- 1Division of Prevention and Community Health, National Center for Cardiovascular Disease, National Clinical Research Center of Cardiovascular Disease, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China
- 2PGY3 General Surgery, Memorial University of Newfoundland, St. John’s, NL, Canada
Background: At present, the association between age at menarche and menopause, reproductive lifespan, and cardiovascular disease (CVD) risk among Chinese postmenopausal women is not clear, and some related researches are contradictory.
Methods: A total of 6,198 Chinese postmenopausal women with a mean age of 63.6 years were enrolled at baseline in 2012–2015 and followed up for 5 years. A standardized questionnaire was used to collect relevant information by well-trained interviewers. Physical examination of the participants was performed by trained medical staff. CVD events were observed during follow-up. Cox proportional hazards models were used to estimate hazard ratios between reproductive characteristics and CVD events.
Results: Age at menarche was positively associated with CVD events (HR, 1.106; 95%CI, 1.047–1.167). There was a negative association between age at menopause and CVD risk in postmenopausal women with comorbidity (HR, 0.952; 95%CI, 0.909–0.996). Reproductive lifespan was negatively associated with CVD events (HR, 0.938; 95%CI, 0.880–0.999). The CVD risk increased by 10.6% for every 1-year increase in age at menarche. The CVD risk reduced by 6.2% for every 1-year increase in age at menopause in women with comorbidity. The CVD risk reduced by 3.8% for every 1-year increase in reproductive lifespan.
Conclusions: Based on the large prospective study with a nationally representative sample, Chinese postmenopausal women with late age at menarche and shorter reproductive lifespan have higher risk of CVD events.
Introduction
Cardiovascular disease (CVD) is the dominant cause of death throughout the world (1), accounting for 32% of the total deaths (2). In China, CVD is the leading cause of death and disability (1), accounting for approximately 45% of all deaths (3). Meanwhile, CVD as the principle cause of death in women is still under-recognized and undertreated (4). Therefore, comprehensive recognition of CVD risk factors and timely implementation of appropriate interventions for women is of tremendous public health importance.
Menarche is a marker of puberty and the onset of ovarian and other endocrine functions relating to reproduction (5). The results of previous studies on the relationship between age at menarche, menopause, reproductive lifespan, and the risk of CVD are inconsistent (6–11). For example, a systematic review revealed that three out of four studies show a generally linear association between a decrease in age at menarche with an increase in CVD risk (11). However, the UK Million Women Study manifested a significant U-shaped relationship between age at menarche and coronary heart disease (CHD) risk (6). Data from Asian populations were also contradictory and inconclusive (11).
Age at onset of menopause may be a marker for not only reproductive aging but also for general health and somatic aging (12). Adverse changes in CVD risk factors occur around the menopausal transition (13–15). A meta-analysis including 50,000 Western women showed a negative linear association between age at menopause and CVD (16). A follow-up study found a non-significant increased risk of death caused by CHD and stroke among women with earlier natural menopause (12). This discrepancy may be due in part to a variety of confounding variables including genetic background, race, study design, or environmental factors amongst different populations.
The interval between menarche and menopause defines a woman’s natural reproductive span. Due to exposure to different hormonal levels, early or late onset of these events may be associated with increased risk of many chronic health problems. However, the association between duration of reproductive lifespan and CVD risk has not been investigated thoroughly. A shorter duration of reproductive life span was associated with a higher Framingham Risk Score. However, this study was limited by its cross-sectional design to investigate a long-term CVD event risk (17). Results from the Australian Longitudinal Study of Women’s Health showed that an increase in reproductive lifespan per year was associated with a 7% reduction in stroke risk, but this association disappeared when only the duration of endogenous estrogen exposure was considered (18). Further, the association was either null or mixed (19, 20). These findings suggest that the association between reproductive lifespan and CVD is not completely clear and requires further investigation.
Most previous studies addressing this objective are conducted in Western populations. Asian women, including Chinese women, have different menstrual and reproductive patterns as well as different lifestyle factors compared with women living in Western countries. At present, there is a lack of clear and comprehensive researches on the relationship between age at menarche, menopause, reproductive lifespan, and CVD risk in Chinese women. Based on a national representative cohort study, we investigate the associations between age at menarche and menopause, reproductive lifespan, and the CVD risk and examine the relationship in subgroups amongst Chinese postmenopausal women.
Materials and Methods
Study Design and Participants
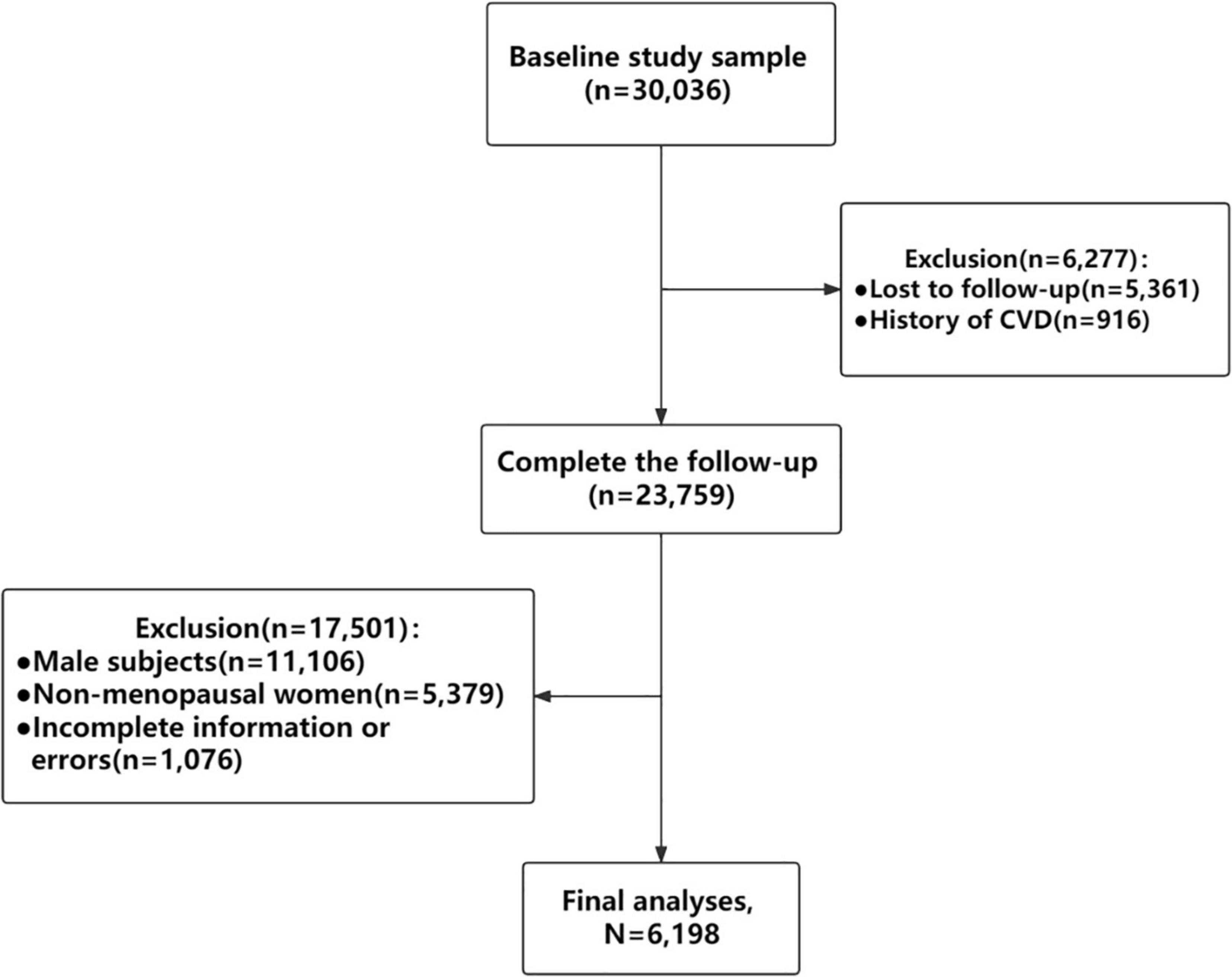
The Study on Prevalence and Key Technologies of Important Cardiovascular Diseases in China conducted from October 2012 to December 2015 was a large-scale representative CVD cross-sectional survey in China. A stratified multistage random sampling method was used to select about 500,000 nationally representative subjects aged ≥15 years from 262 districts and counties in 31 provinces of mainland China to investigate demographic characteristics, lifestyle risk factors, pharmacological treatment, female reproductive related characteristics and so on. In addition, the second sampling was carried out on the project. The selected districts/counties were divided into eastern, central and western region, and then stratified by urban and rural areas, 16 cities and 17 counties were selected by simple random sampling method. Random sampling was carried out in the above 33 districts/counties by stages. In the last stage, only respondents aged 35 and above were randomly selected from eligible regions to obtain blood samples for investigation of fasting plasma glucose (FPG), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels. Finally, those 30,036 individuals who completed the baseline survey were followed up in 2018–2019. 5,361 people lost to follow-up within the 5 years and the overall rate of follow-up was 82.15%. A total of 6,198 postmenopausal women were included in the final analysis after exclusions of 916 people with history of CVD, 11,106 male participants, 5,379 non-menopausal women and 1,076 postmenopausal women with incomplete information or errors (Figure 1).
The written informed consent was obtained from each participant. The Ethics Committee of Fuwai Hospital (Beijing, China) approved this study.
Baseline Measurement and Data Collection
A standardized questionnaire was used to collect information on demographic characteristics, lifestyle risk factors, medical history, and characteristics related to female reproduction by well-trained interviewers in 2012–2015. Height and weight were measured using a standardized cartesian and an OMRON body fat and weight meter (V-body HBF-371, Omron, Japan) with participants wearing thin clothing and no shoes. Blood pressure of the participant was measured using an Omron HBP-1300 professional portable sphygmomanometer (Omron, Kyoto, Japan) on the right arm in the sitting position after resting for at least 5 min. Laboratory examinations were performed on venous blood samples collected after at least 8 h of fasting at night. FPG, TC, TG, LDL-C, and HDL-C levels were measured by a central core laboratory (Beijing Adicon Clinical Labora-tories, Inc., Beijing, China).
Follow-Up and Outcome Measures
During 2018–2019, we tracked CVD events via interviewing participants or their proxies in-person or via telephone or mail questionnaires, and further examined medical records for reconfirmation. Participants’ CVD events were initially recorded by local investigators, and then hospital records were reviewed by the central adjudication committee of Fuwai Hospital (Beijing, China) to determine the final diagnosis. CVD events were defined as CHD, stroke, chronic heart failure, and death due to CVD. The criteria for stroke included non-fatal and fatal stroke (subarachnoid hemorrhage, ischemic stroke, intracerebral hemorrhage, and unspecified stroke). CHD was defined as non-fatal (including myocardial infarction, coronary artery bypass graft surgery, or percutaneous coronary intervention) and fatal CHD (such as fatal myocardial infarction and other coronary deaths).
Variable Definition
At baseline, each participant was asked her age at the time of her first period, which was recorded as age at menarche. Menopausal status was defined based on the World Health Organization’s definition of menopause as the absence of menstruation for ≥12 months, and the age at which menopause occurred was recorded at baseline. The reproductive lifespan was defined as the interval between age at menarche and menopause.
Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). Criteria for overweight and obese were defined as BMI between 24.0 and 27.9 kg/m2 and BMI ≥ 28.0 kg/m2, respectively (21). Alcohol drinking was defined as consuming an alcoholic beverage at least once per week in the past month. Smoking was defined as people who have smoked at least 20 packets of cigarettes in their lifetime and were still smoking (22). Diabetes was defined as fasting blood glucose ≥7 mmol/dL or was diagnosed with diabetes by a doctor or was prescribed hypoglycemic drugs within 2 weeks (23). According to the Guidelines for Dyslipidemia in China, TG ≥ 2.26mmol/L or TC ≥ 6.22mmol/L or HDL-C < 1.04 mmol/L or LDL-C ≥ 4.14 mmol/L or prior lipid-lowering medication or prior diagnosis of dyslipidemia (24). Have pharmacological treatment is defined as taking any one of anti-hypertensive, hypoglycemic, or anti-hyperlipidemia drugs. Comorbidity refers to any combination of two or more of three diseases: diabetes, hypertension and hypercholesterolemia following previous study (25). Parity was defined as the number of live babies a woman gives birth to, whether or not the baby dies after delivery (26).
Statistical Analysis
Baseline characteristics of participants were presented as mean and standard deviation (SD) for normally distributed data or as a proportion for categorical data. The t-test, χ2 test, and variance analysis were used to compare variables between the different groups. Logistic linear regression model was used to explore the trend of variables. Multivariable Cox proportional hazard model was used to estimate Hazard ratios (HRs) and 95% confidence intervals (CIs) for CVD events associated with age at menarche (classified as ≤13, 14, 15, 16, and ≥17 years) with 15 years as the reference group, age at menopause (classified as ≤44, 45–46, 47–48, 49–50, and ≥51 years) with 47–48 years as the reference group, and reproductive lifespan (classified as ≤28, 29–31, 32–34, 35–37, and ≥38 years) with 32–34 years as the reference group.
This study further investigated the associations by additionally adjusting for age at recruitment (continuous), BMI (continuous), waist circumference (continuous), ethnicity (Han or other ethnicities), region (urban or rural), marital status (unmarried/widowed, married/cohabiting), education level (elementary or below, junior high school, high school or above), alcohol drinking (yes or no), smoking (yes or no), pharmacological treatment (yes or no), comorbidity (yes or no), family history of CVD (yes or no), ever pregnant (yes or no), contraceptive use (yes or no), breastfeeding experience (yes or no), and parity (0–1, 2, ≥3). We also examined the CVD risk by age at menarche and menopause and reproductive lifespan in subgroups of women defined by region, age at recruitment, marital status, BMI, education level, comorbidity, pharmacological treatment, alcohol drinking status, and family history of CVD, use of contraceptives, parity.
R 3.6.2 software were used to conduct analyses. The threshold of statistical significance was set at p < 0.05.
Results
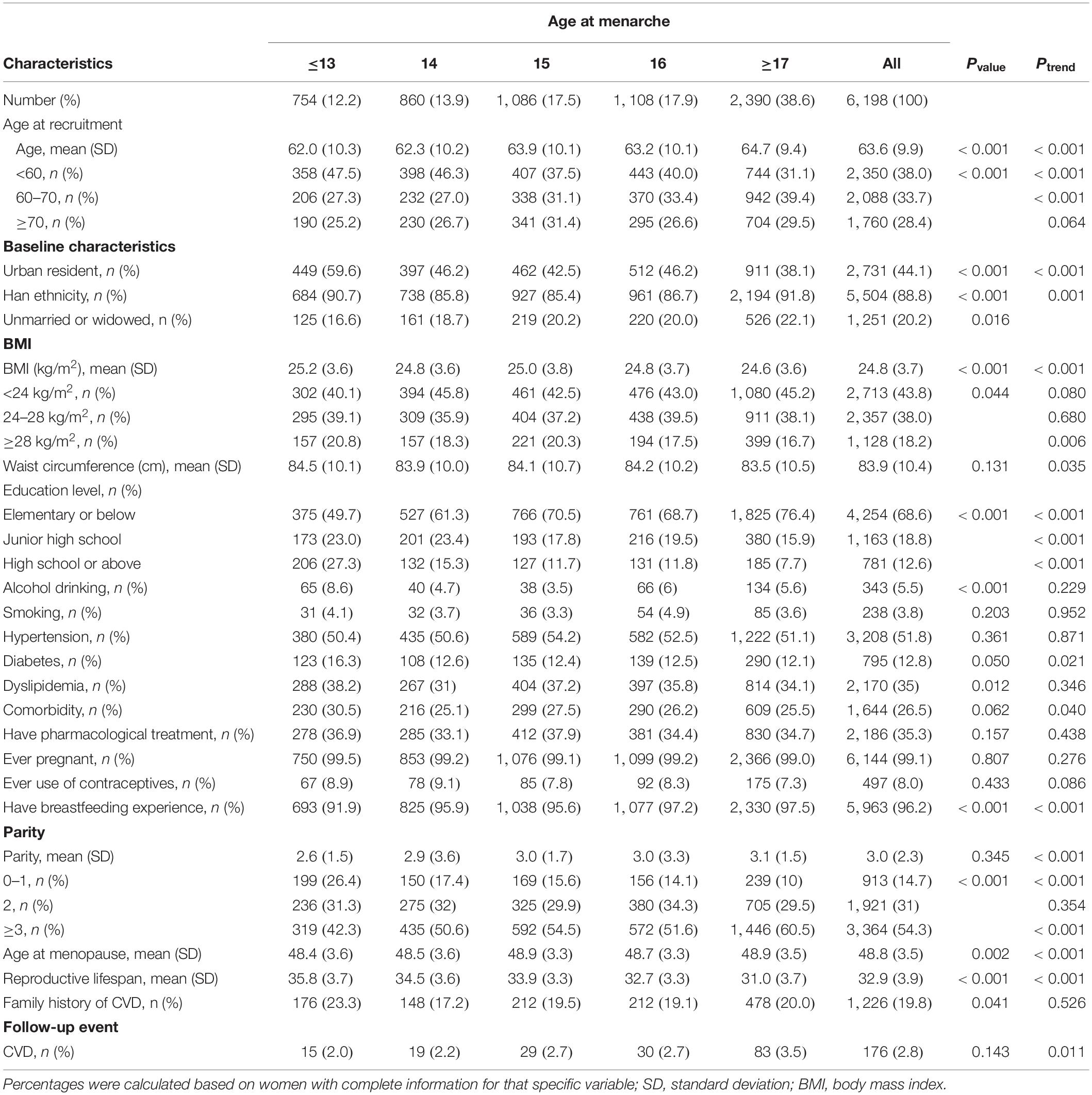
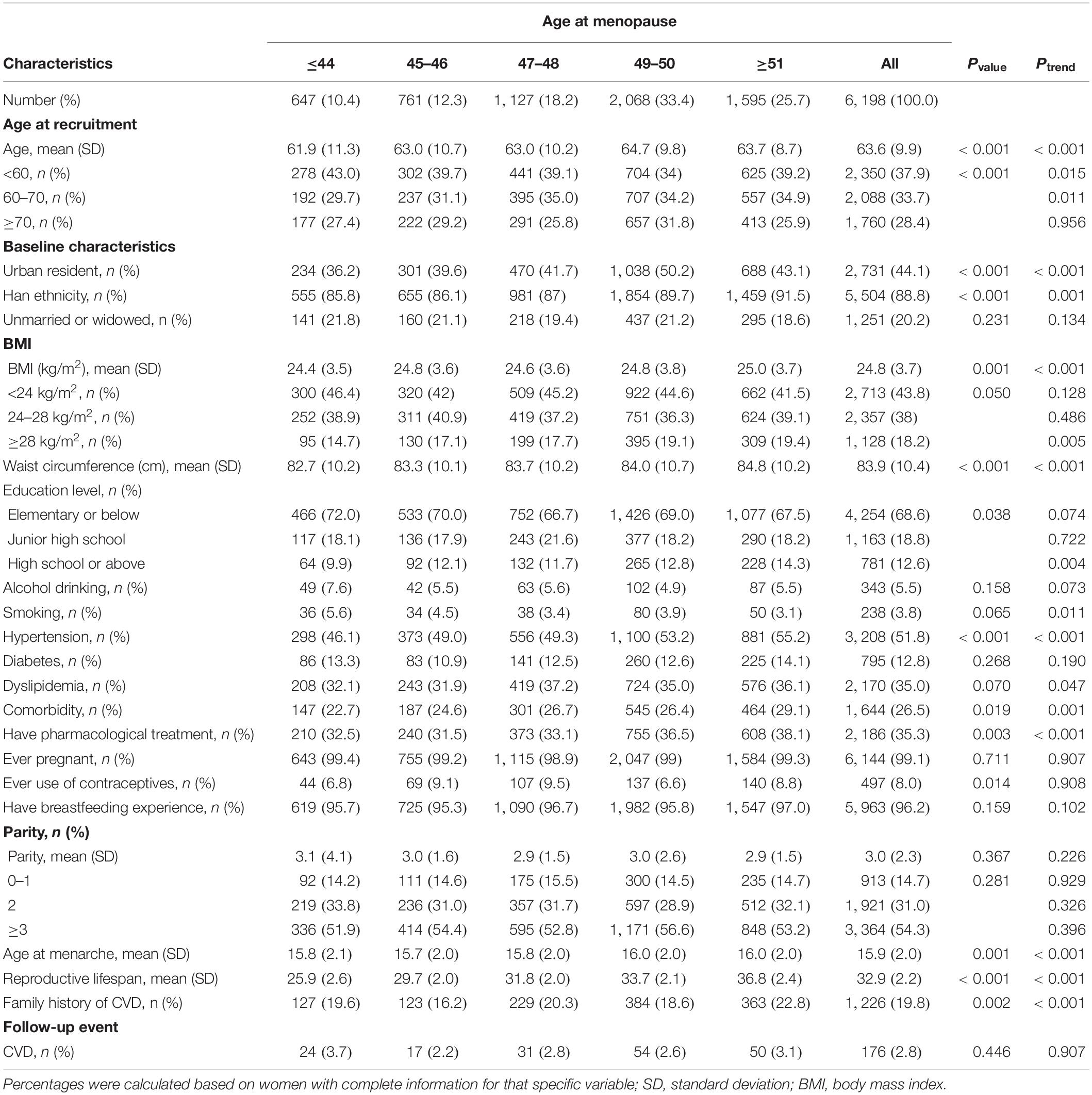
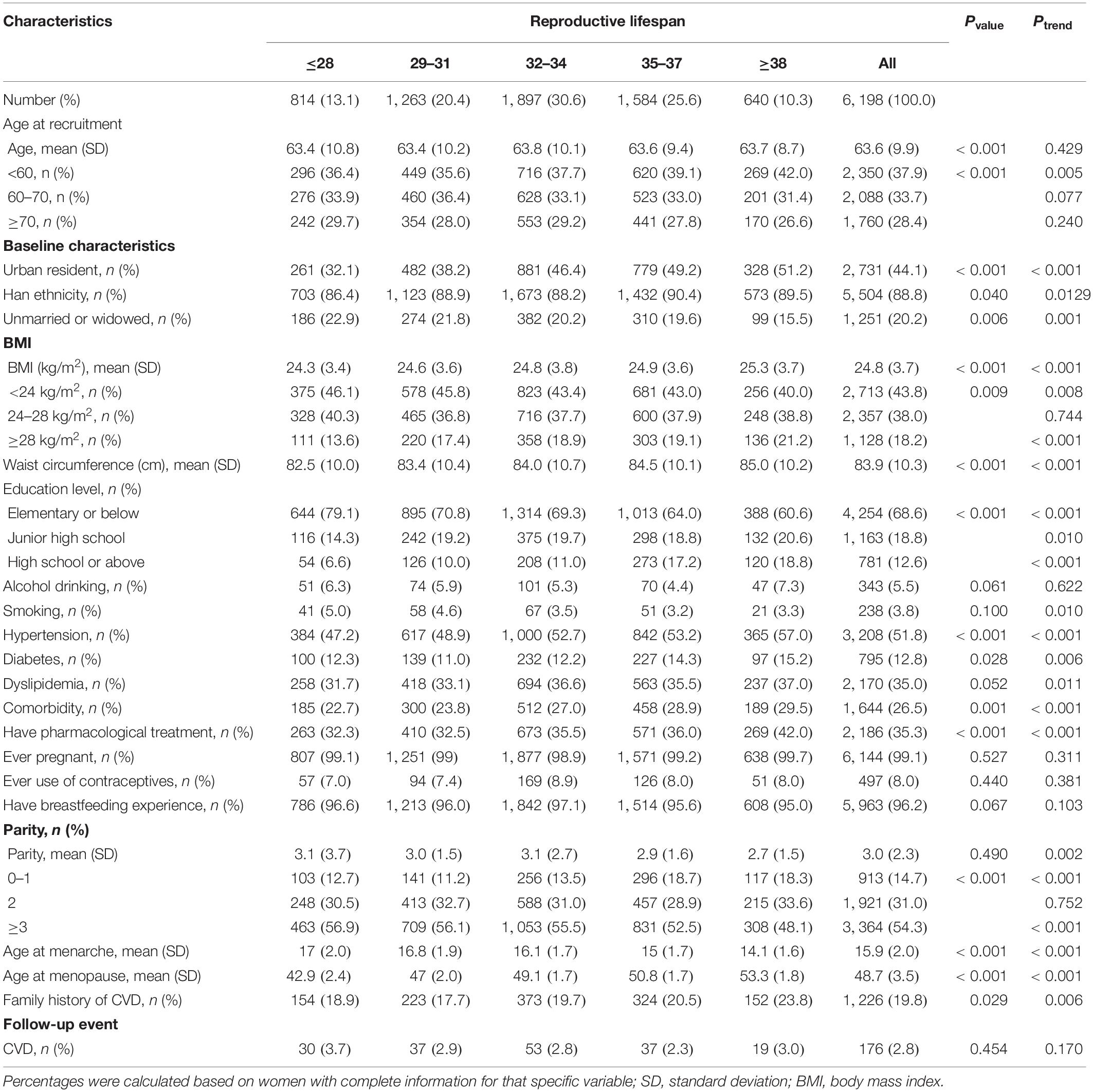
The characteristics of the study participants by age at menarche and menopause, and reproductive lifespan were listed in Tables 1–3, respectively. The mean (SD) age of study participants at recruitment was 63.6 (9.9) years, and the mean age at menarche and menopause, and reproductive lifespan were 15.9 (2.0), 48.7 (3.5), and 32.9 (3.9) years, respectively. The mean (SD) BMI, waist circumference, and parity were 24.8 (3.7) kg/m2, 83.9 (10.4) cm, and 3.0 (2.3), respectively. A minority of women were smokers (3.8%), alcohol drinkers (5.5%), and users of contraceptives (8.0%). Most women had previously pregnant (99.1%) and breastfeeding experience (96.2%). Women with earlier age at menarche had higher educational level, had lower BMI, waist circumference and parity, had higher percent of urban resident. Women with later age at menopause had higher educational level, BMI, and waist circumference, had higher percent of comorbidity, Women with lower reproductive lifespan had higher parity, had lower educational level, BMI and waist circumference, had lower percent of comorbidity.
Figure 2 showed the HRs for CVD events by age at menarche, age at menopause, and reproductive lifespan amongst postmenopausal women. In model 1, the association between age at menarche and CVD was significant, and the association between age menopause, reproductive lifespan and CVD was not significant. In model 2 and 3, the association between age at menarche, reproductive lifespan and CVD was significant, and the association between age menopause and CVD was not significant. Model 3 showed that for those with age at menarche ≤ 13, 14, 15 (reference), 16, and ≥ 17 years, the HRs (95%CIs) were respectively 0.887 (0.489–1.609), 0.993 (0.560–1.762) 1.000 (reference), 1.157 (0.699–1.915), and 1.589 (1.041–2.424), for those with age at menopause ≤44, 45–46, 47–48 (reference), 49–50, and ≥51 years, the HRs (95%CIs) were respectively 1.388n(0.821–2.346), 0.861 (0.482–1.537), 1.000 (reference), 0.909 (0.587–1.405), and 1.111. (0.716–1.726), for those with reproductive lifespan ≤28, 29–31, 32–34 (reference), 35–37, and ≥38, the HRs (95%CIs) were respectively 1.368 (0.896–2.090), 1.059 (0.700–1.602), 1.000 (reference), 0.746 (0.492–1.132), and 0.926 (0.538–1.549). The highest risk was seen in those with menarche at age ≥17 years, and those with reproductive lifespan ≤28 years.
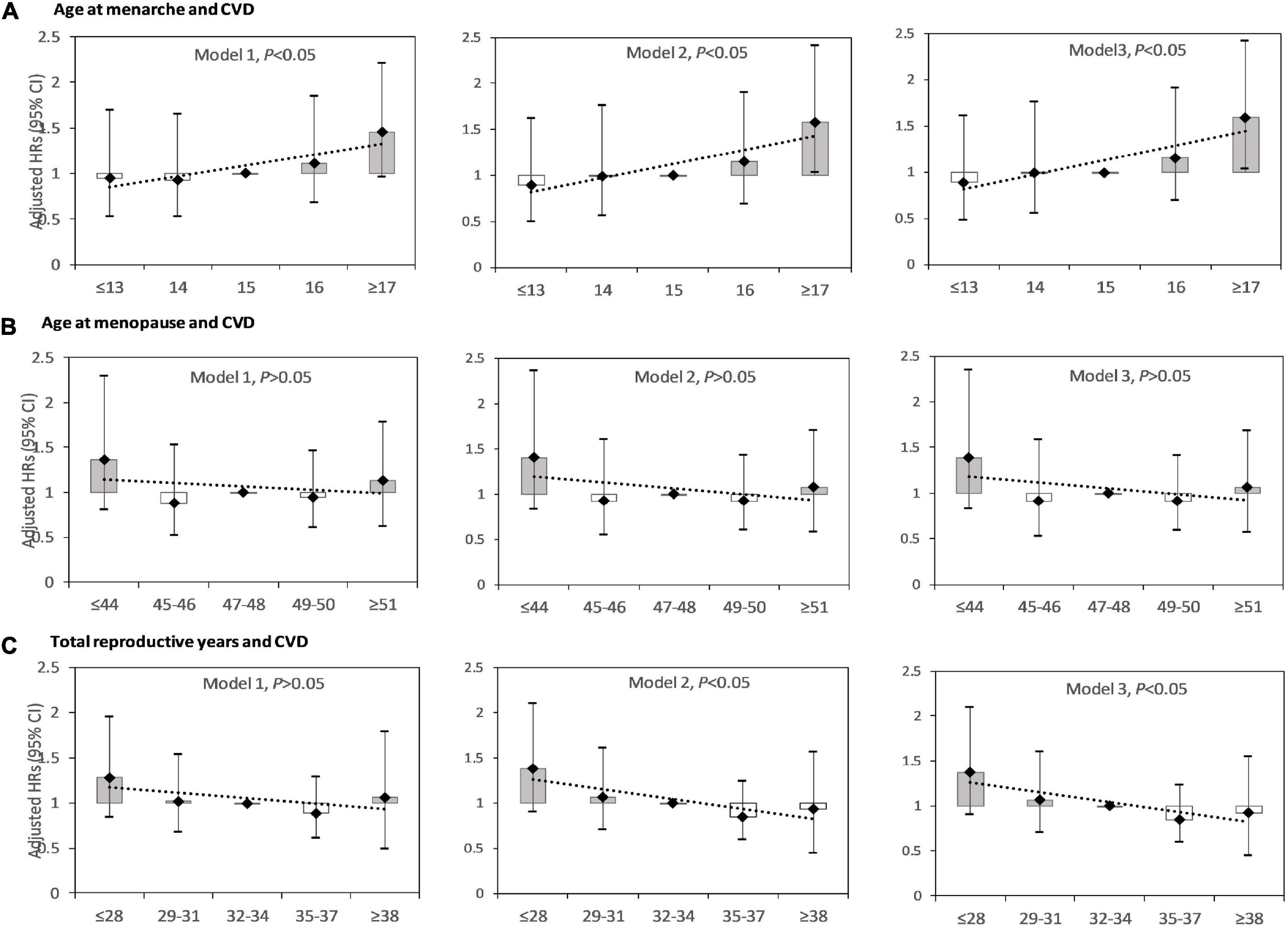
Figure 2. Hazard ratios (HRs) for cardiovascular disease (CVD) by age at menarche, age at menopause, and reproductive lifespan among postmenopausal women. (A) HRs for CVD by age at menarche; (B) HRs for CVD by age at menopause; (C) HRs for CVD by reproductive lifespan. Model 1: adjusted for age at recruitment. Model 2: model 1 plus region, ethnicity, marital status, body mass index, waist circumference, education level, alcohol drinking, smoking, comorbidity, pharmacological treatment, family history of CVD. Model 3: model 2 plus pregnant, contraceptive use status, and breastfeeding experience, parity.
Age at menarche was positively associated with CVD events, with an adjusted HR (95%CI) of 1.106 (1.047–1.167) per year. For every 1-year increase in age at menarche, the risk of CVD increased by 10.6%. The association between age at menopause and risk of CVD was not significant, with an adjusted HR (95%CI) of 0.982 (0.943–1.024) per year. Reproductive lifespan was negatively associated with CVD events, with an adjusted HR (95%CI) of 0.962 (0.929–0.996) per year. For every 1-year increase in reproductive lifespan, the risk of CVD was reduced by 3.8%.
Subgroup analyses were also conducted according to region, age at recruitment, marital status, BMI, education level, pharmacological treatment, comorbidity, alcohol drinking status, use of contraceptives, parity, and family history of CVD (Figure 3). There was a positive association between age at menarche and risk of CVD events in postmenopausal women within the following subgroups: living in urban areas, 70 years old and above, low BMI, low education level, have no comorbidity, have pharmacological treatment, non-drinkers, no previous contraceptive use, and 0–2 live births. There was a negative association between reproductive lifespan and risk of CVD events among the following subgroups: married, 60–70 years old, low BMI, low education level, have comorbidity, have pharmacological treatment, non-drinkers, no previous contraceptive use, and 2 live births. There was a negative association between age at menopause and CVD risk in postmenopausal women with comorbidity, with an adjusted HR (95%CI) of 0.938 (0.880–0.999) per year. The risk of CVD reduced by 6.2% for every 1-year increase in age at menopause in postmenopausal women with comorbidity.
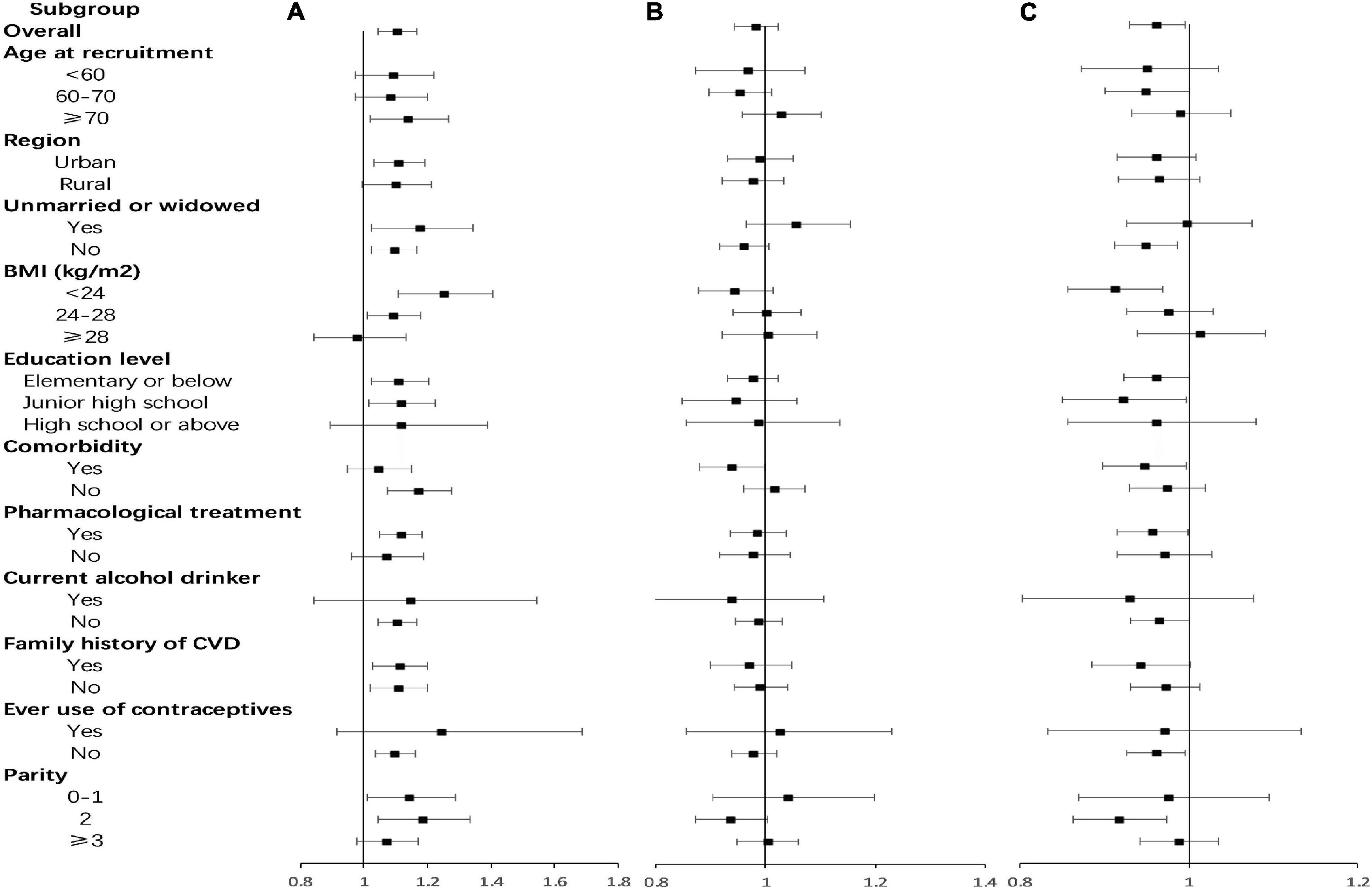
Figure 3. Adjusted hazard ratios (HRs) for cardiovascular disease (CVD) per year by age at menarche, age at menopause, and reproductive lifespan within various subgroups. (A) Age at menarche; (B) age at menopause; (C) reproductive lifespan. Analyses were adjusted for age at recruitment, region, ethnicity, marital status, body mass index, waist circumference, education level, alcohol drinking, smoking, comorbidity, pharmacological treatment, family history of CVD, pregnant, contraceptive use status, and breastfeeding experience, parity.
Discussion
To our knowledge, this was the first large prospective study that comprehensively investigated the associations between age at menarche and menopause, reproductive lifespan, and risk of CVD events in Chinese postmenopausal women using a nationally representative sample and evaluating multiple subgroups. Based on 6,198 Chinese postmenopausal women without prior history of CVD, we found that late age at menarche and shorter reproductive span is significantly associated with increased risk in CVD events among women. These associations also appeared to be similar amongst subgroups. There was also a negative association between age at menopause and CVD risk in postmenopausal women with comorbidity. For every 1-year increase in age at menarche, the risk of CVD increased by 10.6%, and for every 1-year increase in reproductive lifespan, the risk of CVD reduced by 3.8%. The risk of CVD reduced by 6.2% for every 1-year increase in age at menopause in women with comorbidity.
Several studies have previously investigated the relationship of age of menarche and CVD events, but reported inconsistent findings. A systematic review revealed that for Caucasian populations, eight out of twelve studies show a generally linear association between a decrease in age at menarche with an increase in CVD risk (11). But the UK Million Women Study showed a significant U-shaped relationship between age at menarche and the outcomes CHD (6). This discrepancy may be due in part to small sample sizes, geographic limits to one city or province, or only rural areas, age or ethnic differences, or residual confounding from unmeasured or other potential risk factors. The results from studies with Asian populations are inconclusive, with either early or late menarche increasing the CVD risk. One cohort study reported an increased risk of ischemic stroke for women with an age at menarche ≤13 years compared to those with 15 years, but the participants were selected from a rural town in northern Japan and therefore not representativeness of the general population (27). The same U-shape between age at menarche and CVD risk was found in data from the Chinese Kadoori Biobank (CKB), but the associations seemed to differ by birth cohort (28). A case-control study reported that higher age at menarche was associated with an increased ischemic stroke risk, which was consistent with our study (29).
Some mechanisms may explain the positive correlation between age of menarche and risk of CVD events in our results. Studies have shown that late menarche is associated with low estrogen levels (30, 31). Estrogen affects the elasticity of blood vessels and regulates the level of metabolic mediators such as lipids, inflammatory markers and the coagulation system (4). Estrogen has a protective effect on CVD in women by stimulating endothelial nitric oxide synthase, which mediates vasodilation and maintains cardiovascular health (32, 33). Women with early age at menarche are more likely to have higher levels of ovarian hormone, which can protect women from hypertension and atherosclerosis (34). Several studies agree about the different impact of testosterone on males and females (35, 36). Low endogen levels of this hormone in males can be associated with CVD and coronary stenosis severity. High levels of circulating androgens in women appear to be associated with higher cardiovascular risk (37, 38). In addition, age at menarche is associated with the higher adiposity both in childhood and in adulthood (39, 40). Adiposity indicators mediated the relationship between age at menarche and blood pressure in women. Adiposity is a risk factor of CVD, so the association between age at menarche and CVD could a result of higher estrogen levels or higher adiposity in early maturing women (40).
Additionally, the relationships between age at menopause and CVD risk in previous research are contradictory. Two meta-analysis indicated women with early menopausal age have a higher risk of incident CVD (16, 41). However, the result might have introduced some heterogeneity or has certain limitations about extrapolation due to the limited racial population included. Results of UK Biobank on early natural menopause were associated with a increased risk for a composite of CVD may have a “healthy participant” selection bias (42). The following studies are consistent with our findings on no convincing relationship between early menopausal age and cardiovascular disease (12, 43–47). Differences in population and study design and the influence of confounding factors may be the main reasons for the inconsistent results.
Persuasive evidence demonstrated that bilateral oophorectomy in premenopausal women increases the risk of CHD unless exogenous hormones are administered (48). However, there is no convincing evidence to support the hypothesis that natural menopause is a risk factor for CHD. When women with natural, surgical, and medicated menopause were analyzed together, the risk increased significantly with decreasing age at menopause, but this inverse association was limited to current non-smokers and not statistically significant amongst women with natural menopause (49). In a previous report from the Nurses’ Health Study, with further adjustment for smoking and age the relative risk of CHD in postmenopausal women diminished from significantly elevated to insignificant (48). These results might reflect the need to closely control the residual confounding factors of smoking and age, which have been adjusted for in our study. Furthermore, we found that there was a negative association between age at menopausal and CVD risk in postmenopausal women with comorbidity. As far as we know, diabetes, hypertension and dyslipidemia are independent risk factors for CVD events and show a additive association with the risk of CVD (50). Our finding suggests that this association may be more striking to particularly attention in postmenopausal women.
Previous studies investigating the association between reproductive lifespan and CVD risk were inconsistent. A systematic review and meta-analysis found that women with a shorter reproductive lifespan was associated with a higher risk of CVD events, with a 31% increased risk of stroke in particular (19). The Nurse’s Health Study found that the CVD risk was 25% higher amongst those with <30 years of reproductive lifespan compared to 34–37 years (51). One cross-sectional study from the United States showed that each yearly increase in reproductive lifespan was associated with 3% reduction in the risk for overall CVD and stroke events (20). The findings from this and other studies were consistent for CHD (34, 51, 52). A cohort study from the Women’s Health Initiative manifested that a shorter total reproductive duration in postmenopausal women resulted in a modestly increased risk of any heart failure (53). In Asia, the study of CKB found that total reproductive years were inversely associated with risks of both fatal and non-fatal CVD events, with 1.4% lower risk of CVD events death per each additional reproductive year (52). For stroke mortality, a large prospective study from Japan showed a decreased risk for longer reproductive lifespan (54). The above relationship between reproductive lifespan and CVD risk is consistent with our current study. However, some studies have found that there is no statistically significant association between the number of reproductive years and CVD mortality (9, 46, 55, 56). In addition, Jung et al. found a U-shaped association between reproductive lifespan and ischemic heart disease; the risk of which increased in both reproductive lifespan <30 and 40 years when 36–39 years were used as a reference (34). In one prospective study, it was significantly associated with 5% higher mortality for every additional year of reproductive lifespan (57).
Timing of menarche and menopause were correlated in that women who experienced early menarche were at higher risk of early menopause (51). In addition, reproductive lifespan can serve as a simple way to estimate estrogen exposure (51). Therefore, the underlying mechanism linking a longer reproductive lifespan with reduced risk of CVD events can be attributed to prolonged exposure to endogenous estrogen, which is accordant with our previous explanatory mechanism. These findings suggest that reproductive factors may play an important role in maintaining and improving cardiovascular health, which can be used to assess populations with poor cardiovascular health for targeted interventions.
Strengths and Limitations
Important strengths of our study are the prospective design and nationally representative sample. The study has several other strengths, including the standardized approaches and stringent quality control measures taken to ensure data quality and reliability. We adjusted for potential risk factors for CVD and conducted a detailed subgroup analysis to improve the reliability of our results, which allowed us to simultaneously adjust for potential confounders and reliably assess the associations.
Several limitations need to be taken into account. First, CVD is composed of multiple diseases, so it is unclear whether role in our study is specific for certain CVD. Second, age at menarche is known to vary by ethnicity, with girls of African and Asian descent experiencing earlier menarche than Caucasian girls (58, 59). However, it is unknown whether ethnicity plays a role in the association between age at menarche and CVD events. Furthermore, our study participants were Chinese women, which minimized the confounding effects by ethnic background, but might reduce the generalization of our results to other ethnic groups. Thirdly, ages at the time of menarche and menopause were self-reported which raises the possibility of misclassification due to recall bias. However, previous studies have reported that the recall of age at menarche (60, 61) and menopause is relatively accurate (62, 63). Therefore, self-reported age at menarche and menopause were reasonably valid and reproducible. If recall bias existed, it would be non-differential, tending to attenuate the real strengths of the associations that we observed. Lastly, although we have adjusted for a comprehensive range of potential confounders, the possibility of residual confounding from other known or unknown risk factors cannot be completely ruled out.
Conclusion
Based on 6,198 Chinese postmenopausal women who had no prior history of CVD, we found that late age at menarche and shorter reproductive span are both significantly associated with increased risk of CVD events. These associations also appeared to be similar amongst subgroups. In addition, there was a negative association between age at menopause and CVD risk in postmenopausal women with comorbidity. Our findings have important public health implications for early detection and timely implementation of appropriate interventions in women at high risk of CVD.
Data Availability Statement
Data from this study cannot be used publicly. Requests to access these datasets should be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Fuwai Hospital (Beijing, China). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LC: methodology, formal analysis, software, and writing – original draft preparation partly. ZH: conceptualization and writing – original draft preparation partly. XW and YS: investigation and writing – original draft preparation partly. ZC, LZ, and CZ: data curation, formal analysis, and software. HZ, XC, YT, JC, RG, and YH: investigation. JV: language polishing. ZW: conceptualization, funding acquisition, and writing—reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the CAMS Innovation Fund for Medical Sciences (grant number: 2017-I2M-1–004), the China National Science & Technology Pillar Program (grant number: 2011BAI11B01), the surveillance of cardiovascular and its risk factors among Chinese Residents, and the National Key R&D Program of China during the Thirteen Five-Year Plan Period (grant number: 2018YFC1315303).
Acknowledgments
This study was accomplished through the fine work of the staff at the national level, we thank all of colleagues involved in the survey and we also gratefully acknowledge Suning Li for maintaining the data. The authors are grateful to OMRON Corporation, Kyoto, Japan, for supporting the Blood Pressure Monitor (HBP- 1300) and body fat and weight measurement device (Vbody HBF-371); Henan Huanan Medical Science & Technology Co., Ltd., China, for Digital ECG device (GY- 5000); Microlife, Taipei, Taiwan, for Automated ABI device (Watch BP Office device). OMRON Corporation, Henan Huanan Medical Science & Technology Co., Ltd., and Microlife were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CVD, cardiovascular disease; CHD, coronary heart disease; BMI, body mass index; SD, standard deviation; HRs, hazard ratios; CIs, confidence intervals; CKB: Chinese Kadoori Biobank.
References
1. Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, et al. Rapid health transition in China, 1990-2010: findings from the Global burden of disease study 2010. Lancet. (2013) 381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1
2. Abubakar I, Tillmann T, Banerjee A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2015) 385:117–71. doi: 10.1016/S0140-6736(14)61682-2
3. World Health Organization [WHO]. Noncommunicable Diseases. Country Profiles 2018. (2018). Available online at: https://www.who.int/publications/i/item/9789241514620 (accessed March 29, 2022).
4. Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J. (2010) 18:598–603.
6. Canoy D, Beral V, Balkwill A, Wright FL, Kroll ME, Reeves GK, et al. Age at menarche and risks of coronary heart and other vascular diseases in a large UK Cohort. Circulation. (2015) 131:237–44. doi: 10.1161/CIRCULATIONAHA.114.010070
7. Charalampopoulos D, McLoughlin A, Elks CE, Ong KK, et al. Age at menarche and risks of all-cause and cardiovascular death: a systematic review and meta-analysis. Am J Epidemiol. (2014) 180:29–40. doi: 10.1093/aje/kwu113
8. Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. A prospective study of age at menarche, parity, age at first birth, and coronary heart disease in women. Am J Epidemiol. (1987) 126:861–70.
9. Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, et al. Relationships of age at menarche and menopause, and reproductive year with mortality from cardiovascular disease in Japanese postmenopausal women: the JACC study. J Epidemiol. (2006) 16:177–84. doi: 10.2188/jea.16.177
10. Zhu D, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. (2019) 4:e553–64. doi: 10.1016/S2468-2667(19)30155-0
11. Luijken J, van der Schouw YT, Mensink D, Onland-Moret NC. Association between age at menarche and cardiovascular disease: a systematic review on risk and potential mechanisms. Maturitas. (2017) 104:96–116.
12. Snowdon DA, Kane RL, Beeson WL, Burke GL, Sprafka JM, Potter J, et al. Is early natural menopause a biologic marker of health and aging? Am J Public Health. (1989) 79:709–14.
13. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. (2003) 88:2404–11.
14. Agrinier A, Cournot M, Dallongeville J, Arveiler D, Ducimetière P, Ruidavets JB, et al. Menopause and modifiable coronary heart disease risk factors: a population based study - ScienceDirect. Maturitas. (2010) 65:237–43. doi: 10.1016/j.maturitas.2009.11.023
15. Toth MJ, Chernof A, Sites CK, Poehlman ET. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord. (2000) 24:226–31.
16. Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. (2016) 1:767–76. doi: 10.1001/jamacardio.2016.2415
17. Kim SH, Sim MY, Park SB. Association between duration of reproductive lifespan and Framingham risk score in postmenopausal women. Maturitas. (2015) 82:431–5. doi: 10.1016/j.maturitas.2015.07.011
18. Mishra SR, Waller M, Chung HF, Mishra GD. Association of the length of oestrogen exposure with risk of incident stroke in postmenopausal women: insights from a 20-year prospective study. Int J Cardiol. (2021) 328:206–14. doi: 10.1016/j.ijcard.2020.12.022
19. Mishra SR, Hung HF, Waller M, Mishra GD. Duration of estrogen exposure during reproductive years, age at menarche and age at Menopause, and risk of cardiovascular disease events, all−cause and cardiovascular mortality: a systematic review and meta−analysis. BJOG. (2021) 128:809–21. doi: 10.1111/1471-0528.16524
20. Mansoor H, Elgendy IY, Segal R, Hartzema A. Duration of reproductive years and the risk of cardiovascular and cerebrovascular events in older women: insights from the national health and nutrition examination survey. J Womens Health. (2017) 26:1047–52. doi: 10.1089/jwh.2016.6013
21. Katzmarzyk PT, Church TS, Janssen I, Ross R, Blair SN. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care. (2005) 28:391–7.
22. Dong Y, Wang X, Zhang L, Chen Z, Zheng C, Wang J, et al. High-sensitivity C reactive protein and risk of cardiovascular disease in China-CVD study. J Epidemiol Community Health. (2019) 73:188–92.
23. Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, et al. Status of hypertension in china: results from the China hypertension survey, 2012-2015. Circulation. (2018) 137:2344–56.
24. Joint Committee on Guidelines for Prevention and Treatment of dyslipidemia in adults in China. Chinese guidelines for prevention and treatment of dyslipidemia in adults [J]. Chin J cardiol. (2007) 35:390–419.
25. Zhang D, Tang X, Shen P, Si Y, Liu X, Xu Z, et al. Multimorbidity of cardiometabolic diseases: prevalence and risk for mortality from one million Chinese adults in a longitudinal cohort study. BMJ Open. (2019) 9:e024476. doi: 10.1136/bmjopen-2018-024476
26. Liu G. Relationship between Age of Menarche and Live Birth and Obesity and Hypertension in Women. Chongqing: Chongqing Medical University (2019).
27. Murakami K, Metoki H, Satoh M, Asayama K, Hosaka M, Matsuda A, et al. Menstrual factors and stroke incidence in Japanese postmenopausal women: the Ohasama study. Neuroepidemiology. (2016) 2016:109–16. doi: 10.1159/000452220
28. Yang L, Li L, Millwood IY, Peters SAE, Chen Y, Guo Y, et al. Age at menarche and risk of major cardiovascular diseases: evidence of birth cohort effects from a prospective study of 300,000 Chinese women. Int J Cardiol. (2017) 227:497–502. doi: 10.1016/j.ijcard.2016.10.115
29. Hsieh YC, Hwang L-C, Hsieh F-I, Lien LM, Lin H-J, Hu C-J, et al. Early menarche and ischemic stroke risk among postmenopausal women. Int J Gerontol. (2010) 4:16–22. doi: 10.1212/01.wnl.0000250238.69938.f5
30. Qiu C, Chen H, Wen J, Zhu P, Lin F, Huang B, et al. Associations between age at menarche and menopause with cardiovascular disease, diabetes, and osteoporosis in Chinese women. J Clin Endocrinol Metab. (2013) 98:1612–21. doi: 10.1210/jc.2012-2919
31. Kleijn MJJD, Schouw YTVD, Graaf YVD. Reproductive history and cardiovascular disease risk in postmenopausal women - A review of the literature. Maturitas. (1999) 33:7–36.
32. Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor a mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. (1999) 103:401–6.
33. Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. (2002) 53:688–708.
34. Jung KJ, Kim MR, Yun YD, Kim HC, Jee SH. Duration of ovarian hormone exposure and atherosclerotic cardiovascular disease in Korean women: the Korean Heart Study. Menopause. (2016) 23:60–6. doi: 10.1097/GME.0000000000000489
36. Hu X, Rui L, Zhu T, Xia H, Yang X, Wang X, et al. Low testosterone level in middle-aged male patients with coronary artery disease. Eur J Intern Med. (2011) 22:e133–6.
37. Alexander CJ, Tangchitnob EP, Lepor NE. Polycystic ovary syndrome: a major unrecognized cardiovascular risk factor in women. Rev Cardiovasc Med. (2009) 10:83–90.
38. Christakou CD, Diamanti-Kandarakis E. Role of androgen excess on metabolic aberrations and cardiovascular risk in women with polycystic ovary syndrome. Womens Health. (2008) 4:583–94. doi: 10.2217/17455057.4.6.583
39. Castilho SD, Nucci LB. Age at menarche in schoolgirls with and without excess weight. J Pediatr. (2015) 91:75–80. doi: 10.1016/j.jped.2014.05.008
40. Werneck AO, Oyeyemi AL, Cyrino ES, Ronque ERV, Szwarcwald CL, et al. Association between age at menarche and blood pressure in adulthood: is obesity an important mediator? Hypertens Res. (2018) 41:856–64.
41. Roeters van Lennep JE, Heida KY, Bots ML, Hoek A Collaborators Dutch. Cardiovascular disease risk in women with premature ovarian insufficiency: a systematic review and meta-analysis. Eur J Prev Cardiol. (2016) 23:178–86.
42. Honigberg MC, Zekavat SM, Aragam K, Finneran P, Klarin D, Bhatt DL, et al. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA. (2019) 322:2411–21.
43. Huan L, Deng X, He M, Chen S, Niu W. Meta-analysis: early age at natural menopause and risk for all-cause and cardiovascular mortality. Biomed Res Int. (2021) 2021:6636856.
44. Gong D, Sun J, Zhou Y, Zou C, Fan Y. Early age at natural menopause and risk of cardiovascular and all-cause mortality: a meta-analysis of prospective observational studies. Int J Cardiol. (2016) 203:115–9. doi: 10.1016/j.ijcard.2015.10.092
45. Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. (2006) 13:265–79. doi: 10.1097/01.gme.0000218683.97338.ea
46. Wu X, Cai H, Kallianpur A, Gao YT, Yang G, Chow WH, et al. Age at menarche and natural menopause and number of reproductive years in association with mortality: results from a median follow-up of 11.2 years among 31,955 naturally menopausal Chinese women. PLoS One. (2014) 9:e103673. doi: 10.1371/journal.pone.0103673
47. Gallagher LG, Davis LB, Ray RM, Psaty BM, Gao DL, Checkoway H, et al. Reproductive history and mortality from cardiovascular disease among women textile workers in Shanghai, China. Int J Epidemiol. (2011) 40:1510–8. doi: 10.1093/ije/dyr134
48. Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. (1987) 316:1105–10.
49. van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. (1996) 347:714–8.
50. Wang J, Wang Z, Guo F, Zhang Y, Ji H, Chen G, et al. Individual and Combined Cardiometabolic Morbidities and the Subsequent Risk of Cardiovascular Events in Chinese Adults. J Clin Endocrinol Metab. (2022) 107:e84–94. doi: 10.1210/clinem/dgab609
51. Ley SH, Li Y, Tobias DK, Manson JE, Rosner B, Hu FB, et al. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc. (2017) 6:e006713.
52. Ling Y, Lin L, Kartsonaki C, Guo Y, Chen Y, Bian Z, et al. Menopause characteristics, total reproductive years, and risk of cardiovascular disease among Chinese women. Circ Cardiovasc Qual Outcomes. (2017) 10:e004235.
53. Hall PS, Nah G, Howard BV, Lewis CE, Allison MA, Sarto GE, et al. Reproductive factors and incidence of heart failure hospitalization in the Women’s Health Initiative. J Am Coll Cardiol. (2017) 69:2517–26.
54. Otsuki S, Saito E, Sawada N, Abe SK, Hidaka A, Yamaji T, et al. Female reproductive factors and risk of all-cause and cause-specific mortality among women: the Japan public health center–based prospective study (JPHC study). Ann Epidemiol. (2018) 28:597–604.e6. doi: 10.1016/j.annepidem.2018.06.001
55. Chang HS, Odongua N, Ohrr H, Sull JW, Nam CM. Reproductive risk factors for cardiovascular disease mortality among postmenopausal women in Korea: the Kangwha Cohort Study, 1985-2005. Menopause. (2011) 18:1205–12. doi: 10.1097/gme.0b013e31821adb43
56. Jansen SC, Temme EH, Schouten EG. Lifetime estrogen exposure versus age at menopause as mortality predictor. Maturitas. (2002) 43:105–12. doi: 10.1016/s0378-5122(02)00183-4
57. Jaspers L, Kavousi M, Erler NS, Hofman A, Laven JS, Franco OH. Fertile lifespan characteristics and all-cause and cause-specific mortality among postmenopausal women: the Rotterdam Study. Fertil Steril. (2017) 107:448–456.e1 doi: 10.1016/j.fertnstert.2016.11.006
58. Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics. (2002) 110:e43. doi: 10.1542/peds.110.4.e43
59. Yermachenko A, Dvornyk V. Nongenetic determinants of age at menarche: a systematic review. BioMed Res Int. (2014) 2014:371583. doi: 10.1155/2014/371583
60. Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. (2002) 155:672–9. doi: 10.1093/aje/155.7.672
61. Casey V, Dwyer JT, Coleman KA, Krall EA, Gardner J, Valadian I, et al. Accuracy of recall by middle-aged participants in a longitudinal study of their body size and indices of maturation earlier in life. Ann Hum Biol. (1991) 18:155–66. doi: 10.1080/03014469100001492
62. Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, et al. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol. (1987) 126:319–25. doi: 10.1093/aje/126.2.319
Keywords: age at menarche, age at menopause, reproductive lifespan, cardiovascular events, postmenopausal women
Citation: Chen L, Hu Z, Wang X, Song Y, Chen Z, Zhang L, Zheng C, Vallis J, Zhou H, Cao X, Tian Y, Cai J, Gu R, Huang Y and Wang Z (2022) Age at Menarche and Menopause, Reproductive Lifespan, and Risk of Cardiovascular Events Among Chinese Postmenopausal Women: Results From a Large National Representative Cohort Study. Front. Cardiovasc. Med. 9:870360. doi: 10.3389/fcvm.2022.870360
Received: 06 February 2022; Accepted: 13 June 2022;
Published: 09 September 2022.
Edited by:
Liesl Joanna Zühlke, South African Medical Research Council, South AfricaReviewed by:
Iwona Wronka, Jagiellonian University, PolandMarco Matteo Ciccone, University of Bari Aldo Moro, Italy
Copyright © 2022 Chen, Hu, Wang, Song, Chen, Zhang, Zheng, Vallis, Zhou, Cao, Tian, Cai, Gu, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zengwu Wang, d2FuZ3plbmd3dUBmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work
 Lu Chen1†
Lu Chen1† Zhen Hu
Zhen Hu Zengwu Wang
Zengwu Wang