- 1Health Behaviour Research Collaborative, College of Health Medicine and Wellbeing, University of Newcastle, Callaghan, NSW, Australia
- 2Hunter Medical Research Institute, New Lambton Heights, NSW, Australia
- 3Northern Sydney Local Health District (NSLHD) Supportive and Palliative Care Network, St Leonards, NSW, Australia
- 4Northern Clinical School, The University of Sydney, Darlington, NSW, Australia
- 5Northern Sydney Cancer Centre, Royal North Shore Hospital, St Leonards, NSW, Australia
Background: The impetus to develop and implement tools for non-malignant patient groups is reflected in the increasing number of instruments being developed for heart failure and chronic respiratory diseases. Evidence syntheses of psychometric quality and clinical utility of these tools is required to inform research and clinical practice.
Aims: This systematic review examined palliative care needs tools for people diagnosed with advanced heart failure or chronic respiratory diseases, to determine their: (1) psychometric quality; and (2) acceptability, feasibility and clinical utility when implemented in clinical practice.
Methods: Systematic searches of MEDLINE, CINAHL, Embase, Cochrane and PsycINFO from database inception until June 2021 were undertaken. Additionally, the reference lists of included studies were searched for relevant articles. Psychometric properties of identified measures were evaluated against pre-determined and standard criteria.
Results: Eighteen tools met inclusion criteria: 11 were developed to assess unmet patient palliative care needs. Of those, 6 were generic, 4 were developed for heart failure and 1 was developed for interstitial lung disease. Seven tools identified those who may benefit from palliative care and include general and disease-specific indicators. The psychometric qualities of the tools varied. None met all of the accepted criteria for psychometric rigor in heart failure or respiratory disease populations. There is limited implementation of needs assessment tools in practice.
Conclusion: Several tools were identified, however further validation studies in heart failure and respiratory disease populations are required. Rigorous evaluation to determine the impact of adopting a systematic needs-based approach for heart failure and lung disease on the physical and psychosocial outcomes of patients and carers, as well as the economic costs and benefits to the healthcare system, is required.
Introduction
Practice guidelines from multiple societies and international policy documents emphasize the importance of delivering equitable and appropriate palliative care to people diagnosed with advanced heart failure (HF) and chronic respiratory diseases, such as chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD) [e.g., (1–5)]. People living with these progressive conditions will eventually experience physical function decline, as well as changes to their psychological, social and spiritual functioning and wellbeing (6–8). Despite comparable mortality rates and symptom burden, fewer people with these conditions are referred to palliative care services and when they are it is typically later compared to those with a cancer diagnosis (9–11). For instance, a Canadian retrospective population-based study reported that significantly fewer patients with COPD received specialist palliative care (SPC) compared to those with lung cancer (20 vs. 57%) (12). A UK population based study of over 92,000 patients with COPD found only 7.8% of the cohort received SPC (13). A systematic review of studies with patients with ILD reported palliative care involvement ranging from 0 to 38% (14). Similar data have been reported for patients with HF in the USA (15, 16), UK (17), Australia (18), Canada (19) and Europe.
A range of patient-, provider- and system- related factors contribute to non-referrals, late or crisis referrals to palliative care for patients with chronic HF and chronic respiratory disease. Patients and families have identified denial, misperception about the potential benefits and purpose of palliative care, and negative previous experiences with services (20, 21). While some providers report feeling comfortable providing a palliative approach (22), for others there is uncertainty about the role of palliative care and when this approach should be introduced (23, 24). Health care providers' poor recognition of their patient's palliative care needs can be impacted by time constraints, a lack of education or training, and awareness or availability of standardized tools and referral pathways (20, 22–25). Some health care providers perceive palliative care is not as useful for non-malignant conditions or that SPC services prioritize patients with cancer (26). Limited availability of SPC services and workforce shortages also limit timely referrals (20, 23, 25, 27). Poor integration of palliative care and cardiology and respiratory services has been reported (28).
In addition to the aforementioned factors, one of the most pertinent barriers to palliative care referrals remains the ongoing reliance on diagnosis and estimated prognosis as the main trigger for palliative care referral (9, 27). Diagnosis-based approaches have contributed to the over-representation of cancer patients in SPC services. Prognosis as a prompt for palliative care is also problematic, given the unpredictable trajectory (29) and evidence of inaccurate estimates by clinicians for patients with progressive chronic diseases (9). For instance, respiratory providers and general practitioners report reliance on the “surprise question” (SQ), which asks clinicians “Would you be surprised if this patient died in the next 12 months?”, to promote referrals (30), despite reports of poor to modest prognostic accuracy across studies of patients diagnosed with organ failure, cancer and those attending general practice (31, 32).
A shift from prognosis and diagnosis-based approaches to a needs-based approach for guiding delivery of care has been advocated by international bodies such as Palliative Care Australia and the World Health Organization (WHO). Underpinning this approach is the timely recognition of needs and the delivery of holistic care by non-palliative care specialists to all those with a life-limiting illness. Studies highlight high levels of unmet needs across physical, psychological, social, practical and information domains for patients with HF and COPD, and their carers (33, 34). Therefore, a key component to support the successful integration of a needs-based approach in clinical practice requires the rigorous development, testing and implementation of tools that can accurately assess palliative care needs across a range of settings and diseases (35). Needs assessment tools have been broadly categorized into two groups: those developed to assist in the early identification of individuals who would benefit from palliative care; and those developed to identify and monitor unmet palliative and supportive care needs (35). Factors to consider in tool selection include the: (i) purpose, context and target population being assessed; (ii) the acceptability of the tool to patients, families and health care professionals; (iii) and the psychometric qualities of the instrument (35). Introduction of these tools requires a structured approach, given the potential impact on patients and services, with a particular emphasis on acceptability, feasibility and cost-effectiveness.
Aims
This systematic review examined palliative care needs tools for people diagnosed with advanced HF or chronic respiratory diseases, to determine their: (1) psychometric quality; and (2) acceptability, feasibility and clinical utility when implemented in clinical practice.
Methods
Literature Search
The electronic databases Medline, CINAHL, Embase, Psycinfo and Cochrane were searched using a combination of Medical Subject Headings (MeSH) and keywords (see Supplementary Table 1 for the full search strategy). Major search terms included: “needs assessment,” “unmet needs,” “palliative care,” “hospice and palliative care,” in addition to general and more specific search terms for advanced HF and the major types of chronic respiratory disease. Searches were limited to studies published from the earliest records for each database until June 2021 and studies conducted with humans. The reference lists of included studies and the reference lists of relevant review articles were also manually searched to identify any additional studies.
Inclusion and Exclusion Criteria
Studies were included if they: (i) focused on people diagnosed with HF or chronic respiratory disease (e.g., COPD, ILD); (ii) included a tool that aimed to identify individuals for whom a palliative approach is required or assess palliative care needs; (iii) examined psychometric properties, acceptability, feasibility or clinical utility of a palliative care tool; and (iv) included primary collected data. Studies that included a heterogeneous sample of patients including HF and/or chronic respiratory disease patients, were included if they reported outcomes separately for the target population(s); or reported on a sample comprising at least 50% of the target populations.
Studies were excluded if they: (i) were published in a language other than English; (ii) examined tools assessing aspects of health or care other than needs, such as symptoms (e.g., Edmonton Symptom Assessment Scale, St George Respiratory Questionnaire), quality of life (e.g., Minnesota Living with Heart Failure), functional status (e.g., Australian Karnofsky Performance Scale), satisfaction with care (e.g., Quality Care Questionnaire- Palliative Care); (iii) focused on one needs domain, and (iv) were reviews, case studies, commentaries, theses, conference abstracts, protocols or editorials.
Study Screening
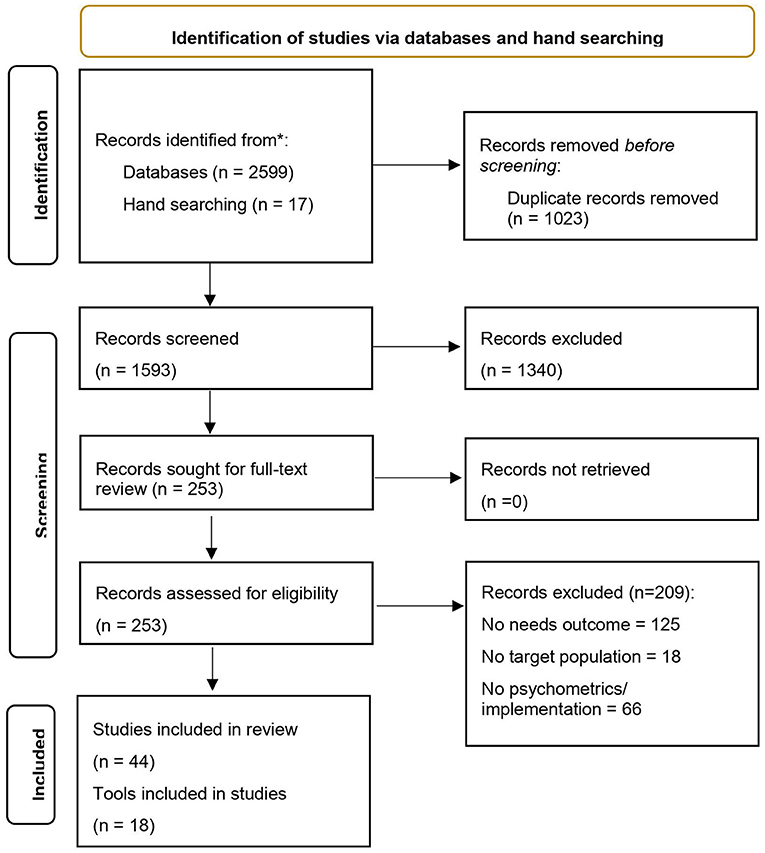
Article screening and coding was conducted using the reference management system Covidence. Following removal of duplicate citations, reviewers (AW, BH, KF) independently screened the titles and abstracts of all retrieved studies according to inclusion and exclusion criteria. Discrepancies were resolved by consensus between reviewers, or where there was insufficient detail available to exclude on the basis of study title and abstract, these studies progressed to full-text review. Pairs of reviewers (AW, BH and KF) independently assessed full-text articles for their eligibility for inclusion. Reasons for excluding studies at full-text review were documented (Figure 1 PRISMA flow diagram of included studies). If discrepancies between reviewers for study inclusion could not be resolved by consensus, a field expert (KC) was consulted as a fourth reviewer to determine inclusion. Three authors (AW, BH and KF) undertook data extraction. Discrepancies were resolved by consensus.
Data Extraction
Characteristics for Studies Examining Psychometric Properties of Existing Tools
Study characteristics and the sample used to develop and/or validate each of the included tools were extracted for all psychometric articles: (a) population; (b) country and setting, (c) purpose; (d) tool completion; (e) domains and items; (f) question format and (g) psychometrics. The psychometric properties were evaluated against pre-determined and generally accepted criteria including: reliability (internal consistency, inter-rater reliability and test-retest); validity (face, content, construct, and criterion); responsiveness; and cross-cultural adaptation, summarized in Table 2.
Characteristics of Studies Examining Implementation of Existing Tools
Study data extracted from each study implementing the included tools: (a) study design and aims; (b) setting and sample characteristics; (c) evaluation/intervention strategies; and (d) summary of outcomes.
Data Synthesis
A narrative approach was taken to synthesis the psychometric and implementation data of studies examining the included tools.
Results
Search Results
An overview of the search results and study coding process is outlined in Figure 1 using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram. The initial search yielded 2,616 articles. After removing 1,023 duplicates, 1,593 articles were included in the title and abstract screen. A total of 253 studies were included in the full-text review, of which, 44 met inclusion criteria (30 studies of 18 tools assessing psychometric properties with the target populations; 14 implementation studies) (see Tables 1, 2).
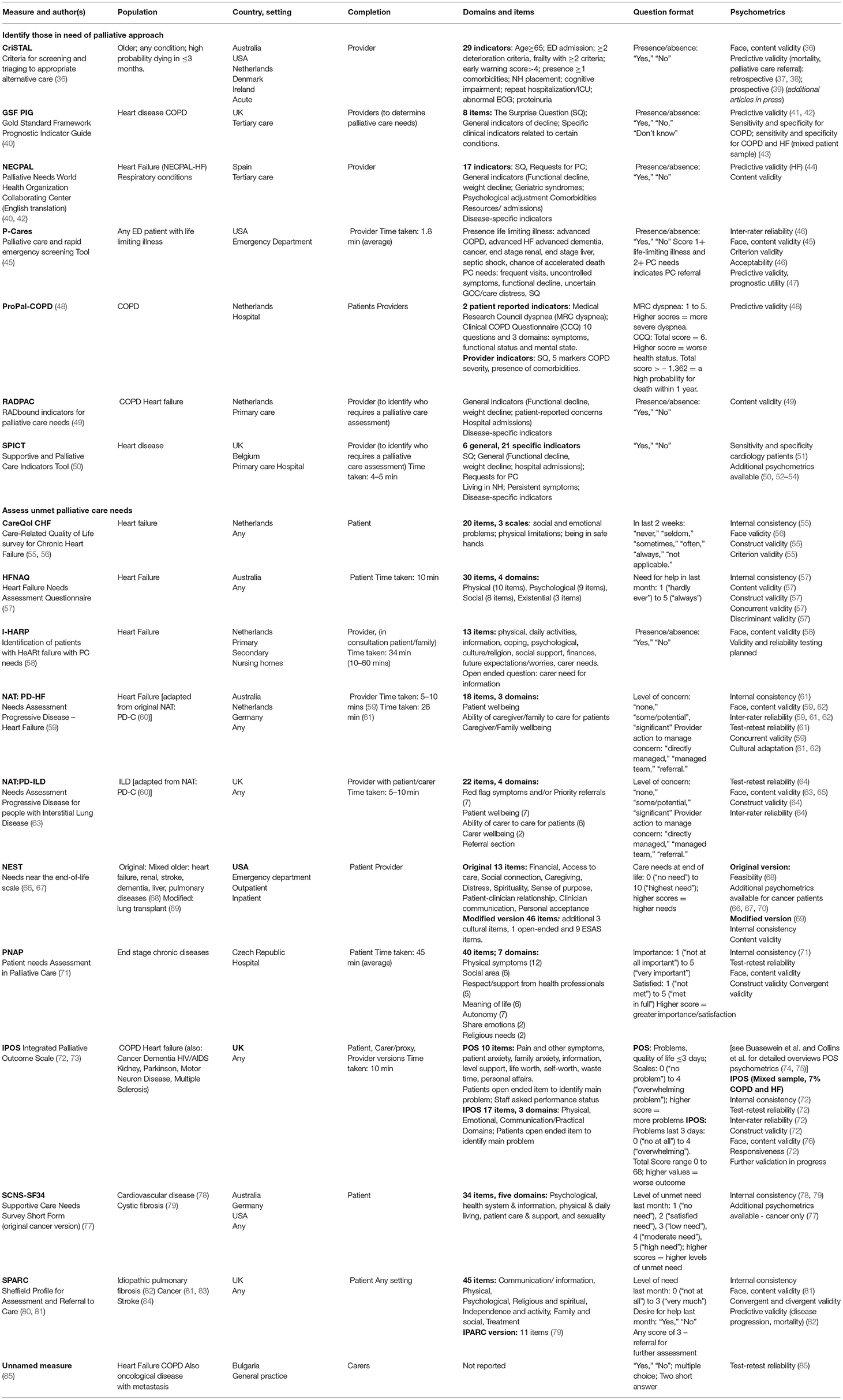
Table 1. Study and sample characteristics used to develop and/or psychometrically test identified tools.
Properties of Identified Tools
Purpose, Population and Context
As seen in Table 1, 11 tools have been developed with the primary aim of assessing and monitoring unmet needs across the spectrum of palliative care domains. Generic measures suitable for assessment of needs across a range of chronic diseases included the Integrated Palliative Outcome Scale (IPOS), which is one of the most established and well-validated tools in palliative care, as well as the Needs near the end-of-life scale (NEST); Patient Needs Assessment in Palliative Care (PNAP); Supportive Care Needs Survey Short Form (SCNS-SF34); Sheffield Profile for Assessment and Referral to Care (SPARC) and an unnamed proxy-completed measure. The remaining tools were developed and tested among people diagnosed with HF (Care-Related Quality of Life survey for Chronic Heart Failure [CareQol CHF]; Needs Assessment Tool: Progressive Disease – Heart Failure [NAT: PD-HF]; Heart Failure Needs Assessment Questionnaire [HFNAQ]; and Identification of patients with HeARt failure with PC needs [I-HARP]) or chronic respiratory diseases (Needs Assessment Progressive Disease for people with Interstitial Lung Disease [NAT: PD-ILD]). Two of these tools, the NAT: PD-HF and NAT: PD-ILD, included items that assessed the needs of both patients diagnosed with HF or ILDs and their carers within the same instrument.
The remaining seven tools incorporate broader assessments that include general and disease-specific indicators with the primary aim of identifying people with progressive chronic diseases who are at risk of deteriorating and may benefit from palliative care across a range of settings. These include the Supportive and Palliative Care Indicators Tool (SPICT) for application across care settings (50); the Gold Standard Framework Prognostic Indicator Guide (GSF PIG) tested in tertiary care (40); the RADbound indicators for Palliative Care Needs (RADPAC) tool developed to support general practitioners (GPs) (49); and the Palliative Needs WHO Collaborating Center (NECPAL- CCOMs) tool, adapted from the SPICT and GSF PIG (40). Hospital-specific tools include the Criteria for Screening and Triaging to Appropriate alternative care (CrisTAL) tool for older person likely to die within the next 3 months (36); and the Palliative Care and Rapid Emergency Screening (P- CaRES) tool (45). The ProPal-COPD was developed for application for patients with COPD (48).
Reliability
Internal Consistency
Eight tools assessed the internal consistency of the scale. Of these, four reported adequate Cronbach's alphas [exceeding 0.70 (96)] for the total scale and each domain (CareQol CHF, HFNAQ, SCNS-SF34 [in cardiovascular population], PNAP). For the IPOS, SCNS-SF34 (in cystic fibrosis population) and NEST, internal consistency was partially confirmed (Cronbach's alpha of <0.70 for at least one domain).
Test-Retest Reliability
Only four tools examined test-retest reliability. One met the criteria (k > 0.60) for the total scale and each domain (PNAP); for the remainder (IPOS, NAT: PD-ILD, unnamed measure) test-retest reliability was partially confirmed (k <0.60 for at least one domain).
Inter-rater Reliability
Inter-rater reliability was assessed for three tools, including the P-Cares, NAT: PD-HF and NAT: PD-ILD using hypothetical case vignettes and video simulated consultations. Inter-rater reliability was confirmed (IRR cutoff of Gwet's AC1 = 0.8) for the P-Cares. At least moderate agreement was found across all items in the NAT: PD-HF (prevalence and bias-adjusted kappa range 0.54-0.90); while inter-rater reliability was partially confirmed for the NAT: PD-ILD (5/16 items had moderate agreement, 11/16 had fair agreement). Inter-rater reliability was explored for the IPOS using patient-staff and staff-staff ratings of 376 patients receiving palliative care in a range of settings in two countries (72). Kappa scores (at least ≥= 0.4) were reported for 11 of 17 IPOS items.
Validity
Face and Content Validity
Face and/or content validity was reported for 12 tools. To establish face and content validity, approaches included reviewing previous literature on palliative care needs (CrisTAL, P-Cares, PNAP, RADPAC), adapting items derived from existing tools (CrisTAL, NECPAL, NAT:PD-HF, NAT: PD-ILD, NEST, PNAP, IPOS); and using expert panels and/or focus groups and interviews with health care providers, patients and/or caregivers to derive or refine selected items (CareQol CHF, CrisTAL, NEST, NAT:PD-HF, NAT: PD-ILD, P-Cares, PNAP, IPOS, RADPAC). Some studies employed multiple strategies to select and refine items (HFNAQ, I-HARP, P-Cares, SPARC).
Construct Validity
Adequate construct validity was demonstrated for four tools, with mixed results reported for the NAT: PD-HF. Convergent and divergent validity were examined against other existing tools (CareQol CHF, NAT: PD-HF, NAT: PD-ILD, IPOS, PNAP). Factor analysis was performed to examine construct validity (CareQol CHF, IPOS). Construct validity has also been established for original versions of some tools (e.g., POS, NEST, SPARC, SCNS-SF34). While evidence for construct validity in HF and chronic respiratory disease populations were not available for all tools and all disease-specific subscales reviewed, some authors reported that additional data is forthcoming (e.g., IPOS, I-HARP).
Criterion Validity
Some tools assessing level of unmet need examined criterion validity through comparison with established measures. Adequate criterion validity was established for the CareQol CHF and P-Cares. Three studies of the NAT: PD-HF demonstrated mixed results in relation to construct and criterion validity (17, 59, 61). Other studies focused primarily on examining the predictive validity of tools used to identify those in need of palliative care, particularly in relation to predicting disease progression, mortality and/or palliative care referral (CrisTAL, GSF-PIG, NECPAL, P-Cares, ProPal-COPD, SPARC and SPICT tools).
Responsiveness
There was limited evidence found for tool responsiveness (or sensitivity) to change over time, with only one study examining this psychometric property. A change of 5 points in the total IPOS score was reported to represent a moderate effect size in a mixed palliative population (72).
Administration Mode and Acceptability
Nine tools were completed by health care providers, two included both patient and provider assessment, and seven were self-completed by patients and/or their family or carer proxies. Acceptability was typically evaluated by assessing the length of time taken to complete the tool, reading ease and number of missing items. Where reported, average completion time ranged between 2 min (e.g., P-Cares) and 45 min (e.g., PNAP). Readability was reported for the IPOS, however, no further details were provided in relation to how this was examined (90). Only one study reported the proportion of missing items. A non-response rate of 6% was reported for the IPOS questionnaire, a value greater than the 5% threshold for acceptability (90). Respondent feedback was also obtained about ease of use, clarity, and comprehensiveness of the items for some tools. Further evidence of acceptability, feasibility and clinical utility of tools when implemented in clinical practice is summarized below and in Table 2.
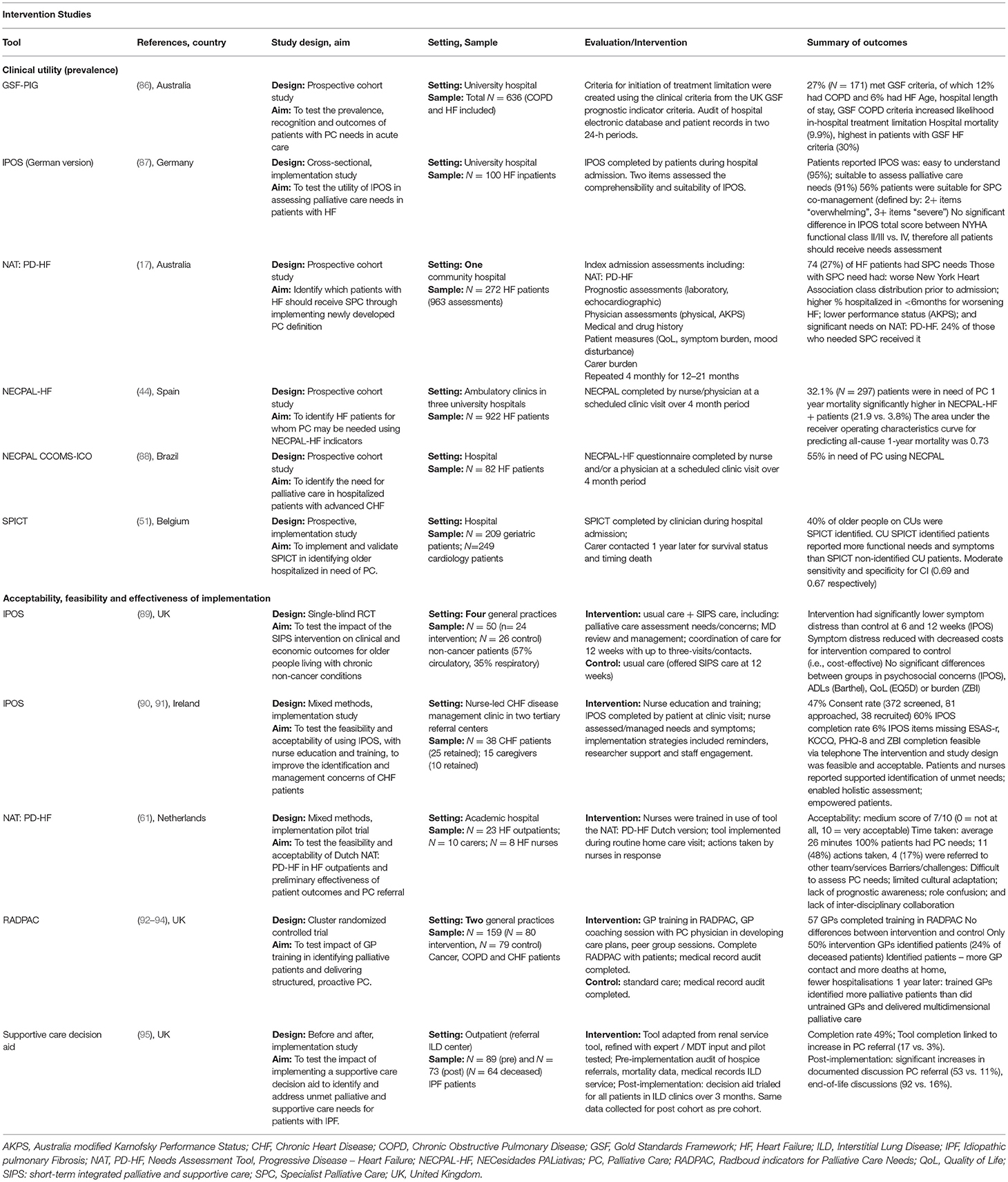
Table 2. Summary of studies summarizing the implementation of tools to identify and assess palliative care needs.
Acceptability, Feasibility and Clinical Utility of Implemented Tools
The feasibility of using tools to identify patients in need of palliative care in a range of settings was explored (Table 2). Tools such as the GSF-PIG (86), SPICT (51) and NECPAL (44, 88) were used to identify the proportion of HF and COPD patients in need of palliative care across general practice, hospital and outpatient settings. A prospective cohort study incorporated the NAT: PD-HF in a battery of assessment tools to test a newly developed definition of need for SPC in patients hospitalized with HF (17). Palliative care needs were identified for 27% of patients, however NAT: PD-HF score alone did not significantly predict PC needs. Utility of the German version of the IPOS was reported in a study of hospitalized HF patients, with 56% patients identified as suitable for palliative care (87).
Five studies examined the implementation of the tool(s) alone or as part of a broader intervention on care processes and services outcomes. A pilot implementation trial of a NAT: PD-HF intervention combined with nurse training did not improve communication about PC needs (61). No improvements in symptom burden, physical functioning, care dependency, or caregiver burden, end of life documentation or health care utilization were recorded, however, the intervention was not adequately powered for efficacy testing. In a mixed-methods, implementation study, use of the IPOS in a HF clinic, supported by nurse education and implementation strategies (reminders, staff engagement and research support), was found to be acceptable and feasible (90). Patients and nurses reported the approach improved recognition of needs, facilitated a more holistic assessment and empowered patients; however, some nurses reported uncertainty when it came to addressing identified needs (91). A small before and after study reported benefits of a shared care pathway and supporting tools for patients with HF, including improved access to palliative care, preferred place of death and access to a holistic HF service from point of care to the end of life (97). A cluster randomized controlled trial involved training GPs in identifying patients in need of palliative care and care planning using the RADPAC (93). Among deceased patients in both study groups, no differences were found for out-of-hours contact, GP contacts, place of death, or hospitalisations (93). However, the sub-group of identified patients had more GP contacts, less hospitalisations and were more likely to die at home. Longer-term outcomes, assessed 12-months later found trained GPs identified more palliative patients (most with a cancer diagnosis) and delivered multidimensional palliative care more often than untrained GPs (94). Another before and after implementation study of a supportive care decision aid with ILD patients found that completion was linked to increase in palliative care referral (17 vs. 3%) (95). Significant increases in documented discussions of palliative care referral (53 vs. 11%) and end-of-life discussions (92 vs. 16%) were reported for the post-implementation cohort. Effectiveness and cost-effectiveness were reported in only one trial of a needs-based palliative and supportive care intervention, with significant reductions in symptom distress (measured by IPOS) of older people living with chronic non-cancer (89).
Discussion
This systematic review examined the psychometric quality, acceptability and clinical utility of needs assessment tools in identifying and addressing the palliative care needs of people with HF and chronic respiratory diseases. None of the tools included in this review met all psychometric criteria. Evidence for the acceptability and clinical utility of using the tools in these populations in clinical practice is limited.
A two stage process for needs assessment in routine practice has been proposed in the literature (35, 98). The first stage requires a pragmatic method of identifying those who are currently experiencing, or are likely to develop, palliative care needs (35, 98). Brief tools may be most appropriate for this purpose, particularly in busy settings with limited resources. These tools may also be more feasible for these patients, given the expected gradual, abrupt or intermittent functional decline as they progress toward the end of life. However, no tools identified in this review were designed to provide a brief snapshot of the needs of the target population (i.e., <5 min). The IPOS, NAT: PD-HF and NAT: PD-ILD, with an estimated completion time of 10 min, offer opportunity for development in this area. Alternatively, short provider-completed tools, such as the SPICT, NECPAL and RADPAC, may be useful as a first step in identifying those for whom a palliative approach may be beneficial (98–100). Disadvantages of these tools include their generic nature, that they do not quantify the severity or nature of the palliative care needs, and a lack of action prompts to address needs. Instead, these tools focus primarily on disease-related indicators (98, 99). This could result in under-recognition of holistic needs across psychological, social, cultural, and spiritual domains as defined by the WHO (101).
The second stage should involve the use of tools that facilitate a more comprehensive assessment of the nature and severity of needs patients may experience across domains (35, 98, 100). The mode of administration and potential burden remain important considerations for selection. Self-report tools, such as the HFNAQ, CareQol CHF and PNAP, place the individual patient as the expert, potentially promoting a person-centered approach to care. However, some self-report tools may be too burdensome for patients who are facing the end of their life and/or experiencing severe exacerbations. For instance, the estimated completion time for the PNAP is 45 min. Self-report tools are also challenging to implement with patients who are acutely unwell or close to death. Tools that rely on proxy ratings, in contrast, can minimize patient burden, but ratings may not always accurately reflect patients' perceptions of what is most important to them or where they want support. Some tools, such as the IPOS, NAT: PD-HF, I-HARP and NAT: PD-ILD were developed to provide a combination of patient-proxy ratings, either through the completion of different versions of the tool (IPOS) or by completing the tool during consultations with patients and/or family members (NATs and IHARP). While the former enables a comparison of ratings to inform care planning, an advantage of the latter approach is that it enables a real-time discussion of what is most important to the patient, as well as the acceptability of actions that providers may suggest to address identified needs. This, however, has implications for time burden, highlighting the importance of exploring impact on time and resources.
Underpinning the development of needs assessment tools, is the perception that these tools can be feasibily implemented so that patients with identified needs can receive appropriate care, leading to an improvement in outcomes. Our review identified few studies examining the acceptability, feasibility and clinical utility of tools in routine practice. This suggests to date, few data report work in this area for for HF and respiratory disease when compared with measures development and descriptive research. Many were single-center, cross-sectional studies aimed at estimating prevalence. The settings in which these tools were implemented varied considerably, with the majority focused on a heterogeneous population in which people with HF or respiratory diseases comprised a smaller proportion. Data on acceptability from the perspective of the health care team implementing the tool, as well as level of burden and additional support and resources required to successfully implement care plans developed as a consequence, were rarely examined. To date, the RADPAC is the only identification tool which has been tested in a methodologically rigorous controlled trial. Despite being introduced within the context of a multi-component package that included GP education and training, no significant differences were found between the intervention and control group. The finding that a sub-group of identified patients reported more home deaths and fewer hospitalizations, and that trained GPs identified more palliative patients and delivered more palliative care, suggests utility and effectiveness warrants further examination. Organizations such as the European Association for Palliative Care Task Force have recommended the SPICT for use in HF populations (99), however, acknowledge further work is needed to validate this tool.
Most studies involved implementing tools without consideration of actions to be taken to address recognized needs. As part of this, a key challenge for needs-based approach is to determine the most appropriate methods for scoring unmet needs surveys and determining what constitutes a clinically significant change. Further, a lack of education and training for the providers involved was highlighted as an important limitation. For instance, in a Dutch study involving nurses implementing the NAT: PD-HF, nurses reported lacking the knowledge and training to address identified needs (61). In the case of the NAT: PD-HF and NAT: PD-ILD, these actions are largely based on clinical judgement, without clear criteria for referral. Evidence of effectiveness and cost-effectiveness for improving outcomes is also lacking.
Implications for Research, Practice and Policy
There is emerging evidence that palliative care is an effective approach for people diagnosed with HF and chronic respiratory conditions. Traditional palliative care approaches rely on prognosis and diagnosis as triggers for referral (102). However, the poor utility of available prognostic tools and the ambiguous relationship between prognosis and palliative care needs suggest that prognostication may not be an appropriate trigger (9, 26, 31, 99, 103). Implementing approaches confirmed as efficacious in one patient cohort, such as cancer, and translating them into practice with other non-malignant cohorts is insufficient given their unique burden and complexities (102). A needs-based approach offers a promising alternative, but the rigor of the approach must be established before such processes are accepted and widely implemented. The limited evidence for successful implementation and the psychometric shortcomings of existing tools, demonstrates the importance of psychometrically robust tools to progress the field. Further validation of tools that can reliably and repeatedly assess unmet needs across the broad range of palliative care domains, as well as identify changes in needs over time, is required. The interpretation and utility of these tools with HF and chronic respiratory populations also requires further development of criteria defining clinical significance and clinically important changes in needs.
Identification of needs must also be supported by care processes and actions that are informed by best available evidence, align with needs and do not cause undue harm. Structured care processes (e.g., care bundles) potentially have numerous benefits for delivering good clinical care, while also facilitating measurement and feedback processes (104, 105). Studies quantifying the nature, severity and trajectory of unmet needs for HF and chronic respiratory conditions can inform the selection of care processes with which to intervene. Generalist and specialist providers should receive targeted education and training to ensure they are equipped with the skills to: recognize palliative care needs; appropriately communicate this with patients; and provide appropriate care (102). Promoting earlier identification of palliative care needs and appropriate care planning, tailored to medical conditions, has the potential to achieve hospital avoidance, death in place of choice, better symptom control and less family distress. Improvements in planning and clinical care can also potentially reduce the distress experienced by health professionals in this field.
Study Limitations
A strength of this review include the systematic literature search that encompassed a wide range of broad search terms and multiple databases. However, gray literature, dissertations or policy documents were not included, as while this literature contributes important information, it is not peer-reviewed. Publications were also restricted to English language, which may have resulted in some studies being missed.
Conclusion
The impetus to develop and implement tools for palliative care is reflected in the increasing number of needs assessment tools being developed and tested with HF and chronic respiratory disease populations. However, further evidence of psychometric quality is needed, particularly test-retest reliability, predictive validity, responsiveness, and clinical utility of these tools. Further, relying on “needs” as the recommended criterion must be supported by a systematic approach that incorporates structured care processes; improved community awareness of the potential benefits offered by palliative care; and education and training for providers across care settings. Rigorous evaluation to determine the impact of adopting a systematic needs-based approach for HF and chronic respiratory disease on the physical and psychosocial outcomes of patients and carers, as well as the economic costs and benefits to the healthcare system, is required.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
AW: conceptualization. AW, KF, and BH: screening of articles and data extraction (methodology). AW, BH, KF, and KC: analysis, interpretation, and writing (original draft preparation). All authors contributed to the final version of the article and approved the submitted version.
Funding
BH is supported by a Colin Dodds Australian Rotary Health Postdoctoral Fellowship (G1801108). This research was supported by the National Health and Medical Research Council via a Dementia Research Team grant (1095078) and also infrastructure funding from the University of Newcastle and Hunter Medical Research Institute.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge the assistance of Angela Smith from Hunter New England Health Libraries who assisted with refining and conducting the literature search.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.878428/full#supplementary-material
References
1. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur Respir J. (2017) 49:1700214. doi: 10.1183/13993003.00214-2017
2. Lanken P, Terry P, Delisser H, Fahy B, Hansen-Flaschen J, Heffner J, et al. An Official American Thoracic Society Clinical Policy Statement: Palliative Care for Patients with Respiratory Diseases and Critical Illnesses. Am J Respir Crit Care Med. (2008) 177:912–27. doi: 10.1164/rccm.200605-587ST
3. Ponikowski P, Voors A, Anker S, Bueno H, Cleland J, Coats A, et al. Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. (2017) 70:776–803. doi: 10.1016/j.jacc.2017.04.025
5. Prasad JD, Mahar A, Bleasel J, Ellis SJ, Chambers DC, Lake F, et al. The interstitial lung disease multidisciplinary meeting: a position statement from the Thoracic Society of Australia and New Zealand and the Lung Foundation Australia. Respirology. (2017) 22:1459–72. doi: 10.1111/resp.13163
6. Lee JYT, Tikellis G, Corte TJ, Goh NS, Keir GJ, Spencer L, et al. The supportive care needs of people living with pulmonary fibrosis and their caregivers: a systematic review. Eur Respir Rev. (2020) 29:190125. doi: 10.1183/16000617.0125-2019
7. Carvajalino S, Reigada C, Johnson MJ, Dzingina M, Bajwah S. Symptom prevalence of patients with fibrotic interstitial lung disease: a systematic literature review. BMC Pulm Med. (2018) 18:78. doi: 10.1186/s12890-018-0651-3
8. Bierle RS, Vuckovic KM, Ryan CJ. Integrating Palliative Care Into Heart Failure Management. Crit Care Nurse. (2021) 41:e9–e18. doi: 10.4037/ccn2021877
9. Rajnoveanu RM, Rajnoveanu AG, Fildan AP, Todea DA, Man MA, Motoc NS, et al. Palliative Care Initiation in Chronic Obstructive Pulmonary Disease: Prognosis-Based, Symptoms-Based or Needs-Based? Int J Chron Obstruct Pulmon Dis. (2020) 15:1591–600. doi: 10.2147/COPD.S254104
10. Siouta N, van Beek K, Preston N, Hasselaar J, Hughes S, Payne S, et al. Towards integration of palliative care in patients with chronic heart failure and chronic obstructive pulmonary disease: a systematic literature review of European guidelines and pathways. BMC Palliat Care. (2016) 15:18. doi: 10.1186/s12904-016-0089-4
11. Fadol AP, Patel A, Shelton V, Krause KJ, Bruera E, Palaskas NL. Palliative care referral criteria and outcomes in cancer and heart failure: a systematic review of literature. Cardiooncology. (2021) 7:32. doi: 10.1186/s40959-021-00117-8
12. Kendzerska T, Nickerson JW, Hsu AT, Gershon AS, Talarico R, Mulpuru S, et al. End-of-life care in individuals with respiratory diseases: a population study comparing the dying experience between those with chronic obstructive pulmonary disease and lung cancer. Int J Chron Obstruct Pulmon Dis. (2019) 14:1691–701. doi: 10.2147/COPD.S210916
13. Bloom CI, Slaich B, Morales DR, Smeeth L, Stone P, Quint JK. Low uptake of palliative care for COPD patients within primary care in the UK. Eur Respir J. (2018) 51:1701879. doi: 10.1183/13993003.01879-2017
14. Palmer E, Kavanagh E, Visram S, Bourke AM, Forrest I, Exley C. Which factors influence the quality of end-of-life care in interstitial lung disease? A systematic review with narrative synthesis. Palliat Med. (2021) 36:237–53. doi: 10.1177/02692163211059340
15. Liu AY, O'Riordan DL, Marks AK, Bischoff KE, Pantilat SZ. A Comparison of Hospitalized Patients With Heart Failure and Cancer Referred to Palliative Care. JAMA Network Open. (2020) 3:e200020-e. doi: 10.1001/jamanetworkopen.2020.0020
16. Warraich HJ, Wolf SP, Mentz RJ, Rogers JG, Samsa G, Kamal AH. Characteristics and Trends Among Patients With Cardiovascular Disease Referred to Palliative Care. JAMA Netw Open. (2019) 2:e192375. doi: 10.1001/jamanetworkopen.2019.2375
17. Campbell RT, Petrie MC, Jackson CE, Jhund PS, Wright A, Gardner RS, et al. Which patients with heart failure should receive specialist palliative care? Eur J Heart Fail. (2018) 20:1338–47. doi: 10.1002/ejhf.1240
18. Rosenwax L, Spilsbury K, McNamara BA, Semmens JB. A retrospective population based cohort study of access to specialist palliative care in the last year of life: who is still missing out a decade on? BMC Palliat Care. (2016) 15:46. doi: 10.1186/s12904-016-0119-2
19. Seow H, O'Leary E, Perez R, Tanuseputro P. Access to palliative care by disease trajectory: a population-based cohort of Ontario decedents. BMJ Open. (2018) 8:e021147. doi: 10.1136/bmjopen-2017-021147
20. Abedini NC, Guo G, Hummel SL, Bozaan D, Beasley M, Cowger J, et al. Factors influencing palliative care referral for hospitalised patients with heart failure: an exploratory, randomised, multi-institutional survey of hospitalists and cardiologists. BMJ Open. (2020) 10:e040857. doi: 10.1136/bmjopen-2020-040857
21. Kim JW, Atkins C, Wilson AM. Barriers to specialist palliative care in interstitial lung disease: a systematic review. BMJ Support Palliat Care. (2019) 9:130–8. doi: 10.1136/bmjspcare-2018-001575
22. Smallwood N, Currow D, Booth S, Spathis A, Irving L, Philip J. Attitudes to specialist palliative care and advance care planning in people with COPD: a multi-national survey of palliative and respiratory medicine specialists. BMC Palliat Care. (2018) 17:115. doi: 10.1186/s12904-018-0371-8
23. Crimmins RM, Elliott L, Absher DT. Palliative Care in a Death-Denying Culture: Exploring Barriers to Timely Palliative Efforts for Heart Failure Patients in the Primary Care Setting. Am J Hosp Palliat Care. (2021) 38:77–83. doi: 10.1177/1049909120920545
24. Kavalieratos D, Mitchell EM, Carey TS, Dev S, Biddle AK, Reeve BB, et al. “Not the ‘grim reaper service”': an assessment of provider knowledge, attitudes, and perceptions regarding palliative care referral barriers in heart failure. J Am Heart Assoc. (2014) 3:e000544. doi: 10.1161/JAHA.113.000544
25. Bonares MJ, Mah K, MacIver J, Hurlburt L, Kaya E, Rodin G, et al. Referral Practices of Cardiologists to Specialist Palliative Care in Canada. CJC Open. (2021) 3:460–9. doi: 10.1016/j.cjco.2020.12.002
26. Philip J, Crawford G, Brand C, Gold M, Miller B, Hudson P, et al. A conceptual model: Redesigning how we provide palliative care for patients with chronic obstructive pulmonary disease. Palliat Support Care. (2018) 16:452–60. doi: 10.1017/S147895151700044X
27. Romanò M. Barriers to Early Utilization of Palliative Care in Heart Failure: a Narrative Review. Healthcare (Basel). (2020) 8:36. doi: 10.3390/healthcare8010036
28. Hill L, Prager Geller T, Baruah R, Beattie JM, Boyne J, de Stoutz N, et al. Integration of a palliative approach into heart failure care: a European Society of Cardiology Heart Failure Association position paper. Eur J Heart Fail. (2020) 22:2327–39. doi: 10.1002/ejhf.1994
29. Maddocks M, Lovell N, Booth S, Man WDC, Higginson IJ. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet. (2017) 390:988–1002. doi: 10.1016/S0140-6736(17)32127-X
30. Broese JMC, van der Kleij RMJJ, Verschuur EML, Kerstjens HAM, Engels Y, Chavannes NH. Provision of Palliative Care in Patients with COPD: a survey among pulmonologists and general practitioners. Int J Chron Obstruct Pulmon Dis. (2021) 16:783–94. doi: 10.2147/COPD.S293241
31. White N, Kupeli N, Vickerstaff V, Stone P. How accurate is the 'Surprise Question' at identifying patients at the end of life? A systematic review and meta-analysis. BMC Med. (2017) 15:139. doi: 10.1186/s12916-017-0907-4
32. Downar J, Goldman R, Pinto R, Englesakis M, Adhikari NK. The “surprise question” for predicting death in seriously ill patients: a systematic review and meta-analysis. CMAJ. (2017) 189:E484–e93. doi: 10.1503/cmaj.160775
33. Clari M, Ivziku D, Casciaro R, Matarese M. The unmet needs of people with chronic obstructive pulmonary disease: a systematic review of qualitative findings. COPD. (2018) 15:79–88. doi: 10.1080/15412555.2017.1417373
34. DeGroot L, Koirala B, Pavlovic N, Nelson K, Allen J, Davidson P, et al. Outpatient palliative care in heart failure: an integrative review. J Palliat Med. (2020) 23:1257–69. doi: 10.1089/jpm.2020.0031
35. Girgis A, Waller A, Hobden B. Palliative care needs assessment tools. In: Cherny NI, Fallon M, Kaasa S, Portenoy RK, Currow DC, editors. Oxford Textbook of Palliative Medicine. 6th ed. Oxford: Oxford University Press (2021).
36. Cardona-Morrell M, Hillman K. Development of a tool for defining and identifying the dying patient in hospital: Criteria for Screening and Triaging to Appropriate aLternative care (CriSTAL). BMJ Support Palliat Care. (2015). doi: 10.1136/bmjspcare-2014-000770
37. Cardona-Morrell M, Chapman A, Turner RM, Lewis E, Gallego-Luxan B, Parr M, et al. Pre-existing risk factors for in-hospital death among older patients could be used to initiate end-of-life discussions rather than Rapid Response System calls: a case-control study. Resuscitation. (2016) 109:76–80. doi: 10.1016/j.resuscitation.2016.09.031
38. Williams M, Cardona-Morrell M, Stevens P, Bey J, Smith Glasgow ME. Timing of palliative care team referrals for inpatients receiving rapid response services: A retrospective pilot study in a US hospital. Int J Nurs Stud. (2017) 75:147–53. doi: 10.1016/j.ijnurstu.2017.07.017
39. Cardona M, Lewis ET, Turner RM, Alkhouri H, Asha S, Mackenzie J, et al. Efficacy of a tool to predict short-term mortality in older people presenting at emergency departments: Protocol for a multi-centre cohort study. Arch Gerontol Geriatr. (2018) 76:169–74. doi: 10.1016/j.archger.2018.02.014
40. Gomez-Batiste X, Martinez-Munoz M, Blay C, Amblas J, Vila L, Costa X, et al. Identifying patients with chronic conditions in need of palliative care in the general population: development of the NECPAL tool and preliminary prevalence rates in Catalonia. BMJ Support Palliat Care. (2013) 3:300–8. doi: 10.1136/bmjspcare-2012-000211
41. Moretti C, Iqbal J, Murray S, Bertaina M, Parviz Y, Fenning S, et al. Prospective assessment of a palliative care tool to predict one-year mortality in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. (2017) 6:272–9. doi: 10.1177/2048872616633841
42. Gómez-Batiste X, Martínez-Muñoz M, Blay C, Amblàs J, Vila L, Costa X, et al. Utility of the NECPAL CCOMS-ICO© tool and the Surprise Question as screening tools for early palliative care and to predict mortality in patients with advanced chronic conditions: a cohort study. Palliat Med. (2017) 31:754–63. doi: 10.1177/0269216316676647
43. Noppe D, Veen HI, Mooren K. COPD patients in need of palliative care: Identification after hospitalization through the surprise question. Chron Respir Dis. (2019) 16:1479972318796219. doi: 10.1177/1479972318796219
44. Gastelurrutia P, Zamora E, Domingo M, Ruiz S, González-Costello J, Gomez-Batiste X. Palliative Care Needs in Heart Failure. A Multicenter Study Using the NECPAL Questionnaire. Rev Esp Cardiol (Engl Ed). (2019) 72:870–2. doi: 10.1016/j.recesp.2019.01.019
45. George N, Barrett N, McPeake L, Goett R, Anderson K, Baird J. Content Validation of a Novel Screening Tool to Identify Emergency Department Patients With Significant Palliative Care Needs. Acad Emerg Med. (2015) 22:823–37. doi: 10.1111/acem.12710
46. Bowman J, George N, Barrett N, Anderson K, Dove-Maguire K, Baird J. Acceptability and Reliability of a Novel Palliative Care Screening Tool Among Emergency Department Providers. Acad Emerg Med. (2016) 23:694–702. doi: 10.1111/acem.12963
47. Paske JRT, DeWitt S, Hicks R, Semmens S, Vaughan L. Palliative care and rapid emergency screening tool and the palliative performance scale to predict survival of older adults admitted to the hospital from the emergency department. Am J Hosp Palliat Care. (2021) 38:800–6. doi: 10.1177/1049909120960713
48. Duenk RG, Verhagen C, Bronkhorst EM, Djamin RS, Bosman GJ, Lammers E, et al. Development of the ProPal-COPD tool to identify patients with COPD for proactive palliative care. Int J Chron Obstruct Pulmon Dis. (2017) 12:2121–8. doi: 10.2147/COPD.S140037
49. Thoonsen B, Engels Y, van Rijswijk E, Verhagen S, van Weel C, Groot M, et al. Early identification of palliative care patients in general practice: development of RADboud indicators for PAlliative Care Needs (RADPAC). Br J General Pract. (2012) 62:625–31. doi: 10.3399/bjgp12X654597
50. Highet G, Crawford D, Murray SA, Boyd K. Development and evaluation of the Supportive and Palliative Care Indicators Tool (SPICT): a mixed-methods study. BMJ Support Palliat Care. (2014) 4:285–90. doi: 10.1136/bmjspcare-2013-000488
51. Piers R, De Brauwer I, Baeyens H, Velghe A, Hens L, Deschepper E, et al. Supportive and Palliative Care Indicators Tool prognostic value in older hospitalised patients: a prospective multicentre study. BMJ Support Palliat Care. (2021). doi: 10.1136/bmjspcare-2021-003042. [Epub ahead of print].
52. Afshar K, Feichtner A, Boyd K, Murray S, Junger S, Wiese B, et al. Systematic development and adjustment of the German version of the Supportive and Palliative Care Indicators Tool (SPICT-DE). BMC Palliat Care. (2018) 17:27. doi: 10.1186/s12904-018-0283-7
53. De Bock R, Van Den Noortgate N, Piers R. Validation of the supportive and palliative care indicators tool in a geriatric population. J Palliat Med. (2018) 21:220–4. doi: 10.1089/jpm.2017.0205
54. Mudge AM, Douglas C, Sansome X, Tresillian M, Murray S, Finnigan S, et al. Risk of 12-month mortality among hospital inpatients using the surprise question and SPICT criteria: a prospective study. BMJ Support Palliat Care. (2018) 8:213–20. doi: 10.1136/bmjspcare-2017-001441
55. van Kessel P, de Boer D, Hendriks M, Plass AM. Measuring patient outcomes in chronic heart failure: psychometric properties of the Care-Related Quality of Life survey for Chronic Heart Failure (CaReQoL CHF). BMC Health Serv Res. (2017) 17:536. doi: 10.1186/s12913-017-2452-4
56. Van Kessel P, Hendriks M, Van der Hoek L, Plass A. Development of the Care-Related Quality of life Measure for Chronic Heart Failure (CaReQoL CHF)(In Dutch: Ontwikkeling van de Care Related Quality of Life voor Chronisch Hartfalen (CaReQoL CHF)). Utrecht: Nivel (2015).
57. Davidson PM, Cockburn J, Newton PJ. Unmet needs following hospitalization with heart failure: implications for clinical assessment and program planning. J Cardiovasc Nurs. (2008) 23:541–6. doi: 10.1097/01.JCN.0000338927.43469.35
58. Ament SMC, van den Beuken-Everdingen M, Maessen JMC, Boyne J, Schols J, Stoffers H, et al. Professionals guidance about palliative medicine in chronic heart failure: a mixed-method study. BMJ Support Palliat Care. (2020). doi: 10.1136/bmjspcare-2020-002580. [Epub ahead of print].
59. Waller A, Girgis A, Davidson PM, Newton PJ, Lecathelinais C, Macdonald PS, et al. Facilitating needs-based support and palliative care for people with chronic heart failure: preliminary evidence for the acceptability, inter-rater reliability, and validity of a needs assessment tool. J Pain Symptom Manage. (2013) 45:912–25. doi: 10.1016/j.jpainsymman.2012.05.009
60. Waller A, Girgis A, Currow D, Lecathelinais C. Development of the palliative care needs assessment tool (PC-NAT) for use by multi-disciplinary health professionals. Palliat Med. (2008) 22:956–64. doi: 10.1177/0269216308098797
61. Janssen DJ, Boyne J, Currow DC, Schols JM, Johnson MJ, La Rocca HB. Timely recognition of palliative care needs of patients with advanced chronic heart failure: a pilot study of a Dutch translation of the needs assessment tool: progressive disease–heart failure (NAT: PD-HF). Eur J Cardiovasc Nurs. (2019) 18:375–88. doi: 10.1177/1474515119831510
62. Gonzalez-Jaramillo V, Guyer J, Luethi N, Sobanski P, Zbinden R, Rodriguez E, et al. Validation of the German version of the needs assessment tool: progressive disease-heart failure. Health Qual Life Outcomes. (2021) 19:214. doi: 10.1186/s12955-021-01817-6
63. Boland JW, Reigada C, Yorke J, Hart SP, Bajwah S, Ross J, et al. The adaptation, face, and content validation of a needs assessment tool: Progressive disease for people with interstitial lung disease. J Palliat Med. (2016) 19:549–55. doi: 10.1089/jpm.2015.0355
64. Johnson MJ, Jamali A, Ross J, Fairhurst C, Boland J, Reigada C, et al. Psychometric validation of the needs assessment tool: progressive disease in interstitial lung disease. Thorax. (2018) 73:880–3. doi: 10.1136/thoraxjnl-2017-210911
65. Reigada C, Papadopoulos A, Boland JW, Yorke J, Ross J, Currow DC, et al. Implementation of the Needs Assessment Tool for patients with interstitial lung disease (NAT:ILD): facilitators and barriers. Thorax. (2017) 72:1049–51. doi: 10.1136/thoraxjnl-2016-209768
66. Emanuel LL, Alpert HR, Baldwin DC, Emanuel EJ. What terminally ill patients care about: toward a validated construct of patients' perspectives. J Palliat Med. (2000) 3:419–31. doi: 10.1089/jpm.2000.3.4.419
67. Emanuel LL, Alpert HR, Emanuel EE. Concise screening questions for clinical assessments of terminal care: the needs near the end-of-life care screening tool. J Palliat Med. (2001) 4:465–74. doi: 10.1089/109662101753381601
68. Grudzen CR, Richardson LD, Morrison M, Cho E, Morrison RS. Palliative care needs of seriously ill, older adults presenting to the emergency department. Acad Emerg Med. (2010) 17:1253–7. doi: 10.1111/j.1553-2712.2010.00907.x
69. Pawlow PC, Blumenthal NP, Christie JD, Matura LA, Courtright KR, Aryal S, et al. The palliative care needs of lung transplant candidates. Clin Transplant. (2020) 34:e14092. doi: 10.1111/ctr.14092
70. Scandrett KG, Reitschuler-Cross EB, Nelson L, Sanger JA, Feigon M, Boyd E, et al. Feasibility and effectiveness of the NEST13+ as a screening tool for advanced illness care needs. J Palliat Med. (2010) 13:161–9. doi: 10.1089/jpm.2009.0170
71. Buzgova R, Kozakova R, Sikorova L, Zelenikova R, Jarosova D. Development and psychometric evaluation of patient needs assessment in palliative care (PNAP) instrument. Palliat Support Care. (2016) 14:129–37. doi: 10.1017/S1478951515000061
72. Murtagh FE, Ramsenthaler C, Firth A, Groeneveld EI, Lovell N, Simon ST, et al. A brief, patient- and proxy-reported outcome measure in advanced illness: Validity, reliability and responsiveness of the Integrated Palliative care Outcome Scale (IPOS). Palliat Med. (2019) 33:1045–57. doi: 10.1177/0269216319854264
73. Higghinson I,. Palliative Care Outcome Scale (2012). Available online at: http://pos-pal.org/maix/ (accessed January 03, 2022).
74. Bausewein C, Le Grice C, Simon S, Higginson I. The use of two common palliative outcome measures in clinical care and research: a systematic review of POS and STAS. Palliat Med. (2011) 25:304–13. doi: 10.1177/0269216310395984
75. Collins ES, Witt J, Bausewein C, Daveson BA, Higginson IJ, Murtagh FE. A Systematic Review of the Use of the Palliative Care Outcome Scale and the Support Team Assessment Schedule in Palliative Care. J Pain Symptom Manage. (2015) 50:842–53.e19. doi: 10.1016/j.jpainsymman.2015.07.015
76. Schildmann EK, Groeneveld EI, Denzel J, Brown A, Bernhardt F, Bailey K, et al. Discovering the hidden benefits of cognitive interviewing in two languages: the first phase of a validation study of the integrated palliative care outcome scale. Palliat Med. (2016) 30:599–610. doi: 10.1177/0269216315608348
77. Boyes A, Girgis A, Lecathelinais C. Brief assessment of adult cancer patients' perceived needs: development and validation of the 34-item Supportive Care Needs Survey (SCNS-SF34). J Eval Clin Pract. (2009) 15:602–6. doi: 10.1111/j.1365-2753.2008.01057.x
78. Kohlmann S, Kilbert MS, Ziegler K, Schulz KH. Supportive care needs in patients with cardiovascular disorders. Patient Educ Couns. (2013) 91:378–84. doi: 10.1016/j.pec.2013.01.002
79. Trandel ET, Pilewski JM, Dellon EP, Jeong K, Yabes JG, Moreines LT, et al. Prevalence of unmet palliative care needs in adults with cystic fibrosis. J Cyst Fibros. (2020) 19:394–401. doi: 10.1016/j.jcf.2019.11.010
80. Hughes P, Ahmed N, Winslow M, Walters SJ, Collins K, Noble B. Consumer views on a new holistic screening tool for supportive and palliative-care needs: Sheffield Profile for Assessment and Referral for Care (SPARC): a survey of self-help support groups in health care. Health Expect. (2015) 18:562–77. doi: 10.1111/hex.12058
81. Ahmed N, Bestall JC, Payne SA, Noble B, Ahmedzai SH. The use of cognitive interviewing methodology in the design and testing of a screening tool for supportive and palliative care needs. Support Care Cancer. (2009) 17:665–73. doi: 10.1007/s00520-008-0521-2
82. Stewart I, McKeever T, Braybrooke R, Oballa E, Simpson JK, Maher TM, et al. Patient-reported distress can aid clinical decision-making in idiopathic pulmonary fibrosis: analysis of the PROFILE cohort. Eur Respir J. (2019) 53:1801925. doi: 10.1183/13993003.01925-2018
83. Wilcock A, Klezlova R, Coombes S, Rawson A, Bentley R, Hooper D, et al. Identifying supportive and palliative care needs in people with a recent diagnosis of thoracic cancer: acceptability of the SPARC questionnaire. Thorax. (2010) 65:937–8. doi: 10.1136/thx.2009.131243
84. Burton CR, Payne S, Addington-Hall J, Jones A. The palliative care needs of acute stroke patients: a prospective study of hospital admissions. Age Ageing. (2010) 39:554–9. doi: 10.1093/ageing/afq077
85. Foreva G, Assenova R. Hidden patients: the relatives of patients in need of palliative care. J Palliat Med. (2014) 17:56–61. doi: 10.1089/jpm.2013.0333
86. Milnes S, Orford NR, Berkeley L, Lambert N, Simpson N, Elderkin T, et al. A prospective observational study of prevalence and outcomes of patients with Gold Standard Framework criteria in a tertiary regional Australian Hospital. BMJ Support Palliat Care. (2019) 9:92–9. doi: 10.1136/bmjspcare-2015-000864
87. Roch C, Palzer J, Zetzl T, Störk S, Frantz S, van Oorschot B. Utility of the integrated palliative care outcome scale (IPOS): a cross-sectional study in hospitalised patients with heart failure. Eur J Cardiovasc Nurs. (2020) 19:702–10. doi: 10.1177/1474515120919386
88. Orzechowski R, Galvão AL, Nunes TDS, Campos LS. Palliative care need in patients with advanced heart failure hospitalized in a tertiary hospital. Rev Esc Enferm USP. (2019) 53:e03413. doi: 10.1590/s1980-220x2018015403413
89. Evans CJ, Bone AE, Yi D, Gao W, Morgan M, Taherzadeh S, et al. Community-based short-term integrated palliative and supportive care reduces symptom distress for older people with chronic noncancer conditions compared with usual care: a randomised controlled single-blind mixed method trial. Int J Nurs Stud. (2021) 120:103978. doi: 10.1016/j.ijnurstu.2021.103978
90. Kane PM, Daveson BA, Ryan K, Ellis-Smith CI, Mahon NG, McAdam B, et al. Feasibility and acceptability of a patient-reported outcome intervention in chronic heart failure. BMJ Support Palliat Care. (2017) 7:470–9. doi: 10.1136/bmjspcare-2017-001355
91. Kane PM, Ellis-Smith CI, Daveson BA, Ryan K, Mahon NG, McAdam B, et al. Understanding how a palliative-specific patient-reported outcome intervention works to facilitate patient-centred care in advanced heart failure: a qualitative study. Palliat Med. (2018) 32:143–55. doi: 10.1177/0269216317738161
92. Thoonsen B, Groot M, Engels Y, Prins J, Verhagen S, Galesloot C, et al. Early identification of and proactive palliative care for patients in general practice, incentive and methods of a randomized controlled trial. BMC Fam Pract. (2011) 12:123. doi: 10.1186/1471-2296-12-123
93. Thoonsen B, Vissers K, Verhagen S, Prins J, Bor H, van Weel C, et al. Training general practitioners in early identification and anticipatory palliative care planning: a randomized controlled trial. BMC Fam Pract. (2015) 16:126. doi: 10.1186/s12875-015-0342-6
94. Thoonsen B, Gerritzen SHM, Vissers KCP, Verhagen S, van Weel C, Groot M, et al. Training general practitioners contributes to the identification of palliative patients and to multidimensional care provision: secondary outcomes of an RCT. BMJ Support Palliat Care. (2019) 9:e18. doi: 10.1136/bmjspcare-2015-001031
95. Sharp C, Lamb H, Jordan N, Edwards A, Gunary R, Meek P, et al. Development of tools to facilitate palliative and supportive care referral for patients with idiopathic pulmonary fibrosis. BMJ Support Palliat Care. (2018) 8:340–6. doi: 10.1136/bmjspcare-2017-001330
96. Streiner DL, Norman GR, Cairney J. Health Measurement Scales: A Practical Guide to Their Development and Use. Oxford University Press. (2015). pp. 2015–01.
97. Smith D. Development of an end-of-life care pathway for patients with advanced heart failure in a community setting. Int J Palliat Nurs. (2012) 18:295–300. doi: 10.12968/ijpn.2012.18.6.295
98. Remawi BN, Gadoud A, Murphy IMJ, Preston N. Palliative care needs-assessment and measurement tools used in patients with heart failure: a systematic mixed-studies review with narrative synthesis. Heart Fail Rev. (2021) 26:137–55. doi: 10.1007/s10741-020-10011-7
99. Sobanski PZ, Alt-Epping B, Currow DC, Goodlin SJ, Grodzicki T, Hogg K, et al. Palliative care for people living with heart failure: European Association for Palliative Care Task Force expert position statement. Cardiovasc Res. (2020) 116:12–27. doi: 10.1093/cvr/cvz200
100. ElMokhallalati Y, Bradley SH, Chapman E, Ziegler L, Murtagh FE, Johnson MJ, et al. Identification of patients with potential palliative care needs: A systematic review of screening tools in primary care. Palliat Med. (2020) 34:989–1005. doi: 10.1177/0269216320929552
101. World Health Organisation. Definition of Palliative Care. (2022). Available online at: https://www.who.int/health-topics/palliative-care
102. Kavalieratos D, Gelfman LP, Tycon LE, Riegel B, Bekelman DB, Ikejiani DZ, et al. Palliative Care in Heart Failure: Rationale, Evidence, and Future Priorities. J Am Coll Cardiol. (2017) 70:1919–30. doi: 10.1016/j.jacc.2017.08.036
103. Philip J, Collins A, Smallwood N, Chang YK, Mo L, Yang IA, et al. Referral criteria to palliative care for patients with respiratory disease: a systematic review. Eur Respir J. (2021) 58:2004307. doi: 10.1183/13993003.04307-2020
104. Clark K, Byfieldt N. Improving the quality of care delivered to people imminently dying in hospital by implementing a care bundle: an observational before and after feasibility study. Int J Care Coord. (2015) 18:18–26. doi: 10.1177/2053434515574788
Keywords: palliative care, lung disease, heart failure, needs assessment, psychometrics
Citation: Waller A, Hobden B, Fakes K and Clark K (2022) A Systematic Review of the Development and Implementation of Needs-Based Palliative Care Tools in Heart Failure and Chronic Respiratory Disease. Front. Cardiovasc. Med. 9:878428. doi: 10.3389/fcvm.2022.878428
Received: 18 February 2022; Accepted: 25 March 2022;
Published: 13 April 2022.
Edited by:
Piotr Z. Sobanski, Schwyz Hospital, SwitzerlandReviewed by:
Philip Larkin, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandWojciech Leppert, Poznan University of Medical Sciences, Poland
Copyright © 2022 Waller, Hobden, Fakes and Clark. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy Waller, YW15LndhbGxlckBuZXdjYXN0bGUuZWR1LmF1
 Amy Waller
Amy Waller Breanne Hobden2
Breanne Hobden2