
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 10 March 2022
Sec. Cardiovascular Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.875078
This article is part of the Research Topic Frozen Elephant Trunk Surgery in Aortic Dissection View all 22 articles
 Matti Jubouri1
Matti Jubouri1 Fatima Kayali2
Fatima Kayali2 Priyanshu Saha3
Priyanshu Saha3 Daniyal M. Ansari3
Daniyal M. Ansari3 Yousef Rezaei4
Yousef Rezaei4 Sven Z. C. P. Tan5
Sven Z. C. P. Tan5 Mostafa Mousavizadeh4
Mostafa Mousavizadeh4 Saeid Hosseini4
Saeid Hosseini4 Idhrees Mohammed6
Idhrees Mohammed6 Mohamad Bashir7*
Mohamad Bashir7*Background: The introduction of the frozen elephant trunk (FET) technique for total arch replacement (TAR) has revolutionized the field of aortivascular surgery by allowing hybrid repair of complex aortic pathologies in a single step through combining an open surgical approach with an endovascular one. FET has been associated with favorable aortic remodeling, however, its is also associated with development of distal stent graft induced new entry (dSINE) tears postoperatively. The rate of aortic remodeling and the incidence of dSINE have been linked together, in addition, there seems to be a relationship between these two variables and FET insetion length as well as graft size.
Aims: The scope of this review is to highlight the rate of aortic remodeling as well the incidence of dSINE associated with different FET devices available commercially. This review also aimed to investigate the relationship between aortic remodeling, dSINE, FET insertion length and FET graft size.
Methods: We conducted a comprehensive literature search using multiple electronic databases including PubMed, Ovid, Scopus and Embase in order to collate all research evidence on the above mentioned variables.
Results: Thoraflex™ Hybrid Plexus seems to yield optimum aortic remodeling by promoting maximum false thrombosis as well true lumen expansion. Thoraflex Hybrid™ is also associated with the lowest incidence of dSINE post-FET relative to the other FET devices on the market. Aortic remodeling and dSINE do influence each other and are both linked with FET graft length and size.
Conclusion: The FET technique for TAR shows excellent aortic remodeling but is associated with a considerable risk of dSINE development. However, Thoraflex™ Hybrid has demonstrated itself to be the superior FET device on the aortic arch prostheses market. Since aortic remodeling, dSINE, FET insertion length and stent graft size are all interconnect, the choice of FET device length and size must be made with great care for optimum results.
The frozen elephant trunk (FET) technique for total arch replacement (TAR) was introduced in 2003 and has been in use to treat a wide range of complex aortic pathologies (1). This marked an evolution from the two-stage elephant trunk (ET) procedure which involved replacing the ascending aorta and arch with a graft, followed by placement of an “elephant trunk” prosthetic that extended into the descending aorta. The conventional ET impeded full aortic remodeling and potentially caused injury to the aortic intima, thus negatively influencing clinical outcomes. Therefore, necessitating a second surgical intervention to either extend the graft or attach it to the relative aortic segment (2). Hence, FET ameliorated the two-stage procedure through innovation and permitted combined open surgical replacement of the aortic arch along with endovascular intervention in the descending aorta during a single operation in hybrid theaters.
The FET procedure has been associated with favorable aortic remodeling outcomes (3, 4). Aortic remodeling, however, has had a multitude of definitions across the literature. Different studies have calculated this significant prognostic tool in varying ways; however, it is essentially a late effect characterized by the occlusion of the primary entry tear site subsequently promoting false lumen thrombosis. This led to a decrease in false lumen (FL) diameter and a re-expansion of the true lumen (TL) (5). It is well-established within the literature that aortic remodeling has proved to be an accurate prognostic tool for patients (6). Additionally, aortic remodeling has been linked to improved survival rates and form an overall good measure of clinical success (7). Failure of false lumen thrombosis has been linked to aneurysm dilatation, rupture of the thoracic aorta, and higher reintervention rates (8).
One of the noted complications of FET is development of distal stent graft induced new entry (dSINE) tears, typically due to the use of endovascular manipulation as well as stent graft and distal TL size mismatch. The choice of FET devices as well as its size and length has shown to be linked with the incidence of dSINE. Evidence in the literature also suggest that there is an inverse relationship between dSINE and the rate of aortic remodeling distally (9).
This review aimed to highlight the incidence of distal stent graft induced new entry (dSINE) as well as the rate of aortic remodeling associated with TAR with FET. Another scope of this review was to assess the efficacy of the Thoraflex™ Hybrid device in achieving optimal remodeling and dSINE outcomes. Finally, we investigated the relationship between aortic remodeling, dSINE, and FET graft length and size.
The revolutionary FET procedure is well-established in the literature with excellent aortic remodeling, including favorable FL thrombosis rates and significant positive changes in TL and FL diameters (3, 4). A 2019 study on total arch replacement reported that using the FET technique proved to have a high rate of TL expansion and FL thrombosis at the level of bronchial carina, in addition to the descending aorta. It was also thought that FET reduced the sequalae of vascular complications resulting from failed aortic remodeling (3). These results are also not expected to differ between acute and chronic aortic dissection presentations (10). In a meta-analyis that included 1,279 patients, FL thrombosis was achieved in 96.8% of patients (95% CI, 90.7–98.9%) (11). Similarly, these findings were supported in a review by Di Bartolomeo et al. (12), where complete or partial FL thrombosis was expected in 90% of patients. This figure is incomparable to conservative management, wherein FL thrombosis occurred in between 33.3 and 77.8% of patients (12). Unfortunately, these promising results have not been seen to the same extent at the level of the abdominal aorta and further management has been recommended for this subset (10).
The Thoraflex™ Hybrid Plexus (THP™) (Terumo Aortic, Inchinnan, Scotland, UK) device has been designed with a special interrupted pattern, which is thought to protect the aortic wall from the substantial forces of blood flow to achieve excellent aortic remodeling (13). Mehanna et al. (13), who investigated aortic remodeling following FET with THP™, carried out Spearman rank correlation tests to assess the correlation of their results. Aortic remodeling ratios, prior to and following the procedure, had a moderately positive correlation. This was calculated as 0.566 and 0.582 for TL expansion and FL diameter decrease, respectively, with p values of 0.006 and 0.004, respectively, indicating strong statistical significance. A significant expansion of the TL ratio was seen using Thoraflex™ Hybrid post-operatively, with a median increase from 0.31 to 0.4 mm (p = 0.042). This trend matched the significant FL ratio decrease from 0.66 to 0.54 mm (p = 0.02). The study included a total on 20 patients out of whom 8 were acute and 12 were chronic, with no significant difference found when comparing aortic remodeling in both groups (p = 0.26). The authors stressed that Thoraflex™ Hybrid is the optimal FET device as it is the safest to use due to its interrupted pattern as mentioned earlier (13).
In the previous studies described, the authors used a Computed Tomography (CT) scan to assess the aortic remodeling diameters. In a 2021 study by Usai et al. (14), volume measurements were used instead to overcome the 2D view limitations of CT images, allowing the authors to gauge a more accurate measure of lumen patency using the Thoraflex™ Hybrid device. Here, surfaces and volumes were measured using CT angiography prior to and following FET, in addition to 12- and 24-month follow-up. The mean true lumen (in cm3) prior to the FET was 77.03 cm3 (± 47.96 cm3). At discharge, this mean increased by 10.96 cm3. This growth was sustained and the TL measurements 12 and 24 months after were 113.83 and 133.84 cm3, respectively. This long-term analysis of TL volumetric expansion evidenced a statistically significant growth even after 2 years of the FET procedure (p = 0.047). Similarly, the mean FL measurement was 158.33 cm3 (± 68.24 cm3), 167.56 cm3 (± 90.24 cm3), 164.36 cm3 (± 59.72 cm3) and 157.20 cm3 (± 78.0 cm3) prior to the procedure, following and at 12-month and 24-month follow-up (14). These long-term outcomes seem to be congruent a previous 2018 study, where chronic dissection patients showed significant TL expansion (15 ± 17 mm to 28 ± 2 mm) (p = 0.001) and FL diameter drop (40 ± 11 mm to 32 ± 17 mm) (p = 0.026) 2 years following the procedure (15). Additionally, Usai et al. (14) confirmed that in their study almost all Thoraflex Hybrid™ patients had significant TL expansion and reported only 1 patient required reintervention, due to a FL aneurysm. Furthermore, the authors also noted that the most significant growth in the surface TL measurement was at the level of the diaphragm (p = 0.00193) (14).
There appears to be some controversy in the literature regarding the impact of presentation on the aortic remodeling following FET procedures. It was initially expected that there is no difference between acute and chronic dissections, however, in a study involving 100 patients who had Thoraflex Hybrid™ implanted, FL thrombosis was achieved at a much more accelerated pace in acute, rather than chronic dissection patients. Despite this, full FL thrombosis was achieved in 100% of patients within 24 months at the proximal segment of the aorta. Additionally, as expected, distal segments of the aorta had less successful rates (16). An international multicentre registry which used E-Vita in 137 patients (65 acute and 72 chronic) also confirmed that acute dissections are associated with greater aortic remodeling rates (10). When comparing the FL thrombosis rates in both studies together, Thoraflex™ Hybrid came out on top with improved results.
As reported by Shrestha et al. (17), the 100% rate of FL thrombosis achieved using Thoraflex™ shows its superiority to other devices as seen by the 82.1% and 89.4% rates reported by Kobayashi et al. (16) and Chen et al. (18), respectively. Yet it is important to take into consideration the differences amongst these 3 independent studies. Kobayashi et al. (16) carried out a simplified FET technique in 34 patients with complicated acute type A aortic dissections. The “simplified” approach involves using gelatine-resorcinol adhesive to attach the dissected aortic arch wall layers, followed by an antegrade open arch approach to deploy the aortic stent-graft into the arch or proximal descending aorta. Post-operatively, only 82.1% of patients had FL thrombosis seen in the aortic arch using the TAG, Talent and E-Vita stent-grafts (16). On the other hand, Chen et al. (18) reported complete FL thrombosis in 89.4% of patients around the triple-branched stent-graft, created by Yuhengjia Science and Technology. In this study, 122 patients underwent total arch replacement for acute type A aortic dissection, with open placement of this triple-branched stent graft (18). Similarly, in a study involving 41 acute type A dissection patients who underwent TAR with FET using E-Vita, a FL thrombosis rate of 93.9% at the pulmonary trunk level was achieved (19). The above evidence shows beyond doubt that Thoraflex Hybrid™ offers the highest rate of FL thrombosis.
The Thoraflex™ Hybrid Plexus has also demonstrated TL expansion not only at the level of pulmonary bifurcation (covered by the stent), but also at the distal descending aorta, with complete FL thrombosis in 73.1% of cases at the former site (20). Similar to other findings in the literature, there was a small, albeit significant, expansion of aortic diameter at the level of the abdominal aorta. Further benefit yielded Terumo Aortic's Thoraflex™ Hybrid was highlighted in a study by Fiorentino et al. (20), where pre-planned second-stage procedures were prevented in 18.2% of patients due to the promising aortic remodeling rates. Additionally, when comparing Thoraflex™ Hybrid to other devices, the need for endoprosthetic extensions due to incomplete FL thrombosis was less by 6% with the Thoraflex™ Hybrid in comparison with the E-Vita HP (21).
In a more detailed study on aortic remodeling after FET with Thoraflex Hybrid™ for acute (n = 31) and chronic dissections (n = 34) at the stent, thoracoabdominal transition and coeliac trunk levels, significant TL and FL changes were noted (15). A summary of the findings showing the favorable aortic remodeling associated with Thoraflex Hybrid™ is illustrated in Tables 1, 2.
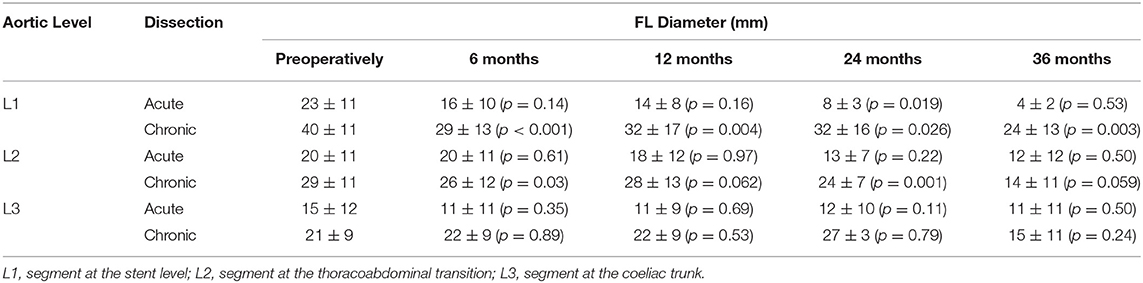
Table 1. Summary of TL diameter results from Berger et al. (Thoraflex Hybrid) (15).
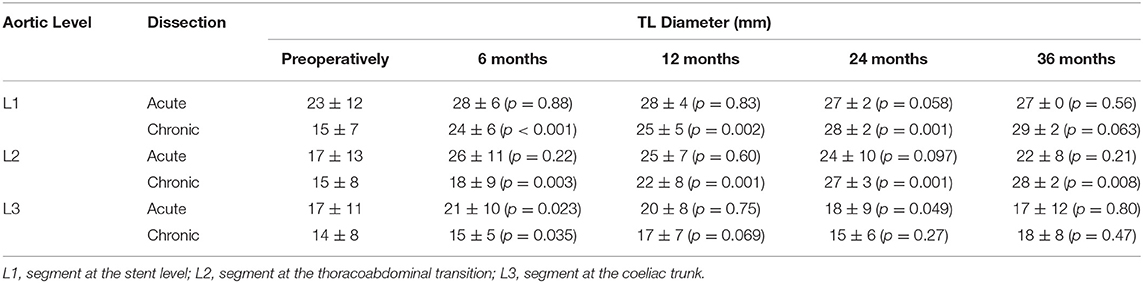
Table 2. Summary of FL diameter results from Berger et al. (Thoraflex Hybrid) (15).
The same aforementioned international multicentre registry which used E-Vita in 137 patients (65 acute and 72 chronic) also measured TL diameter preoperatively and at follow-up, which had a median of 32 months (21–53 months) (10). A summary of the results can be found in Table 3. Additionally, in a different study using the Cronus device, authors reported no change in TL diameter at the level of the aortic arch (22). Comparing the results from the three devices, Thoraflex Hybrid™ clearly yields the optimum aortic remodeling.
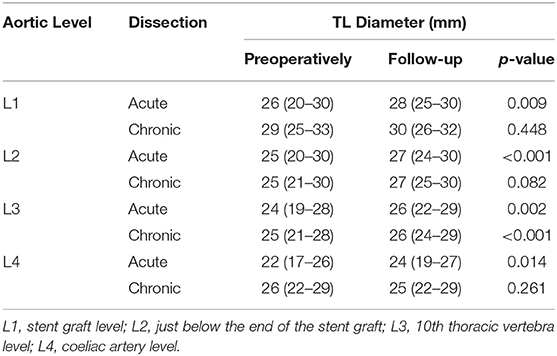
Table 3. Summary of TL diameter results from Iafrancesco et al. (E-Vita) (10).
Variations of reported aortic remodeling data in the literature could be attributed to multiple factors. For instance, differences in results could be a result of the varying pathologies that the FET procedure is used to treat, in addition to the heterogenous population included in these studies. Such variance in demographics and comorbidities, such as advanced age, can have a negative prognostic effect on the procedure outcomes (14). It is also important to note that there was an evident lack of large cohort, multicentre trials and comparative studies involving the Thoraflex Hybrid™ device and further studies must take place to evidence the promising results of this novice tool. Table 4 Summarizes all the findings in Distal Stent Induced New Entry.
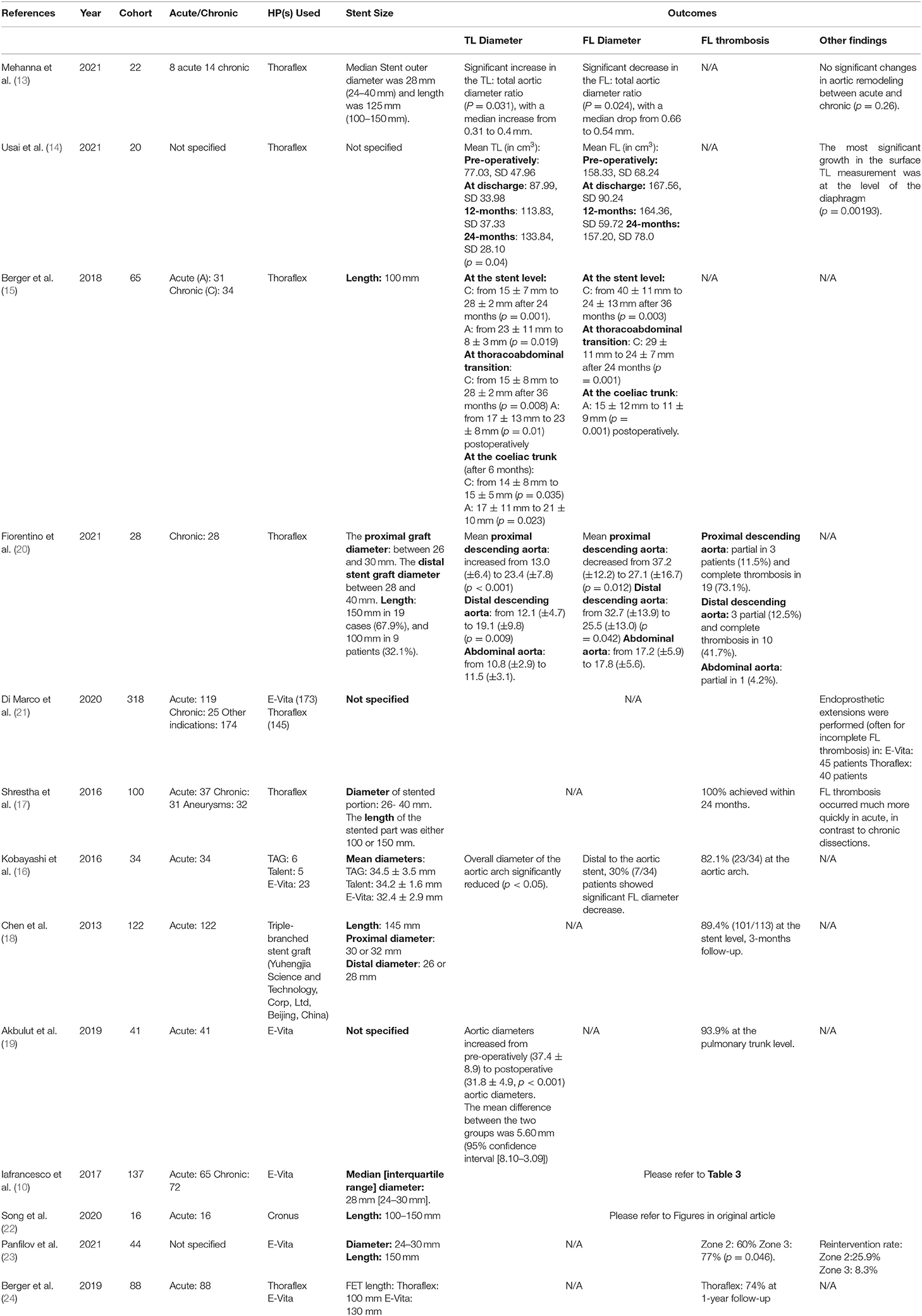
Table 4. Summary of studies included in Distal Stent Induced New Entry and their aortic remodeling data reported.
As mentioned earlier, dSINE is a serious complication associated with FET where a new intimal entry tear develops distally due to the stent graft portion of the FET HP. Untreated dSINE has showed a mortality rate of up to 25% following endovascular aortic intervention (9, 25). The distal landing zone of the stent is often located in the diseased zone of the aorta, as a result, the stent graft portion of the FET device can induce injury to the intima of the aorta due to the fragile and mobile dissection membranes mismatching with a stiff stent graft (26, 27). dSINE necessitates secondary intervention which negates the benefit of FET being a 1-stage process. Nevertheless, secondary endovascular reintervention has shown to have excellent clinical outcome (25). Therefore, oversizing the FET graft is strongly suggested to increase the risk of dSINE forming, however, this has been challenged (25, 27, 28). This relationship between dSINE occurrence and the size of the graft will be discussed further on in this review. As will also be discussed later in this review, formation of dSINE perfuses the FL and can lead to a new patent FL, which significantly impede positive aortic remodeling (26, 29, 30).
The incidence of dSINE for any device is varied between different studies using the same device, this is likely to be associated with the difference in the study design, population (baseline morbidity), and surgical technique or device size and length used. For example, the incidence of dSINE reported in the studies identified ranged from 0 to 14.5% with Thoraflex Hybrid™, 1–18.2% with E-Vita, and 0–27.3% with Frozenix™. The size and length of device used are interconnected with the incidence of dSINE (9, 20, 24, 25, 31–37). A summary of all the dSINE findings to-be discussed in this section can be found in Table 5.
Terumo Aortic's Thoraflex™ Hybrid is an excellent FET device with very favorable survival outcomes that are to some extent superior to those offered by other commercially available devices. The incidence of dSINE with Thoraflex Hybrid™ can be considered the lowest within the aortic arch prostheses market as demonstrated above (9, 20, 24, 25, 31–37).
One of the many studies that reported a relatively low incidence of dSINE is Kreibich et al. (26), with 12.7% of patients developing dSINEs 27 months following FET with Thoraflex Hybrid™. Other than the aforementioned fact that the dSINE group of patients had smaller true lumen diameters at the level of the stent graft (L1; p = 0.251) and the level of thoracoabdominal transition (L2; p = 0.44), there was no difference in type of dissection or clinical characteristics between patients that did and did not develop dSINE. Another study by the same authors (26) showed the safety, efficacy and consistency of THP™ by reporting a dSINE rate of 11.4%.
Charchyan et al. (32) compared the rate of dSINE in different operative stent devices. Group one was treated with distal Z shaped nitinol stent grafts (E-Vita), group two was treated with ring shaped nitinol stent graft (Thoraflex™ Hybrid) and group three used distal dissection-specific stent grafts (Valiant™ retrograde stent graft, Medtronic Vascular, Santa Rosa, CA, USA). Upon univariate and multivariate risk factor analysis, the study determined two statistically significant predictors of developing dSINE, which included connective tissue disorder and stent graft diameter. Univariate risk factor analysis of connective tissue disorder being associated with developing dSINE was derived to have a hazard ratio of 4.02 (95% CI, 0.89–18.27; p = 0.072). Moreover, multivariate analysis showed connective tissue disorder to have a hazard ratio of 5.62 (95% CI, 0.68–46.59; p = 0.110). Univariate risk factor analysis of the association of stent graft diameter with developing dSINE showed to have a hazard ratio of 1.36 (95%CI,1.08–1.71; p = 0.009). Furthermore, multivariate analysis derived a hazard ratio of 1.37 (95% CI, 1.02–1.83; p = 0.034). This study is helpful as it provides significant evidence supporting the numerical trends suggested by Kreibich et al. (9). Charchyan et al. (32) also suggested using a dissection specific stent graft with a tapered structure thus preventing oversizing at the distal edge or using a longer stent graft to minise the occurrence of dSINE.
This study proved that with the E-Vita Z-shaped nitinol stent grafts, dSINE occurrence was significantly higher than the Thoraflex Hybrid™ ring shaped nitinol stent graft. In the E-Vita group, 13% of patients developed dSINE whereas in the Thoraflex Hybrid™ group this was only 4.5%, which was significantly less (p = 0.043, Cramer's V = 0.24). The mean follow up time was 16 months and 14.5 months respectively for group one and two. This study contradicts Kreibich et al. (9) study, as it provides a direct comparison between devices and confirms Thoraflex Hybrid™ superiority over E-Vita (32). Only one study looking at E-Vita Open device reported a relatively low dSINE occurrence of 1% of a patient population of 286. However other complications such as endoleak occurred in 5% of patients which is more than any study investigating Thoraflex™ Hybrid (33).
Berger et al. (24) also directly compared Thoraflex™ and E-Vita Open and further proved Thoraflex Hybrid's more favorable results as 14.5% of patients in the Thoraflex Hybrid™ developed dSINE post-FET relative to 18.2% with E-Vita Open (P = 0.19). To extend this claim, a dSINE rate of 7.1% was reported by Fiorentino et al. (20) in their study which used Thoraflex Hybrid™ in 28 patients with chronic dissections/post-dissection aneurysms.
Custom-made graft and J Graft Open Stent Graft (Frozenix™) studies have reported relatively positive dSINE results, however, these are widely varied between studies. Hirano et al. (34) reported that dSINE occurred in 4% of J Graft Open Stent Graft patients. Again, a replicated study found that dSINE occurred in only 1 patient (0.72%) with the J Graft Open Stent graft which required TEVAR reintervention (35). Interestingly, none of the patients 50 in Iino et al. (36) suffered from dSINE post-FET. However, the authors here used the Frozenix graft in combination with the Gelweave™ graft (Terumo Aortic, Scotland, UK). This shows the extent of the positive affect induced by the Terumo Aortic line of aortic arch prostheses (36). On the other hand, Yamane et al. (37) also used Frozenix™ HP alone in 11 patients out of whom 3 developed dSINE (27.3%). Another study reported 9 cases of dSINE amongst 70 patients receiving Frozenix™ HP (12.9%). All 9 patients underwent successful reintervention with TEVAR (25). Finally, Furutachi et al. (31) reported a dSINE rate of 15.8% amongst their Frozenix™ FET group. Spring back force from the stent graft has been established to be one of the underlying mechanisms for dSINE, causing injury to aortic intimal when the spring force straightens its configuration (27). Furutachi et al. (31) also stated that the Frozenix™ graft is said to exert a strong spring back force on the aortic wall which increases the risk of dSINE developing. On the contrary, the Thoraflex Hybrid™ design has been shown to actually reduce the stress on the aortic wall (38).
Very surprisingly, despite a comprehensive and thorough literature search using multiple databases, no evidence on dSINE directly associated with Cronus™ could be identified. Tan et al. (39) is the only FET study identified which used Cronus™ and reported on untreated entry tears (63.6%), however, these were distal to the stent graft itself therefore not directly induced by it. It is worth noting that Cronus™ is geographically confined mainly to China, hence why there is little to no data on it making it difficult to draw a definitive conclusion on its effectiveness regarding dSINE (38).
If dSINE is diagnosed, the initial management involves optimizing blood pressure. If the false lumen continues to grow larger in diameter, pseudoaneurysm formation, malperfusion or development of symptoms occur then secondary endovascular reintervention with TEVAR is necessary. The artery of Adamkiewicz is identified in the preoperative CT and preserved to the best ability to prevent the risk of paraplegia. Secondary reintervention with TEVAR for dSINE has been reported to achieve excellent results (25, 40).
Several preventive procedures can be undertaken to prevent the development of dSINE, firstly endografts with tapered configuration can potentially be used to prevent excessive oversizing. This tapering effect in diameter and area comprises of using a small stent graft distally and a larger stent graft proximally (38). In TEVAR, Hsu et al. (41) demonstrated dSINE occurrence decreased from 34.7 to 8.3% using this endografting technique. This was also supported by Janosi et al. (42), who also demonstrated the effectiveness of this TEVAR technique in reducing the incidence of dSINE. Janosi et al. (42) found that patients who developed dSINE showed a significantly higher taper ratio of the true lumen of the aorta (40.9 ± 14.13% vs. 25.36 ± 20.2%, p < 0.05). The same principle applies in FET as proven by Ma et al. (38), who described tapered stent grafts as the “ideal” ones. In fact, this same fascinating review indicated that Thoraflex™ Hybrid is the only FET HP available commercially with a different proximal and distal diameter of its stent graft portion. Unlike other FET devices, Thoraflex™ Hybrid is unique in that is has a wide and versatile portfolio with varying combinations of stent-graft diameters as well as stent graft lengths. In addition, as aforementioned its interrupted pattern projects minimal radial forces on the diseased aortic wall relative to the other aortic arch hybrid prostheses, confirming Thoraflex Hybrid™ to be the optimal FET device choice for TAR (13, 38). It is essential to correctly choose the FET graft size after careful TL measurement, to avoid aortic remodeling mismatch in the distal transition zone between the stent-covered aorta and non-stent-covered aorta, thereby minimizing the risk of dSINE developing and maximizing the rate of aortic remodeling.
The culmination of the process of aortic remodeling is marked by the complete thrombosis and elimination of the FL with the normalization of the TL diameter. This achieved by the action of the stent graft portion of the hybrid prosthesis (HP) sealing off any entry tears and cutting the perfusion to the FL (29, 30). Developing dSINE means the FL remains perfused and patent, thereby negatively influencing aortic remodeling, this concept has been discussed in several studies (29, 30). Nomura et al. (25) stated that all patients who developed dSINE also developed enlargement of the thoracic aortic diameter, including the FL, whereas thoracic aortic remodeling did not occur. This is significantly incomparable with the 57.3% thoracic aortic remodeling rate in the non-dSINE group (p = 0.0013). Furthermore, a Russian single-center study (32) reported that 11% of their FET patient population who experienced dSINE also showed negative aortic remodeling which necessitated reintervention with thoracic endovascular aortic repair (TEVAR). In addition, Wada et al. (27) stated that the non-stent-covered aorta is unlikely to undergo sufficient remodeling due to the expansion of the FL and the pulsatile wall stress in the FL, both caused by dSINE. Another study which showed an identical trend is Kreibich et al. (9), which found that patients who developed dSINE showed to have smaller true lumen diameters at the level of the stent graft (L1; p = 0.251) and the level of thoracoabdominal transition (L2; p = 0.44). To further prove the relationship between dSINE and aortic remodeling, both Huang et al. and Chen et al. (43, 44) conducted studies on TEVAR in type B aortic dissection (TBAD). Results demonstrated that patients who suffered from dSINE post-TEVAR also showed significantly worse aortic remodeling.
Aortic remodeling and dSINE are connected together, but might also be associated with a FET graft length and size, as some studies study revealed that these two variables strongly influence both of the rate of aortic remodeling and the incidence of dSINE, both concomitantly and independently.
When it comes to aortic remodeling and dSINE associated with FET, in addition to the difference between anatomical sites and the presentation of the dissection, another significant factor to consider is the distal landing site for the prosthetic anastomosis. The landing zone in the FET technique has historically been zone 3, at the proximal descending aorta, after the left subclavian artery. However, novel evidence has suggested that zone 2, between the left common carotid and subclavian artery, yields more benefit (23). The Society of Vascular Surgery/Society of Thoracic Surgeons (SVS/STS) Aortic Dissection Classification System of dissection subtype according to zone location of primary entry tear is well-illustrated in Lombradi et al. (45). Firstly, a 2019 study of 282 patients reported that proximilisation of the anastomosis led to a reduced visceral ischaemia time (p = 0.001) and other complications such as recurrent laryngeal nerve injury (46). Another 2018 study supported this by evidencing significantly lower rates of post-FET renal (p = 0.004) and pulmonary failure (p < 0.001). Additionally, these benefits were observed long-term, where the 5-year survival rates were also superior with zone 2 implantation (p = 0.022) (33). Therefore, we thought it would be of excellent academic merit to evaluate whether aortic remodeling rates would also be influenced by the distal landing zones of the anastomosis, to improve future practice and establish its connection to both aortic remodeling rate and dSINE incidence.
Despite the overarching principal that proximilisation of the distal anastomosis offers superior results in general, a study by Panfilov et al. (23) describes zone 2 anastomosis as a “double-edged sword.” This 2021 study concluded that proximilisation decreases the extent of the FET procedure, in addition to the ischaemic time, and overall post-operative morbidity and mortality (23). However, complete FL thrombosis was significantly higher with zone 3 anastomosis. At 24 months post-FET, FL thrombosis in zone 2 was 60 and 77% in zone 3 (p = 0.046). Additionally, this increased remodeling was thought to explain the lower reintervention rates in zone 3 (8.3 vs. 25.9%). Panfilov et al. (23) hypothesized that failure of FL thrombosis may be due to the reduced length of coverage in the aorta, causing the higher rates of reintervention. This is congruent to Berger et al. (24) findings that aortic remodeling could be improved with longer coverage of the descending aorta and hence further stabilization.
This notion to use longer FET stent grafts was also fully supported by Yamane et al. (37), who discovered a strong association between the FET insertion length and dSINE incidence as a short insertion length was much more common in the dSINE group, thereby further confirming our earlier statement on this relationship. However, for future practice using the Thoraflex™ Hybrid, Leone et al. (46) made a different recommendation of using the shorter graft length of 100 mm for better aortic remodeling outcomes. The authors suggested that the 100 mm endograft can sufficiently stabilize the dissection flap and that a large intimal tear is often located proximally near the left subclavian artery. Moreover, a recent study has shown that elongated grafts were associated with a 13% increase in freedom from negative remodeling (47). Nevertheless, the majority of evidence favors greater FET insertion lengths for improved remodeling. Regarding graft size, a 2015 review noted that oversized stents in acute dissection could attribute to new intimal tears and subsequently impair aortic wall healing and remodeling (12, 21). As shown in Table 4, varying lengths and diameters of the Thoraflex™ Hybrid were used in different studies.
Kreibich et al. (9) used the 100 mm Thoraflex Hybrid™ graft used in 123 patients, 14 (11.4%) developed dSINE, and out of 3 patients treated with the 150 mm graft, 2 (66.7%) developed dSINE. The authors also found that larger stent graft diameters and oversizing were more common in dSINE patients. This is another study that supported using longer FET stent grafts to increase the insertion length and extend the stent graft coverage of the aorta more distally, to reduce the risk of dSINE in this case, in addition to Berger et al. (24), Yamane et al. (37), and Panfilov et al. (23) mentioned above. On the other hand, Nishi et al. presented a counter argument that some surgeons recommend using a short FET stent to decrease the direct coverage to the segmental arteries and ensure the blood supply to the spinal cord. However, this was countered by Tan et al. (39), who stated that this alone is insufficient to avoid paraplegia and that the stent should be extended distally to treat any distal entry tears. Thoraflex™ Hybrid is licencesed to be implanted across the entire arch (zone 0–4), thus the distal anastomosis zone will determine the length of graft needed (48).
The compelling evidence in this review strongly establishes the connection between dSINE, aortic remodeling, and the insertion length of the FET stent graft. Kreibich et al. (9), Yamane et al. (24), Berger et al. (37), Tan et al. (39), and Panfilov et al. (23) alls agreed that using longer FET stent grafts is very likely to reduce the incidence of dSINE or boost aortic remodeling. With the proven negative relationship between the latter two, the nature of the relationship between these three variables is a three-way one.
Excessive oversising of the FET graft distally has also been strongly associated with dSINE occurrence. Several studies have established that oversizing is a risk factor for dSINE development, however, a few other studies have argued against this. Di Marco et al. (1), Wada et al. (27), Berger et al. (24), Tsagakis et al. (28) and Canaud et al. (49) all recommended against unnecessary graft oversizing as they argued that excessive oversizing is a significant risk factor for development of dSINE. Janosi et al. (42), Jang et al. (50) and Huang et al. (51) studied the relationship between distal oversizing and dSINE and presented solid evidence showing a significant difference in oversizing ratios between patients who did and did not develop dSINE, further extending the validity of the above argument. On the other hand, although Kreibich et al. (9) also recommended avoidance of oversizing, there was a numeric but not statistically significant trend toward oversizing in their dSINE patient group (P = 0.613). In addition, in contrast to the majority of reports both Nomura et al. (25) and Yamane et al. (37) did not find a significant different in oversizing between patients with and without dSINE post-FET. Finally, Tochii et al. (3) presented both sides of the argument by stating that although graft oversizing may induce intimal damage, undersizing the FET stent graft may also increase the risk of type 1b endoleak which can negatively influence aortic remodeling through impeding FL thrombosis. Damberg et al. (52) also supported the use of 10–20% oversized grafts to reduce the risk of type Ib endoleak by allowing a tighter distal stent-graft anastomosis. It is worth noting the studies highlighted used different definitions and calculations for oversizing. Yet, all evidence considered, it is clearcut that excessively oversizing the stent-graft, particularly distally, significantly increases the risk of forming dSINE. Given the already established relationship between dSINE and aortic remodeling, it is safe to say that FET graft size is in turn associated with aortic remodeling.
In conclusion, aortic remodeling has been a significant indicator for patient prognosis following the FET procedure. The Thoraflex™ Hybrid device has evidenced significantly increased TL diameter, decreased FL diameter, and favorable FL thrombosis in both CT angiography and volumetric measurement studies. Moreover, these effects were superior to the other HP available commercially and were also maintained long-term. Not only does the Thoraflex™ Hybrid show excellent aortic remodeling, but it has also shown reduced dSINE rates in comparison with other HPs, encouraging further studies into the effectiveness of this device and its increased implementation in future practice given its favorable outcomes and high versatility. With dSINE negatively influencing aortic remodeling distally and with FET insertion length interconnecting with both outcomes, the choice of both FET graft size and length must be made with great care to achieve optimal results.
MJ, FK, PS, and DA were involved in literature review design, literature search, and manuscript writing. YR was involved in literature search. ST, MM, SH, IM, and MB involved in manuscript revision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Di Marco L, Murana G, Fiorentino M, Amodio C, Mariani C, Leone A, et al. The frozen elephant trunk surgery: a systematic review analysis. Indian J Thorac Cardiovasc Surg. (2019) 35:118–26. doi: 10.1007/s12055-019-00815-0
2. Karck M, Chavan A, Hagl C, Friedrich H, Galanski M, Haverich A. The frozen elephant trunk technique: A new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg. (2003) 125:1550–3. doi: 10.1016/S0022-5223(03)00045-X
3. Tochii M, Takami Y, Ishikawa H, Ishida M, Higuchi Y, Sakurai Y, et al. Aortic remodeling with frozen elephant trunk technique for Stanford type A aortic dissection using Japanese J-graft open stent graft. Heart Vessels. (2019) 34:307–15. doi: 10.1007/s00380-018-1246-x
4. Roselli EE, Idrees JJ, Bakaeen FG, Tong MZ, Soltesz EG, Mick S, et al. Evolution of Simplified Frozen Elephant Trunk Repair for Acute DeBakey Type I Dissection: Midterm Outcomes. Ann Thorac Surg. (2018) 105:749–55. doi: 10.1016/j.athoracsur.2017.08.037
5. Watanabe Y, Shimamura K, Yoshida T, Daimon T, Shirakawa Y, Torikai K, et al. Aortic remodeling as a prognostic factor for late aortic events after thoracic endovascular aortic repair in type B aortic dissection with patent false lumen. J Endovasc Ther. (2014) 21:517–25. doi: 10.1583/13-4646R.1
6. Heijmen R, Fattori R, Thompson M, Dai-Do D, Eggebrecht H, Degrieck I, et al. Mid-term Outcomes and Aortic Remodelling After Thoracic Endovascular Repair for Acute, Subacute, and Chronic Aortic Dissection: The VIRTUE Registry. Eur J Vasc Endovasc Surg. (2014) 48:363–71. doi: 10.1016/j.ejvs.2014.05.007
7. Mani K, Clough RE, Lyons OTA, Bell RE, Carrell TW, Zayed HA, et al. Predictors of Outcome after Endovascular Repair for Chronic Type B Dissection. Eur J Vasc Endovasc Surg. (2012) 43:386–91. doi: 10.1016/j.ejvs.2012.01.016
8. Stanley GA, Murphy EH, Knowles M, Ilves M, Jessen ME, Dimaio JM, et al. Volumetric analysis of type B aortic dissections treated with thoracic endovascular aortic repair. J Vasc Surg. (2011) 54:985–92. doi: 10.1016/j.jvs.2011.03.263
9. Kreibich M, Bünte D, Berger T, Vötsch A, Rylski B, Krombholz-Reindl P, et al. Distal Stent Graft–Induced New Entries After the Frozen Elephant Trunk Procedure. Ann Thorac Surg. (2020) 110:1271–9. doi: 10.1016/j.athoracsur.2020.02.017
10. Iafrancesco M, Goebel N, Mascaro J, Franke UFW, Pacini D, Di Bartolomeo R, et al. Aortic diameter remodelling after the frozen elephant trunk technique in aortic dissection: results from an international multicentre registry. Eur J Cardiothorac Surg. (2017) 52:310–8. doi: 10.1093/ejcts/ezx131
11. Takagi H, Umemoto T. A Meta-Analysis of Total Arch Replacement with Frozen Elephant Trunk in Acute Type A Aortic Dissection. Vasc Endovascular Surg. (2016) 50:33–46. doi: 10.1177/1538574415624767
12. Di Bartolomeo R, Pantaleo A, Berretta P, Murana G, Castrovinci S, Cefarelli M, et al. Frozen elephant trunk surgery in acute aortic dissection. J Thorac Cardiovasc Surg. (2015) 149:S105–9. doi: 10.1016/j.jtcvs.2014.07.098
13. Mehanna M, Elhamami M, Abolkasem A, Ramadan B, Almaghraby A, Mascaro J. Aortic remodelling and false lumen changes after the frozen elephant trunk technique using the thoraflex hybrid stented graft for aortic dissection. Egypt Hear J. (2021) 73:1–6. doi: 10.1186/s43044-021-00198-x
14. Usai MV, Ibrahim A, Oberhuber A, Dell'Aquila AM, Martens S, Motekallemi A, et al. Quantification of volume changes in the descending aorta after frozen elephant trunk procedure using the Thoraflex hybrid prosthesis for type A aortic dissection. J Thorac Dis. (2021) 13:60. doi: 10.21037/jtd-20-2356
15. Berger T, Kreibich M, Morlock J, Kondov S, Scheumann J, Kari FA, et al. True-lumen and false-lumen diameter changes in the downstream aorta after frozen elephant trunk implantation. Eur J Cardio-Thoracic Surg. (2018) 54:375–81. doi: 10.1093/ejcts/ezy031
16. Kobayashi M, Chaykovska L, van der Loo B, Nguyen TDL, Puippe G, Salzberg S, et al. Long-term results of simplified frozen elephant trunk technique in complicated acute type A aortic dissection: a case–control study. Vascular. (2016) 24:523–30. doi: 10.1177/1708538115627728
17. Shrestha M, Kaufeld T, Beckmann E, Fleissner F, Umminger J, Abd Alhadi F, et al. Total aortic arch replacement with a novel 4-branched frozen elephant trunk prosthesis: Single-center results of the first 100 patients. J Thorac Cardiovasc Surg. (2016) 152:148–59. doi: 10.1016/j.jtcvs.2016.02.077
18. Chen LW, Lu L, Dai XF, Wu XJ, Zhang GC, Yang GF, et al. Total arch repair with open triple-branched stent graft placement for acute type A aortic dissection: experience with 122 patients. J Thorac Cardiovasc Surg. (2014) 148:521–8. doi: 10.1016/j.jtcvs.2013.10.021
19. Akbulut M, Ak A, Arslan Ö, Çekmecelioglu D, Taş S, Dönmez AA, et al. Early and mid-term results of frozen elephant trunk procedure for acute type A aortic dissection. Turkish J Thorac Cardiovasc Surg. (2019) 27:135. doi: 10.5606/tgkdc.dergisi.2019.16879
20. Fiorentino M, de Beaufort HWL, Sonker U, Heijmen RH. Thoraflex hybrid as frozen elephant trunk in chronic, residual type A and chronic type B aortic dissection. Interact Cardiovasc Thorac Surg. (2021) 32:566–72. doi: 10.1093/icvts/ivaa305
21. Di Marco L, Votano D, Leone A, Pacini D. Frozen elephant trunk: assets and liabilities of a challenging technique. Vessel Plus. (2020) 4:32. doi: 10.20517/2574-1209.2020.23
22. Song SB, Wu XJ, Sun Y, Cai SH, Hu PY, Qiang HF. A modified frozen elephant trunk technique for acute Stanford type A aortic dissection. J Cardiothorac Surg. (2020) 15:1–8. doi: 10.1186/s13019-020-01306-9
23. Panfilov DS, Kozlov BN, Pryakhin AS, Kopeva K V. Frozen elephant trunk technique with different proximal landing zone for aortic dissection. Interact Cardiovasc Thorac Surg. (2021) 33:286–92. doi: 10.1093/icvts/ivab086
24. Berger T, Weiss G, Voetsch A, Arnold Z, Kreibich M, Rylski B, et al. Multicentre experience with two frozen elephant trunk prostheses in the treatment of acute aortic dissection. Eur J Cardio-thoracic Surg. (2019) 56:572–8. doi: 10.1093/ejcts/ezz037
25. Nomura Y, Tonoki S, Kawashima M, Fujisue J, Uchino G, Miyahara S, et al. Distal Stent Graft-Induced New Entry after Total Arch Replacement with Frozen Elephant Trunk for Aortic Dissection. Ann Vasc Dis. (2021) 14:362–7. doi: 10.3400/avd.oa.21-00105
26. Kreibich M, Berger T, Rylski B, Chen Z, Beyersdorf F, Siepe M, et al. Aortic reinterventions after the frozen elephant trunk procedure. J Thorac Cardiovasc Surg. (2019) 159:392–9. doi: 10.1016/j.jtcvs.2019.02.069
27. Wada T, Yamamoto H, Kadohama T, Takagi D. Aortic remodeling mismatch: a potential risk factor of late distal stent graft-induced new entry after frozen elephant trunk deployment. JTCVS Tech. (2021) 8:46–8. doi: 10.1016/j.xjtc.2021.04.036
28. Tsagakis K, Jakob H. Which Frozen Elephant Trunk Offers the Optimal Solution? Reflections From Essen Group Semin. Thorac Cardiovasc Surg. (2019) 31:679–85. doi: 10.1053/j.semtcvs.2019.05.038
29. Katayama A, Uchida N, Katayama K, Arakawa M, Sueda T. The frozen elephant trunk technique for acute type A aortic dissection: Results from 15 years of experience. Eur J Cardio-thoracic Surg. (2014) 47:355–60. doi: 10.1093/ejcts/ezu173
30. Dohle DS, Jakob H, Schucht R, Janosi RA, Schlosser T, El Gabry M, et al. The impact of entries and exits on false lumen thrombosis and aortic remodelling. Eur J Cardio-thoracic Surg. (2017) 52:508–15. doi: 10.1093/ejcts/ezx236
31. Furutachi A, Takamatsu M, Nogami E, Hamada K, Yunoki J, Itoh M, et al. Early and mid-term outcomes of total arch replacement with the frozen elephant trunk technique for type A acute aortic dissection. Interact Cardiovasc Thorac Surg. (2019) 29:753–60. doi: 10.1093/icvts/ivz154
32. Charchyan ER, Breshenkov DG, Belov YV. Hybrid aortic repair in patients with type III aortic dissection and concomitant proximal aortic lesion. Khirurgiya Zhurnal im NI Pirogova. (2020) 9:28−37. doi: 10.17116/hirurgia202009128
33. Tsagakis K, Wendt D, Dimitriou AM, Thielmann M, Sharaf-Eldin S, Gabry M. El, et al. The frozen elephant trunk treatment is the operation of choice for all kinds of arch disease. J Cardiovasc Surg (Torino). (2018) 59:540–6. doi: 10.23736/S0021-9509.18.10597-0
34. Hirano K, Tokui T, Nakamura B, Inoue R, Inagaki M, Hirano R, et al. Impact of the Frozen Elephant Trunk Technique on Total Aortic Arch Replacement. Ann Vasc Surg. (2020) 65:206–16. doi: 10.1016/j.avsg.2019.10.075
35. Yoshitake A, Tochii M, Tokunaga C, Hayashi J, Takazawa A, Yamashita K, et al. Early and long-term results of total arch replacement with the frozen elephant trunk technique for acute type A aortic dissection. Eur J Cardio-thoracic Surg. (2020) 58:707–13. doi: 10.1093/ejcts/ezaa099
36. Iino K, Takago S, Saito N, Ueda H, Yamamoto Y, Kato H, et al. Total arch replacement and frozen elephant trunk for acute type A aortic dissection. J Thorac Cardiovasc Surg. (2020). doi: 10.1016/j.jtcvs.2020.10.135
37. Yamane Y, Katayama K, Furukawa T, Shimizu H, Okazaki T, Takasaki T, et al. Mid-Term Results of Frozen Elephant Trunk Technique for Chronic Aortic Dissection. Ann Vasc Dis. (2020) 13:137–43. doi: 10.3400/avd.oa.19-00131
38. Ma WG, Zheng J, Sun LZ, Elefteriades JA. Open Stented Grafts for Frozen Elephant Trunk Technique: Technical Aspects and Current Outcomes. Aorta (Stamford, Conn). (2015) 3:122–35. doi: 10.12945/j.aorta.2015.14.062
39. Tan L, Xiao J, Zhou X, Shen K, Li F, Luo J, et al. Untreated distal intimal tears may be associated with paraplegia after total arch replacement and frozen elephant trunk treatment of acute Stanford type A aortic dissection. J Thorac Cardiovasc Surg. (2019) 158:343–50. doi: 10.1016/j.jtcvs.2018.08.111
40. Weng SH, Weng CF, Chen WY, Huang CY, Chen IM, Chen CK, et al. Reintervention for distal stent graft-induced new entry after endovascular repair with a stainless steel-based device in aortic dissection. J Vasc Surg. (2013) 57:64–71. doi: 10.1016/j.jvs.2012.07.006
41. Hsu H, Shih C. Distal Stent Graft-Induced New Entry – Endovascular Today [Internet]. Endovascular Today. (2016). Available online at: https://evtoday.com/articles/2016-jan-supplement2/distal-stent-graftinduced-new-entry
42. Jánosi RA, Tsagakis K, Bettin M, Kahlert P, Horacek M, Al-Rashid F, et al. Thoracic aortic aneurysm expansion due to late distal stent graft-induced new entry. Catheter Cardiovasc Interv. (2015) 85:E43–53. doi: 10.1002/ccd.25614
43. Huang CY, Hsu HL, Chen PL, Chen IM, Hsu CP, Shih CC. The Impact of Distal Stent Graft–Induced New Entry on Aortic Remodeling of Chronic Type B Dissection. Ann Thorac Surg. (2018) 105:785–93. doi: 10.1016/j.athoracsur.2017.08.039
44. Chen IM, Chen PL, Huang CY, Weng SH, Chen WY, Shih CC. Factors Affecting Optimal Aortic Remodeling After Thoracic Endovascular Aortic Repair of Type B (IIIb) Aortic Dissection. Cardiovasc Intervent Radiol. (2017) 40:671–81. doi: 10.1007/s00270-017-1563-y
45. Lombardi JV, Hughes GC, Appoo JJ, Bavaria JE, Beck AW, Cambria RP, et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) Reporting Standards for Type B Aortic Dissections. Ann Thorac Surg. (2020) 109:959–81. doi: 10.1016/j.athoracsur.2019.10.005
46. Leone A, Di Marco L, Coppola G, Amodio C, Berardi M, Mariani C, et al. Open distal anastomosis in the frozen elephant trunk technique: initial experiences and preliminary results of arch zone 2 versus arch zone 3†. Eur J Cardiothorac Surg. (2019) 56:564–71. doi: 10.1093/ejcts/ezz103
47. Kozlov BN, Panfilov DS, Saushkin VV, Nasrashvili GG, Kuznetsov MS, Nenakhova AA, et al. Distal aortic remodelling after the standard and the elongated frozen elephant trunk procedure. Interact Cardiovasc Thorac Surg. (2019) 29:117–23. doi: 10.1093/icvts/ivz026
49. Canaud L, Gandet T, Sfeir J, Ozdemir B, Solovei L, Alric P. Risk factors for distal stent graft-induced new entry tear after endovascular repair of thoracic aortic dissection. J Vasc Surg. (2019) 69:1610–4. doi: 10.1016/j.jvs.2018.07.086
50. Jang H, Kim MD, Kim GM, Won JY, Ko YG, Choi D, et al. Risk factors for stent graft-induced new entry after thoracic endovascular aortic repair for Stanford type B aortic dissection. J Vasc Surg. (2017) 65:676–85. doi: 10.1016/j.jvs.2016.09.022
51. Huang CY, Chen CW, Chen PL, Chen WY, Chen IM, Hsu CP, et al. Association between aortic remodeling and stent graft-induced new entry in extensive residual type A dissecting aortic aneurysm after hybrid arch repair. Ann Vasc Surg. (2016) 31:60–9. doi: 10.1016/j.avsg.2015.08.022
Keywords: aortic dissection, aortic aneurysm, aortic surgery, frozen elephant trunk, total arch replacement
Citation: Jubouri M, Kayali F, Saha P, Ansari DM, Rezaei Y, Tan SZCP, Mousavizadeh M, Hosseini S, Mohammed I and Bashir M (2022) Incidence of Distal Stent Graft Induced New Entry vs. Aortic Remodeling Associated With Frozen Elephant Trunk. Front. Cardiovasc. Med. 9:875078. doi: 10.3389/fcvm.2022.875078
Received: 13 February 2022; Accepted: 21 February 2022;
Published: 10 March 2022.
Edited by:
Amer Harky, Liverpool Heart and Chest Hospital, United KingdomReviewed by:
Ali Bashir, Carol Davila University of Medicine and Pharmacy, RomaniaCopyright © 2022 Jubouri, Kayali, Saha, Ansari, Rezaei, Tan, Mousavizadeh, Hosseini, Mohammed and Bashir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamad Bashir, ZHJtb2Jhc2hpckBvdXRsb29rLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.