- 1Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, South Korea
- 2Department of Preventive Medicine, Yonsei University College of Medicine, Seoul, South Korea
- 3Cancer Big Data Center, National Cancer Control Institute, National Cancer Center, Goyang, South Korea
- 4Seoul Hospital, Ewha Womans University College of Medicine, Seoul, South Korea
- 5Myongji Hospital, Hanyang University College of Medicine, Goyang, South Korea
- 6CHA Bundang Medical Center, CHA University College of Medicine, Seongnam, South Korea
Background: It is unclear whether beta-blocker treatment is advantageous in patients with stable coronary artery disease (CAD) who underwent percutaneous coronary intervention (PCI). We evaluated the clinical impact of long-term beta-blocker maintenance in patients with stable CAD after PCI with drug-eluting stent (DES).
Methods: From a nationwide cohort database, we identified the stable CAD patients without current or prior history of myocardial infarction or heart failure who underwent DES implantation. An intention-to-treat principle was used to analyze the impact of beta-blocker treatment on long-term outcomes of major adverse cardiovascular events (MACE) composed of cardiovascular death, myocardial infarction, and hospitalization with heart failure.
Results: After stabilized inverse probability of treatment weighting, a total of 78,380 patients with stable CAD was enrolled; 45,746 patients with and 32,634 without beta-blocker treatment. At 5 years after PCI with a 6-month quarantine period, the adjusted incidence of MACE was significantly higher in patients treated with beta-blockers [10.0 vs. 9.1%; hazard ratio (HR) 1.11, 95% CI 1.06–1.16, p < 0.001] in an intention-to-treat analysis. There was no significant difference in all-cause death between patients treated with and without beta-blockers (8.1 vs. 8.2%; HR 0.99, 95% CI 0.94–1.04, p = 0.62). Statistical analysis with a time-varying Cox regression and rank-preserving structure failure time model revealed similar results to the intention-to-treat analysis.
Conclusions: Among patients with stable CAD undergoing DES implantation, long-term maintenance with beta-blocker treatment might not be associated with clinical outcome improvement.
Trial Registration: ClinicalTrial.gov (NCT04715594).
Introduction
Beta-blockers are considered the primary choice in long-term maintenance drug therapy in patients with coronary artery disease, based on positive evidence for improving clinical outcomes in patients with acute myocardial infarction (MI) (1, 2) or heart failure (3). Long-term beta-blocker maintenance is associated with reduced mortality after percutaneous coronary intervention (PCI) in patients with acute MI (4). However, there is a lack of evidence supporting the beneficial impact of long-term beta-blocker treatment in patients with stable coronary artery disease (CAD) (5). Randomized clinical studies, which usually enroll small patient numbers (6), and observational studies (7, 8) have found no significant benefit to beta-blocker treatment in reducing mortality or ischemic events among patients with stable CAD. Furthermore, published data evaluating the clinical benefits of beta-blocker treatment in patients with stable CAD under the specific situation of post-PCI with drug-eluting stents (DES) is very rare. Using a nationwide cohort database, we sought to investigate the clinical impact of long-term beta-blocker maintenance in patients with stable CAD after PCI with DES.
Materials and Methods
Study Design and Data
This study was a nationwide retrospective analysis of the National Health Claims database established by the National Health Insurance Service (NHIS) of Korea, which contains claimed medical costs, detailed information on prescribed drugs including the number of pills and drug dosage, and medical history presented as International Classification of Diseases, Tenth Revision (ICD-10) codes. A majority (97.1%) of the Korean population is required to subscribe to the NHIS, which is the sole insurer managed by the Korean government. Considering that the NHIS also covers information for the remaining population (2.9%) categorized as medical aid subjects, this cohort is considered to represent the entire Korean population (9). This study was approved by the Institutional Review Board of our institute. Informed consent was waived because personal information was masked after cohort generation according to strict confidentiality guidelines of the Korean Health Insurance Review and Assessment Service. This study is registered at ClinicalTrial.gov (NCT04715594). We were also provided with death certificates including ICD-10 codes from the National Institute of Statistics of Korea.
Study Population
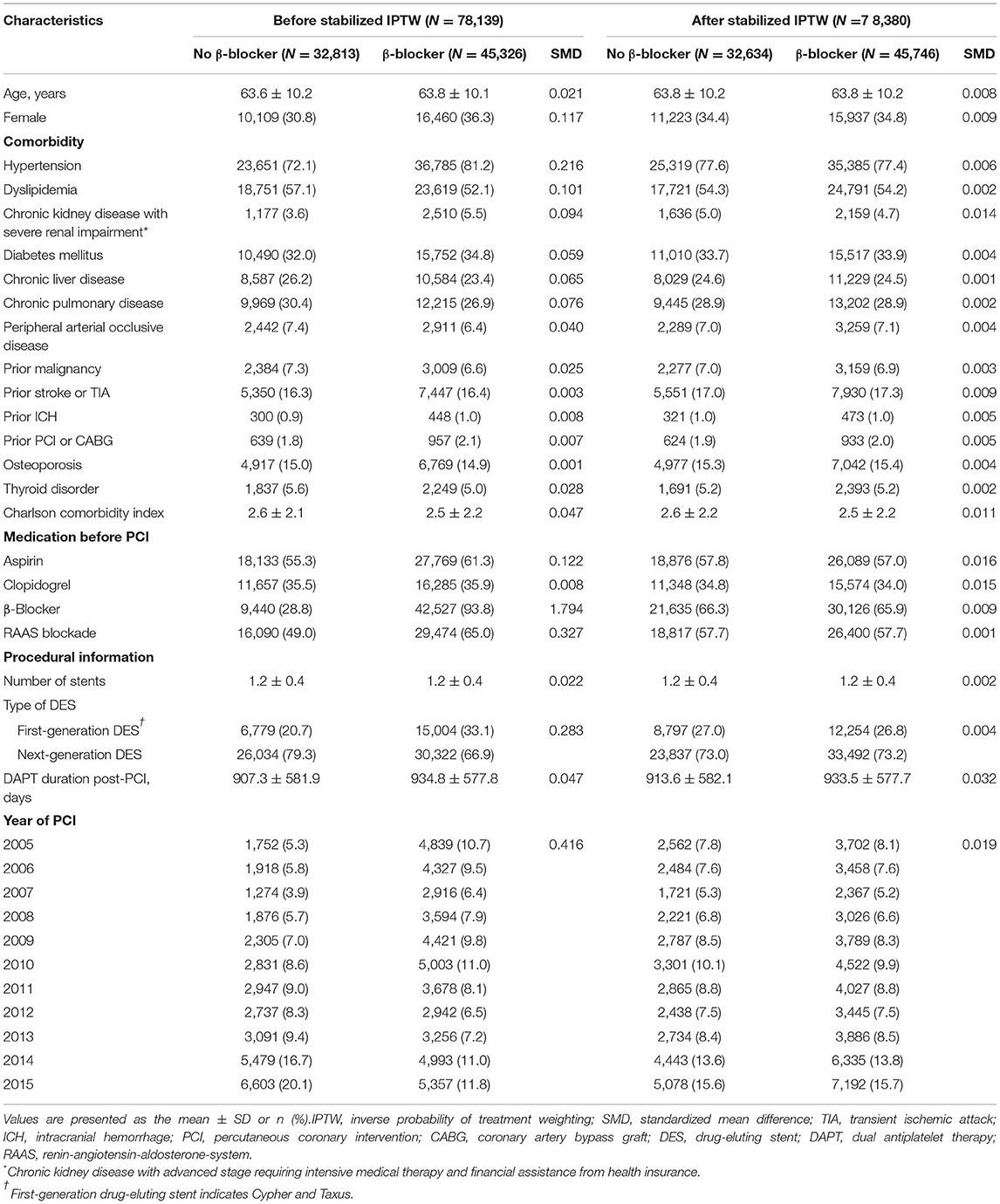
Among the 52 million citizens included in the NHIS database, we identified 214,340 patients (≥20 years old) who underwent DES implantation between January 2005 and December 2015, in Korea (CONNECT DES cohort registry). Patients with current (n = 22,079) or prior (n = 43,637) history of MI, history of heart failure (n = 31,310), or history of atrial fibrillation (n = 4,671) were excluded from this study. Furthermore, patients with missing covariates were excluded (n = 376). Patients with an insufficient period of beta-blocker prescription (<90 days, n = 32,573) or those with any clinical event (n = 1,555) during 180 days of quarantine were also excluded from the analyses. Consequently, the remaining 78,139 patients with stable CAD that was treated with DES implantation were included in the analysis of this study (Figure 1).
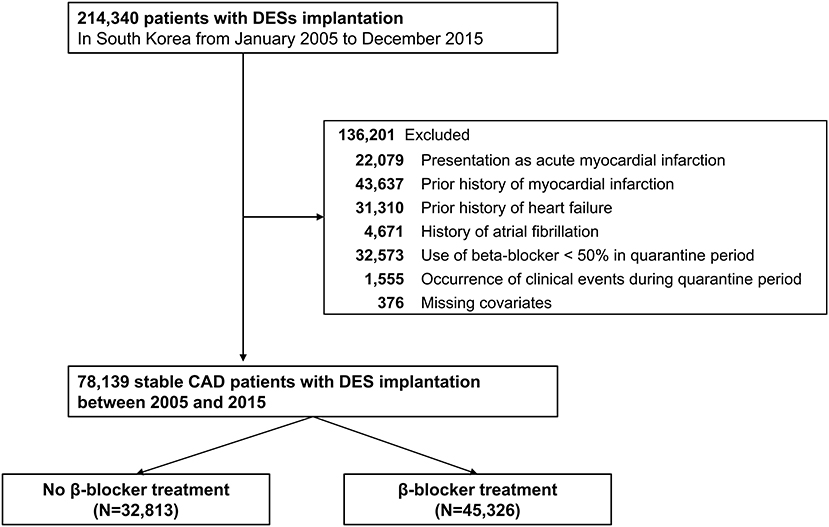
Figure 1. Flow chart of the study population. CAD, coronary artery disease; DES, drug-eluting stent.
Study Procedures and Outcomes
To emulate a randomized clinical trial that compares the impact of long-term beta-blocker treatment in patients with stable CAD, we used an intention-to-treat design for beta-blocker treatment, defined as a prescription of more than a 90-day supply of beta-blocker during 180 days of quarantine since index PCI. Types of prescribed beta-blockers are presented in Supplementary Table 1. We utilized ICD-10 codes, fee-for-service, and prescribed medication codes provided by the NHIS database and death certificates provided by the National Statistical Office. The primary outcome of interest was major adverse cardiovascular events (MACE) composed of cardiovascular death, MI, and hospitalization with heart failure for 5 years after PCI with 6 months of quarantine. Secondary outcomes were all-cause death and the individual MACE components. Cardiovascular death was ascertained from the National Statistical Office of Korea, which provided death certificates with an accuracy of 92% for the specific causes of death (9–11). Cardiovascular death was identified by a death certificate with at least one cardiovascular-related diagnosis (acute MI, stroke, heart failure, or sudden cardiac death). MI was defined by the ICD-10 codes corresponding to acute MI (10) and satisfaction of one or more of the following conditions: (1) concurrent presence of claims for coronary angiography, (2) admission via the emergency department, or (3) cardiac biomarkers tested more than 4 times. A detailed description of each clinical outcome, including the definition of hospitalization due to heart failure, is presented in Supplementary Table 2. Additionally, we included baseline comorbidities and drug prescription status before PCI for propensity score calculation, and stabilized inverse probability of treatment weights (IPTW) was used to accounting for differences in baseline characteristics, medical history, and confounding bias (Supplementary Table 3).
Statistical Analysis
Continuous variables are reported as mean and SD, and dichotomous variables are presented as frequency and percentage. To minimize the effect of confounding bias, we calculated the IPTW using the propensity score, which was calculated by logistic regression with covariates of age, sex, history of comorbidities and medications, and year of PCI (Supplementary Table 4). We also stabilized IPTW by multiplying it by the marginal probability of receiving treatment. The effect size difference between the two groups for all comorbidities and medications was calculated using standardized mean difference and Kernel density plots. Standardized mean difference values above 0.2 were regarded as a potential imbalance between the two groups. Cumulative incidence curves and the rate of clinical outcomes of interest during follow-up were plotted using the Kaplan–Meier method. The adjusted hazard ratio (HR) for each clinical outcome of interest was calculated using a Cox proportional hazard regression model. A cause-specific hazard model was used to consider death as a competing risk when comparing the incidences of cardiovascular death, MI, and hospitalization due to heart failure. We further conducted sensitivity analyses to assess the robustness of the main results. First, the heterogeneity of treatment effects in subgroups was assessed using interaction terms in a Cox proportional hazard model. Second, to estimate the effect of continuous treatment, the rank-preserving structural failure times (RPSFT) model was used (12). This method estimates counterfactual event times that would have occurred if patients had not switched treatments (13). It also uses a counterfactual framework to estimate the common causal effect of the treatment using a grid search method and may be associated with low bias when a large number of patients switch treatments (14, 15). Since the RPSFT model was designed originally for analysis of a randomized controlled trial with frequent the crossover between treatment groups (15), our observational study utilized the RPSFT model after propensity score-matching to establish homogenous covariate balance at baseline between patients treated with and without beta-blocker (Supplementary Table 4). Third, we performed a time-varying Cox regression in which treatment (with or without beta-blocker) was a time-dependent variable considering switch between treatments in real-world practice. Among the patients who were assigned to the beta-blocker treatment group during a quarantine period, those with discontinuation of beta-blocker for ≥90 days were considered unexposed during the interval. Fourth, we conducted an intention-to-treat analysis by assigning patients treated with beta-blockers for more than 1 day during the quarantine period, instead of 90 days, to the treatment group. Fifth, we defined the intention-to-treat group as a prescription of more than a 16-day supply of beta-blocker in the 30-day quarantine period after PCI because the 180-day observational period used in the main analysis could have masked the occurrence of adverse clinical events early after DES implantation.
All tests were two-sided and a p-value < 0.05 was considered statistically significant. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.6 with “RPSFTM” and “survival” packages (The R Foundation, www.R-project.org).
Results
Baseline demographics and medical history of the cohort population before and after stabilized IPTW are presented in Table 1. After weighting, 78,380 DES-treated patients were included: 45,746 with and 32,634 without beta-blocker treatment. After stabilized IPTW, there was no evidence of inequality in baseline demographic characteristics or medical history between the two groups (all standardized mean difference <0.1, Supplementary Figures 1, 2). The incidence and relative hazards of primary and secondary outcomes are presented in Table 2, Figure 2, and Supplementary Figure 3. At 5 years after PCI with 6 months of quarantine, the adjusted incidence rate of MACE was significantly higher in patients treated with beta-blockers (10.0 vs. 9.1% in those without beta-blocker treatment; HR 1.11, 95% CI 1.06–1.16, p < 0.001, Figure 2) in an intention-to-treat analysis (Table 2 and Supplementary Table 5). There was no significant difference in all-cause death between patients treated with and without beta-blocker (8.1 vs. 8.2%; HR 0.99, 95% CI 0.94–1.04, p = 0.62, Supplementary Figure 3), As for the individual components of MACE, there was no significant association between beta-blocker treatment and risk of cardiovascular death (5.9 vs. 5.9% in those without beta-blocker treatment; HR 1.00, 95% CI 0.94–1.06, p = 0.88) or MI (3.8 vs. 3.6% in those without beta-blocker treatment; HR 1.03, 95% CI 0.96–1.11, p = 0.42), while the adjusted hospitalization rate due to heart failure was significantly higher in patients treated with beta-blockers (4.1 vs. 3.1% in those without beta-blocker treatment; HR 1.32, 95% CI 1.23–1.43, p < 0.001) (Table 2). Consistent findings were observed regardless of DAPT duration (Supplementary Table 6). In a subgroup analysis, there was no significant interaction between the baseline comorbidities and beta-blocker treatment for a 5-year occurrence of MACE (Figure 3) or all-cause mortality (Supplementary Figure 4). There was no significant difference in treatment effect according to the generation of beta-blockers (Supplementary Table 7). During a 5-year follow-up period, there were frequent changes in the prescribed beta-blocker status during follow-up (Figure 4). Among 45,326 patients initially treated with beta-blocker before stabilized IPTW weighting, administration of beta-blocker was discontinued in 6,162 (13.6%) without adverse cardiovascular events such as death, MI, or heart failure. Of the 32,813 patients initially treated without beta-blocker, 3,622 (11.0%) started taking beta-blocker during the 5-year follow-up period and showed no MI or heart failure. To take into account frequent cross-over between the treatment groups, we performed additional statistical analyses with time-varying Cox regression (Figure 5A) and RPSFT models (Figure 5B), which demonstrated no statistically significant impact of beta-blocker treatment on the occurrence of all-cause death, cardiovascular death, or MI, whereas beta-blocker treatment was associated with a higher incidence rate of MACE or hospitalization for heart failure. Consistent findings were obtained when patients treated with beta-blocker for more than 1 day during 180 days of quarantine were considered as a treatment group (Figure 5C) or when the prescription status of beta-blocker within a 30-day period, instead of a 180-day quarantine period, after index PCI was applied (Figure 5D).
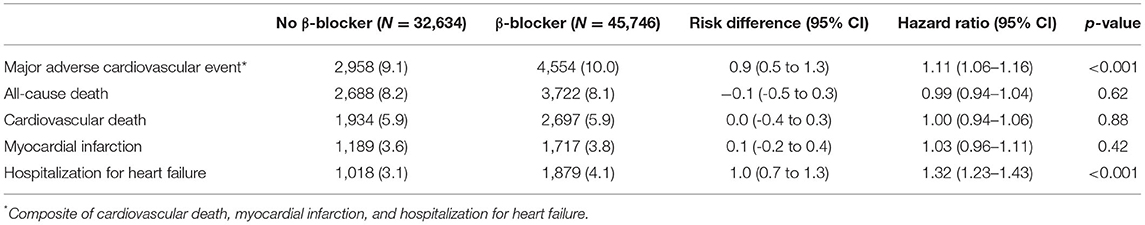
Table 2. Risks of primary and secondary outcomes at 5 years after percutaneous coronary intervention between patients prescribed with or without β-blocker after stabilized inverse probability of treatment weighting.
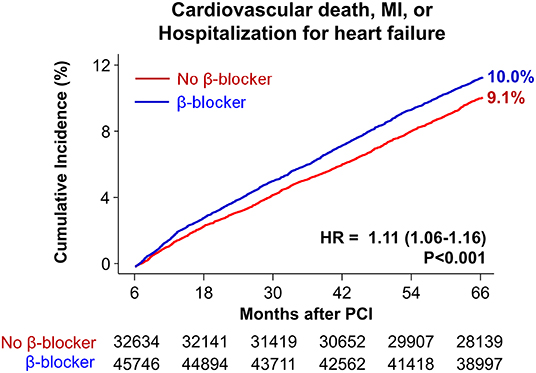
Figure 2. Time-to-event curves for major adverse cardiovascular events for 5 years after PCI. The cumulative incidence of major adverse cardiovascular events for 5 years after PCI. HR, hazard ratio; MI, myocardial infarction; PCI, percutaneous coronary intervention.
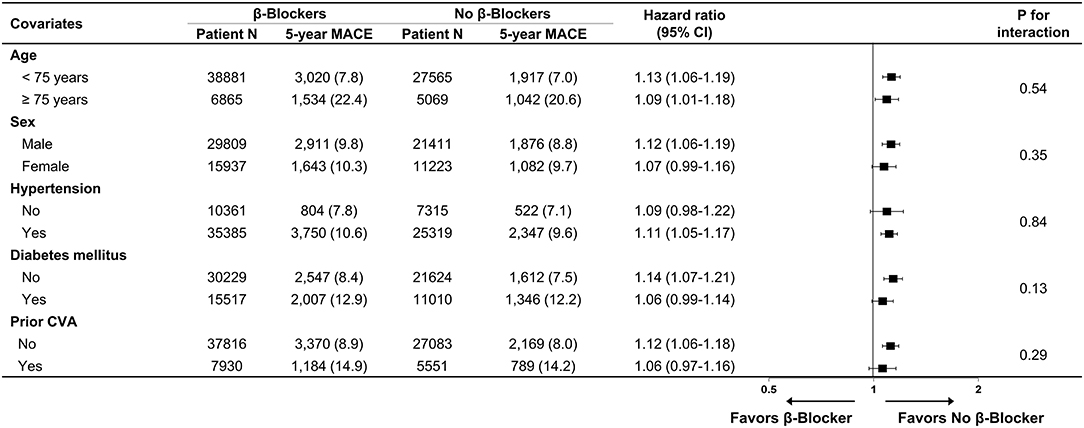
Figure 3. Subgroup analysis for major adverse cardiovascular events. Numbers and percentages show the number of patients at risk, number of events, and the incidence rate of major adverse cardiac events 5 years after drug-eluting stent implantation. CI, confidence interval; CVA, cerebrovascular accidents; MACE, major adverse cardiovascular events.
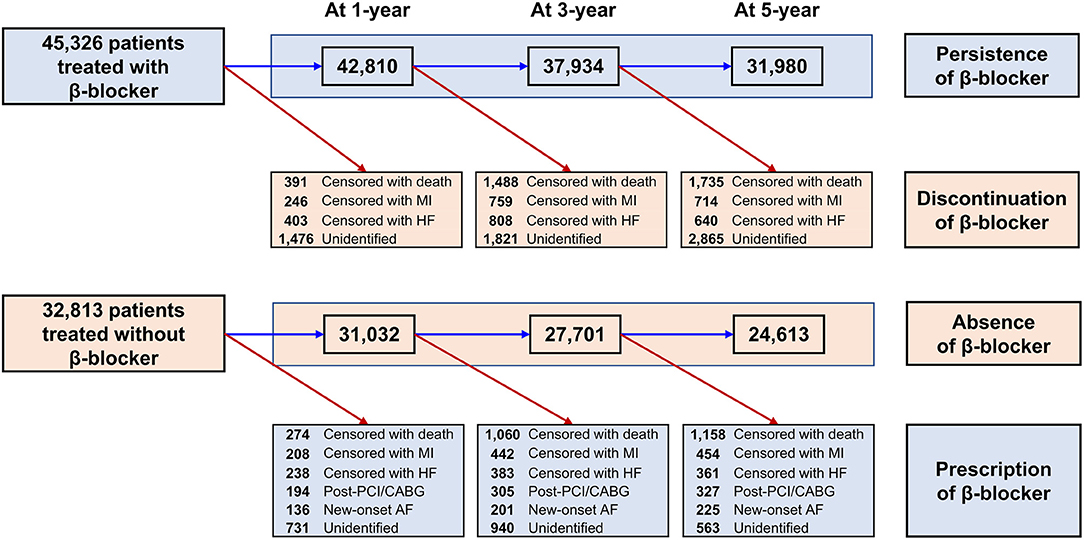
Figure 4. Temporal trends in change of beta-blocker prescription. AF, atrial fibrillation; CABG, coronary artery bypass graft surgery; HF, heart failure; MI, myocardial infarction; PCI, percutaneous coronary intervention.
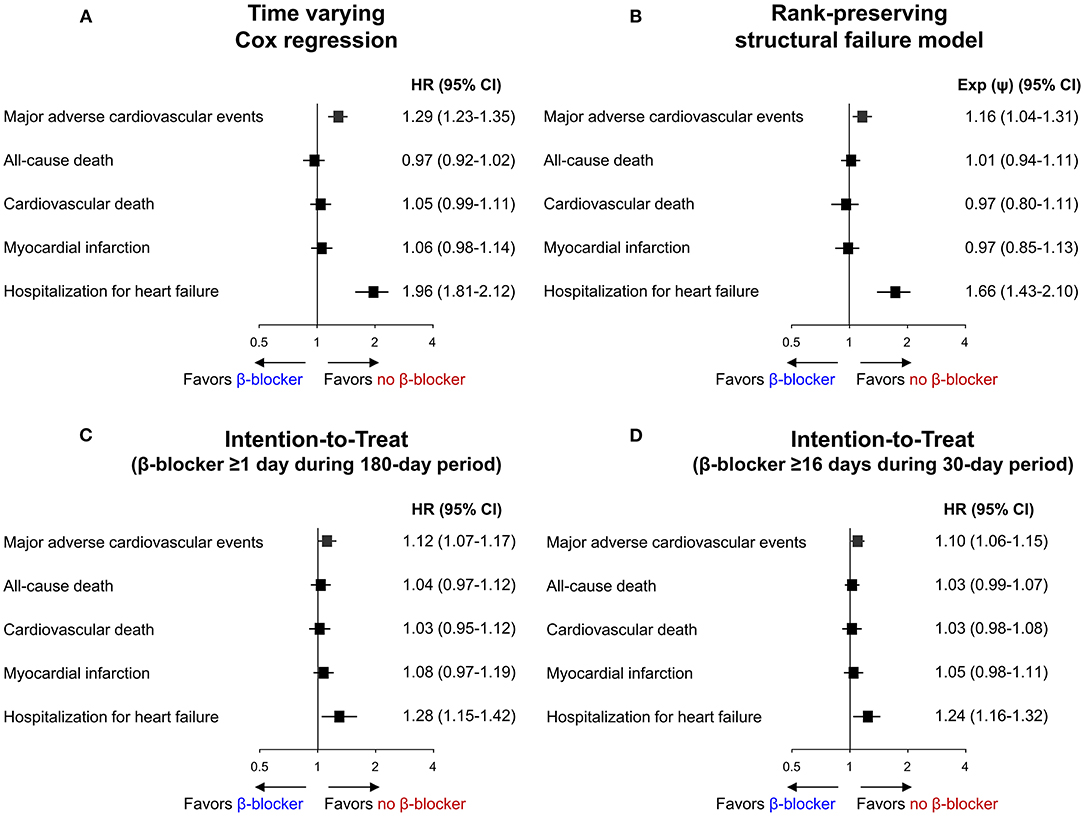
Figure 5. Sensitivity analysis for primary and secondary outcomes. Risk of primary and secondary outcomes according to beta-blocker treatment analyzed by (A) Time varying Cox regression, (B) Rank-preserving structural failure model, and (C,D) Intention-to-Treat method. CI, confidence interval; HR, hazard ratio. Exp (ψ) indicates an increase/decrease in survival in the non-treatment group.
Discussion
This nationwide cohort analysis evaluated the association between long-term beta-blocker treatment and clinical outcomes including mortality in patients with stable CAD after DES implantation. Taking advantage of the unique feature of the Korean NHIS database that accurately tracks all medication information over the entire study period, we could analyze the clinical impact of long-term beta-blocker administration in real-world practice by emulating the intention-to-treat manner of a randomized controlled trial. Furthermore, we applied time-varying Cox regression analysis and the RPSFT model to account for switching between treatment strategies, which is typical in real-world practice. The major findings of our study are as follows: (1) in patients with stable CAD after DES implantation, long-term maintenance treatment with beta-blockers was not associated with improvement of clinical outcomes, and (2) sensitivity analyses that considered switching between treatment strategies revealed consistent findings of a negligible impact of beta-blocker on clinical outcomes.
Long-term maintenance beta-blocker treatment after PCI in patients with MI or heart failure is recommended highly based on a large body of evidence that the treatment reduces mortality and morbidity (4, 16). This benefit mainly relies on the heart rate lowering property that decreases oxygen requirements, and negative inotropic effects that mitigate adverse cardiac remodeling and ventricular arrhythmia (17). Furthermore, in a COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial, which demonstrated comparable effects of optimal medical therapy to PCI in stable CAD patients, beta-blockers were a mainstay drug treatment prescribed in 87% of patients enrolled in the trial (18).
There are two published studies that evaluated the association between beta-blocker treatment at discharge and clinical outcomes in stable CAD patients undergoing PCI without prior history of MI or heart failure. One registry study (n = 5,288) reported that beta-blocker treatment at discharge was associated with a significantly increased risk of cardiac death/MI during a 3-year follow-up after index PCI (HR 1.48, 95% CI 1.05–2.10, p =0.02) (19). Another registry study with a larger number of patients (n = 122,734) reported no significant association between beta-blocker treatment at discharge and mortality or MI at 3-year follow-up (8). In addition, discharge with beta-blocker treatment was associated with more frequent readmission due to heart failure (8). However, these two studies did not provide detailed information on the prescribed beta-blocker status during the 3-year follow-up (8, 19). Because continuous beta-blocker prescription status after discharge during long-term follow-up was not evaluated clearly, the impact of drug switch during the follow-up period was not addressed (8, 19).
To minimize potential sources for bias, we excluded patients with concomitant indications for beta-blocker treatment such as MI, heart failure, or atrial fibrillation. Next, we emulated randomized controlled trials using intention-to-treat analysis with a 180-day quarantine period to assign treatment groups and the stabilized IPTW model to adjust for baseline differences. Furthermore, taking advantage of the unique strength of the NHIS database of Korea, which enables tracking and tracing of complete medication information during an entire follow-up period, we compared the main results with those of sensitivity analyses using the time-varying Cox regression and RPSFT model, which consider switches between treatment groups during follow-up. Finally, to compensate for the possible immortal time bias caused by 6 months of quarantine without clinical events, we set a quarantine period of 1 month as a sensitivity analysis. Results from observational studies cannot be used to establish causality, and residual perturbations can persist after propensity score weighting. However, despite the heterogeneity of treatment groups, various sensitivity analyses confirmed consistency compared with the main analysis.
In our analysis, 14% of patients initially treated with beta-blocker after DES implantation eventually discontinued the drug without the occurrence of significant clinical events. In fact, patients who are prescribed beta-blocker can complain of numerous side effects such as fatigue, bradycardia, depression, hypotension, bronchospasm, peripheral vasoconstriction, or postural hypotension, which usually leads to discontinuation of beta-blocker treatment. Furthermore, chronic beta-blocker use has been associated with lipid profile deterioration and new-onset diabetes (20–22). One study reported that beta-blocker treatment increased serum triglyceride level, decreased HDL cholesterol level, and increased plasma small dense LDL, resulting in an atherogenic lipoprotein phenotype (23). A meta-analysis that included 94,492 hypertensive patients treated with beta-blocker has suggested a positive association between beta-blocker treatment and new-onset diabetes (21). Furthermore, non-selective beta-blockers can cause coronary artery spasms by inhibiting β-adrenergic mediated vasodilation (24). Concerns for possible side effects of long-term beta-blocker administration weaken the rationale for routine use of beta-blocker in specific patients with stable CAD after PCI with DES.
Limitations
This study has several limitations. First, in this nationwide cohort based on claims data, the systolic function of the left ventricle before PCI and during the follow-up period are not included; thus, patients with borderline left ventricular systolic function or reduced left ventricular systolic function without heart failure diagnosis could be included. Furthermore, in the administrative database, heart failure with preserved ejection fraction could have been underdiagnosed (25). Second, considering the nature of retrospective data based on claims, the findings presented in this study cannot be used to establish causal associations, and residual confounding variables could persist even after stabilized IPTW. Third, we adopted the RPSFT model to correct for frequent changes in beta-blocker prescription. Since the RPSFT is a statistical model typically applied to the analysis of randomized controlled trials, caution is needed in interpreting the findings obtained through this analysis despite the 1:1 propensity score matching for the establishment of RPSFT.
Conclusions
Among patients with stable CAD undergoing DES implantation, long-term maintenance with beta-blocker treatment was associated with an increased occurrence of MACE. Beta-blocker treatment may not be recommended as a maintenance drug therapy in specific patients with stable CAD after index PCI.
Data Availability Statement
The datasets generated for the analyses are not publicly available because of strict government restrictions. Requests to access these datasets should be directed to M-KH, bWtob25nNjFAeXVocy5hYw==.
Ethics Statement
The studies involving human participants were reviewed and approved by Yonsei University Health System Institutional Review Board. The Ethics Committee waived the requirement of written informed consent for participation.
Author Contributions
S-JL, D-WC, YS, CK, S-JH, C-MA, J-SK, B-KK, Y-GK, DC, E-CP, YJ, C-MN, and M-KH contributed to the conception and design. S-JL and M-KH wrote the study protocol. D-WC and C-MN performed the programming to extract the data from the NHIS database. C-MN and M-KH had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. S-JL, D-WC, C-MN, and M-KH verified the data and conducted all analyses. YS, CK, S-JH, C-MA, J-SK, B-KK, Y-GK, DC, E-CP, and YJ provided a critical review of the manuscript. All authors read and approved the final publication.
Funding
This work was supported by the Cardiovascular Research Center, Seoul, Korea.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.878003/full#supplementary-material
Abbreviations
CAD, coronary artery disease; CI, confidence interval; DES, drug-eluting stent; HR, hazard ratio; IPTW, inverse probability of treatment weights; MACE, major adverse cardiovascular events; MI, myocardial infarction; NHIS, national health insurance service; PCI, percutaneous coronary intervention; RPSFT, rank-preserving structural failure times.
References
1. Puymirat E, Riant E, Aissaoui N, Soria A, Ducrocq G, Coste P, et al. β blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ. (2016) 354i4801 doi: 10.1136/bmj.i4801
2. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. (2017) 70:776–803. doi: 10.1161/CIR.0000000000000509
4. Kim J, Kang D, Park H, Kang M, Park TK, Lee JM, et al. Long-term beta-blocker therapy and clinical outcomes after acute myocardial infarction in patients without heart failure: nationwide cohort study. Eur Heart J. (2020) 41:3521–29. doi: 10.1093/eurheartj/ehaa376
5. Ohman EM. CLINICAL PRACTICE. Chronic stable angina. N Engl J Med. (2016) 374:1167–76. doi: 10.1056/NEJMcp1502240
6. Huang HL, Fox KA. The impact of beta-blockers on mortality in stable angina: a meta-analysis. Scott Med J. (2012) 57:69–75. doi: 10.1258/smj.2011.011274
7. Bangalore S, Steg G, Deedwania P, Crowley K, Eagle KA, Goto S, et al. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. (2012) 308:1340–49. doi: 10.1001/jama.2012.12559
8. Motivala AA, Parikh V, Roe M, Dai D, Abbott JD, Prasad A, et al. Predictors, trends, and outcomes (among older patients≥ 65 years of age) associated with beta-blocker use in patients with stable angina undergoing elective percutaneous coronary intervention: insights from the NCDR registry. JACC Cardiovasc Interv. (2016) 9:1639–48. doi: 10.1016/j.jcin.2016.05.048
9. Choi EK. Cardiovascular research using the Korean national health information database. Korean Circ J. (2020) 50:754–72. doi: 10.4070/kcj.2020.0171
10. You SC, Rho Y, Bikdeli B, Kim J, Siapos A, Weaver J, et al. Association of ticagrelor vs clopidogrel with net adverse clinical events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA. (2020) 324:1640–50. doi: 10.1001/jama.2020.16167
11. Won TY, Kang BS, Im TH, Choi HJ. The study of accuracy of death statistics. J Korean Soc Emerg Med. (2007) 18:256–62.
12. Toh S, Hernán MA. Causal inference from longitudinal studies with baseline randomization. Int J Biostat. (2008) 4:22 doi: 10.2202/1557-4679.1117
13. Morden JP, Lambert PC, Latimer N, Abrams KR, Wailoo AJ. Assessing methods for dealing with treatment switching in randomised controlled trials: a simulation study. BMC Med Res Methodol. (2011) 11:4. doi: 10.1186/1471-2288-11-4
14. Gold MR, Padhiar A, Mealing S, Sidhu MK, Tsintzos SI, Abraham WT. Long-term extrapolation of clinical benefits among patients with mild heart failure receiving cardiac resynchronization therapy: analysis of the 5-year follow-up from the REVERSE study. JACC Heart Fail. (2015) 3:691–700. doi: 10.1016/j.jchf.2015.05.005
15. Jönsson L, Sandin R, Ekman M, Ramsberg J, Charbonneau C, Huang X, et al. Analyzing overall survival in randomized controlled trials with crossover and implications for economic evaluation. Value Health. (2014) 17:707–13. doi: 10.1016/j.jval.2014.06.006
16. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa909
17. McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: β-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. (2009) 150:784–94. doi: 10.7326/0003-4819-150-11-200906020-00006
18. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. (2007) 356:1503–16. doi: 10.1056/NEJMoa070829
19. Ozasa N, Morimoto T, Bao B, Furukawa Y, Nakagawa Y, Kadota K, et al. β-blocker use in patients after percutaneous coronary interventions: one size fits all? Worse outcomes in patients without myocardial infarction or heart failure. Int J Cardiol. (2013) 168:774–9. doi: 10.1016/j.ijcard.2012.10.001
20. Weir MR, Moser M. Diuretics and beta-blockers: is there a risk for dyslipidemia? Am Heart J. (2000) 139:174–83. doi: 10.1016/S0002-8703(00)90325-9
21. Bangalore S, Parkar S, Grossman E, Messerli FH. A meta-analysis of 94,492 patients with hypertension treated with beta blockers to determine the risk of new-onset diabetes mellitus. Am J Cardiol. (2007) 100:1254–62. doi: 10.1016/j.amjcard.2007.05.057
22. Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. (2007) 369:201–7. doi: 10.1016/S0140-6736(07)60108-1
23. Rosenblit PD. Common medications used by patients with type 2 diabetes mellitus: what are their effects on the lipid profile? Cardiovasc Diabetol. (2016) 15:95. doi: 10.1186/s12933-016-0412-7
24. Japanese beta-Blockers and Calcium Antagonists Myocardial Infarction (JBCMI) investigators. Comparison of the effects of beta blockers and calcium antagonists on cardiovascular events after acute myocardial infarction in Japanese subjects. Am J Cardiol. (2004) 93:969–73. doi: 10.1016/j.amjcard.2004.01.006
Keywords: percutaneous coronary intervention, coronary artery disease, beta-blocker, drug-eluting stents, treatment outcome
Citation: Lee S-J, Choi D-W, Kim C, Suh Y, Hong S-J, Ahn C-M, Kim J-S, Kim B-K, Ko Y-G, Choi D, Park E-C, Jang Y, Nam C-M and Hong M-K (2022) Long-Term Beta-Blocker Therapy in Patients With Stable Coronary Artery Disease After Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 9:878003. doi: 10.3389/fcvm.2022.878003
Received: 11 March 2022; Accepted: 15 April 2022;
Published: 17 May 2022.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Yifan Lu, Temple University, United StatesJing-Song Ou, The First Affiliated Hospital of Sun Yat-sen University, China
Copyright © 2022 Lee, Choi, Kim, Suh, Hong, Ahn, Kim, Kim, Ko, Choi, Park, Jang, Nam and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Myeong-Ki Hong, bWtob25nNjFAeXVocy5hYw==; Chung-Mo Nam, Y21uYW1AeXVocy5hYw==
†These authors have contributed equally to this work
 Seung-Jun Lee
Seung-Jun Lee Dong-Woo Choi2,3†
Dong-Woo Choi2,3† Choongki Kim
Choongki Kim Yongsung Suh
Yongsung Suh Sung-Jin Hong
Sung-Jin Hong Jung-Sun Kim
Jung-Sun Kim Byeong-Keuk Kim
Byeong-Keuk Kim Donghoon Choi
Donghoon Choi Eun-Cheol Park
Eun-Cheol Park Myeong-Ki Hong
Myeong-Ki Hong