 84, 86). Three to five criteria were fulfilled by four studies (46, 70, 83, 103). The three domains most frequently not met were (1) method reported for distinguishing the initial and recurrent VTE (37, 39–41, 46, 48, 63, 65, 67, 70, 71, 83, 103), (2) follow-up longer than 2 years (17, 69, 75, 83, 84, 103), and (3) follow-up rate ≥90% of patients (17, 40, 41, 46, 48, 67, 83, 103).
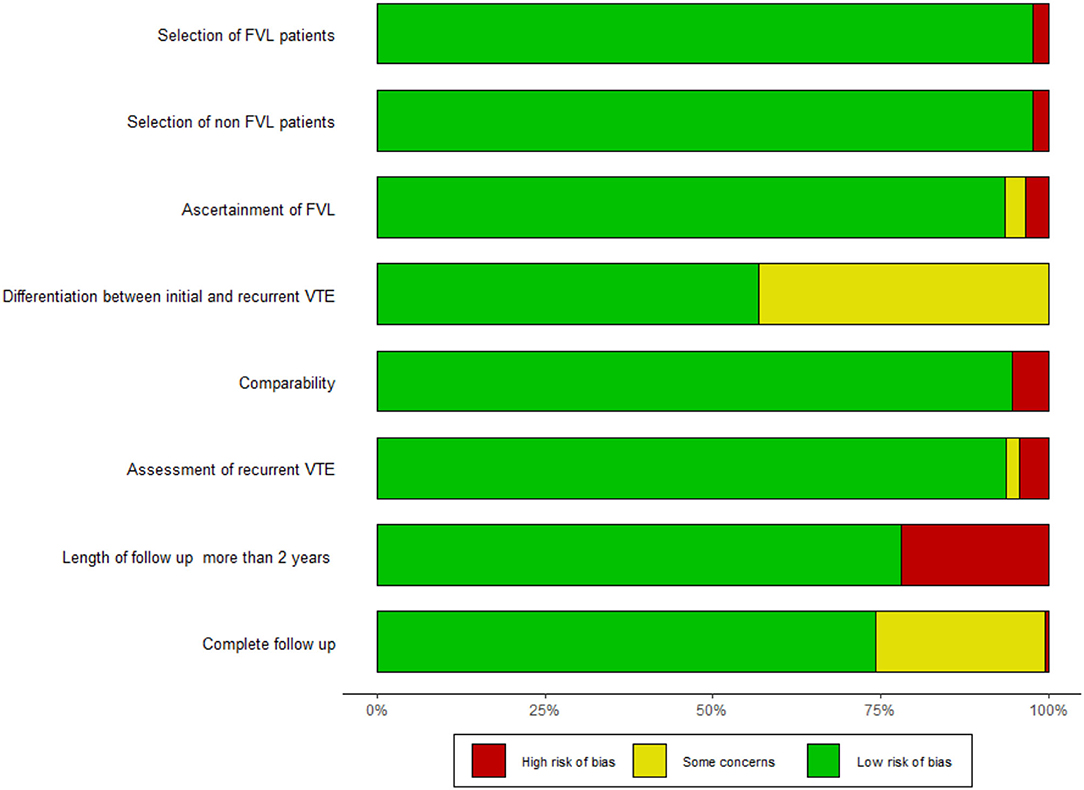
Figure 2. Summary of methodological quality. Rating according to the NOS questionnaire. The detailed questionnaire is shown in the Supplementary List 2.
Assessing all studies for potential influential outliers using statistical criteria (105), we identified the study from Franco Moreno et al. (103) (Supplementary Table S2; Supplementary Figure S2). Thus, this study was excluded for the purpose of the overall analysis. A recurrent event was recorded in 1,867 individuals (14%). Recurrent events were observed in 18% of the FVL mutation patients and in 13% of the non-FVL mutation patients. Details are reported in Table 2. The relative risk was 1.46 (95% CI: 1.31, 1.64, I2 = 0.17; 95% prediction interval 1.10, 1.94) (Figure 3).
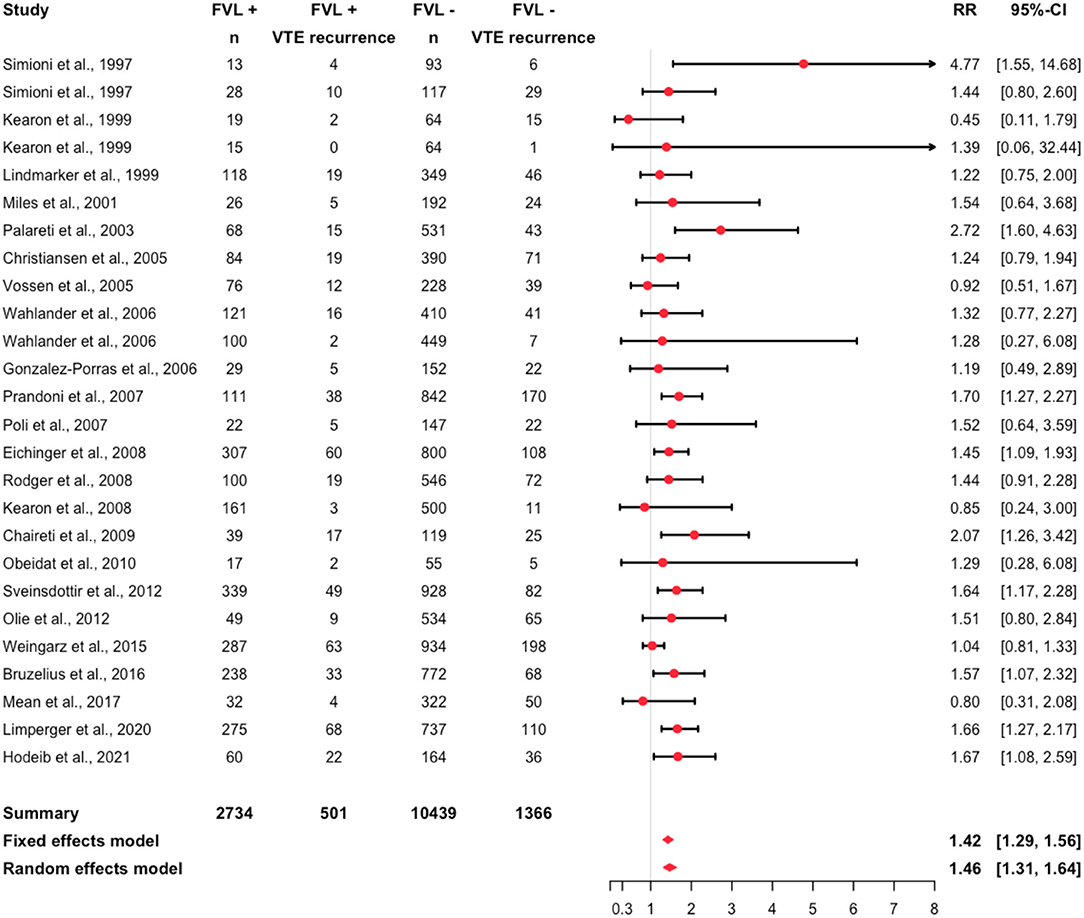
Figure 3. Forest plot summarizing the relative risk of recurrent VTE among heterozygous FVL mutation patients (I2 = 0.17).
In several sensitivity analyses, we assessed the risk in specific subgroups. Among the primary studies, the RR varied between 0.45 (95% CI: 0.11, 1.79) (69) and 4.77 (95% CI: 1.55, 14.68) (67). A RR smaller than one was calculated in four primary studies (48, 69, 70, 73). Focusing on different anticoagulants, the RR was 1.65 (95% CI: 1.33, 2.04) in patients treated with VKA, and 1.28 (95% CI: 0.27, 6.08) in patients treated with DOAC (Supplementary Figure S3). Pooling studies with unprovoked VTE only, the RR was 1.53 (95% CI: 0.99, 2.35) (Supplementary Figure S4). It was 1.47 (95% CI: 1.27, 1.71) in studies including both, patients with provoked and unprovoked VTE. In one study group (67), patients with a first provoked VTE only were analyzed, resulting in a RR of 4.77 (95% CI: 1.55, 14.68). Considering different localizations of the initial event, the RR was 1.29 (95% CI: 0.28, 6.08) in patients with PE (Supplementary Figure S5), and 1.52 (95% CI: 1.2, 1.93) in patients with proximal DVT or PE. It was 1.6 (95% CI: 1.32, 1.95) in patients with proximal DVT/PE or distal DVT. Excluding patients with cancer, the RR was 1.59 (95% CI: 1.27, 1.99) (Supplementary Figure S6). The RR was 1.69 (95% CI: 1.14, 2.51) in studies published after 2011, 1.52 (95% CI: 1.33, 1.75) in studies published between 2001 and 2011, and 1.44 (95% CI: 0.77, 2.68) in studies published before 2001 (Supplementary Figure S7).
Analysis of Helsana health care claims data in Switzerland showed that 46,522 APCR tests and 49,625 polymerase-chain reaction (PCR) for FVL mutation were recorded between 2014 and 2020 (Supplementary Table S3; Supplementary Figure S8). For APCR, the frequency of testing varied between 6,206 (0.1% of the population, 2014) and 7,206 (0.1%, 2016). For PCR, the frequency ranged between 6,793 (0.1%, 2017) and 7,614 (0.1%, 2019). Considering patients having any test, the total number of patients with APCR and/or PCR varied between 9,661 (0.2%, 2017) and 10,614 (0.2%, 2016). The frequency of testing was stable between 2014 and 2020.
We conducted a comprehensive systematic review retrieving all high-quality epidemiological data investigating the association of heterozygous FVL mutation and recurrent VTE. Thirty-one prospective cohort studies were identified and 24 publications summarizing 13,571 patients were included in the meta-analysis. Overall, a 42% increased risk of recurrence was found in patients with heterozygous FVL mutation. Various subgroup analyses did not identify a population with a significantly modified risk. However, a significant proportion of the analyzed Swiss population was tested for FVL mutation each year.
The present work is the most comprehensive systematic review to date. Considering all currently available data, we were able to analyze various subgroups of patients. However, our results are essentially consistent with previous investigations (24–26). Segal et al. (26) included 13 prospective studies summarizing 4,730 patients, reporting an overall odds ratio of 1.56. Marchiori et al. (25) included 10 prospective studies with 3,203 patients concluding on a relative risk of 1.39. Ho et al. (24) summarized two retrospective studies and eight prospective studies, reporting an odds ratio of 1.41. We analyzed a number of patient subgroups (type of anticoagulation, triggering risk factors, VTE localization, presence of cancer, and year of publication) and none of these analyses revealed statistically significant differences in the recurrence risk (Supplementary Figures S3–S7). However, a remarkable higher recurrence risk was reported in the only study including patients with provoked VTE (67). However, this was a small study published in 1997 and the results were never confirmed in other settings.
Our investigation has several strengths. First, we conducted a comprehensive literature search and applied strict inclusion criteria to include high-quality data only. Secondly, we pooled three times more patients compared to the latest systematic review. Thirdly, most of the studies had a low risk of bias and the between-study heterogeneity is low. Fourthly, we were able to conduct several subgroup analyses, thus strengthening the interpretation. Of course, our study has limitations as well. First, inherent with any meta-analytic approach, our investigation relies on data retrieved from primary studies. However, only four studies were estimated to have a high risk of bias. One of those studies was classified as a potentially influential outlier and thus excluded for overall analysis. The remaining three studies affected only 4% of the patients. Thus, we do not believe that this might have influenced our results. Secondly, the number of patients were limited in certain subgroups; patients with provoked VTE, cancer, DOAC, and PE were underrepresented. Thirdly, it was impossible to retrieve separate data for hetero- and homozygous patients in few studies. However, we do not believe that this might have influenced our results because only few patients are included in the large number of patients. Fourthly, one might argue that the proportion of patients with unprovoked VTE varies considerably among studies. However, as long as the between-study heterogeneity was low, this might be regarded as a strength of our study, increasing external validity.
Our data confirm that the presence of FVL mutation represents a minor risk factor only. Compared to the much stronger risk factors unprovoked VTE, proximal DVT/PE, male sex, elevated D-Dimers, high factor VIII plays the presence of FVL mutation only a marginal role (9, 11, 78, 106–109). Consistently, several prediction models for recurrent VTE were developed and FVL mutation was not identified as a relevant predictor in any of the models including clinical characteristics (11, 84, 107, 110, 111). Thus, an important task is to translate this evidence into clinical practice. Determination of FVL mutation shall be challenged and the reimbursement of these analyses might be questioned. However, some authors argue that the presence of FVL mutation might contribute to a significantly elevated risk if combined with other (high risk) thrombophilia. To date, the data supporting this hypothesis are not sufficient. Individual patient-data meta-analyses are a promising tool to study this research question.
Summarizing all currently available high-quality epidemiological data, the risk of recurrent VTE was only moderately increased. This observation was consistent among various subgroups. Our data confirm that the presence of FVL mutation plays only a marginal role in the risk assessment for recurrent VTE. Efforts should be made to reduce the still very frequent determination in clinical practice.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
DE developed the search strategy, conducted the literature search, retrieved the data, interpreted the results, wrote the manuscript, and contributed to the study protocol. HN wrote the analysis plan, conducted the analysis, and interpreted the data. BA developed the search strategy and contributed to the literature search. CH collected the data (health care claims) and contributed to the study protocol and the interpretation of the data. MN developed the study protocol, conducted the literature search, contributed to the analysis plan, interpreted the results, and wrote the manuscript. All authors contributed to and approved the final manuscript.
MN was supported by a research grant of the Swiss National Science Foundation (#179334).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.883986/full#supplementary-material
1. Connors JM. Thrombophilia testing and venous thrombosis. N Engl J Med. (2017) 377:1177–87. doi: 10.1056/NEJMra1700365
2. Middeldorp S. Inherited thrombophilia: a double-edged sword. Hematology Am Soc Hematol Educ Program. (2016) 2016:1–9. doi: 10.1182/asheducation-2016.1.1
3. Nilius H, Mertins T, Boss R, Knuchel M, Blozik E, Kremer Hovinga JA, et al. Long-term survival after venous thromboembolism: a prospective cohort study. Front Cardiovasc Med. (2021) 8:749342. doi: 10.3389/fcvm.2021.749342
4. ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to the global disease burden. J Thromb Haemost. (2014) 12:1580–90. doi: 10.1111/jth.12698
5. Tagalakis V, Patenaude V, Kahn SR, Suissa S. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. (2013) 126:832.e13–21. doi: 10.1016/j.amjmed.2013.02.024
6. Verso M, Agnelli G, Ageno W, Imberti D, Moia M, Palareti G, et al. Long-term death and recurrence in patients with acute venous thromboembolism: the MASTER registry. Thromb Res. (2012) 130:369–73. doi: 10.1016/j.thromres.2012.04.003
7. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. (2012) 379:1835–46. doi: 10.1016/S0140-6736(11)61904-1
8. Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. (2016) 41:3–14. doi: 10.1007/s11239-015-1311-6
9. Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for recurrent venous thrombosis. Lancet. (2010) 376:2032–9. doi: 10.1016/S0140-6736(10)60962-2
10. Ahmad A, Sundquist K, Palmer K, Svensson PJ, Sundquist J, Memon AA. Risk prediction of recurrent venous thromboembolism: a multiple genetic risk model. J Thromb Thrombolysis. (2019) 47:216–26. doi: 10.1007/s11239-018-1762-7
11. Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. (2010) 121:1630–6. doi: 10.1161/CIRCULATIONAHA.109.925214
12. Zhu T, Martinez I, Emmerich J. Venous thromboembolism: risk factors for recurrence. Arterioscler Thromb Vasc Biol. (2009) 29:298–310. doi: 10.1161/ATVBAHA.108.182428
13. Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. (2007) 98:756–64. doi: 10.1160/TH07-03-0212
14. Sundquist K, Sundquist J, Svensson PJ, Zoller B, Memon AA. Role of family history of venous thromboembolism and thrombophilia as predictors of recurrence: a prospective follow-up study. J Thromb Haemost. (2015) 13:2180–6. doi: 10.1111/jth.13154
15. Zöller B, Ohlsson H, Sundquist J, Sundquist K. Family history of venous thromboembolism (VTE) and risk of recurrent hospitalization for VTE: a nationwide family study in Sweden. J Thromb Haemost. (2014) 12:306–12. doi: 10.1111/jth.12499
16. Hron G, Eichinger S, Weltermann A, Minar E, Bialonczyk C, Hirschl M, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med. (2006) 119:50–3. doi: 10.1016/j.amjmed.2005.04.043
17. Wahlander K, Eriksson H, Lundstrom T, Clason SB, Wall U, Nystrom P, et al. Risk of recurrent venous thromboembolism or bleeding in relation to thrombophilic risk factors in patients receiving ximelagatran or placebo for long-term secondary prevention of venous thromboembolism. Br J Haematol. (2006) 133:68–77. doi: 10.1111/j.1365-2141.2006.05960.x
18. Anderson FA Jr. Spencer FA. Risk factors for venous thromboembolism. Circulation. (2003) 107(23 Suppl. 1):I9–I16. doi: 10.1161/01.CIR.0000078469.07362.E6
19. Stevens SM, Woller SC, Bauer KA, Kasthuri R, Cushman M, Streiff M, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. (2016) 41:154–64. doi: 10.1007/s11239-015-1316-1
20. De Stefano V, Rossi E. Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives: a review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost. (2013) 110:697–705. doi: 10.1160/TH13-01-0011
21. Baglin T, Gray E, Greaves M, Hunt BJ, Keeling D, Machin S, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. (2010) 149:209–20. doi: 10.1111/j.1365-2141.2009.08022.x
22. NICE. Venous Thromboembolic Diseases: Diagnosis, Management and Thrombophilia Testing [NG158]. National Institute for Health and Care Excellence (2012).
23. Kyrle PA, Eichinger S. Deep vein thrombosis. Lancet. (2005) 365:1163–74. doi: 10.1016/S0140-6736(05)71880-8
24. Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. (2006) 166:729–36. doi: 10.1001/archinte.166.7.729
25. Marchiori A, Mosena L, Prins MH, Prandoni P. The risk of recurrent venous thromboembolism among heterozygous carriers of factor V Leiden or prothrombin G20210A mutation. A systematic review of prospective studies. Haematologica. (2007) 92:1107–14. doi: 10.3324/haematol.10234
26. Segal JB, Brotman DJ, Necochea AJ, Emadi A, Samal L, Wilson LM, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. (2009) 301:2472–85. doi: 10.1001/jama.2009.853
27. Legnani C, Palareti G, Antonucci E, Poli D, Cosmi B, Falanga A, et al. Thrombophilia testing in the real-world clinical setting of thrombosis centres taking part in the Italian Start 2-Register. Blood Transfus. (2021) 19:244–52.
28. Kudo M, Lee HL, Yang IA, Masel PJ. Utility of thrombophilia testing in patients with venous thrombo-embolism. J Thorac Dis. (2016) 8:3697–703. doi: 10.21037/jtd.2016.12.40
29. Favaloro EJ, McDonald D. Futility of testing for factor V Leiden. Blood Transfus. (2012) 10:260–3. doi: 10.2450/2012.0097-12
30. Tientadakul P, Chinthammitr Y, Sanpakit K, Wongwanit C, Nilanont Y. Inappropriate use of protein C, protein S, and antithrombin testing for hereditary thrombophilia screening: an experience from a large university hospital. Int J Lab Hematol. (2011) 33:593–600. doi: 10.1111/j.1751-553X.2011.01332.x
31. Blinkenberg EØ, Kristoffersen A-H, Sandberg S, Steen VM, Houge G. Usefulness of factor V Leiden mutation testing in clinical practice. Eur J Hum Genet. (2010) 18:862–6. doi: 10.1038/ejhg.2010.33
32. Laberge AM, Psaty BM, Hindorff LA, Burke W. Use of Factor V Leiden genetic testing in practice and impact on management. Genet Med. (2009) 11:750–6. doi: 10.1097/GIM.0b013e3181b3a697
33. Middeldorp S, Van Hylckama Vlieg A. Does thrombophilia testing help in the clinical management of patients? Br J Haematol. (2008) 143:321–35. doi: 10.1111/j.1365-2141.2008.07339.x
34. Zöller B, Svensson PJ, Dahlbäck B, Lind-Hallden C, Hallden C, Elf J. Genetic risk factors for venous thromboembolism. Expert Rev Hematol. (2020) 13:971–81. doi: 10.1080/17474086.2020.1804354
35. Hotoleanu C. Genetic risk factors in venous thromboembolism. In: Islam MS, editor. Thrombosis and Embolism: From Research to Clinical Practice, Vol. 1. Cham: Springer International Publishing (2017). p. 253–72.
36. Olie V, Zhu T, Martinez I, Scarabin PY, Emmerich J. Sex-specific risk factors for recurrent venous thromboembolism. Thromb Res. (2012) 130:16–20. doi: 10.1016/j.thromres.2011.10.026
37. Sveinsdottir SV, Saemundsson Y, Isma N, Gottsater A, Svensson PJ. Evaluation of recurrent venous thromboembolism in patients with Factor V Leiden mutation in heterozygous form. Thromb Res. (2012) 130:467–71. doi: 10.1016/j.thromres.2012.03.020
38. Weingarz L, Schindewolf M, Schwonberg J, Hecking C, Wolf Z, Erbe M, et al. Thrombophilia and risk of VTE recurrence according to the age at the time of first VTE manifestation. VASA. (2015) 44:313–23. doi: 10.1024/0301-1526/a000447
39. Bruzelius M, Ljungqvist M, Bottai M, Bergendal A, Strawbridge RJ, Holmstrom M, et al. F11 is associated with recurrent VTE in women. A prospective cohort study. Thromb Haemost. (2016) 115:406–14. doi: 10.1160/th15-06-0459
40. Limperger V, Kenet G, Kiesau B, Kother M, Schmeiser M, Langer F, et al. Role of prothrombin 19911 A>G polymorphism, blood group and male gender in patients with venous thromboembolism: Results of a German cohort study. J Thromb Thrombolysis. (2020) 51:494–501. doi: 10.1007/s11239-020-02169-6
41. Hodeib H, Youssef A, Allam AA, Selim A, Tawfik MA, Abosamak MF, et al. Genetic risk profiling associated with recurrent unprovoked venous thromboembolism. Genes. (2021) 12:874. doi: 10.3390/genes12060874
42. Eichinger S, Pabinger I, Stumpflen A, Hirschl M, Bialonczyk C, Schneider B, et al. The risk of recurrent venous thromboembolism in patients with and without factor V Leiden. Thromb Haemost. (1997) 77:624–8. doi: 10.1055/s-0038-1656023
43. Eichinger S, Weltermann A, Mannhalter C, Minar E, Bialonczyk C, Hirschl M, et al. The risk of recurrent venous thromboembolism in heterozygous carriers of factor V Leiden and a first spontaneous venous thromboembolism. Arch Intern Med. (2002) 162:2357–60. doi: 10.1001/archinte.162.20.2357
44. Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. (2003) 362:523–6. doi: 10.1016/S0140-6736(03)14111-6
45. Mansilha A, Araujo F, Severo M, Sampaio SM, Toledo T, Albuquerque R. Genetic polymorphisms and risk of recurrent deep venous thrombosis in young people: prospective cohort study. Eur J Vasc Endovasc Surg. (2005) 30:545–9. doi: 10.1016/j.ejvs.2005.05.038
46. Gonzalez-Porras JR, Garcia-Sanz R, Alberca I, Lopez ML, Balanzategui A, Gutierrez O, et al. Risk of recurrent venous thrombosis in patients with G20210A mutation in the prothrombin gene or factor V Leiden mutation. Blood Coagul Fibrinolysis. (2006) 17:23–8. doi: 10.1097/01.mbc.0000201488.33143.09
47. Zee RY, Bubes V, Shrivastava S, Ridker PM, Glynn RJ. Genetic risk factors in recurrent venous thromboembolism: a multilocus, population-based, prospective approach. Clin Chim Acta. (2009) 402:189–92. doi: 10.1016/j.cca.2009.01.011
48. Mean M, Limacher A, Stalder O, Angelillo-Scherrer A, Alberio L, Fontana P, et al. Do Factor V Leiden and Prothrombin G20210A mutations predict recurrent venous thromboembolism in older patients? Am J Med. (2017) 130:1220.e17–e22. doi: 10.1016/j.amjmed.2017.05.026
49. Kapoor S, Opneja A, Gollamudi J, Nayak LV. Prior history of venous thromboembolism is a significant risk factor for recurrence of thrombosis after cancer diagnosis. Blood. (2020) 136(Suppl. 1):32–3. doi: 10.1182/blood-2020-141961
50. Pabinger I, Ay C, Dunkler D, Thaler J, Reitter EM, Marosi C, et al. Factor V Leiden mutation increases the risk for venous thromboembolism in cancer patients - results from the Vienna Cancer And Thrombosis Study (CATS). J Thromb Haemost. (2015) 13:17–22. doi: 10.1111/jth.12778
51. Mohammed AI, Abdulqader AMR, Jalal SD, Mahmood SN. ABO blood groups and thrombophilia markers in patients with unstimulated thrombosis in Kurdistan Region of Iraq. Clin Appl Thromb Hemost. (2020) 26:1076029620922913. doi: 10.1177/1076029620922913
52. van Hylckama Vlieg A, Baglin CA, Bare LA, Rosendaal FR, Baglin TP. Proof of principle of potential clinical utility of multiple SNP analysis for prediction of recurrent venous thrombosis. J Thromb Haemost. (2008) 6:751–4. doi: 10.1111/j.1538-7836.2008.02920.x
53. Timp JF, Braekkan SK, Lijfering WM, Van Hylckama Vlieg A, Hansen JB, Rosendaal FR, et al. Prediction of recurrent venous thrombosis in all patients with a first venous thrombotic event: The Leiden Thrombosis Recurrence Risk Prediction model (L-TRRiP). PLoS Medicine. (2019) 16:e1002883. doi: 10.1371/journal.pmed.1002883
54. van Hylckama Vlieg A, Flinterman LE, Bare LA, Cannegieter SC, Reitsma PH, Arellano AR, et al. Genetic variations associated with recurrent venous thrombosis. Circ Cardiovasc Genet. (2014) 7:806–13. doi: 10.1161/CIRCGENETICS.114.000682
55. Moher D Liberati A Tetzlaff J Altman DG The The PG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
56. Lim W, Le Gal G, Bates SM, Righini M, Haramati LB, Lang E, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. (2018) 2:3226–56. doi: 10.1182/bloodadvances.2018024828
57. Bates SM, Jaeschke R, Stevens SM, Goodacre S, Wells PS, Stevenson MD, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2012) 141(2 Suppl.):e351S–e418S. doi: 10.1378/chest.11-2299
58. Bounameaux H, Perrier A, Righini M. Diagnosis of venous thromboembolism: an update. Vasc Med. (2010) 15:399–406. doi: 10.1177/1358863X10378788
59. Wells GA, Shea B, O'connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute (2014).
60. Huber CA, Schwenkglenks M, Rapold R, Reich O. Epidemiology and costs of diabetes mellitus in Switzerland: an analysis of health care claims data, 2006 and 2011. BMC Endocr Disord. (2014) 14:44. doi: 10.1186/1472-6823-14-44
61. Haller E, Watzke B, Blozik E, Rosemann T, Reich O, Huber CA, et al. Antidepressant prescription practice and related factors in Switzerland: a cross-sectional analysis of health claims data. BMC Psychiatry. (2019) 19:196. doi: 10.1186/s12888-019-2178-4
62. Huber CA, Nagler M, Rosemann T, Blozik E, Näpflin M, Markun S. Trends in micronutrient laboratory testing in Switzerland: a 7-year retrospective analysis of healthcare claims data. Int J Gen Med. (2020) 13:1341–8. doi: 10.2147/IJGM.S275406
63. Miles JS, Miletich JP, Goldhaber SZ, Hennekens CH, Ridker PM. G20210A mutation in the prothrombin gene and the risk of recurrent venous thromboembolism. J Am Coll Cardiol. (2001) 37:215–8. doi: 10.1016/S0735-1097(00)01080-9
64. Lindmarker P, Schulman S, Sten-Linder M, Wiman B, Egberg N, Johnsson H. The risk of recurrent venous thromboembolism in carriers and non-carriers of the G1691A allele in the coagulation factor V gene and the G20210A allele in the prothrombin gene. Thromb Haemost. (1999) 81:684–9. doi: 10.1055/s-0037-1614554
65. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. (2005) 293:2352–61. doi: 10.1001/jama.293.19.2352
66. Lijfering WM, Christiansen SC, Rosendaal FR, Cannegieter SC. Contribution of high factor VIII, IX and XI to the risk of recurrent venous thrombosis in factor V Leiden carriers. J Thromb Haemost. (2009) 7:1944–6. doi: 10.1111/j.1538-7836.2009.03580.x
67. Simioni P, Prandoni P, Lensing AW, Scudeller A, Sardella C, Prins MH, et al. The risk of recurrent venous thromboembolism in patients with an Arg506–>Gln mutation in the gene for factor V (factor V Leiden). N Engl J Med. (1997) 336:399–403. doi: 10.1056/NEJM199702063360602
68. Simioni P, Prandoni P, Lensing AW, Manfrin D, Tormene D, Gavasso S, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood. (2000) 96:3329–33. doi: 10.1182/blood.V96.10.3329.h8003329a_3329_3333
69. Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, Anderson DR, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. (1999) 340:901–7. doi: 10.1056/NEJM199903253401201
70. Vossen CY, Walker ID, Svensson P, Souto JC, Scharrer I, Preston FE, et al. Recurrence rate after a first venous thrombosis in patients with familial thrombophilia. Arterioscler Thromb Vasc Biol. (2005) 25:1992–7. doi: 10.1161/01.ATV.0000174806.76629.7b
71. Chaireti R, Jennersjö C, Lindahl TL. Thrombin generation and D-dimer concentrations in a patient cohort investigated for venous thromboembolism. Relations to venous thrombosis, factor V Leiden and prothrombin G20210A: The LIST study. Thromb Res. (2009) 124:178–84. doi: 10.1016/j.thromres.2008.12.033
72. Chaireti R, Jennersjo C, Lindahl TL. Is thrombin generation at the time of an acute thromboembolic episode a predictor of recurrence? The LInkoping Study on Thrombosis (LIST)–a 7-year follow-up. Thromb Res. (2013) 131:135–9. doi: 10.1016/j.thromres.2012.11.015
73. Kearon C, Julian JA, Kovacs MJ, Anderson DR, Wells P, MacKinnon B, et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. (2008) 112:4432–6. doi: 10.1182/blood-2008-06-163279
74. Baglin T, Luddington R, Brown K, Baglin C. High risk of recurrent venous thromboembolism in men. J Thromb Haemost. (2004) 2:2152–5. doi: 10.1111/j.1538-7836.2004.01050.x
75. Palareti G, Legnani C, Cosmi B, Valdre L, Lunghi B, Bernardi F, et al. Predictive value of D-dimer test for recurrent venous thromboembolism after anticoagulation withdrawal in subjects with a previous idiopathic event and in carriers of congenital thrombophilia. Circulation. (2003) 108:313–8. doi: 10.1161/01.CIR.0000079162.69615.0F
76. Cristina L, Benilde C, Michela C, Mirella F, Giuliana G, Gualtiero P. High plasma levels of factor VIII and risk of recurrence of venous thromboembolism. Br J Haematol. (2004) 124:504–10. doi: 10.1046/j.1365-2141.2003.04795.x
77. Cosmi B, Legnani C, Cini M, Guazzaloca G, Palareti G. D-dimer levels in combination with residual venous obstruction and the risk of recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Haemost. (2005) 94:969–74. doi: 10.1160/TH05-02-0095
78. Prandoni P, Noventa F, Ghirarduzzi A, Pengo V, Bernardi E, Pesavento R, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. (2007) 92:199–205+III–IV. doi: 10.3324/haematol.10516
79. Prandoni P, Tormene D, Spiezia L, Pesavento R, Simioni P. Duration of anticoagulation and risk of recurrent thromboembolism in carriers of factor V Leiden or prothrombin mutation. J Thromb Haemost. (2008) 6:2223–4. doi: 10.1111/j.1538-7836.2008.03173.x
80. Prandoni P, Prins MH, Lensing AW, Ghirarduzzi A, Ageno W, Imberti D, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med. (2009) 150:577–85. doi: 10.7326/0003-4819-150-9-200905050-00003
81. Nemeth B, Lijfering WM, Nelissen RGHH, Schipper IB, Rosendaal FR, Le Cessie S, et al. Risk and risk factors associated with recurrent venous thromboembolism following surgery in patients with history of venous Thromboembolism. JAMA Netw Open. (2019) 2:e193690. doi: 10.1001/jamanetworkopen.2019.3690
82. Poli D, Antonucci E, Ciuti G, Abbate R, Prisco D. Anticoagulation quality and the risk of recurrence of venous thromboembolism. Thromb Haemost. (2007) 98:1148–50. doi: 10.1160/TH07-05-0348
83. Obeidat NM. The effect of genetically related risk factors on the recurrence rate of acute pulmonary embolism in a Tertiary Teaching Hospital in Jordan. Jordan Med J. (2010) 44:398–403.
84. Rodger MA, Kahn SR, Wells PS, Anderson DA, Chagnon I, Le Gal G, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ. (2008) 179:417–26. doi: 10.1503/cmaj.080493
85. Gandara E, Kovacs MJ, Kahn SR, Wells PS, Anderson DA, Chagnon I, et al. Non-OO blood type influences the risk of recurrent venous thromboembolism: a cohort study. Thromb Haemost. (2013) 110:1172–9. doi: 10.1160/TH13-06-0488
86. Eichinger S, Hron G, Bialonczyk C, Hirschl M, Minar E, Wagner O, et al. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch Intern Med. (2008) 168:1678–83. doi: 10.1001/archinte.168.15.1678
87. Hron G, Eichinger S, Weltermann A, Quehenberger P, Halbmayer WM, Kyrle PA. Prediction of recurrent venous thromboembolism by the activated partial thromboplastin time. J Thromb Haemost. (2006) 4:752–6. doi: 10.1111/j.1538-7836.2006.01868.x
88. Lechner D, Wiener C, Weltermann A, Eischer L, Eichinger S, Kyrle PA. Comparison between idiopathic deep vein thrombosis of the upper and lower extremity regarding risk factors and recurrence. J Thromb Haemost. (2008) 6:1269–74. doi: 10.1111/j.1538-7836.2008.02998.x
89. Eichinger S, Hron G, Kollars M, Kyrle PA. Prediction of recurrent venous thromboembolism by endogenous thrombin potential and D-dimer. Clin Chem. (2008) 54:2042–8. doi: 10.1373/clinchem.2008.112243
90. Kyrle PA, Minar E, Bialonczyk C, Hirschl M, Weltermann A, Eichinger S. The risk of recurrent venous thromboembolism in men and women. N Engl J Med. (2004) 350:2558–63. doi: 10.1056/NEJMoa032959
91. Eichinger S, Pecheniuk NM, Hron G, Deguchi H, Schemper M, Kyrle PA, et al. High-density lipoprotein and the risk of recurrent venous thromboembolism. Circulation. (2007) 115:1609–14. doi: 10.1161/CIRCULATIONAHA.106.649954
92. Schonauer V, Kyrle PA, Weltermann A, Minar E, Bialonczyk C, Hirschl M, et al. Superficial thrombophlebitis and risk for recurrent venous thromboembolism. J Vasc Surg. (2003) 37:834–8. doi: 10.1067/mva.2003.157
93. Hoke M, Kyrle PA, Minar E, Bialonzcyk C, Hirschl M, Schneider B, et al. Tissue factor pathway inhibitor and the risk of recurrent venous thromboembolism. Thromb Haemost. (2005) 94:787–90. doi: 10.1160/TH05-06-0412
94. Eichinger S, Weltermann A, Minar E, Stain M, Schonauer V, Schneider B, et al. Symptomatic pulmonary embolism and the risk of recurrent venous thromboembolism. Arch Intern Med. (2004) 164:92–6. doi: 10.1001/archinte.164.1.92
95. Kyrle PA, Minar E, Hirschl M, Bialonczyk C, Stain M, Schneider B, et al. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med. (2000) 343:457–62. doi: 10.1056/NEJM200008173430702
96. Kyrle PA, Eichinger S, Pabinger I, Stumpflen A, Hirschl M, Bialonczyk C, et al. Prothrombin fragment F1+2 is not predictive for recurrent venous thromboembolism. Thromb Haemost. (1997) 77:829–33. doi: 10.1055/s-0038-1656062
97. Ahmad A, Sundquist K, Zoller B, Dahlback B, Svensson PJ, Sundquist J, et al. Identification of polymorphisms in Apolipoprotein M gene and their relationship with risk of recurrent venous thromboembolism. Thromb Haemost. (2016) 116:432–41. doi: 10.1160/TH16-03-0178
98. Ahmad A, Sundquist K, Zoller B, Svensson PJ, Sundquist J, Memon AA. Thrombomodulin gene c.1418C>T polymorphism and risk of recurrent venous thromboembolism. J Thromb Thrombolysis. (2016) 42:135–41. doi: 10.1007/s11239-015-1328-x
99. Sundquist K, Wang X, Svensson PJ, Sundquist J, Hedelius A, Larsson Lonn S, et al. Plasminogen activator inhibitor-1 4G/5G polymorphism, factor V Leiden, prothrombin mutations and the risk of VTE recurrence. Thromb Haemost. (2015) 114:1156–64. doi: 10.1160/TH15-01-0031
100. Strandberg K, Svensson PJ, Ohlin AK. Venous thromboembolism in carriers of the Factor V Leiden mutation and in patients without known thrombophilic risk factor; prediction of recurrence and APC-PCI complex concentration and/or soluble thrombomodulin antigen and activity. Thromb Res. (2007) 121:145–51. doi: 10.1016/j.thromres.2007.03.020
101. Le Moigne E, Delluc A, Tromeur C, Nowak E, Mottier D, Lacut K, et al. Risk of recurrent venous thromboembolism among young women after a first event while exposed to combined oral contraception versus not exposed to: a cohort study. Thromb Res. (2013) 132:51–5. doi: 10.1016/j.thromres.2013.05.028
102. Moigne EL, Delluc A, Novak E, Mottier D, Gal GL. Risk of recurrence after contraception-related venous thrombosis: Cohort study. J Thromb Haemost. (2011) 2:174. doi: 10.1016/S0049-3848(11)70136-0
103. Franco Moreno AI, Garcia Navarro MJ, Ortiz Sanchez J, Martin Diaz RM, Madronal Cerezo E, de Ancos Aracil CL, et al. A risk score for prediction of recurrence in patients with unprovoked venous thromboembolism (DAMOVES). Eur J Intern Med. (2016) 29:59–64. doi: 10.1016/j.ejim.2015.12.010
104. Bizien N, Noel-Savina E, Tromeur C, Delluc A, Mottier D, Leroyer C, et al. Age is a major risk factor of venous thromboembolism (VTE). Eur Respir J. (2011) 38(Suppl. 55):p3936.
105. Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing Meta-Analysis With R: A Hands-On Guide, 1st Edn. Boca Raton, FL; London: Chapman & Hall/CRC Press (2021).
106. Douketis J, Tosetto A, Marcucci M, Baglin T, Cosmi B, Cushman M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. (2011) 342:d813. doi: 10.1136/bmj.d813
107. Nagler M, Van Kuijk SMJ, Ten Cate H, Prins MH, Ten Cate-Hoek AJ. Predicting recurrent venous thromboembolism in patients with deep-vein thrombosis: development and internal validation of a potential new prediction model (Continu-8). Front Cardiovasc Med. (2021) 8:655226. doi: 10.3389/fcvm.2021.655226
108. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. (2016) 149:315–52. doi: 10.1016/j.chest.2015.11.026
109. Bruinstroop E, Klok FA, Van de Ree MA, Oosterwuk FL, Huisman MV. Elevated d-dimer levels predict recurrence in patients with idiopathic venous thromboembolism: a meta-analysis. J Thromb Haemost. (2009) 7:611–8. doi: 10.1111/j.1538-7836.2009.03293.x
110. Ensor J, Riley RD, Moore D, Snell KI, Bayliss S, Fitzmaurice D. Systematic review of prognostic models for recurrent venous thromboembolism (VTE) post-treatment of first unprovoked VTE. BMJ Open. (2016) 6:e011190. doi: 10.1136/bmjopen-2016-011190
Keywords: heterozygous factor V Leiden mutation, recurrent venous thromboembolism, prospective cohort studies, systematic review, risk factors
Citation: Eppenberger D, Nilius H, Anagnostelis B, Huber CA and Nagler M (2022) Current Knowledge on Factor V Leiden Mutation as a Risk Factor for Recurrent Venous Thromboembolism: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 9:883986. doi: 10.3389/fcvm.2022.883986
Received: 25 February 2022; Accepted: 17 March 2022;
Published: 07 April 2022.
Edited by:
Tzu-Fei Wang, University of Ottawa, CanadaReviewed by:
Radhika Gangaraju, University of Alabama at Birmingham, United StatesCopyright © 2022 Eppenberger, Nilius, Anagnostelis, Huber and Nagler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Nagler, bWljaGFlbC5uYWdsZXJAaW5zZWwuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.