
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 05 July 2022
Sec. Lipids in Cardiovascular Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.871011
This article is part of the Research Topic Cardiovascular Diseases in Autoimmune Diseases: Dyslipidemia and vascular inflammation View all 8 articles
 Yuzhou Gan1,2†
Yuzhou Gan1,2† Yawei Zhao1,2,3†
Yawei Zhao1,2,3† Gongming Li1,2,4
Gongming Li1,2,4 Hua Ye1,2
Hua Ye1,2 Yunshan Zhou1,2
Yunshan Zhou1,2 Chang Hou5
Chang Hou5 Lan Wang5
Lan Wang5 Jianping Guo1,2
Jianping Guo1,2 Chun Li1,2*
Chun Li1,2*Background: Antiphospholipid syndrome (APS) is a disorder associated with thromboembolic diseases, including acute myocardial infarction (AMI). Given that AMI is a relatively common condition with poor prognostic features, identification of risk factors for AMI in APS is important.
Methods: A retrospective cohort study was performed consisting of 332 patients with APS, and 239 patients with thrombotic APS were finally included. Patients were followed up in the outpatient department for 5 years. Clinical data and laboratory parameters were analyzed to identify the risk factors for AMI in APS. The primary and secondary clinical outcomes were all-cause mortality and recurrence of thrombosis, respectively.
Results: AMI was observed in 12.1% (29/239) of patients with APS. Compared to patients without AMI, patients with AMI had multiple organ thrombosis (55.1 vs. 34.3%, p = 0.029), recurrent thrombosis (58.6 vs. 34.3%, p = 0.011), a higher incidence of atherosclerosis (62.1 vs. 23.8%, p < 0.001), higher neutrophil count (×109/L) [4.68 (3.25, 8.17) vs. 3.71 (2.64, 5.80), p = 0.036], longer QT interval (ms) [438 ms (423, 454) vs. 425 ms (410, 446), p = 0.016], and fewer venous thrombosis events (27.6 vs. 63.3%, p < 0.001). Multivariate logistic regression analysis (adjusted for age and gender) identified several factors that were positively associated with AMI, including multiple organ thrombosis [odds ratio (OR) 8.862, 95% confidence interval (CI): 1.817–43.212, p = 0.007), atherosclerosis (OR 5.397, 95%CI: 1.943–14.994, p = 0.001), and elevated neutrophil count (>6.3×109/L) (OR 3.271, 95%CI: 1.268–8.440, p = 0.014). The venous thrombosis was negatively associated with AMI (OR 0.106, 95%CI: 0.036–0.314, p < 0.001). Kaplan–Meier analysis revealed that the recurrence rates of arterial thrombosis differed significantly between patients with AMI and those without AMI [hazard ratio (HR) = 3.307, p = 0.038].
Conclusion: Atherosclerosis, multiple organ thrombosis, an increased number of neutrophils are variables positively associated with AMI in APS, and venous thrombosis had a negative association with AMI. AMI only predicts the subsequent recurrence of arterial thrombosis. These findings suggest that distinct pathophysiological mechanisms may exist and contribute to the development of venous or arterial thrombotic APS.
Antiphospholipid syndrome (APS) is a prothrombotic autoimmune disease characterized by recurrent thrombosis and/or obstetric events in the presence of antiphospholipid antibodies. Coronary artery disease is one of the main cardiac manifestations of APS, and 2.8–5.5% of patients with acute myocardial infarction (AMI) are young individuals with AMI secondary to APS (1). In one study of patients with APS with a low pre-test probability for cardiovascular events, the prevalence of myocardial scarring detected by cardiac MRI was surprisingly high (11%) (2). Furthermore, Cervera et al. reported that 10% of patients with primary APS died of AMI (3). In addition, poor outcomes of AMI in autoimmune diseases have been confirmed in numerous studies (4, 5). Thus, AMI is a relatively common condition with a poor prognosis in APS. However, to date, there has been no cohort study focusing on the clinical and laboratory features and prognosis of APS patients with AMI, including subsequent death and recurrent thrombosis. Although the control of traditional cardiovascular disease (CVD) risk factors in APS has been emphasized by recommendations as overarching principles (6), it remains unclear whether CVD risk factors fully contribute to AMI in APS. In addition, the accelerated atherosclerosis and increased prevalence of CVD cannot be completely explained by traditional risk factors or the use of glucocorticoids in other autoimmune diseases (7). The aim of the present study was to characterize and identify the risk factors for AMI in APS.
This was a single-center retrospective cohort study performed at the Department of Rheumatology, Peking University People's Hospital. A total of 332 patients with APS were consecutively enrolled between July 2009 and January 2021. The diagnosis of APS was confirmed by two rheumatologists (YZG and YWZ) according to the 2006 Sapporo criteria (8) or catastrophic antiphospholipid syndrome (CAPS) according to the current diagnostic criteria (9). Another inclusion criterion was the disease onset age, which was >18 years. The study was approved by the ethics committee of Peking University People's Hospital (2019PHB253-01) and complied with the Declaration of Helsinki guidelines.
Baseline demographics and clinical and laboratory characteristics were obtained from the electronic medical records at the time of APS diagnosis. Venous thromboembolic events (e.g., deep venous thrombosis of the upper limbs of the legs, visceral venous thrombosis, and/or pulmonary embolism) were confirmed by limb ultrasound, pulmonary computed tomography (CT) or scintigraphy (ventilation/perfusion), abdominal pelvic CT scan, and vessel angiography as indicated. Arterial thrombotic events (e.g., peripheral arterial thrombosis, acute cerebral infarction, and/or visceral arterial thrombosis) were diagnosed using typical clinical pictures with positive arteriography [e.g., leg or upper limb ultrasound, CT, or magnetic resonance angiography (MRA)] and surgery. Multiple organ thrombosis was defined as the involvement of at least two organ systems during the course of the disease. The adjusted global antiphospholipid syndrome score (aGAPSS) was calculated for each patient by adding the points corresponding to the risk factors, excluding antibodies to phosphatidylserine/prothrombin (aPS/PT) that are not routinely tested in most clinical laboratories, as previously described (10). The aGAPSS ranged from 0 to 17.
Atherosclerosis was defined as the presence of any plaque in the carotid or femoral arteries, which were tested using B-mode and Doppler ultrasound examination with an Aplio 500 system (Canon Medical Systems Corp., Tochigi, Japan) with a 14L5 transducer or a Logic E9 system (GE Healthcare, Chicago, IL, USA) with a 9L transducer. Electrocardiography (ECG) was performed using a MAC 5500 HD resting ECG system (GE Healthcare, Wauwatosa, USA). Transthoracic echocardiogram (TTE) evaluation was performed using either an iE33 (S5-1 probe, Philips Medical Systems, Andover, Massachusetts, USA) or a Vivid E9 (GE Vingmed, Horten, Norway, UK) scanner with a 2.5–3.5 MHz transducer. AMI was diagnosed according to the fourth universal definition of myocardial infarction and classified as ST segment elevation myocardial infarction (STEMI), non-ST segment elevation myocardial infarction (NSTEMI), and unstable angina (UA) (11). Myocardial infarction with non-obstructive coronary arteries (MINOCA) was defined as no coronary artery stenosis > 50% in any potential infarct-related artery without other clinically overt specific causes for the acute presentation (12).
Patients were followed up for 5 years or monitored up to 31 December 2021 if the patients were enrolled after 30 December 2016 in outpatient services. Follow-up information was also obtained from electronic medical records or regular medical examination reports. Medication data were recorded, including sustained anticoagulation treatment, antiplatelet therapies, corticosteroids, immunosuppressants, and statins. If patients received warfarin, the international normalized ratio (INR) was documented every 3 months and the mean INR was calculated. The primary clinical outcome was all-cause mortality (defined as the time from recruitment to death from any cause). The second clinical outcome was the recurrence of thrombosis. Thrombotic events were independently adjudicated by two investigators (YZG and YWZ).
Continuous data with normal distribution were expressed as the mean ± standard deviation, and differences between groups were analyzed using one-way ANOVA. Continuous data with skewed distribution were expressed as medians (P25, P75), and differences between groups were analyzed using the Kruskal–Wallis test. Dichotomous variables were reported as frequencies (percentages), and differences between groups were compared using the chi-square test (or Fisher exact test when appropriate). Univariate and multivariate logistic regression analyses with an imputed dataset were adopted to identify risk factors for AMI, adjusted for age and sex. The variables assessed in the group differences were entered as independent variables in the univariate logistic regression analysis when the p < 0.1. The variables assessed in the univariate regression analysis were entered as independent variables in the multivariate logistic regression analysis when the p < 0.1. Survival and recurrence of thrombosis were estimated using the Kaplan–Meier method, and differences were evaluated using a stratified log-rank test. Data analyses were performed using SPSS 23.0 for Windows. Two-sided p < 0.05 was considered statistically significant.
Of the 332 APS patients, 93 had isolated obstetric APS, 207 had isolated thrombotic APS, and 32 had thrombotic APS with obstetric complications. A flow diagram of the individuals at each stage is shown in Figure 1. A total of 239 patients with thrombotic APS (207 isolated thrombotic and 32 thrombotic APS with obstetrical complications) were enrolled in our cohort. Follow-up data were available for 196 patients (82.0%) with an overall median follow-up time of 4.5 years. Of the 196 patients, 102 (52.0%) completed a 5-year follow-up and 21 (10.7%) died within 5 years.
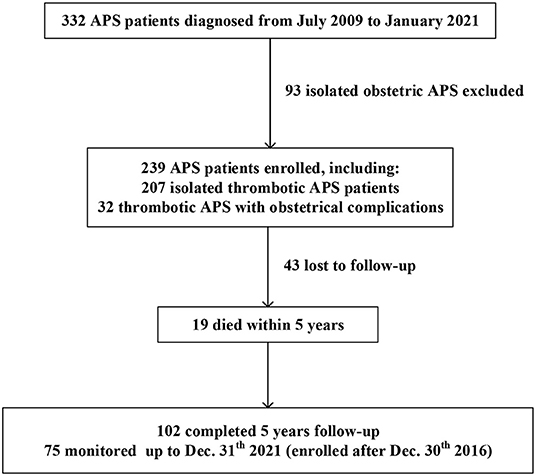
Figure 1. A flow chart of our retrospective study design. Of the 332 APS patients recruited, 239 thrombotic APS patients were enrolled.
The incidence of AMI was 12.1% (29/239) and detailed clinical profiles are shown in Table 1. Nine patients with AMI were male, and the average age of AMI onset was 44.6 years (Table 2). Of the 29 patients with AMI, 14 (48.3%) had STEMI, 5 (17.2%) had NSTEMI, and 10 (34.5%) had UA. In total, 13 patients with APS (44.8%) developed AMI before APS confirmation (range, 7 months to 30 years), 9 patients (31.0%) developed AMI after APS diagnosis (range, 1 month to 5 years), and 7 patients (24.2%) presented with AMI simultaneously with APS (confirmations were performed after 12 weeks). In total, 1 patient (3.4%) underwent coronary artery bypass grafting, 1 patient (3.4%) underwent thrombolysis, 9 patients (31.0%) underwent percutaneous coronary intervention, and the remaining 18 patients (62.2%) received conservative medical treatment. Of the 22 patients who underwent coronary angiography, 7 (31.8%) were MINOCA, including UA, STEMI, and NSTEMI (5, 2, and 1, respectively). One of the patients with AMI died within the first 30 days.
Next, we compared baseline demographics, clinical characteristics, and laboratory parameters between patients with and without AMI (Table 2). Patients with AMI had multiple organ thrombosis (55.1 vs. 34.3%, p = 0.029), recurrent thrombosis (58.6 vs. 34.3%, p = 0.011), higher incidence of atherosclerosis (62.1 vs. 23.8%, p < 0.001), higher neutrophil count (×109/L) [4.68 (3.25, 8.17) vs. 3.71 (2.64, 5.80), p = 0.036], and fewer venous thrombosis events (27.6 vs. 63.3%, p < 0.001). Demographic data, other clinical or laboratory features, and aGAPSS scores did not show any significant differences between the two groups. Supplementary Table 1 showed the detailed profiles of accompanied autoimmune diseases.
For cardiac manifestations, the QTc interval (ms) was significantly longer in APS patients with AMI [438 (423, 454) vs. 425 (410, 446), p = 0.016]. However, significant differences were not observed in other cardiac features, including arrhythmia, myocardial hypertrophy, echocardiographic parameters, and percentage of hydroxychloroquine usage (shown in Table 3).
In logistic regression analysis, the variables assessed in the previous analysis were entered as independent variables with a cut-off p < 0.1. Results of the univariate and multivariate logistic analyses are shown in Table 4. In univariate logistic analysis, several variables were positively associated with AMI, including multiple organ thrombosis [odds ratio (OR) 2.715, 95% confidence interval (CI): 1.230–5.995, p = 0.013], recurrent thrombosis (OR 2.359, 95%CI: 1.076–5.174, p = 0.032), atherosclerosis (OR 5.236, 95%CI: 2.319–11.82, p < 0.001), and elevated neutrophil count (>6.3 ×109/L) (OR 3.000, 95%CI: 1.328–6.780, p = 0.008). Interestingly, venous thrombosis was negatively associated with AMI (OR 0.221, 95%CI: 0.093–0.522, p = 0.001). With age and sex adjustment, the multivariate logistic models revealed that multiple organ thrombosis (OR 8.862, 95%CI: 1.817–43.212, p = 0.007), atherosclerosis (OR 5.397, 95%CI: 1.943–14.994, p = 0.001), and elevated neutrophil count (>6.3 ×109/L) (OR 3.271, 95%CI: 1.268–8.440, p = 0.014) might be risk factors for AMI, and venous thrombosis (OR 0.106, 95%CI: 0.036–0314, p < 0.001) might be protective factors for AMI. Traditional CVD risk factors (smoking, diabetes mellitus, hypertension, dyslipidemia, chronic kidney disease, and hyperuricemia) were not associated with AMI in APS.
The median follow-up duration was 4.8 years in the AMI group and 4.5 years in the non-AMI group. At the last follow-up visit, 4/27 patients in the AMI group and 17/167 in the non-AMI group died, and overall survival rates between the two groups were not significantly different (Figure 2A). Detailed causes of death in these 21 patients with APS were shown in Table 5. In the AMI group, two patients died of pulmonary embolism and the other two patients died of severe infection. In the non-AMI group, the patients died of multiple causes, including pulmonary embolism (n = 5), severe infection (n = 2), malignant tumors (n = 2), severe thrombocytopenia (n = 1), disseminated intravascular coagulation (n = 1), and macrophage activation syndrome (n = 1).
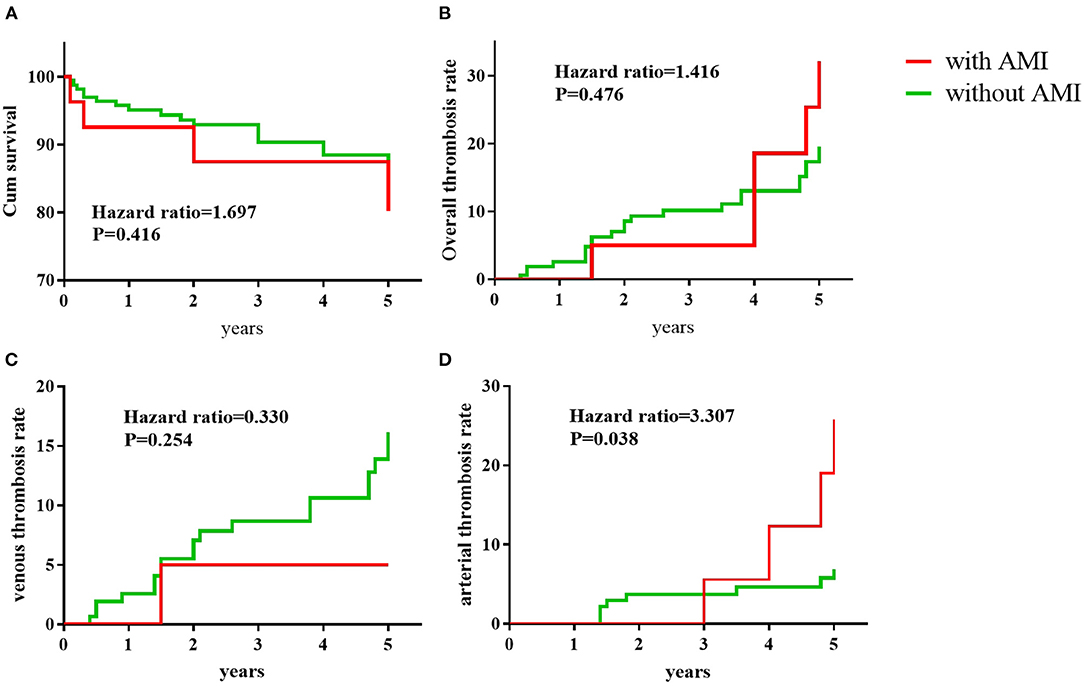
Figure 2. Clinical outcomes during the 5-year follow-up. Kaplan–Meier analysis of the portion of APS patients remaining survival (A), with overall recurrent thrombosis (B), recurrent venous thrombosis (C), and recurrent arterial thrombosis (D). P-values were calculated with the use of the stratified log-rank test.
As shown in Table 6, APS patients with AMI received more antiplatelet treatments [17 (63.0%) vs. 44 (26.3%), p < 0.001] and fewer immunosuppressants [10 (37.0%) vs. 123 (73.6%), p < 0.001] compared to APS patients without AMI. The application of anticoagulants, hydroxychloroquine, and corticosteroids was not significantly different between the two groups. The mean INR of APS patients who took the vitamin K antagonist was not significantly different between the AMI group and non-AMI group (2.19 ± 0.89 vs. 2.24 ± 0.68, P = 0.766). During the 5-year follow-up, the recurrence rate of overall thrombosis was 18.6% (5/27) in the AMI group and 13.7% (23/167) in the non-AMI group; recurrence of venous thrombosis was 3.7% (1/27) in the AMI group and 11.3% (19/167) in the non-AMI group, and the recurrence of arterial thrombosis was 14.8% (4/27) in the AMI group and 4.8% (8/167) in the non-AMI group, respectively. Kaplan–Meier analysis showed that there was no significant difference between the AMI group and the non-AMI group in recurrent rates of overall thrombosis (Figure 2B) or venous thrombosis (Figure 2C). However, the recurrence rates of arterial thrombosis differed significantly between the two groups (Figure 2D).
Our study showed that patients with APS had a high risk of AMI due to thromboembolism and accelerated atherosclerosis. To our knowledge, this is the first cohort study to characterize and explore the risk factors for AMI in patients with APS.
Previously, two register-based studies reported that 1.9–2.8% of patients with APS may develop myocardial infarction (3, 13). However, in our cohort, 12.1% of APS patients developed AMI. The explanation for the higher incidence in our cohort could be the different study designs; these two studies only included AMI events occurring after diagnosis of APS, whereas we included all AMI events which occurred both before the diagnosis of APS and during the follow-up. Another explanation could be the difference in ethnic backgrounds. In our study cohort, all patients were Han Chinese but all patients were Caucasians in two previous studies. In addition, Sacré et al. discovered that the prevalence of myocardial scarring detected by cardiac MRI was 11%, indicating that more than one-tenth of the patients with APS underwent ischemic cardiac events. Andreoli et al. estimated that the overall aPL frequencies in MI were 11% (14). Taken together, we confirmed an increased incidence of AMI in patients with APS.
Early studies reported that 90% of patients with AMI have obvious coronary artery obstruction (12). Recently, MINOCA has been increasingly recognized owing to the common use of high-sensitivity troponins and coronary angiography. Although the American Heart Association emphasized that it is necessary to exclude causes of spontaneous thromboembolism, such as APS (15), the exact incidence of MINOCA in APS remains unclear. In our cohort, MINOCA was seen in one-third of AMI patients, but a systematic study showed that normal coronaries were seen in 75% of APS patients with AMI by cardiac catheterization (16). Such a high incidence might partially be because the case series included in the review mainly focused on MINOCA. In addition, Tornvall et al. discovered that the incidence of MINOCA was similar in systemic lupus erythematosus (SLE) and non-SLE controls (4). Moreover, we found that atherosclerosis, with a higher percentage in the AMI group, could serve as a unique risk factor for AMI in patients with APS. Taken together, our present study suggests that despite MINOCA being common, the majority of AMI cases in APS are attributed to atherosclerosis.
Apart from atherosclerosis, we did not observe any other traditional CVD risk factors (smoking, diabetes mellitus, hypertension, dyslipidemia, chronic kidney disease, and hyperuricemia) that contributed to AMI in our APS cohort. Such phenomena are also found in SLE. Increased risk of ischemic cardiac events in SLE cannot be solely explained by traditional Framingham cardiovascular risk factors (7, 17). These observations prompt us to focus on disease-related risk factors instead of traditional CVD risk factors. It is known that antiphospholipid antibodies are associated with hypercoagulability and myocardial infarction (8, 14, 18, 19). We discovered that patients with AMI had multiple organ thrombosis and recurrent thrombosis, and recurrent thrombosis was a risk factor for AMI, indicating that AMI in APS might be associated with a more severe thrombophilic condition (1, 20). Despite a higher percentage of antiplatelet treatment, the incidence of subsequent arterial thrombosis was significantly higher in the AMI group. Therefore, our study highlights the importance of sustained anticoagulants for the treatment of AMI in APS in addition to controlling cardiovascular risk factors and antiplatelet therapy (6, 21).
Interestingly, by multiple logistic analyses, we revealed that neutrophil elevation was the only laboratory index associated with AMI. Active immunological responses and inflammation have been proposed as important triggers for endothelial dysfunction, leading to accelerated atherosclerosis and cardiovascular diseases in autoimmune diseases (1, 7, 17). In APS patients, neutrophils display an activated phenotype with increased aggregation and mitochondrial dysfunction by increasing mitochondrial reactive oxygen species (ROS) production and enhanced spontaneous neutrophil extracellular trap release, which leads to hyper-inflammation and over-production of antiphospholipid antibodies. It indicates that neutrophils play an important role in the pathogenesis of APS (22, 23). A recent study also found that incased neutrophil count could serve as a biomarker for APS in SLE (24). In addition, compelling evidence indicates a pivotal pathogenic role for neutrophils in acute coronary syndrome (25–27). These findings suggest that elevated neutrophil levels could serve as biomarkers for predicting AMI in APS. However, Pabinger et al. recently reported different phenotypes between neutrophil subpopulations [high and low-density neutrophils (HDNs/LDNs)] in APS (28), suggesting that further work might focus on different subtypes of neutrophils. In addition, decreased neutrophil levels appear to have a better prognosis in myocardial infarction (29, 30), suggesting that neutrophils could be a target for the prevention of AMI in APS. Patients with AMI would be expected to have higher C reactive protein (CRP) levels. However, CRP levels are more elevated in the group without AMI, without statistical significance. This phenomenon might be due to several reasons. Firstly, some patients were not in the acute phase of AMI when they were enrolled in our cohort (31). In addition, some previous studies assumed that hsCRP is more accurate in predicting CV events than CRP. Unfortunately, the hsCRP is not a routine test in our Department.
It is well-accepted that the hallmark of APS is the presence of thrombotic events (8). However, the majority of previous studies only focus on the characteristics and risk factors for overall thrombosis events (32–34), rarely subdividing thrombotic APS into arterial and venous. It is recognized that venous and arterial thrombotic disorders are mechanistically and pathophysiologically distinct entities (35). A systematic review revealed the types of antiphospholipid antibodies that were different in arterial thrombosis and venous thrombosis, thus arterial thrombosis showed higher levels of anticardiolipin antibodies and the lupus anticoagulant (36). Recently, Savino et al. found that levels of IgG anti-high-density lipoproteins antibodies were only associated with arterial thrombosis (37). Moreover, Freire et al. discovered that thrombotic microangiopathy in APS was characterized by an increased frequency of arterial events and stroke and less deep venous thrombosis (38). Similarly, we discovered that the pre-venous thrombosis event was negatively associated with AMI, and AMI predicted a higher incidence of subsequent arterial thrombosis. In addition, prevention strategies have shown some differences between arterial and venous thrombosis (39). These findings suggest that distinct pathophysiological mechanisms may exist and contribute to the development of venous or arterial thrombotic APS, and we may need to pay more attention to arterial thrombotic APS in cases of CVD.
This study has several limitations. First, due to the relatively small number of patients with AMI, we did not compare the clinical and laboratory differences among UA, STEMI, and NSTEMI, and the effectiveness of different therapies in the acute phase of AMI, despite the lower percentage of invasive treatment. Second, because of the limitations of this retrospective study, we did not discuss the monitoring function of neutrophils or the therapeutic role of neutrophil-targeting therapy in AMI. Therefore, prospective cohort or multicenter studies are needed in the future.
In the present study, we investigated clinical and laboratory features of AMI in patients with APS and discovered that atherosclerosis, multiple organ thrombosis, and elevated neutrophil levels may be risk factors for AMI. Arterial thrombotic disorders might be different from venous thrombosis in APS, as venous thrombosis is negatively associated with AMI. AMI only predicts the subsequent recurrence of arterial thrombosis. Future prospective or multicenter studies are desired to validate our findings.
The datasets presented in this article are not readily available because it contains sensitive information that could compromise patient anonymity and contain sensitive medical information. Requests to access the datasets should be directed to ZmlvbmFfbGVlY2h1bkAxNjMuY29t.
The studies involving human participants were reviewed and approved by Ethics Committee of Peking University People's Hospital. The patients/participants provided their written informed consent to participate in this study.
YZG and YWZ: data interpretation and analysis, writing of the original draft, review, and editing. GML: clinical data collection. JPG: editing. HY and YSZ: follow-up of participants. CH and LW: interpretation and collection of cardiac parameter data. CL: conceptualization, methodology, investigation, resources, data curation, supervision, manuscript editing, and funding acquisition. All authors have read and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (Nos. 81801615, 81871289, and 82071814), University of Michigan Medical School (UMMS) and Peking University Health Science Center (PUHSC) Joint Institute (JI) Projects (No. BMU2020JI003), Peking University Medicine Fund of Fostering Young Scholars' Scientific and Technological Innovation and Fundamental Research Funds for the Central Universities (BMU2022PY004), and Peking University People's Hospital Research and Development Funds (RDY 2019-04).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Dr. Feiwen Jin for her support with the statistics of the project.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.871011/full#supplementary-material
1. Kolitz T, Shiber S, Sharabi I, Winder A, Zandman-Goddard G. Cardiac manifestations of antiphospholipid syndrome with focus on its primary form. Front Immunol. (2019) 10:941. doi: 10.3389/fimmu.2019.00941
2. Sacré K, Brihaye B, Hyafil F, Serfaty JM, Escoubet B, Zennaro MC, et al. Asymptomatic myocardial ischemic disease in antiphospholipid syndrome: a controlled cardiac magnetic resonance imaging study. Arthritis Rheum. (2010) 62:2093–100. doi: 10.1002/art.27488
3. Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoenfeld Y, de Ramón E, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. (2015) 74:1011–8. doi: 10.1136/annrheumdis-2013-204838
4. Tornvall P, Göransson A, Ekman J, Järnbert-Pettersson H. Myocardial infarction in systemic lupus erythematosus: incidence and coronary angiography findings. Angiology. (2021) 72:459–64. doi: 10.1177/0003319720985337
5. Yazdani K, Xie H, Avina-Zubieta JA, Zheng Y, Abrahamowicz M, Lacaille D. Has the excess risk of acute myocardial infarction in rheumatoid arthritis relative to the general population declined? A population study of trends over time. Semin Arthritis Rheum. (2021) 51:442–9. doi: 10.1016/j.semarthrit.2021.03.003
6. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. (2019) 78:1296–304. doi: 10.1136/annrheumdis-2019-215213
7. López P, Rodríguez-Carrio J, Martínez-Zapico A, et al. Low-density granulocytes and monocytes as biomarkers of cardiovascular risk in systemic lupus erythematosus. Rheumatology. (2020) 59:1752–64. doi: 10.1093/rheumatology/keaa016
8. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. (2006) 4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x
9. Asherson RA, Cervera R, de Groot PG, Erkan D, Boffa MC, Piette JC, et al. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. (2003) 12:530–4. doi: 10.1191/0961203303lu394oa
10. Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. The global anti-phospholipid syndrome score in primary APS. Rheumatology. (2015) 54:134–8. doi: 10.1093/rheumatology/keu307
11. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72:2231–64. doi: 10.1016/j.jacc.2018.08.1038
12. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
13. Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. (2002) 46:1019–27. doi: 10.1002/art.10187
14. Andreoli L, Chighizola CB, Banzato A, Pons-Estel GJ, Ramire de Jesus G, Erkan D. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis Care Res. (2013) 65:1869–73. doi: 10.1002/acr.22066
15. Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American heart association. Circulation. (2019) 139:e891–908. doi: 10.1161/CIR.0000000000000670
16. Nazir S, Tachamo N, Lohani S, Hingorani R, Poudel DR, Donato A. Acute myocardial infarction and antiphospholipid antibody syndrome: a systematic review. Coron Artery Dis. (2017) 28:332–5. doi: 10.1097/MCA.0000000000000476
17. Kirchler C, Husar-Memmer E, Rappersberger K, Thaler K, Fritsch-Stork R. Type I interferon as cardiovascular risk factor in systemic and cutaneous lupus erythematosus: a systematic review. Autoimmun Rev. (2021) 20:102794. doi: 10.1016/j.autrev.2021.102794
18. Danowski A, de Azevedo MN, de Souza Papi JA, Petri M. Determinants of risk for venous and arterial thrombosis in primary antiphospholipid syndrome and in antiphospholipid syndrome with systemic lupus erythematosus. J Rheumatol. (2009) 36:1195–9. doi: 10.3899/jrheum.081194
19. Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML, et al. The global anti-phospholipid syndrome score. Rheumatology. (2013) 52:1397–403. doi: 10.1093/rheumatology/kes388
20. Denas G, Jose SP, Bracco A, Zoppellaro G, Pengo V. Antiphospholipid syndrome and the heart: a case series and literature review. Autoimmun Rev. (2015) 14:214–22. doi: 10.1016/j.autrev.2014.11.003
21. Cheng C, Cheng GY, Denas G, Pengo V. Arterial thrombosis in antiphospholipid syndrome (APS): Clinical approach and treatment. A systematic review. Blood Rev. (2021) 48:100788. doi: 10.1016/j.blre.2020.100788
22. Wirestam L, Arve S, Linge P, Bengtsson AA. Neutrophils-important communicators in systemic lupus erythematosus and antiphospholipid syndrome. Front Immunol. (2019) 10:2734. doi: 10.3389/fimmu.2019.02734
23. Bravo-Barrera J, Kourilovitch M, Galarza-Maldonado C. Neutrophil extracellular traps, antiphospholipid antibodies and treatment. Antibodies. (2017). 6:4. doi: 10.3390/antib6010004
24. Idborg H, Zandian A, Sandberg AS, Nilsson B, Elvin K, Truedsson L, et al. Two subgroups in systemic lupus erythematosus with features of antiphospholipid or Sjögren's syndrome differ in molecular signatures and treatment perspectives. Arthritis Res Ther. (2019) 21:62. doi: 10.1186/s13075-019-1836-8
25. Laridan E, Martinod K, De Meyer SF. Neutrophil extracellular traps in arterial and venous thrombosis. Semin Thromb Hemost. (2019) 45:86–93. doi: 10.1055/s-0038-1677040
26. Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. (2020) 17:327–40. doi: 10.1038/s41569-019-0326-7
27. Ma Y. Role of neutrophils in cardiac injury and repair following myocardial infarction. Cells. (2021) 10:1676. doi: 10.3390/cells10071676
28. Mauracher LM, Krall M, Roiß J, Hell L, Koder S, Hofbauer TM, et al. Neutrophil subpopulations and their activation potential in patients with antiphospholipid syndrome and healthy individuals. Rheumatology. (2021) 60:1687–99. doi: 10.1093/rheumatology/keaa532
29. Järemo P, Nilsson O. Interleukin-6 and neutrophils are associated with long-term survival after acute myocardial infarction. Eur J Intern Med. (2008) 19:330–3. doi: 10.1016/j.ejim.2007.06.017
30. Shiyovich A, Gilutz H, Plakht Y. White blood cell subtypes are associated with a greater long-term risk of death after acute myocardial infarction. Tex Heart Inst J. (2017) 44:176–88. doi: 10.14503/THIJ-16-5768
31. Zeltser D, Rogowski O, Mardi T, Justo D, Tolshinsky T, Goldin Y, et al. Clinical and laboratory characteristics of patients with atherothrombotic risk factors presenting with low concentrations of highly sensitive C-reactive protein. Atherosclerosis. (2004) 176:297–301. doi: 10.1016/j.atherosclerosis.2004.04.015
32. Saraiva Sda S, Custódio IF, Mazetto Bde M, Collela MP, de Paula EV, et al. Recurrent thrombosis in antiphospholipid syndrome may be associated with cardiovascular risk factors and inflammatory response. Thromb Res. (2015) 136:1174–8. doi: 10.1016/j.thromres.2015.10.029
33. Bazzan M, Vaccarino A, Stella S, Sciascia S, Montaruli B, Bertero MT, et al. Patients with antiphosholipid syndrome and thrombotic recurrences: a real world observation (the Piedmont cohort study). Lupus. (2016) 25:479–85. doi: 10.1177/0961203315617538
34. Sanchez-Redondo J, Espinosa G, Varillas Delgado D, Cervera R. Recurrent thrombosis with direct oral anticoagulants in antiphospholipid syndrome: a systematic literature review and meta-analysis. Clin Ther. (2019) 41:1839–62. doi: 10.1016/j.clinthera.2019.06.015
35. Franchini M, Mannucci PM. Association between venous and arterial thrombosis: clinical implications. Eur J Intern Med. (2012) 23:333–7. doi: 10.1016/j.ejim.2012.02.008
36. Reynaud Q, Lega JC, Mismetti P, Chapelle C, Wahl D, Cathébras P, et al. Risk of venous and arterial thrombosis according to type of antiphospholipid antibodies in adults without systemic lupus erythematosus: a systematic review and meta-analysis. Autoimmun Rev. (2014) 13:595–608. doi: 10.1016/j.autrev.2013.11.004
37. Sciascia S, Cecchi I, Radin M, Rubini E, Suárez A, Roccatello D, et al. IgG anti-high-density lipoproteins antibodies discriminate between arterial and venous events in thrombotic antiphospholipid syndrome patients. Front Med. (2019) 6:211. doi: 10.3389/fmed.2019.00211
38. Freire de. Carvalho J, Brandão Neto R, Skare T. Thrombotic microangiopathy in primary antiphospholipid syndrome is linked to stroke and less deep venous thrombosis. Eur Rev Med Pharmacol Sci. (2021) 25:7369–74. doi: 10.26355/eurrev_202112_27431
Keywords: acute myocardial infarction, antiphospholipid syndrome, risk factor, thrombosis, atherosclerosis
Citation: Gan Y, Zhao Y, Li G, Ye H, Zhou Y, Hou C, Wang L, Guo J and Li C (2022) Risk Factors and Outcomes of Acute Myocardial Infarction in a Cohort of Antiphospholipid Syndrome. Front. Cardiovasc. Med. 9:871011. doi: 10.3389/fcvm.2022.871011
Received: 07 February 2022; Accepted: 08 June 2022;
Published: 05 July 2022.
Edited by:
Blair Solow, University of Texas Southwestern Medical Center, United StatesReviewed by:
Deepa Jayakody Aarachchillage, Imperial College London, United KingdomCopyright © 2022 Gan, Zhao, Li, Ye, Zhou, Hou, Wang, Guo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun Li, MTM4MTExOTAwOThAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.