
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 16 May 2022
Sec. Cardiac Rhythmology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.879918
This article is part of the Research Topic The Wide Spectrum of Ventricular Arrhythmias: From out-of-hospital cardiac arrest to advanced in-hospital treatment View all 10 articles
 Vincenzo Russo1,2*
Vincenzo Russo1,2* Anna Rago2
Anna Rago2 Vincenzo Ruggiero1
Vincenzo Ruggiero1 Francesca Cavaliere1
Francesca Cavaliere1 Valter Bianchi2
Valter Bianchi2 Ernesto Ammendola2
Ernesto Ammendola2 Andrea Antonio Papa2
Andrea Antonio Papa2 Vincenzo Tavoletta2
Vincenzo Tavoletta2 Stefano De Vivo2
Stefano De Vivo2 Paolo Golino1,2
Paolo Golino1,2 Antonio D'Onofrio2
Antonio D'Onofrio2 Gerardo Nigro1,2
Gerardo Nigro1,2Introduction: In the context of randomized clinical trials, subcutaneous implantable cardiac defibrillators (S-ICDs) are non-inferior to transvenous ICDs (T-ICDs) concerning device-related complications or inappropriate shocks in patients with an indication for defibrillator therapy and not in need of pacing. We aimed at describing the clinical features of patients who underwent S-ICD implantation in our clinical practice, as well as the ICD-related complications and the inappropriate therapies among S-ICD vs. T-ICD recipients during a long-term follow-up.
Materials and Methods: All patients undergoing ICD, both S-ICD and TV-ICD, at Monaldi Hospital from January 1, 2015 to January 1, 2019 and followed up at our institution were included in the present analysis. The clinical variables associated with S-ICD implantation were evaluated by logistic regression analyses. We collected the ICD inappropriate therapies, ICD-related complications (including both pulse generator and lead-related complications), ICD-related infections, appropriate ICD therapies, and overall mortality. Kaplan-Meier (KM) analyses were performed to assess the risk of clinical outcome events between the two subgroups. A time-dependent Cox regression analysis was performed to adjust the results.
Results: Total 607 consecutive patients (mean age 53.8 ± 16.8, male 77.8%) with both TV-ICD (n: 290, 47.8%) and S-ICD (n: 317, 52.2%), implanted and followed at our center for a mean follow-up of 1614 ± 1018 days, were included in the study. At multivariate logistic regression analysis, an independent association between S-ICD implantation and ionic channel disease [OR: 6.01 (2.26–15.87); p < 0.0001] and ischemic cardiomyopathy [OR: 0.20 (0.12–0.35); p < 0.0001] was shown. The KM analysis did not show a significantly different risk of the inappropriate ICD therapies (log rank p = 0.64) between the two subgroups; conversely, a significant increase in the risk of ICD-related complications (log rank p = 0.02) and infections (log rank p = 0.02) in TV-ICD group was shown. The adjusted risk for ICD-related infections [OR: 0.07 (0.009–0.55), p = 0.01] and complications [0.31 (0.12–0.81), p = 0.01] was significantly lower among patients with S-ICD.
Conclusions: The choice to implant S-ICD was mainly driven by younger age and the presence of ionic channel disease; conversely ischemic cardiomyopathy reduces the probability to use this technology. No significant differences in inappropriate ICD therapies were shown among S-ICD vs. TV-ICD group; moreover, S-ICD is characterized by a lower rate of infectious and non-infectious complications leading to surgical revision or extraction.
The subcutaneous implantable cardioverter-defibrillator (S-ICD) is an established therapy for the prevention of sudden cardiac death (SCD) (1) and an alternative to a transvenous implantable cardioverter-defibrillator (T-ICD) system in selected patients (2). S-ICD may be particularly useful in patients with channelopathies (3) since several studies showed a high complication rate in those implanted with T-ICD (4–6). S-ICD is non-inferior to T-ICD concerning device-related complications or inappropriate shocks in patients with an indication for defibrillator therapy and not in need of pacing (7–12); however, these data are limited to short follow-up observational case-control studies (7–10) or the context of the randomized clinical trial (11, 12). Moreover, few data about the clinical drivers of S-ICD vs. T-ICD implantation in clinical practice have been still provided. We therefore aimed at describing the clinical features of patients who underwent S-ICD implantation, as well as the ICD-related complications and the inappropriate therapies among S-ICD vs. T-ICD recipients in the clinical practice of a high-volume implantation center.
Data for this study were sourced from the Monaldi Hospital Rhythm Registry (NCT05072119), which includes all patients who underwent ICD implantation and followed up at our Institution through both outpatient visits, every 3–6 months, and remote device monitoring. During the follow-up, the occurrence and the causes of inappropriate and appropriate ICD therapies, ICD-related complications, and deaths were assessed and recorded in the electronic data management system. For the present analysis, we selected all consecutive patients who received de novo both subcutaneous (S-ICD Group) and transvenous (TV Group), from January 1, 2015 to January 1, 2019, according to the European guidelines and recommendations available at the time of implantation (13, 14). We excluded patients with pacing indications (n: 87), CRT (n: 232), upgrade of an existing device (n: 56), incomplete baseline (n: 36) or follow-up data (n: 48). The local institutional review boards approved the study (ID 553-19), and all patients provided written informed consent for data storage and analysis.
The programming of the parameters for the detection of VT/VF was done according to the guidelines recommendations at the time of implant. In particular, we routinely activate for primary prevention only one VF zone (30 intervals at 250 bpm) and for secondary prevention two windows of detection (VF: 30 intervals at 250 bpm; VT2: 30 intervals at 187 bpm or 10–20 bpm < VT rate) with shocks only in VF zone and up to three ATPs and eight shocks in VT2 zone. S-ICD devices were programmed with a conditional zone, between 200 and 250 bpm, and a shock zone > 250 bpm. The programmed sensing vector was primary (48.42%) or secondary (43.4%) for most patients and alternate in a small percentage of cases (8.18%). The bicycle ergometer test was not routinely performed in patients who implanted S-ICD at our Hospital.
The primary study endpoints were: ICD inappropriate therapies, defined as anti-tachycardia pacing (ATP) and/or shocks for conditions other than ventricular tachycardia (VT) or ventricular fibrillation (VF); ICD-related complications, defined as all pulse generator (PG) or lead-related complications requiring surgical intervention; ICD-related infections, defined as all systemic infections requiring complete removal of the system including the leads extraction. The secondary endpoints were the clinical variables associated to S-ICD implantation, appropriate ICD therapies and all-cause mortality. Moreover, the type and distribution of ICD-related complications, defined as early when appearing during the first 30 days after device implantation or late, when occurred after the first-month post-implantation, were assessed.
Categorical data were expressed as number and percentage, whereas continuous variables were expressed as either median [interquartile range (IQR)]) or mean ± SD, based on their distribution as assessed both by the Kolmogorov–Smirnov and the Shapiro–Wilk tests. Between-group differences, for categorical variables, were assessed by the chi-square test, with the application of Yates correction where appropriate. Either parametric Student's t-test or nonparametric Mann–Whitney U test and Wilcoxon test were instead used to compare continuous variables, according to their distribution. Kaplan–Meier analysis was performed to assess the risk of both inappropriate ICD therapies and ICD-related complications between the two subgroups. Univariate and multivariate logistic regression was used to assess the clinical characteristics associated with S-ICD implantation. A time-dependent Cox univariate (unadjusted) and multivariate (adjusted) regression model was used to evaluate the association between S-ICD and clinical outcome events. The multivariate model was computed on all covariates with a p-value < 0.05. A 2-sided probability p-value < 0.05 was considered statistically significant. All analyses were performed using SPSS statistical software (version 24.0, SPSS, Chicago, Illinois) and STATA 14.0 software (StataCorp, College Station, Texas).
A total of 607 consecutive patients (mean age 53.8 ± 16.8, male 77.8%) with both TV-ICD (n: 290, 47.8%) and S-ICD (n: 317, 52.2%) followed at our center for a mean follow-up of 1,614 ± 1,018 days were included in the study. The indication for ICD implantation was primary prevention in 542 patients (89%) and secondary prevention in 65 patients (11%). S-ICD group showed more likely younger age (49 ± 17 vs. 60 ± 14 years; p< 0.0001), higher left ventricular ejection fraction (LVEF) (41 ± 17 vs. 35 ± 12 %; p < 0.0001), and lower prevalence of cardiovascular comorbidities. Ischemic cardiomyopathy (44.5 vs. 27%; p < 0.0001) and idiopathic dilated cardiomyopathy (32.4 vs. 17.3 %; p < 0.0001) were more frequent in the TV-ICD group; conversely, ionic channel disorders (16.7 vs. 2.75 %; p < 0.0001) were more frequent in S-ICD group. The ionic channel disorders group included patients with long QT syndrome (n: 8) and Brugada syndrome (n: 53). All baseline clinical characteristics of the study population are summarized in Table 1.
We assessed potential clinical variables associated with S-ICD implantation among our study population. At multivariate logistic regression analysis, an independent association between S-ICD implantation and ionic channel disease [OR: 6.01 (2.26–15.87); p < 0.0001], ischemic cardiomyopathy [OR: 0.20 (0.12–0.35); p < 0.0001] was shown. All data are shown in Table 2.
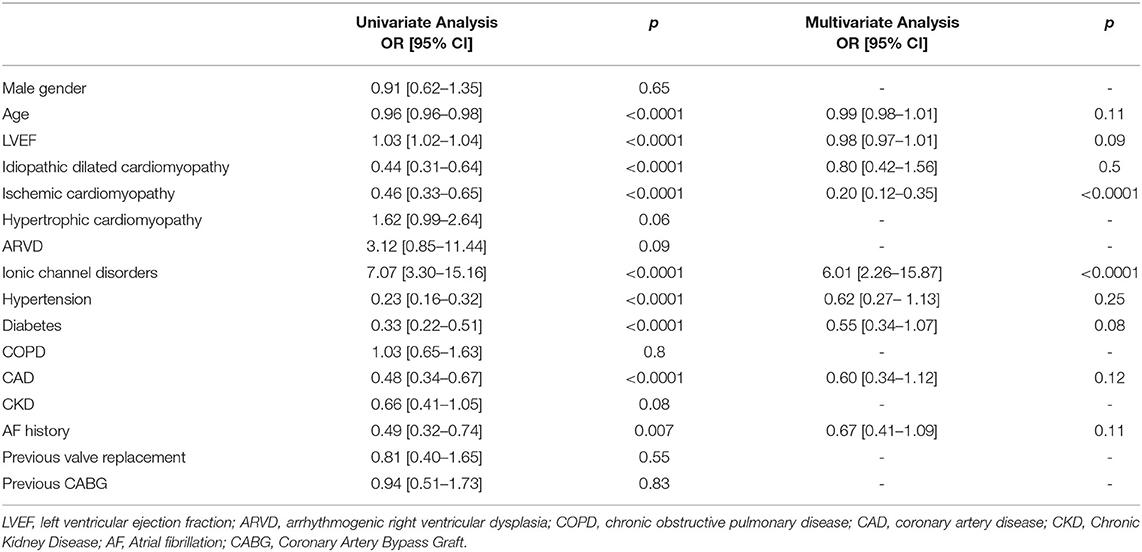
Table 2. Association between S-ICD implantation and clinical covariates: univariate and multivariate analysis.
Among our study population, ICD inappropriate therapies were experienced by 14 patients (2.31%). Out of these, seven patients (2.41%) in the TV-ICD group and seven patients (2.2%) in S-ICD group (p = 0.56). The annual incident rate of ICD inappropriate therapies over the follow-up was 0.6%. The Kaplan–Meier analysis did not show a significantly different risk of inappropriate ICD therapies between the two subgroups (log-rank p = 0.64) (Figure 1). At Cox univariate analysis no baseline patients' characteristic, including the S-ICD (OR: 1.30; 95% CI: 0.43–3.96; p = 0.64), was associated with inappropriate ICD therapies (Supplementary Table 1).
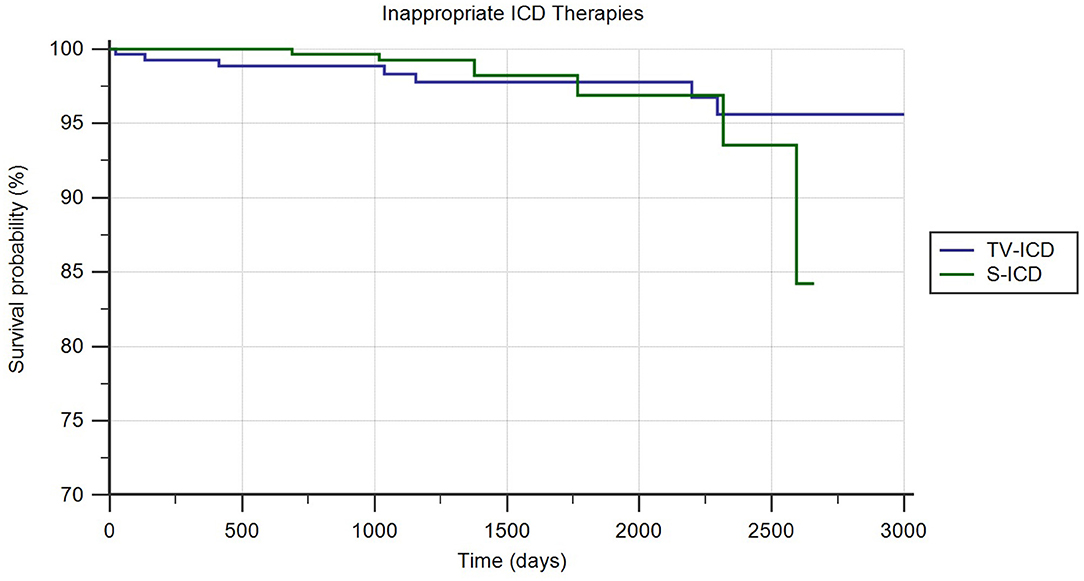
Figure 1. Kaplan-Meier curve comparing survival without ICD-related infections among S-ICD vs. TV-ICD groups.
ICD related complications in need of surgical revision occurred in 24 patients (3.9%); 18 (6.2%) in TV-ICD group and 6 (1.9%) in S-ICD group (p = 0.006); mainly due to increased lead-related complications in TV-ICD vs. S-ICD group (5.9 vs. 0.3%; p = 0.001). In contrast, no significant differences were shown in PG-related complications between the two subgroups (0.34 vs. 1.72%; p = 0.09). The Kaplan–Meier analysis showed a significantly increased risk of ICD-related complications among the TV-ICD group (log-rank p = 0.02) (Figure 2). At Cox multivariate analysis, S-ICD was the only variable significantly associated with a reduction of ICD-related complications (OR: 0.31; 95% CI 0.12–0.83; p < 0.01) (Supplementary Table 2).
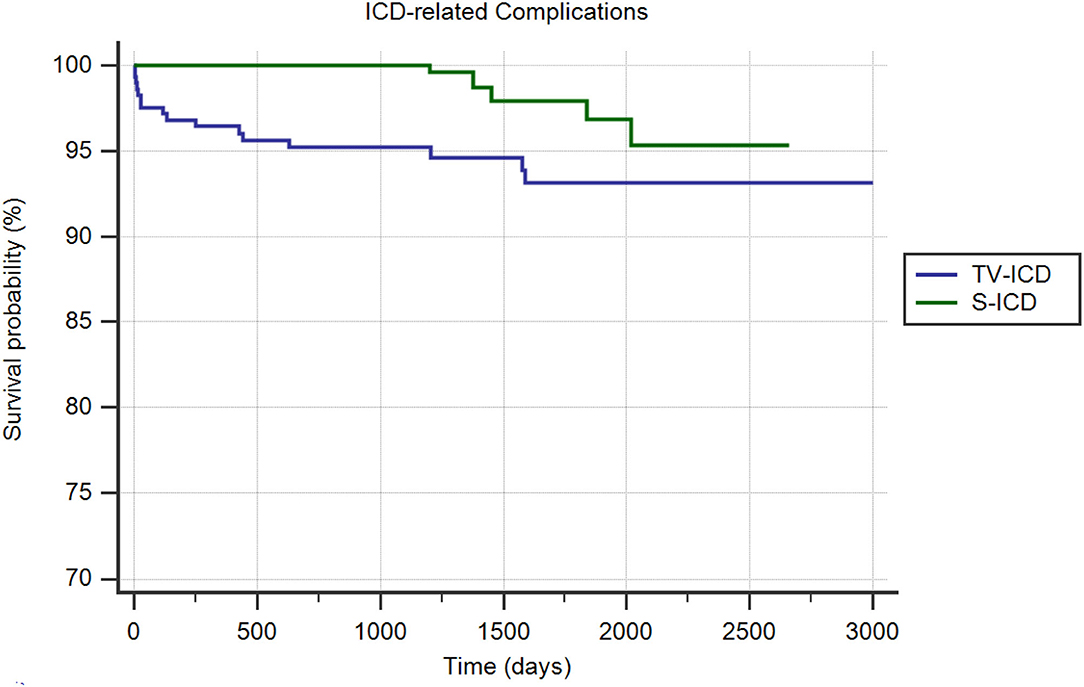
Figure 2. Kaplan-Meier curve comparing survival without ICD related complications among S-ICD vs. TV-ICD groups.
ICD-related infections in need of leads extraction occurred in 11 patients (1.8%); 10 (3.4%) in TV-ICD group and 1 (0.3%) in S-ICD group (p = 0.004). The annual incident rate of ICD-related infections over the follow-up was 0.4%. The Kaplan-Meier analysis showed a significantly increased risk of ICD-related infections among the TV-ICD group (log-rank p = 0.02) (Figure 3). At Cox multivariate analysis, S-ICD was the only variable significantly associated with a reduction of ICD-related infections (OR: 0.07; 95% CI 0.009–0.55; p < 0.01); in contrast previous valve replacement (OR: 7.22; 95% CI 2.34–22.22; p = 0.0006) was associated with an increased risk of ICD-related infections (Supplementary Table 3).
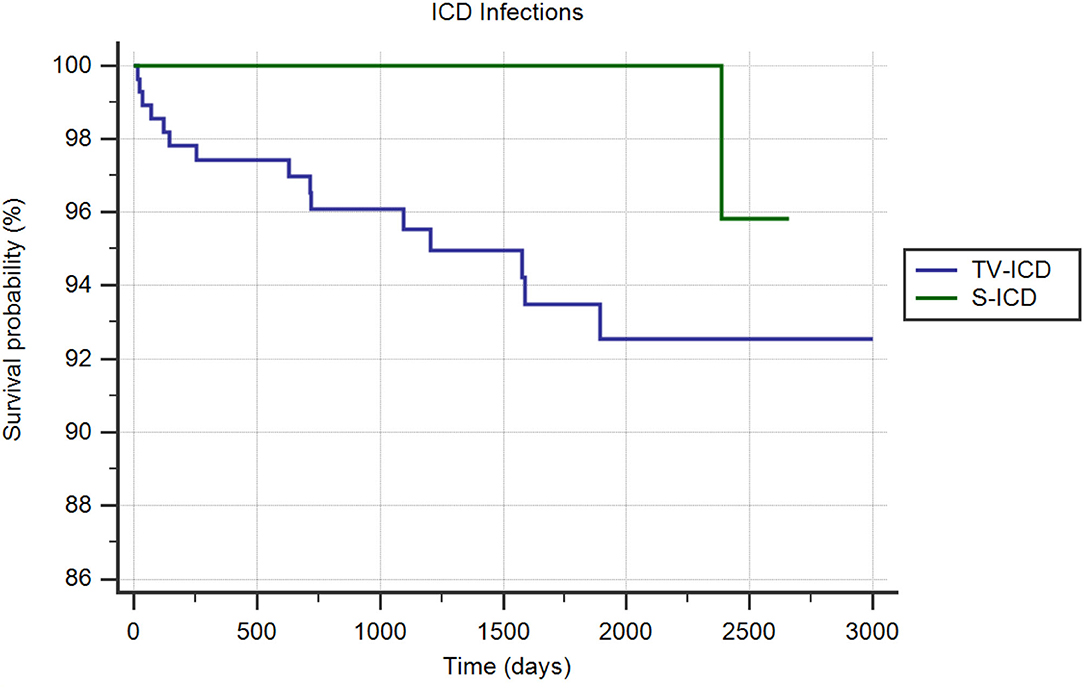
Figure 3. Kaplan-Meier curve comparing survival without ICD infections among S-ICD vs. TV-ICD groups.
In the Table 3 were summarized all primary outcomes events at follow-up among the two subgroups.
Among our study population, ICD appropriate therapies were experienced by 56 patients (9.23%). Out of these, 46 patients (15.86%) in the TV-ICD group and 10 patients (3.15%) in S-ICD group (p = 0.0001). Table 4 shows the number and the underlying disease of patients with at least one appropriate ICD therapy among our study population. The annual incident rate of ICD appropriate therapies over the follow-up was 2.3%. The Kaplan–Meier analysis showed a significantly increased risk of appropriate ICD therapies among the TV-ICD group (log-rank p = 0.04). At Cox multivariate analysis, no clinical variables were independently associated with an increased risk of ICD appropriate therapy (Supplementary Table 4).
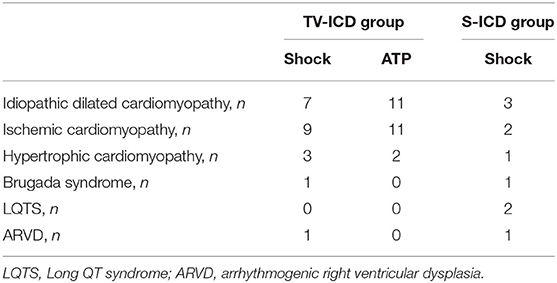
Table 4. Number of patients with at least one appropriate ICD therapy across different patients subgroups.
During the follow-up period, 28 people (4.61 percent) died: 8 patients (2.52%) in the S-ICD group and 20 (6.9%) in the TV-ICD group (p = 0.01). The annual incident rate of mortality over the follow-up was 1.15%. The Kaplan–Meier analysis did not show a significantly different risk of death between the two groups (log-rank p = 0.52) (Figure 4). At Cox multivariate analysis, no clinical variables were independently associated with all-cause mortality (Supplementary Table 5). Table 5 shows the unadjusted and adjusted odds ratio for the association between the clinical outcomes of interest and S-ICD.
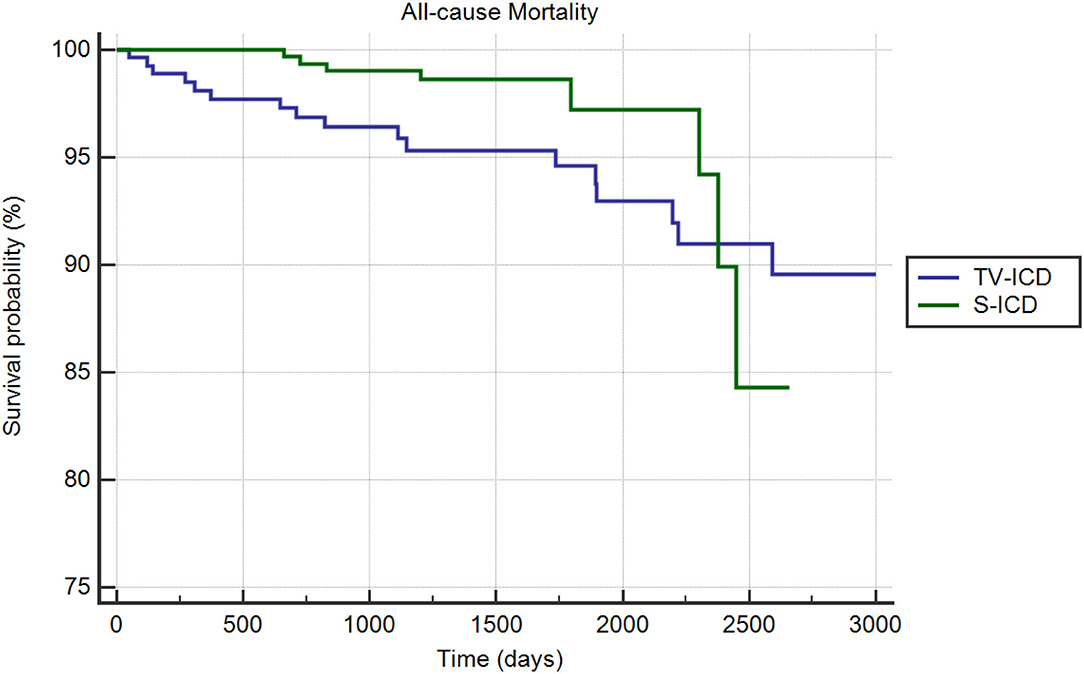
Figure 4. Kaplan-Meier curve comparing survival without all-cause mortality among S-ICD vs. TV-ICD groups.
The main results of our study are the following: younger age and ionic channel diseases are clinical variables independently associated with S-ICD implantation for sudden cardiac death prevention; conversely, ischemic cardiomyopathy reduced the probability to receive S-ICD among our study population. S-ICD patients showed a lower rate of both ICD-related complications and infections and no significant differences in inappropriate ICD therapies compared to TV-ICD patients during the follow-up. Finally, no differences inappropriate ICD therapies and overall mortality have been shown between the two groups. The lower age of the S-ICD group and the higher prevalence of ionic channel disease as clinical drivers of S-ICD implantation among our study population confirm the tendency to consider S-ICD the preferred choice for patients with an active lifestyle and long-life expectance. This is particularly true for inherited genetic arrhythmogenic syndromes (Brugada Syndrome and Long QT syndrome) where clinical arrhythmias are polymorphic VT or VF (not treatable with ATP) and the risk of bradycardia and monomorphic VT is very low (15, 16). The reduced probability for patients with ischemic cardiomyopathy to receive an S-ICD might be due to fair of sustained VT in need of anti-tachycardia pacing (ATP) or incident bradyarrhythmias in need of pacing (17). However, it should be noted that only 15–20% of patients experienced a high rate of monomorphic VT during the first year after the implant with a subsequent risk is 1.8%/year; moreover, the proportions of both monomorphic VT and successful ATP was comparable between patients with ischemic and non-ischemic cardiomyopathy (18). Finally, no studies have still addressed whether the efficacy of ATP translates into hard outcomes such as mortality benefits, prevention of inappropriate shocks, and risks of pro-arrhythmias (19).
Based on this evidence, the choice to implant an ATP-capable ICD should not exclusively be based on the ischemic or non-ischemic cardiomyopathy, but it should have applied a patient's centered tailoring approach which takes into account the potential mechanisms of ventricular arrhythmias and other patient factors such as susceptibility to systemic infections. Our study population included a large cohort of patients with HCM who were more likely treated with S-ICD; this preferred choice may be justified by the low rate of ATP therapies experienced by patients with HCM, with no difference in the rate of shock therapy compared to those with TV-ICD (20). However, the choice to implant an S-ICD should take into account the clinical features of patients with HCM since older age and symptomatic patients seem to be more likely to benefit from T-ICD pacing for the high incidence of symptomatic bradycardia and conduction disturbances in need of pacing, together with monomorphic ventricular tachycardia, as the predominant rhythm triggering successful ATP therapy (21). Similarly regarding ARVD, TV-ICD should be preferred in older patients with an advanced form of the disease, who more often experienced re-entrant VT that could be interrupted by ATP; in contrast, S-ICD is more indicated among younger patients who more likely experienced VF and are particularly prone to lead-related complications requiring device explantation (22). Recently, the prospective randomized comparison of subcutaneous and transvenous implantable cardioverter-defibrillator therapy (PRAETORIAN) trial (12) showed that, among 849 patients with an indication for ICD therapy but not for pacing therapy, the S-ICD was non-inferior to the T-ICD concerning the cumulative incidence of device-related complications or inappropriate shocks. However, there was a higher cumulative incidence of device-related complications in the T-ICD group (9.8 vs. 5.9%) and a higher cumulative incidence of inappropriate shocks in the S-ICD group (9.7 vs. 7.3%) at a median duration of follow-up was 49.1 months. Moreover, S-ICD was associated with a lower risk of lead-related complications, which was counter balanced by an increased risk of pocket hematoma.
A recent metanalysis of 13 randomized clinical trials including 9,073 patients (10) showed that the overall risk of clinically relevant complications and inappropriate shocks was not different between patients treated with S-ICD and TV-ICD. On the contrary, the risk of lead-related complications and major procedural complications was higher in the TV-ICD arm. No significant differences were found in the incidence of appropriate shocks and mortality was comparable between the two devices.
Among our study population, the cumulative incidence of inappropriate therapies was lower than previously reported, mainly due to our strategy to optimize the TV-ICD programming at each follow-up visit or based on remote monitoring reporting. In particular, an approach based on the programming of a VF-only zone (23), a cut-off rate greater than 220–240 bpm (24), longer detection intervals (25), activation of lead noise reduction algorithms (26), and enhanced supraventricular tachycardia discriminators (27) was used in our clinical practice. Moreover, the generation of S-ICD systems implanted at our Institution (EMBLEM A209 and EMBLEM-MRI A219) can apply an additional high-pass filter to the sensing methodology, called SmartPass (SP), designed to reduce inappropriate therapies (28). As previously shown in PRETORIAN trial, we did not observe a significant difference in the cumulative incidence of inappropriate therapies between the S-ICD and TV-ICD groups. The main cause of inappropriate therapies was oversensing in S-ICD group and misdetection of supraventricular arrhythmias in the TV-ICD group.
Regarding the complications, we observed a significant reduction of overall ICD-related complications in the S-ICD group, mainly driven by less frequent lead-related complications; in contrast, the device-related complications were higher in the S-ICD group due to some advisory released by Boston Scientifics for generators.1
Among our population, we reported a low annual rate of ICD infections, confirming the reduced number of infections in high implantation volume centers (29, 30); as we expected, the TV-ICD group showed higher incidence compared to the S-ICD group. This evidence is of pivotal importance since systemic infections represent an important predictor of death for all causes, regardless of the result of the extraction procedure (31).
The present is a single-center observational study mainly including ICD recipients, both TV-ICD and S-ICD, not in need of pacing and CRT. As we expected, the baseline clinical characteristics of the two subgroups were different and a regression analysis was performed to identify which variables have impact on the outcomes of interest; however, due to the observational nature of the study, we cannot exclude residual confounding of unmeasured variables. The results of the present study might be influenced by the high experience in ICD implantation and management of our center. The follow-up is relatively short, about 48 months, however, it is the longest among observational studies. No data about pharmacological therapies have been collected at the time of outcomes events.
In our clinical practice, the choice to implant S-ICD has been mainly driven by younger age and the presence of ionic channel disease; conversely, ischemic cardiomyopathy reduces the probability to use this technology. There were no significant differences in inappropriate ICD therapies between S-ICD and TV-ICD group; moreover, S-ICD has a lower rate of infectious and non-infectious complications leading to surgical revision or extraction.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by University of Campania - Monaldi Hospital. The patients/participants provided their written informed consent to participate in this study.
VRus designed the study. AR, EA, AP, VT, VB, and SD collected the data. VRug, VRus, and FC performed statistical analysis. VRus wrote the manuscript. AD'O, PG, and GN performed critical revision of article. All authors read and revised the manuscript.
The principal investigator, as senior researcher at Department of Medical Translational Sciences of University of Campania, received an unrestricted research grant from Boston Scientific. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.879918/full#supplementary-material
1. ^Available online at: https://www.bostonscientific.com/content/dam/bostonscientific/quality/dlt/reg-code-228/2020Dec_BSC_EmblemPBD_PhysLtr_US_Final.pdf.
1. Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L, et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. (2010) 363:36–44. doi: 10.1056/NEJMoa0909545
2. Kirkfeldt RE, Johansen JB, Nohr EA, Jørgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. (2014) 35:1186–94. doi: 10.1093/eurheartj/eht511
3. Veith M, El-Battrawy I, Roterberg G, Raschwitz L, Lang S, Wolpert C, et al. Long-term follow-up of patients with catecholaminergic polymorphic ventricular arrhythmia. J Clin Med. (2020) 9:903. doi: 10.3390/jcm9040903
4. El-Battrawy I, Roterberg G, Liebe V, Ansari U, Lang S, Zhou X, et al. Implantable cardioverter-defibrillator in Brugada syndrome: long-term follow-up. Clin Cardiol. (2019) 42:958–65. doi: 10.1002/clc.23247
5. El-Battrawy I, Besler J, Ansari U, Liebe V, Schimpf R, Tülümen E, et al. Long-term follow-up of implantable cardioverter-defibrillators in short QT syndrome. Clin Res Cardiol. (2019) 108:1140–6. doi: 10.1007/s00392-019-01449-3
6. Rudic B, Tülümen E, Berlin V, Röger S, Stach K, Liebe V, et al. Low Prevalence of inappropriate shocks in patients with inherited arrhythmia syndromes with the subcutaneous implantable defibrillator single center experience and long-term follow-up. J Am Heart Assoc. (2017) 6:e006265. doi: 10.1161/JAHA.117.006265
7. Brouwer TF, Yilmaz D, Lindeboom R, Buiten MS, Olde Nordkamp LR, Schalij MJ, et al. Long-term clinical outcomes of subcutaneous vs. transvenous implantable defibrillator therapy. J Am Coll Cardiol. (2016) 68:204. doi: 10.1016/j.jacc.2016.08.044
8. Boersma L, Barr C, Knops R, Theuns D, Eckardt L, Neuzil P, et al. Implant and midterm outcomes of the subcutaneous implantable cardioverter- defibrillator registry: the effortless study. J Am Coll Cardiol. (2017) 70:830–41. doi: 10.1016/j.jacc.2017.06.040
9. Mithani AA, Kath H, Hunter K, Andriulli J, Ortman M, Field J, et al. Characteristics and early clinical outcomes of patients undergoing totally subcutaneous vs. transvenous single chamber implantable cardioverter defibrillator placement. Europace. (2018) 20:308–14. doi: 10.1093/europace/eux026
10. Rordorf R, Casula M, Pezza L, Fortuni F, Sanzo A, Savastano S, et al. Subcutaneous vs. transvenous implantable defibrillator: an updated meta-analysis. Heart Rhythm. (2020) 18:P382–91. doi: 10.1016/j.hrthm.2020.11.013
11. Boersma LV, El-Chami MF, Bongiorni MG, Burke MC, Knops RE, Aasbo JD, et al. Understanding outcomes with the EMBLEM S-ICD in primary prevention patients with low EF study (UNTOUCHED): clinical characteristics and perioperative results. Heart Rhythm. (2019) 16:1636–44. doi: 10.1016/j.hrthm.2019.04.048
12. Knops RE, Olde Nordkamp LRA, Delnoy PHM, Boersma LVA, Kuschyk J, El-Chami MF, et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med. (2020) 383:526–36. doi: 10.1056/NEJMoa1915932
13. Pedersen CT, Kay GN, Kalman J, Borggrefe M, Della-Bella P, Dickfeld T, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Europace. (2014) 16:1257–83. doi: 10.1093/europace/euu194
14. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC). Endorsed by: association for european paediatric and congenital cardiology (AEPC). Eur Heart J. (2015) 36:2793–867. doi: 10.1093/eurheartj/ehv316
15. Russo V, Pafundi PC, Caturano A, Dendramis G, Ghidini AO, Santobuono VE, et al. Electrophysiological study prognostic value and long-term outcome in drug-induced type 1 Brugada syndrome: The IBRYD study. JACC Clin Electrophysiol. (2021) 7:1264–73. doi: 10.1016/j.jacep.2021.03.010
16. Dendramis G, D'Onofrio A, Russo V. Prognostic value of electrophysiologic study in drug-induced brugada syndrome: caution is always a must. Am J Cardiol. (2022) 163:143. doi: 10.1016/j.amjcard.2021.10.015
17. Bloch Thomsen PE, Jons C, Raatikainen MJ, Moerch Joergensen R, Hartikainen J, Virtanen V, et al. Cardiac arrhythmias and risk stratification after acute myocardial infarction (CARISMA) study group. Long-term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction: the cardiac arrhythmias and risk stratification after acute myocardial infarction (CARISMA) study. Circulation. (2010) 122:1258–64. doi: 10.1161/CIRCULATIONAHA.109.902148
18. Cheng A, Joung B, Brown ML, Koehler J, Lexcen DR, Sanders P, et al. Characteristics of ventricular tachyarrhythmias and their susceptibility to antitachycardia pacing termination in patients with ischemic and non-ischemic cardiomyopathy: a patient-level meta-analysis of three large clinical trials. J Cardiovasc Electrophysiol. (2020) 31:2720–6. doi: 10.1111/jce.14688
19. Ho G, Birgersdotter-Green U. Antitachycardia pacing: a worthy cause? J Cardiovasc Electrophysiol. (2020) 31:2727–9. doi: 10.1111/jce.14684
20. Jankelson L, Garber L, Sherrid M, Massera D, Jones P, Barbhaiya C, et al. Subcutaneous vs. transvenous implantable defibrillator in patients with hypertrophic cardiomyopathy. Heart Rhythm. (2022) 14:S1547-5271(22)00028-5. doi: 10.1016/j.hrthm.2022.01.013
21. Jiménez-Sánchez D, Castro-Urda V, Toquero-Ramos J, Restrepo-Córdoba MA, Sánchez-García M, García-Izquierdo E, et al. Benefits of cardiac pacing in ICD recipients with hypertrophic cardiomyopathy. J Interv Card Electrophysiol. (2022) 63:165–74. doi: 10.1007/s10840-021-00961-9
22. Olde Nordkamp LR, Wilde AA, Tijssen JG, Knops RE, van Dessel PF, de Groot JR. The ICD for primary prevention in patients with inherited cardiac diseases: indications, use, and outcome: a comparison with secondary prevention. Circ Arrhythm Electrophysiol. (2013) 6:91–100. doi: 10.1161/CIRCEP.112.975268
23. Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. (2013) 10:e85–108. doi: 10.1016/j.hrthm.2013.05.014
24. Clementy N, Challal F, Marijon E, Boveda S, Defaye P, Leclercq C, et al. Very high rate programming in primary prevention patients with reduced ejection fraction implanted with a defibrillator: results from a large multicenter controlled study. Heart Rhythm. (2017) 14: 211–7. doi: 10.1016/j.hrthm.2016.10.024
25. Kutyifa V, Daubert JP, Schuger C, Goldenberg I, Klein H, Aktas MK, et al. Novel ICD programming and inappropriate ICD therapy in CRT-D vs. ICD patients: a MADIT-RIT sub-study. Circ Arrhythm Electrophysiol. (2016) 9:e001965. doi: 10.1161/CIRCEP.114.001965
26. Beau S, Greer S, Ellis CR, Keeney J, Asopa S, Arnold E, et al. Performance of an ICD algorithm to detect lead noise and reduce inappropriate shocks. J Interv Card Electrophysiol. (2016) 45:225–32. doi: 10.1007/s10840-015-0081-6
27. Geller JC, Wöhrle A, Busch M, Elsässer A, Kleemann T, Birkenhauer F et al. Reduction of inappropriate implantable cardioverter-defibrillator therapies using enhanced supraventricular tachycardia discriminators: the ReduceIT study. J Interv Card Electrophysiol. (2021) 61:339–48. doi: 10.1007/s10840-020-00816-9
28. Theuns DAMJ, Brouwer TF, Jones PW, Allavatam V, Donnelley S, Auricchio A et al. Prospective blinded evaluation of a novel sensing methodology designed to reduce inappropriate shocks by the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm. (2018) 15:1515–22. doi: 10.1016/j.hrthm.2018.05.011
29. Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss E, et al. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med. (2019) 380:1895–905. doi: 10.1056/NEJMoa1901111.
30. Russo V, Viani S, Migliore F, Nigro G, Biffi M, Tola G, et al. Lead abandonment and subcutaneous implantable cardioverter-defibrillator (S-ICD) implantation in a cohort of patients with ICD lead malfunction. Front Cardiovasc Med. (2021) 8:692943. doi: 10.3389/fcvm.2021.692943
31. Tarakji KG, Wazni OM, Harb S, Hsu A, Saliba W, Wilkoff BL. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace. (2014) 16:1490–5. doi: 10.1093/europace/euu147
Keywords: subcutaneous ICD (S-ICD), transvenous ICD, complications, infections, inappropriate shock therapy, mortality
Citation: Russo V, Rago A, Ruggiero V, Cavaliere F, Bianchi V, Ammendola E, Papa AA, Tavoletta V, De Vivo S, Golino P, D'Onofrio A and Nigro G (2022) Device-Related Complications and Inappropriate Therapies Among Subcutaneous vs. Transvenous Implantable Defibrillator Recipients: Insight Monaldi Rhythm Registry. Front. Cardiovasc. Med. 9:879918. doi: 10.3389/fcvm.2022.879918
Received: 20 February 2022; Accepted: 06 April 2022;
Published: 16 May 2022.
Edited by:
Enrico Baldi, San Matteo Hospital Foundation (IRCCS), ItalyReviewed by:
Ibrahim El-Battrawy, Ruhr University Bochum, GermanyCopyright © 2022 Russo, Rago, Ruggiero, Cavaliere, Bianchi, Ammendola, Papa, Tavoletta, De Vivo, Golino, D'Onofrio and Nigro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincenzo Russo, dmluY2Vuem8ucnVzc29AdW5pY2FtcGFuaWEuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.