- Department of Cardiovascular Surgery, Dalian Municipal Central Hospital, Dalian, China
Background: The efficacy of dapagliflozin and empagliflozin in sodium-glucose cotransport-2 inhibitors (SGLT-2i) in patients with heart failure (HF) has been discovered. However, which drug could improve varied prognostic outcomes has not been elucidated. Hence, we compared their efficacies on the prognostic improvement of HF.
Methods: Databases including PubMed, EMBASE, Scopus, Google Scholars, and the Cochrane Library were searched for all related randomized controlled trials (RCTs) published from inception to 13 October 2021. Network meta-analyses were performed to generate matrices to show the effect size for pairwise comparison regarding all the interventions.
Results: Eventually a total of 11 RCTs were included in this study. For the primary endpoints, dapagliflozin was comparable with empagliflozin in hospitalization for HF, and empagliflozin (OR=0.70, 95%CI: 0.59–0.84) decreased the risk of exacerbation of HF over dapagliflozin. For the secondary endpoints, dapagliflozin was comparable with empagliflozin in cardiovascular (CV) death /hospitalization for HF, and for CV death, dapagliflozin (OR=0.78, 95%CI: 0.65–0.92) significantly reduced mortality over the placebo. For the tertiary endpoints, dapagliflozin (OR=0.80, 95%CI: 0.66–0.98) significantly decreased the mortality over empagliflozin in all-cause death, and neither drug significantly increased the risk of hypoglycemia.
Recommendations: Overall, 10 mg/day dapagliflozin may be the optimal recommendation for its premium and comprehensive effect on improving the prognosis of patients with HF compared to 10 mg/day empagliflozin.
Introduction
Heart failure (HF), a major public health problem with considerable morbidity and mortality, is often accompanied by various degrees of progressively pathological enlargement of the left ventricular and abnormal cardiac remodeling (1, 2). In recent years, the prevalence of heart failure with preserved ejection fraction (HFpEF) subtype has seized the eyes of scholars in this field. It is generally manifested as left ventricular ejection fraction (LVEF)≥50%, elevated N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels, and definite structural heart disease or diastolic dysfunction in addition to significant signs and symptoms of HF. Its ensuing non-cardiac deaths are more common because of the coexistence of multiple risk factors (age, gender, and BMI) and comorbidities (atrial fibrillation and hypertension) (3). The heart is predominantly remodeled in a centripetal way, so the current triple therapy, angiotensin-converting enzyme inhibitor/angiotensin receptor-neprilysin inhibitor, beta-blockers, and mineralocorticoid receptor antagonist, has limited efficacy in its treatment, and other options are still under exploration (4).
The results of the EMPA-REG OUTCOME trial were the first to demonstrate that empagliflozin in sodium-glucose cotransport-2 inhibitor (SGLT-2i) was not only effective in controlling blood glucose in patients with type 2 diabetes but also in reducing the risk of cardiovascular (CV) death and hospitalization for HF, and the results implied that patients with HF without diabetes may benefit from it as well (5). Undoubtedly, SGLT-2i emerges as a potential therapeutic target for HF. In 2021, the guidelines of the European Society of Cardiology (ESC) and American College of Cardiology (ACC) recommended the addition of dapagliflozin/empagliflozin to the original triple therapy as a new quadruple therapy toward heart failure with reduced ejection fraction (HFrEF), which justifies the two SGLT-2i in treating HFrEF, regardless of diabetes. Moreover, recent studies implied that SGLT-2i might be extended to universal HF more than HFrEF (6).
With its many clinical applications and the publication of large randomized controlled trials (RCTs), SGLT-2i was verified to have CV and renal protective effects in addition to its hypoglycemic mechanism (7). Its diuretic/natriuretic properties may provide additional benefits in terms of reducing circulatory congestion and may reduce the utility of loop diuretics (8). However, there was no head-to-head comparison between dapagliflozin and empagliflozin in terms of improving the prognosis of patients with HF, leaving a paucity of reference in choosing between the two agents. Hence, we conducted a network meta-analysis among dapagliflozin, empagliflozin, and a placebo to find the best agent in benefiting prognosis in patients with universal HF.
Methods
Data Sources and Search Strategy
This meta-analysis was conducted and reported in line with Cochrane and PRISMA (Preferred Reporting Items for Systematic review and Meta-Analyses) guidelines (9). Databases including PubMed, EMBASE, Scopus, Google Scholar, and the Cochrane Library were searched for all related randomized controlled trials (RCTs) published from inception to 13 October 2021. The mesh terms are provided in Supplementary Table S1. Manual screening of the reference list of included trials was used to identify any related studies that may have been missed during the search.
Study Selection and Eligibility Criteria
Inclusion criteria: (1) study subjects were patients with all types of chronic heart failure (CHF, including: HFpEF and HFrEF); (2) the drugs used were dapagliflozin, empagliflozin, and placebo; (3) drug doses of both dapagliflozin and empagliflozin were 10 mg per day; (4) RCTs; and (5) reported at least one prognosis endpoint of interest.
Exclusion criteria: (1) non-RCTs; (2) total number of study cases was <80; (3) drug dosage was inconsistent with the conventional recommended dosage; and (4) full texts were unavailable.
Definition of CHF
Unlike acute HF, CHF is more stable and the clinical symptoms may not be obvious, but there is also a risk of worsening or decompensation. Integrating the diagnostic criteria of CHF from all included studies collectively, CHF was diagnosed as: (1) patients with New York Heart Association functional class (NYHAFC) II-IV symptoms and LVEF ≤ 50%; (2) NYHAFC I symptoms and LVEF ≤ 40%; (3) LVEF > 40% and 6-min walk test distance ≥ 100 m and ≤ 350 m; (4) NT-proBNP level > 300 pg/ml, or NT-proBNP level > 900 pg/ml in patients with atrial fibrillation.
Data Extraction and Bias Assessment
A standardized data form was utilized to extract all data by two reviewers independently and Cochrane risk of bias tools was used for RCTs (10). Discrepancy consultation in data extraction and risk of bias assessment was resolved by a third reviewer. We contacted the study sponsor and the investigator, if necessary, to obtain additional trial-level data and to clarify the definition of the results.
Endpoints of Interest
The primary endpoints were hospitalization for HF and exacerbation of HF [including death/hospitalization for HF, admission to the emergency room, and intravenous diuretics (11)]. The secondary endpoints were CV death/hospitalization for HF and CV death. The tertiary endpoints were all-cause death and hypoglycemia.
Statistical Analysis
All analyses were performed by the application of STATA version 15.0 and Review Manager software version 5.4. Odds ratio (OR) with 95% confidence intervals (95% CI) was calculated to evaluate the binary variables. If I2 < 50% and p > 0.01, a fixed effect model would be adopted, otherwise a random-effect model would be performed. Network meta-analyses (NMA) were executed according to frequentist framework in Stata software by the random-effects model. Matrices (shown as OR and 95% CI) regarding each endpoint were generated to show the pairwise comparison of all interventions. To enhance the stability of the results, the assessment of both gross and loops inconsistency between direct and indirect comparison were performed. Assessment of small sample effect was performed using funnel plots. P < 0.05 was considered to be statistically significant.
Results
Eligible Studies and Study Characteristics
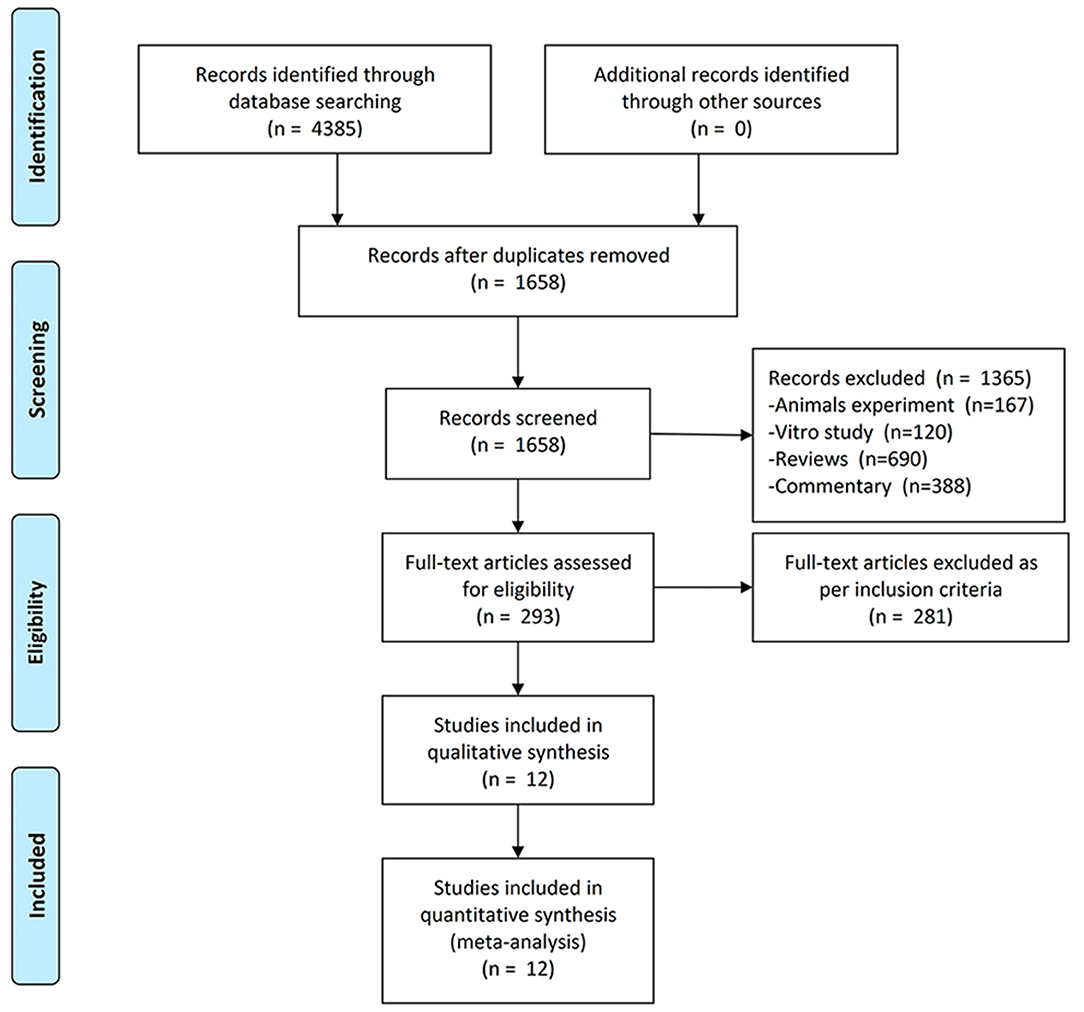
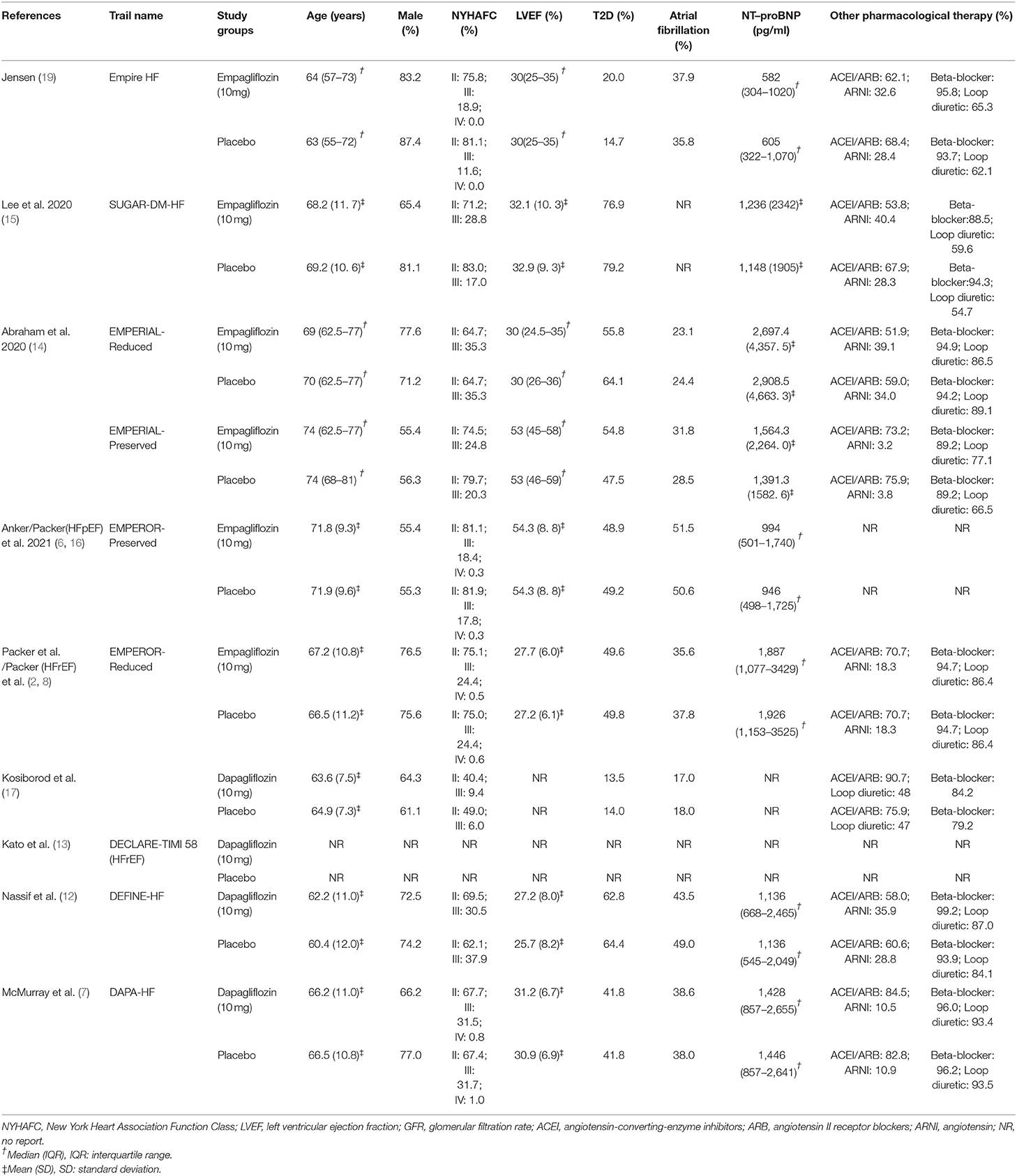
We included the studies from inception to 13 October 2021 and obtained 2,921 publications. After screening and removing duplicates, 293 studies were required to be screened based on inclusion criteria after full-text reading, and finally, 12 studies (2, 6–8, 12–18) containing 11 independent trials were included in this meta-analysis. The screening flow chart is shown in Figure 1. The main characteristics of all included studies have been presented in Table 1 and Supplementary Table S2. Two of the included studies had results from the same clinical trial in a total of two (20) because each study reported results with different tendencies. One study (21) pooled and reported the results of two RCTs.
Risk of Bias and Evidence Network
The specific details of risk-of-bias assessments of the included studies are shown in Supplementary Figure S1. The evidence of a network is shown in Figure 2, with nodes representing different drug interventions and lines representing direct face-to-face comparisons. The width of the lines represents the number of trials. The size of nodes is related to the sample size of the intervention. The combined funnel plots are shown in Figure 3.
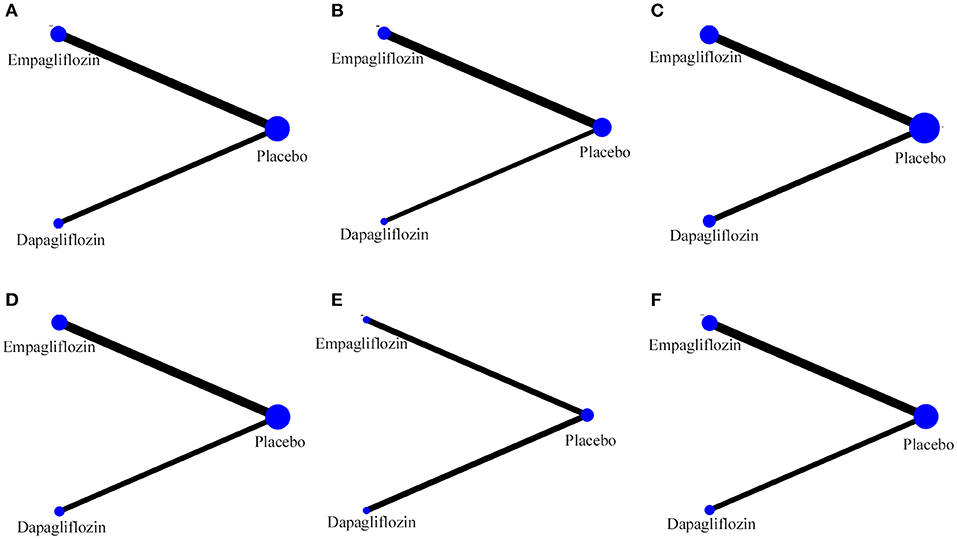
Figure 2. Evidence of network. (A) Hospitalization for HF; (B) exacerbation of HF; (C) all-cause death; (D) CV death; (E) CV death or hospitalization for HF; (F) hypoglycemia.
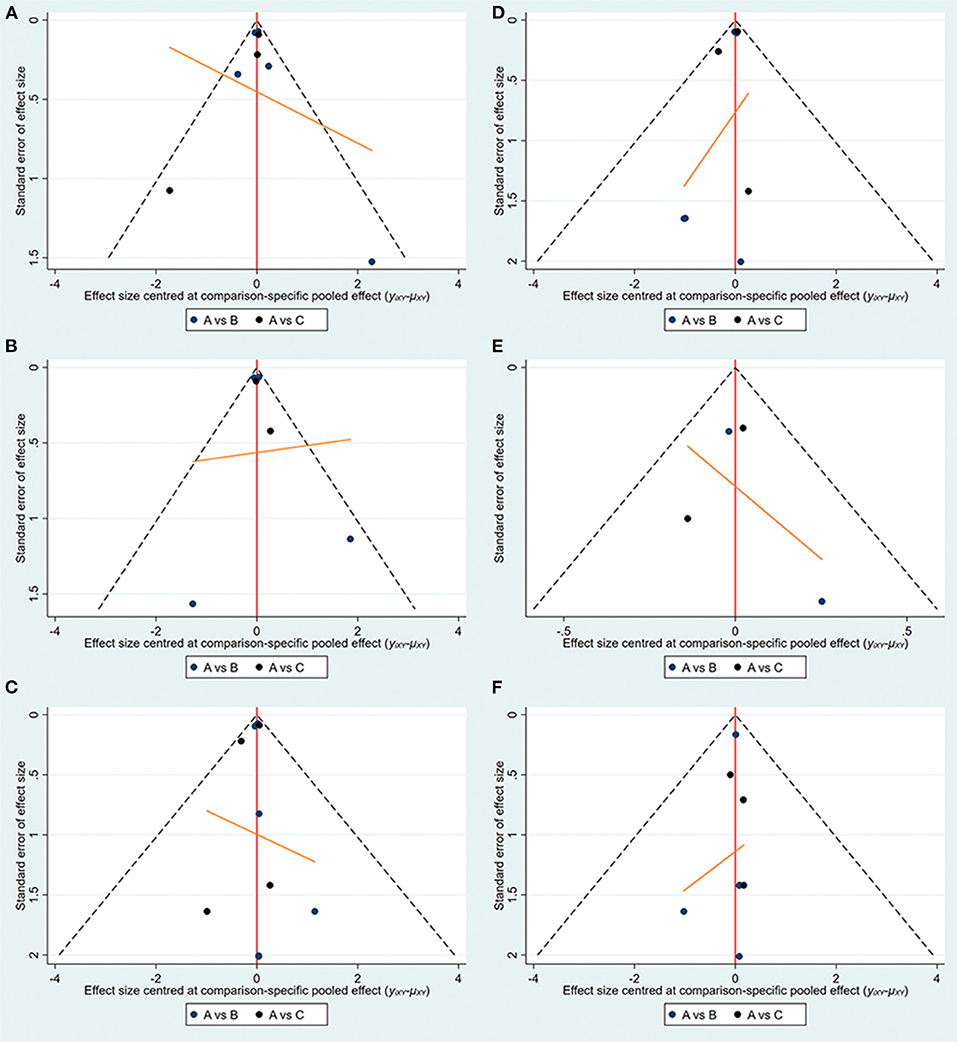
Figure 3. Funnel plots (A:empagliflozin; B:dapagliflozin; C:placebo). (A) Hospitalization for HF; (B) exacerbation of HF; (C) all-cause death; (D) CV death; (E) CV death or hospitalization for HF; (F) hypoglycemia.
Direct Meta-Analysis and Network Meta-Analysis
All direct meta-analyses were the comparison of SGLT-2i with placebo (shown in Supplementary Figure S2), and the full matrix results are presented in detail in Table 2.
Primary Endpoints
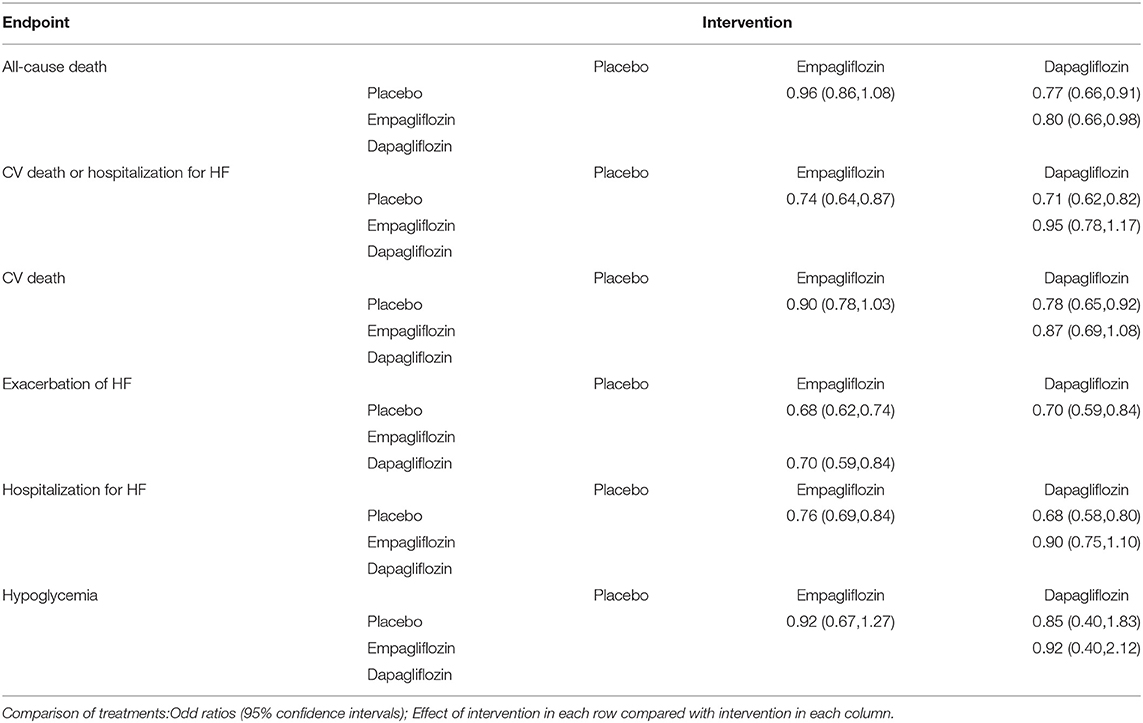
For hospitalization for HF, the OR of empagliflozin vs. placebo was 0.76 (95%CI, 0.69–0.84) and dapagliflozin was 0.68 (95%CI. 0.58–0.80). Network meta-analysis of hospitalization for HF showed that the OR of dapagliflozin vs.empagliflozin was 0.90 (95%CI, 0.75–1.10). For exacerbation of HF, the OR of empagliflozin vs.placebo was 0.68 (95%CI, 0.62–0.74) and dapagliflozin was (0.70; 95%CI, 0.59–0.84). The OR of network meta-analysis of empagliflozin vs.dapagliflozin was 0.70 (95%CI, 0.59–0.84).
Secondary Endpoints
For CV death/hospitalization for HF, the OR of dapagliflozin vs. placebo was 0.71 (95%CI 0.62–0.82) and empagliflozin was 0.74 (95%CI, 0.64–0.87). Network meta-analysis of CV death/hospitalization for HF showed that the OR of dapagliflozin vs.empagliflozin was 0.95 (95%CI, 0.78–1.17). For CV death, the OR of dapagliflozin vs. placebo was 0.78 (95%CI, 0.65–0.92), whereas the OR of empagliflozin was 0.90 (95%CI, 0.78–1.03). The OR of dapagliflozin vs. empagliflozin was 0.87 (95%CI, 0.69–1.08).
Tertiary Endpoints
For all-cause death, the OR of dapagliflozin vs.placebo was 0.77 (95%CI 0.66–0.91), whereas the OR of empagliflozin was 0.96 (95%CI, 0.86–1.08). Network meta-analysis of all-cause death showed that the OR of dapagliflozin vs.empagliflozin was 0.80 (95%CI, 0.66–0.98). As for hypoglycemia, the OR of dapagliflozin vs.placebo was 0.85 (95%CI, 0.40–1.83) and the OR of empagliflozin was 0.92 (95%CI, 0.67–1.27). The OR of dapagliflozin vs.empagliflozin was 0.92 (95%CI, 0.40–2.12).
Consistency and Inconsistency
The overall and loops inconsistency did not exist in all endpoints.
Discussion
To our knowledge, this was the first network meta-analysis comparing the efficacy of dapagliflozin, empagliflozin, and placebo in patients with universal HF. Unlike previous conventional meta-analysis of SGLT-2i with placebo (22–25), we implemented network comparison to materialized pairwise comparison of dapagliflozin vs. empagliflozin with regard to the above endpoints. The dose of the drug was also chosen exclusively as the recommended 10 mg per day. We excluded some studies because they used mixed drug doses and reported confounding results (26). In clinical practice, as the determination of the type of HF was entirely dependent on echocardiographic findings, there were difficulties in classifying patients according to the type of HF when they were in the progress of deteriorating cardiac function. This has a direct impact on the subsequent treatment of the patient and the long-term prognosis. Thus, there is still an urgent need for a new drug that is target specific and has a definite benefit on the prognosis for patients with all types of HF. As mentioned above, recent studies indicated that SGLT-2i might be extended to universal HF more than HFrEF. Thus, this research, as a pilot study to extend them to the full spectrum of HF could be considered as a supplement to the existing guidelines and it is of great clinical value for guiding the medication of all types of HF.
In this work, we compared all the primary endpoints we are concerned about comprehensively. We found that both dapagliflozin and empagliflozin at 10 mg significantly improved the probability of patients being hospitalized for HF compared to the placebo alone and their efficacy was comparable. This might be due to the osmotic diuretic effect of SGLT-2i. It did not inhibit Na-K-2Cl-cotransporter (NKCC2) on macular densa cells and therefore did not activate the RAAS system (27). It also reduced the risk of HF by decreasing urinary magnesium excretion and increasing blood magnesium levels (28).
For exacerbation of HF, we also found that both dapagliflozin and empagliflozin were effective in preventing this type of prognosis in patients relative to the application of placebo. In the meantime, empagliflozin at a dose of 10 mg performed much better. Exacerbation of HF was included in the study as a composite outcome variable, for which part of the mechanism has already been mentioned above. The other part was that SGLT-2i increased hepatic acetone body synthesis, reduced urinary acetone body excretion, increased myocardial acetone body feedstock available, produced more ATP, and reduced myocardial oxygen consumption, which led to a more efficient myocardial energy supply (29). As a result, the activity tolerance of patients with HF can be increased to varying degrees, and the limitations, because of this, in daily living can be effectively reduced.
Abnormalities in blood–lipid metabolism played an important role in predisposing to CV death. Studies have shown that SGLT-2i significantly reduced total cholesterol (TC) and triglyceride (TG) levels and decreased small dense LDL-C, which significantly increased the risk of CV death because of its long intracirculatory cycle, its ability to cross the arterial wall, and its susceptibility to oxidation (30). It had also been established that insulin resistance, circulating levels of leptin, and oxidative stress associated with endothelial cell function were strongly associated with CV death, and SGLT-2i inhibited all of these conditions (31–35). In this meta-analysis, we also found a significant protective effect of dapagliflozin against placebo for CV death, but not for empagliflozin. This was not entirely consistent with the results of previous studies, although it only included two studies (22). We believed that this was because the mean age of empagliflozin was higher than the placebo in the included studies, and that the age factor somewhat reduced its protective effect on CV. Previous studies had identified age as a major risk factor for HF and CV disease, while HF had become one of the leading causes of death in the elderly (36). There was no doubt that the cumulative effect of harmful stimuli (e.g., hypertension and ischemic injury) made the impairment of myocardial reserve more severe in elderly patients due to the limited capacity of the heart to repair and regenerate. Even if this was not the key, the thickening of the ventricular wall, the dilatation of the left atrium, and the increase in the volume of myocardial fibrosis with age all contributed to the objective presence of cardiac dysfunction. Hence, there was a general increase in susceptibility to HF during aging, which in turn drove CV death to some extent. However, at this point in time, after a more objective and comprehensive comparison, the preventive effect of 10 mg of dapagliflozin on CV death is certain. The difference between them suggested that the protective effect of empagliflozin on CV might not be as robust as thought. For the compound variable CV death/hospitalization for HF, we found a significant protective effect of both dapagliflozin and empagliflozin compared to placebo. Otherwise, at the same 10 mg dose, there was no significant difference in the effect of dapagliflozin compared with empagliflozin.
We considered that all-cause death influenced by SGLT-2i was because CV death was so heavily dominant within it, as there was no definitive mechanism to explain its exact effect beyond CV. After analysis, it was found that dapagliflozin at 10 mg was more protective than empagliflozin and placebo. Dapagliflozin showed a stronger protective effect compared to empagliflozin because empagliflozin studies contained a large number of HFpEF patients who suffered from multiple comorbidities (obese, hypertension, atrial fibrillation, etc.) and were often faced with a high risk of non-cardiac deaths (3).
The SGLT-2i lowered blood glucose primarily by lowering the renal glucose threshold and promoting urinary glucose excretion. In patients with HF with normal blood glucose levels, this effect was significantly reduced. In this study, dapagliflozin and empagliflozin at 10 mg orally daily did not increase the risk of severe hypoglycemia compared to placebo, and the effects of both SGLT-2i were generally similar. This was in line with previous research findings (37).
Limitations
Firstly, to reduce within-group variability in the empagliflozin treatment group, we examined only the difference between the 10 mg dose of empagliflozin and placebo. We observed some 25 mg doses mixed in the treatment groups of some studies, but we had to discard the results of this part of the study because we could not break down its effect in some of the studies. This also made this study miss the exploration of the dose-effect. Secondly, the duration of follow-up was not united in some of the included studies, which somewhat increased the occurrence of time-related reporting bias. Thirdly, the proportions of cardiac function classes were not exactly similar within each study endpoint, making the within-group heterogeneity of some of the results high, but the combined effect did not change reversibly when this type of study was excluded. Finally, due to the limited inclusion of results for comparison, the volume of literature reported for some of the analyses is not sufficient, and further expansion of the literature is needed to verify the validity of the results in this study.
Observation
In our study, the priority of dapagliflozin in decreasing all-cause death and CV death over empagliflozin and placebo was obvious. Moreover, dapagliflozin showed equivalent effect with empagliflozin on hospitalization for HF and CV death/hospitalization for HF, and both of them were significantly lower than the placebo, despite the fact that empagliflozin showed a clear superior efficacy over dapagliflozin in exacerbation of HF, and none of them increased the risk of hypoglycemia.
Recommendations
Considering the above results collectively, 10 mg/day dapagliflozin may be the optimal recommendation for its premium and comprehensive effect on improving the prognosis of patients with HF compared to 10 mg/day empagliflozin. However, considering that Empagliflozin study cohorts contained large numbers of HFpEF patients with multiple comorbidities which placed them at high risk for non-CV deaths, further studies are still needed to verify the reliability of the recommendation.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
ZS and WL were responsible for screening articles, extracting all data, and applied the Cochrane Collaboration's tool to assess the risk of bias. XH was responsible for theoretical guidance and decision-making in case of disagreement. ZS, FG, and WL were responsible for article writing and data analysis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.869272/full#supplementary-material
Abbreviations
SGLT-2i, sodium-glucose cotransport-2 inhibitors; HF, heart failure; RCTs, randomized controlled trials; CV, cardiovascular; OR, odds ratio; NYHAFC, New York Heart Association functional class; LVEF, left ventricular ejection fraction; NMA, network meta-analysis; HFpEF, heart failure with preserveded ejection fraction; HFrEF, heart failure with reduced ejection fraction.
References
1. Klein P, Anker SD, Wechsler A, Skalsky I, Neuzil P, Annest LS, et al. Less invasive ventricular reconstruction for ischaemic heart failure. Eur J Heart Fail. (2019) 21:1638–50. doi: 10.1002/ejhf.1669
2. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383:1413–24. doi: 10.1056/NEJMoa2022190
3. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2017) 14:591–602. doi: 10.1038/nrcardio.2017.65
4. Borlaug BA. Evaluation and Management of Heart Failure with Preserved Ejection Fraction. Nat Rev Cardiol. (2020) 17:559–73. doi: 10.1038/s41569-020-0363-2
5. Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. (2018) 137:119–29. doi: 10.1161/CIRCULATIONAHA.117.028268
6. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385:1451–61. doi: 10.1056/NEJMoa2107038
7. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381:1995–2008. doi: 10.1056/NEJMoa1911303
8. Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the emperor-reduced trial. Circulation. (2021) 143:326–36. doi: 10.1161/CIRCULATIONAHA.120.051783
9. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 Esc guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
10. Maddox TM, Januzzi JL Jr., Allen LA, Breathett K, Butler J, Davis LL, et al. 2021 Update to the 2017 Acc Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure with Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. (2021) 77:772–810. doi: 10.1016/j.jacc.2020.11.022
11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical Research ed). (2009) 339:b2700. doi: 10.1136/bmj.b2700
12. Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the define-Hf trial. Circulation. (2019) 140:1463–76. doi: 10.1161/CIRCULATIONAHA.119.042929
13. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. (2019) 139:2528–36. doi: 10.1161/CIRCULATIONAHA.119.040130
14. Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. (2021) 42:700–10. doi: 10.1093/eurheartj/ehaa943
15. Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (Sugar-Dm-Hf). Circulation. (2021) 143:516–25.
16. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: emperor-preserved trial. Circulation. (2021) 144:1284–94. doi: 10.1161/CIRCULATIONAHA.121.056824
17. Kosiborod M, Gause-Nilsson I, Xu J, Sonesson C, Johnsson E. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and concomitant heart failure. J Diabetes Complications. (2017) 31:1215–21. doi: 10.1016/j.jdiacomp.2017.02.001
18. Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. (2021) 77:243–55. doi: 10.1016/j.jacc.2020.11.008
19. Jensen J, Omar M, Kistorp C, Poulsen MK, Tuxen C, Gustafsson I, et al. Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: a double-blinded, randomized, and placebo-controlled trial. Am Heart J. (2020) 228:47–56. doi: 10.1016/j.ahj.2020.07.011
20. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). (2011) 343:d5928. doi: 10.1136/bmj.d5928
21. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research ed). (2019) 366:l4898. doi: 10.1136/bmj.l4898
22. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. Sglt2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the emperor-reduced and dapa-Hf trials. Lancet (London, England). (2020) 396:819–29. doi: 10.1016/S0140-6736(20)31824-9
23. Zheng XD, Qu Q, Jiang XY, Wang ZY, Tang C, Sun JY. Effects of dapagliflozin on cardiovascular events, death, and safety outcomes in patients with heart failure: a meta-analysis. Am J Cardiovasc Drugs. (2021) 21:321–30. doi: 10.1007/s40256-020-00441-x
24. Usman MS, Siddiqi TJ, Memon MM, Khan MS, Rawasia WF, Talha Ayub M, et al. Sodium-glucose co-transporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. (2018) 25:495–502. doi: 10.1177/2047487318755531
25. Gager GM, Gelbenegger G, Jilma B, von Lewinski D, Sourij H, Eyileten C, et al. Cardiovascular outcome in patients treated with sglt2 inhibitors for heart failure: a meta-analysis. Front. Cardiovasc. Med. (2021) 8:691907. doi: 10.3389/fcvm.2021.691907
26. Pellicori P, Ofstad AP, Fitchett D, Zeller C, Wanner C, George J, et al. Early benefits of empagliflozin in patients with or without heart failure: findings from empa-reg outcome. ESC heart failure. (2020) 7:3401–7. doi: 10.1002/ehf2.12891
27. Verma A, Patel AB, Waikar SS. Sglt2 inhibitor: not a traditional diuretic for heart failure. Cell Metab. (2020) 32:13–4. doi: 10.1016/j.cmet.2020.06.014
28. Ray EC. Evolving understanding of cardiovascular protection by Sglt2 inhibitors: focus on renal protection, myocardial effects, uric acid, and magnesium balance. Curr Opin Pharmacol. (2020) 54:11–7. doi: 10.1016/j.coph.2020.06.001
29. Joshi SS, Singh T, Newby DE, Singh J. Sodium-glucose co-transporter 2 inhibitor therapy: mechanisms of action in heart failure. Heart (British Cardiac Society). (2021) 107:1032–8. doi: 10.1136/heartjnl-2020-318060
30. Szekeres Z, Toth K, Szabados E. The Effects of Sglt2 Inhibitors on Lipid Metabolism. Metabolites. (2021) 11. doi: 10.3390/metabo11020087
31. Brown E, Wilding JPH, Alam U, Barber TM, Karalliedde J, Cuthbertson DJ. The expanding role of Sglt2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med. (2021) 53:2072–89. doi: 10.1080/07853890.2020.1841281
32. Wu P, Wen W, Li J, Xu J, Zhao M, Chen H, et al. Systematic review and meta-analysis of randomized controlled trials on the effect of Sglt2 inhibitor on blood leptin and adiponectin level in patients with type 2 diabetes. Horm Metab Res. (2019) 51:487–94. doi: 10.1055/a-0958-2441
33. Katakami N. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. J Atheroscler Thromb. (2018) 25:27–39. doi: 10.5551/jat.RV17014
34. El-Daly M, Pulakazhi Venu VK, Saifeddine M, Mihara K, Kang S, Fedak PWM, et al. Hyperglycaemic impairment of par2-mediated vasodilation: prevention by inhibition of aortic endothelial sodium-glucose-co-transporter-2 and minimizing oxidative stress. Vascul Pharmacol. (2018) 109:56–71. doi: 10.1016/j.vph.2018.06.006
35. Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS One. (2012) 7:e52036. doi: 10.1371/journal.pone.0052036
36. Li H, Hastings MH, Rhee J, Trager LE, Roh JD, Rosenzweig A. Targeting age-related pathways in heart failure. Circ Res. (2020) 126:533–51. doi: 10.1161/CIRCRESAHA.119.315889
37. Ikonomidis I, Pavlidis G, Thymis J, Birba D, Kalogeris A, Kousathana F, et al. Effects of glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with type 2 diabetes mellitus after 12-month treatment. J Am Heart Assoc. (2020) 9:e015716. doi: 10.1161/JAHA.119.015716
Keywords: heart failure, empagliflozin, dapagliflozin, SGLT-2 inhibitor, prognosis
Citation: Shi Z, Gao F, Liu W and He X (2022) Comparative Efficacy of Dapagliflozin and Empagliflozin of a Fixed Dose in Heart Failure: A Network Meta-Analysis. Front. Cardiovasc. Med. 9:869272. doi: 10.3389/fcvm.2022.869272
Received: 04 February 2022; Accepted: 09 March 2022;
Published: 04 April 2022.
Edited by:
Matteo Cameli, University of Siena, ItalyReviewed by:
Mrudula Reddy Munagala, Jackson Health System, United StatesPierre Delanaye, University of Liège, Belgium
Yoshifumi Saisho, Saisho Diabetes Clinic, Japan
Copyright © 2022 Shi, Gao, Liu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuezhi He, aDEzMzUyMjgyMDMwQGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Zepeng Shi†
Zepeng Shi† Xuezhi He
Xuezhi He