- Department of Cardiovascular Surgery, Fuwai Hospital, Chinese Academy of Medical Science, Beijing, China
Objective: The objective of this study was to compare outcomes of re-repair with those of mitral valve replacement (MVR) for failed initial mitral valve repair (MVr).
Methods: We searched the Pubmed, Embase, and Cochrane Library databases for studies that compared mitral valve re-repair with MVR for the treatment of failed initial MVr. Data were extracted by two independent investigators and subjected to a meta-analysis. Odds ratio (OR), risk ratio (RR), hazard ratio (HR), ratio difference (RD), mean difference (MD), and 95% confidence interval (CI) were calculated with the Mantel-Haenszel and inverse-variance methods for mode of repair failure, perioperative outcomes, and follow-up outcomes.
Results: Eight retrospective cohort studies were included, with a total of 938 patients, and mean/median follow-up ranged from 1.8 to 8.9 years. Pooled incidence of technical failure was 41% (RD: 0.41; 95% CI: 0.32 to 0.5; P = 0.00; I2 = 86%; 6 studies, 846 patients). Pooled mitral valve re-repair rate was 36% (RD: 0.36; 95% CI: 0.26–0.46; P = 0; I2 = 91%; 8 studies, 938 patients). Pooled data showed significantly lower perioperative mortality (RR: 0.22; 95% CI: 07 to 0.66; I2 = 0%; P = 0.008; 6 studies, 824 patients) and significantly lower long-term mortality (HR:0.42; 95% CI: 0.3 to 0.58; I2 = 0%; P = 0; 7 studies, 903 patients) in the re-repair group compared with MVR.
Conclusions: Mitral valve re-repair was associated with better immediate and sustained outcomes for failed MVr and should be recommended if technically feasible.
Introduction
Mitral regurgitation (MR) is common, with a prevalence of 0.6–2.7% in the general population (1, 2). Yearly mortality rates with medical treatment in patients ≥ 50 years old are approximately 6% for severe organic MR (3). Surgery has been proven to improve symptoms and survival, and mitral valve repair (MVr) is associated with lower operative mortality and better long-term survival than mitral valve replacement (MVR) (4). Thus, MVr is the treatment of choice recommended by current guidelines, especially for degenerative diseases (5, 6).
In experienced heart centers, a repair rate of nearly 100% for degenerative MR can be achieved (7). However, MVr carries a potential risk for reoperation. The reoperation rate following degenerative MVr is approximately 0.5–1% per year, reducing late survival (5, 6, 8, 9). Re-repair, MVR (10–19), and transcatheter procedures (20–22) have been recommended for failed MVr, but controversies exist regarding optimal treatment strategy. Re-repair and MVR are two strategies for reoperation of failed previous MVr. Some reported similar re-repair results compared with MVR (23), while others adopted an aggressive strategy for re-repair (24).
Thus, we conducted this systematic review and meta-analysis to compare the long-term outcomes of re-repair and MVR for failed previous MVr.
Methods
Search Strategy
The study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (25). On January 14, 2022, a comprehensive literature search was conducted using the PubMed, Embase, and Cochrane Library databases for relevant studies published since 2000 that reported the outcomes of reoperation for failed MVr. The following words found in the title or abstract were used to perform the search: “mitral valve” and “repair” and “recurrent*” or “reoperation.”
Study Selection
Studies that met the following criteria were included: (1) the population consisted of patients who underwent repeat mitral valve surgery (re-repair or MVR) because of failed previous MVr; (2) there were data on perioperative mortality and long-term survival outcomes; (3) studies that reported the outcomes of the re-repair and MVR groups.
The exclusion criteria were as follows: (1) primary surgery including MVR, (2) reoperation due to causes other than repair failure, (3) transcatheter procedures, (4) second cross-clamp during the same surgery, and (5) studies with ≤ 10 patients included, duplicate publications, and review of articles.
Three authors (ZZ, HX, and WS) screened and assessed the studies independently for inclusion. Disagreements regarding the inclusion of articles were resolved via group consensus.
Data Extraction
Two authors (ZZ and HX) reviewed and extracted the reported data from the studies, including details of the study (study design, inclusion criteria, study period, and follow-up duration), baseline demographics, preoperative echocardiographic parameters, causes of repair failure (technique or valve-related), surgical details (re-repair/MVR, and cross-clamp time), perioperative morbidities and mortality, and long-term outcomes (MR recurrence, reoperation, and mortality). For long-term outcomes, the number of observed events and the Kaplan-Meier estimation were extracted and analyzed.
Quality Assessment
Study quality and risk of bias were assessed using the Newcastle-Ottawa Scale. Disagreements were resolved by consensus.
Statistical Analysis and Meta-Analysis
The analyses were performed utilizing Review Manager 5.4 (Cochrane Collaboration, Oxford, United Kingdom). Statistical heterogeneity was assessed using I2 for low (25~49%), moderate (50~ 74%), and high (≥ 75%) heterogeneity. When I2 ≥ 50%, random-effects models were utilized.
For the single-arm meta-analysis (re-repair rate and incidence of technical failure), generic inverse variance methods were used. For binary data (incidence of technical failure), the odds ratio (OR) was calculated using the Mantel-Haenszel method. For continuous data (aortic cross-clamp time and hospital stay time), the mean difference (MD) was calculated using the inverse variance method. For binary data (perioperative and follow-up events), the relative risk (RR) was calculated using the Mantel-Haenszel method. For time-to-event data (the long-term outcomes), the hazard ratio (HR) was calculated using the logarithmic scale generic inverse variance method (26). When HR was not provided, it was calculated using the Kaplan-Meier survival P value and other details if possible (26, 27). When the mean and variance were not provided, they were estimated from the median and quartile range (28–30). Forest plots were generated from the present pooled results.
Results
Study Selection
A total of 2,365 studies were identified using the search criteria. Based on the title and abstract, 17 studies were retrieved for full-text review. Follow-up outcomes comparing re-repair and MVR were not reported in six studies. Two studies by Zegdi et al. used patients from the same institute (AP-HP, Assistance Publique-Hôpitaux de Paris) for two different periods: 1991–2004 (31) and 1987–2006 (14), and the first one was excluded. One study by Ma et al. reported a second cross-clamp under reinstituted cardiopulmonary bypass for incomplete initial repair but not repair failure (32). Kwedar et al. included reoperation after both previous MVr and MVR and did not report outcomes after initial repair specifically (33). The remaining eight studies comprised pooled data (Figure 1).
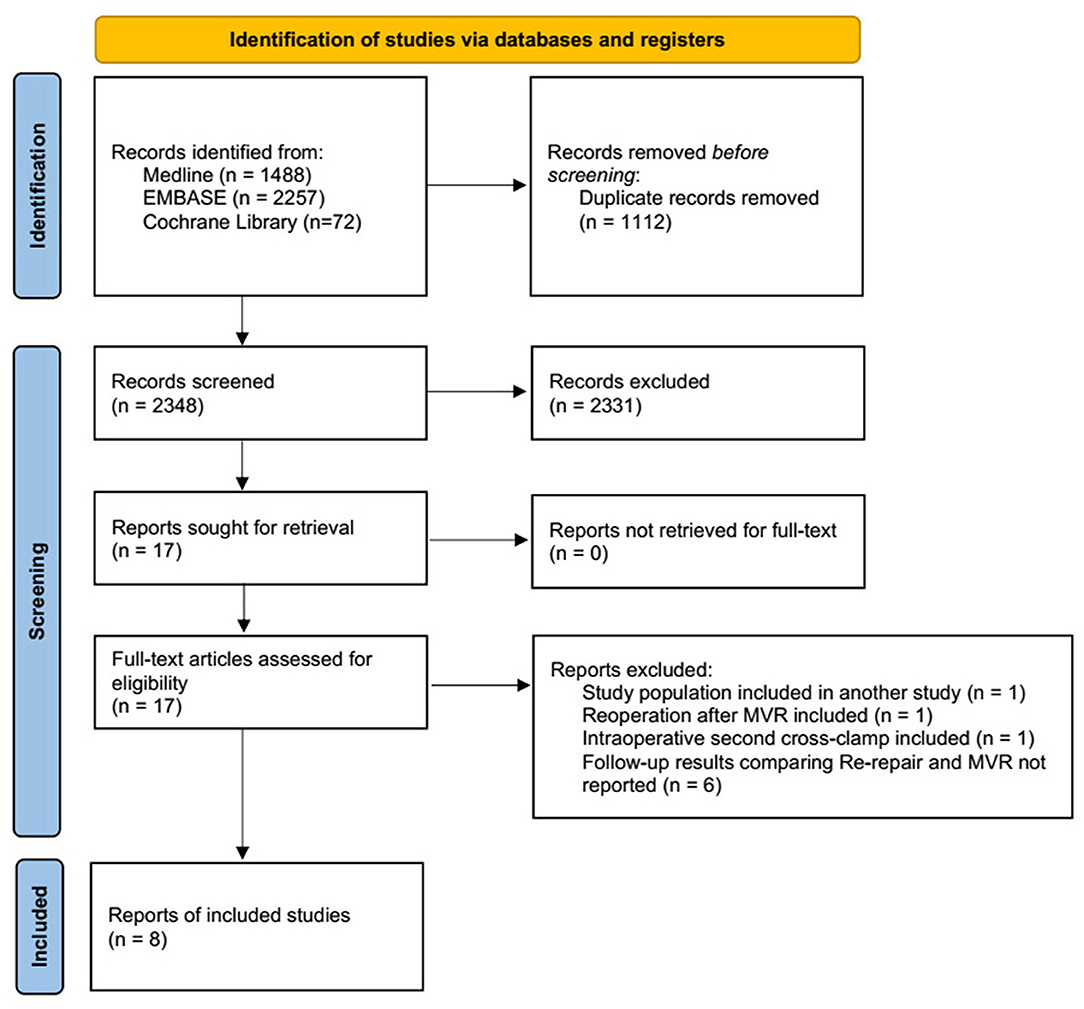
Figure 1. PRISMA flow chart depicting the selection of studies included in the meta-analysis. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; MVR, mitral valve replacement.
Study Characteristics
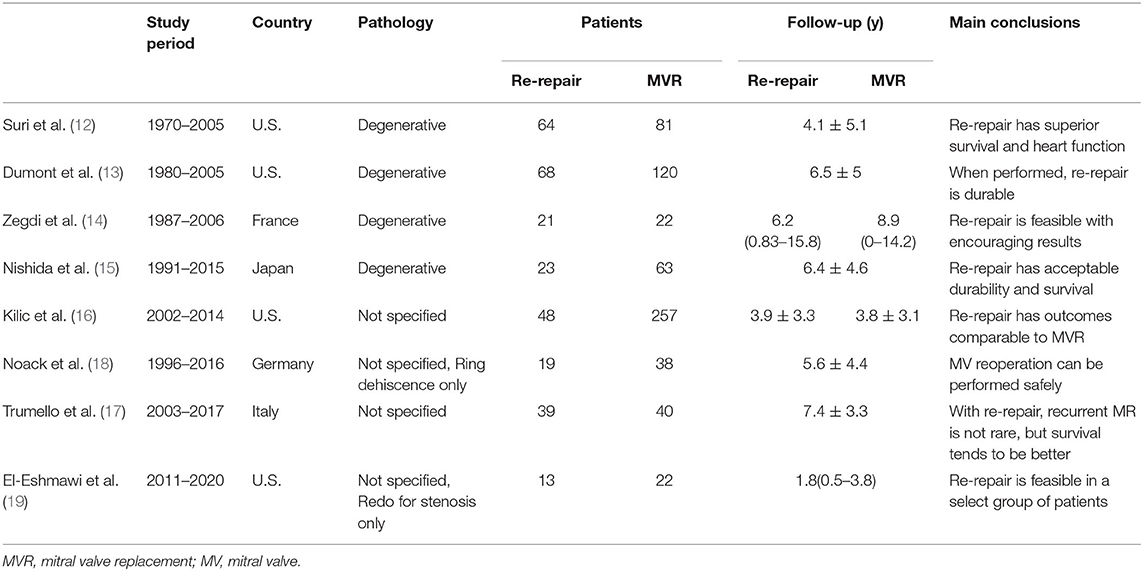
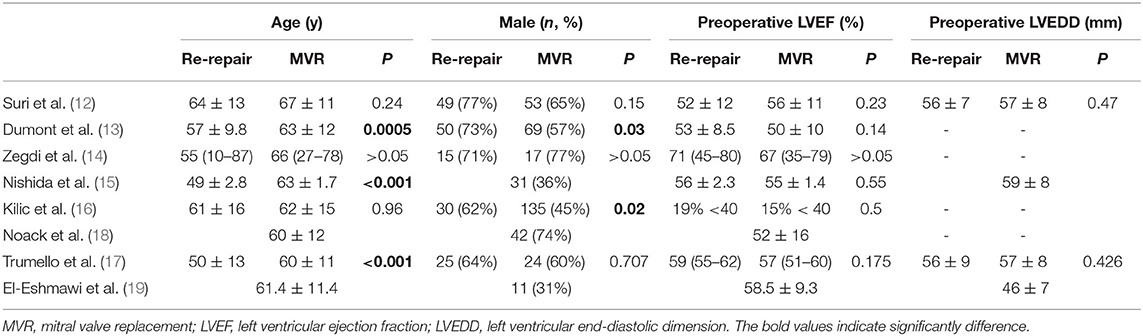
The eight studies were all unmatched retrospective cohort ones. Four studies included only degenerative mitral regurgitation, while the other four studies did not specify the pathological types. A total of 938 patients were included, of whom 295 underwent re-repair and 643 underwent MVR. Six studies compared the preoperative demographic data between the re-repair and MVR groups. All the studies reported follow-up results; mean/median follow-up ranged from 1.8 to 8.9 years. Seven studies reported the Kaplan-Meier survival estimation of the re-repair and MVR groups, while one only reported the follow-up events. The basic characteristics of the studies are listed in Table 1, and the demographic data are shown in Table 2. The Newcastle-Ottawa Scale scores ranged from 7 to 9 out of 9 for the studies. Some points were lost because of a lack of comparability between the groups. The quality assessment of the included studies is listed in Supplementary Table 1.
Mode of Mitral Valve Repair Failure
Failure of MVr can be categorized into technical failure (procedure-related) or progression of disease (valve-related) for recurrent regurgitation (34). Causes of mitral stenosis after MVr included organic and functional (“tunnel effect” due to a small ring) (19).
The proportion of technical failure was reported in six studies (12–17) and showed high heterogeneity (I2 = 86%). One study (18) only included reoperation due to ring dehiscence (technical failure). The pooled incidence of technical failure was 41% (RD: 0.41; 95% CI:0.32–0.5; P = 0; I2 = 86%; 6 studies, 846 patients).
The incidence of technical failure in the re-repair and MVR groups was reported in four studies (12, 15–17). The incidence of technical failure was higher in the re-repair group, but the difference was not significant (OR: 2.66; 95% CI: 1.02–6.93; P =0.05; I2 = 82%; 4 studies, 615 patients) (Figure 2).
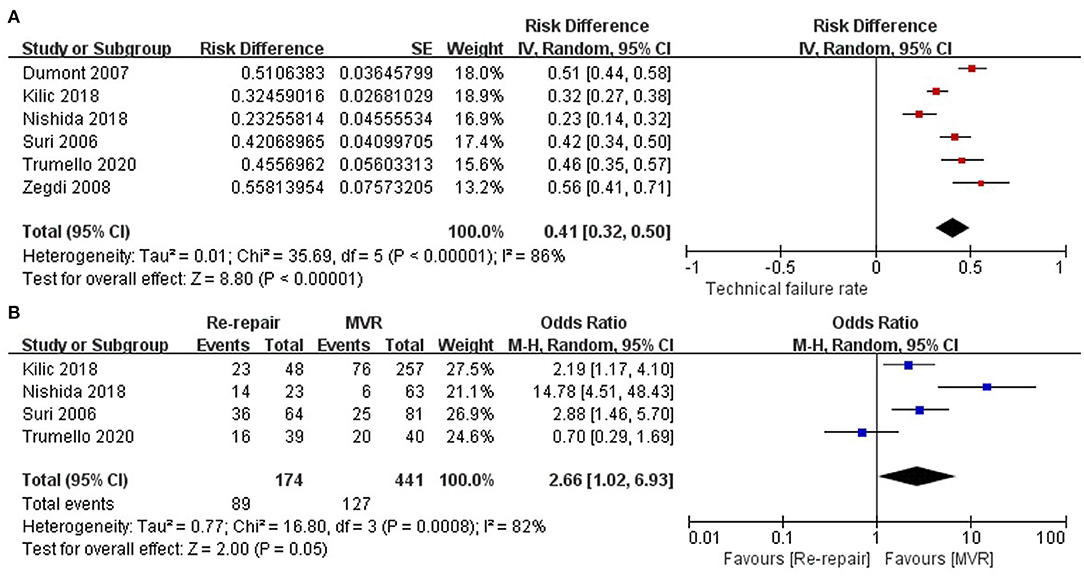
Figure 2. Mode of mitral valve repair failure. (A) Incidence of technical failure of the entire cohort; (B) incidence of technical failure in the re-repair and MVR groups. SE, standard error; IV, inverse variance; CI, confidence interval; M-H, Mantel-Haenszel; MVR, mitral valve replacement.
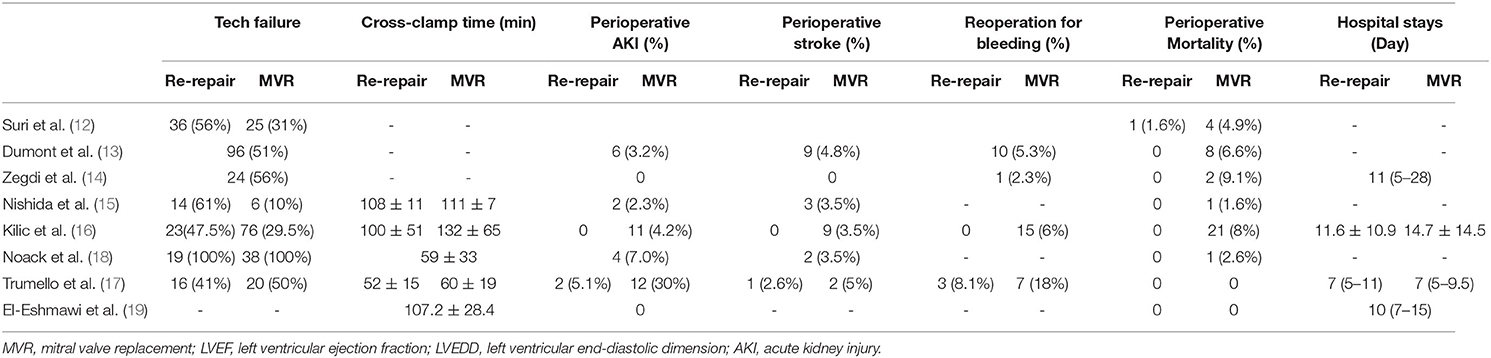
Perioperative Outcomes
The pooled mitral valve re-repair rate was 36% (RD 0.36; 95% CI: 0.26–0.46; P = 0; I2 = 91%; 8 studies; 938 patients).
Mean aortic cross-clamp time was reported by three studies (15–17) and was shorter in the re-repair group, but the difference was not significant (MD = −11.5 min; 95% CI: −23.03–0.02; P =0.05; I2 = 82%; 3 studies, 470 patients).
Perioperative mortality was reported in all the studies, including two studies (17, 19) without perioperative death. The pooled data of the other six studies (12–16, 18) showed significantly lower perioperative mortality in the re-repair group than in the MVR group (RR: 0.22; 95% CI:0.07–0.66; I2 = 0%; P = 0.008; 6 studies, 824 patients).
Major perioperative morbidities were reported in two studies (16, 17). There was a significant difference in the risk of acute kidney injury (AKI) (RR: 0.18; 95% CI:0.05–0.67; I2 = 0%; P = 0.01; 2 studies, 384 patients, but not in stroke (RR: 0.37; 95% CI:0.06–2.34; I2 = 0%; P = 0.29; 2 studies, 384 patients) and reoperation for bleeding (RR: 0.33; 95% CI:0.1–1.09; I2 = 0%; P = 0.07; 2 studies, 384 patients). There was no significant difference in length of hospital stay (MD = −0.96 d; 95% CI: −4.43–2.5; P = 0.59; I2 = 68%; 2 studies, 384 patients) (Table 3; Figure 3). Low cardiac output syndrome, blood transfusion, mediastinitis, and other morbidities were not pooled because of heterogeneity in the diagnostic criteria.
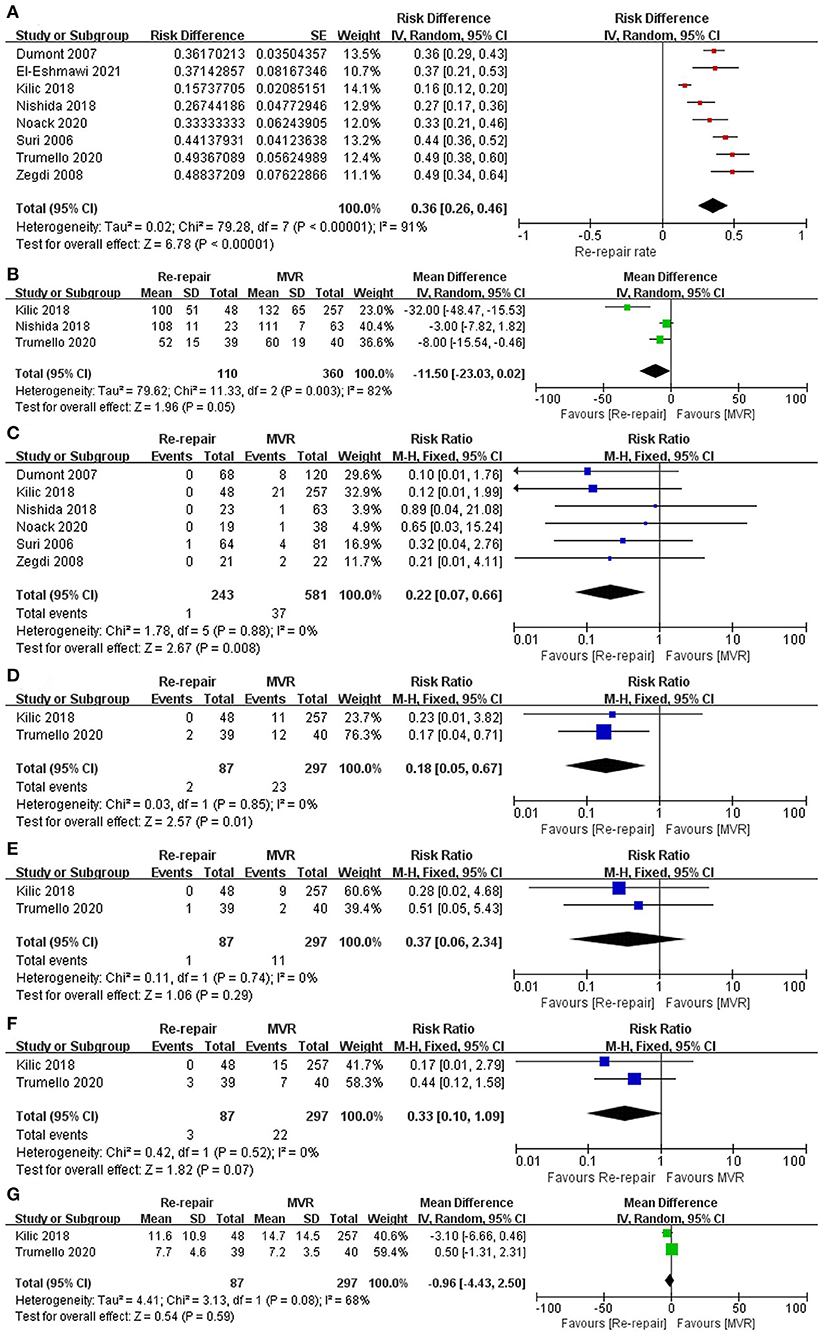
Figure 3. Perioperative outcomes. (A) Re-repair rate for the entire cohort; (B) aortic cross-clamp time; (C) perioperative mortality; (D) AKI; (E) stroke; (F) reoperation for bleeding; (G) postoperative hospital stay. SE, standard error; IV, inverse variance; CI, confidence interval; M-H, Mantel-Haenszel; MVR, mitral valve replacement; AKI, acute kidney injury.
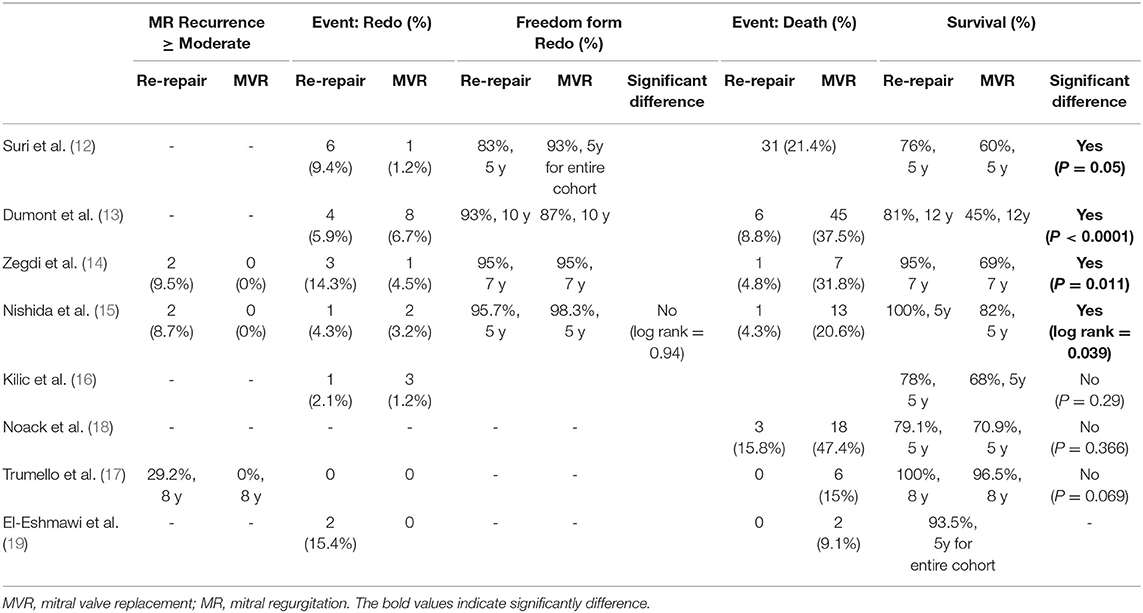
Long-Term Outcomes
Follow-up reoperation was reported in six studies (12–16, 19) and was significantly higher in the re-repair group (RR: 2.11; 95% CI: 1.03–4.28; I2 = 0%; P = 0.04; 6 studies, 802 patients).
Follow-up death was reported in six studies (13–15, 17–19) and was significantly lower in the re-repair group (RR: 0.23; 95% CI: 0.13–0.4; I2 = 0%; P = 0; 6 studies, 488 patients). In addition, re-repair was associated with significantly lower long-term mortality when HR was pooled for analysis (HR: 0.42; 95% CI: 0.3–0.58; I2 = 0%; P = 0; 7 studies, 903 patients). Finally, increase in long-term survival was observed in studies that only reported degenerative diseases (12–15) (HR 0.34; 95% CI: 0.23–0.51; I2 = 12%; P = 0.00; 4 studies, 462 patients). Pooled data from studies reporting mixed pathological types (16–18) showed lower long-term mortality in the re-repair group but without significant difference (HR: 0.62; 95% CI: 0.36–1.07; I2 = 0%; P = 0.09; 3 studies, 441 patients) (Table 4; Figure 4).
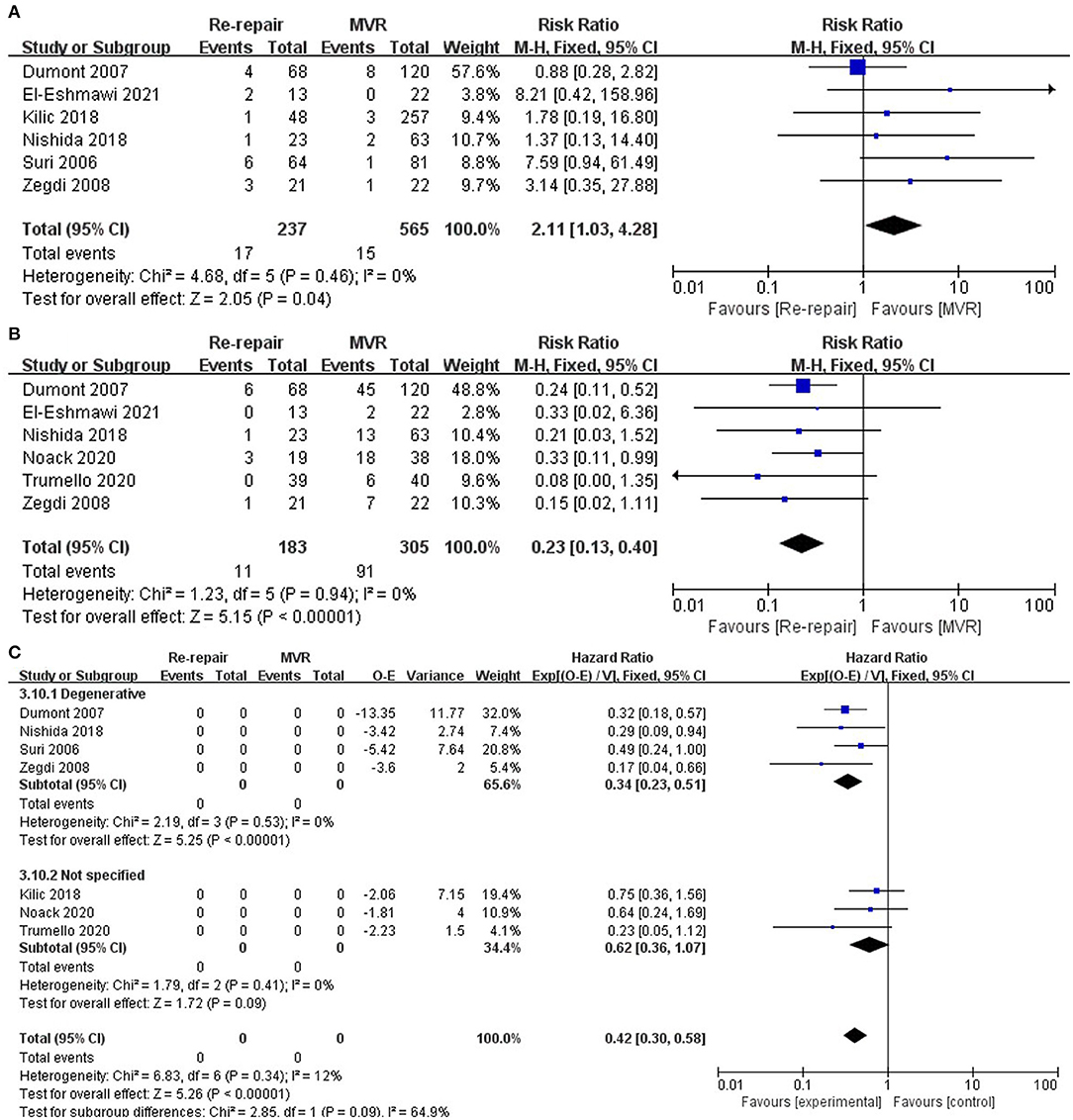
Figure 4. Long-term outcomes. (A) Reoperation; (B) follow-up death, risk ratio pooled; (C) follow-up mortality, hazard ratio pooled. O-E, Observed minus expected events; IV, inverse variance; CI, confidence interval; M-H, Mantel-Haenszel; MVR, mitral valve replacement.
Discussion
In this systematic review and meta-analysis, the major finding was that re-repair for failed previous MVr was associated with significantly improved perioperative and long-term survival. This is the first systematic review and meta-analysis that focused on optimal treatment for failed previous MVr. Veerappan et al. (23) included a second cross-clamp under reinstituted cardiopulmonary bypass (32) and redo after MVR (33).
Advantages of Re-repair
The pooled data in our meta-analysis suggested that mitral valve re-repair was associated with significantly lower perioperative mortality (P = 0.008). For primary surgery, perioperative mortality was significantly lower for MVr than for MVR in both unmatched and matched populations (P < 0.001) (4). This was similar to that of reoperative mitral valve surgery. Kwedar et al. analyzed data from Medicare and found that hospital mortality was 9.8% for re-repair, 12.7% for MVR with bioprosthesis, and 12.2% for MVR with mechanical prosthesis (33). Re-repair has lower perioperative morbidity (16, 17). Relatively simple techniques and preservation of the subvalvular apparatus might contribute to the improved short-term outcomes.
Mitral valve re-repair significantly improved late survival in our meta-analysis (RR and HR, P = 0) despite the higher risk of a third reoperation (P = 0.04). Even in institutes with a “very low threshold for re-repair” and high re-repair rate, re-repair has excellent survival and durability (24). A previous meta-analysis found similar long-term survival between re-repair and MVR, but a second cross-clamp under reinstituted cardiopulmonary bypass was included in the study (23). In a subgroup analysis of studies that did not specify the pathology, the long-term benefit of re-repair was not significant (P = 0.09). However, Aphra et al. reported that patients with rheumatic mitral valve disease showed excellent survival after re-repair, although there was a certain risk of failure over time (35). In addition, patients who underwent re-repair had significantly better ejection fractions and smaller LV end-systolic dimensions during follow-up (12).
Thus, mitral valve re-repair was associated with better immediate and sustained outcomes and should be recommended if technically feasible.
Feasibility of Re-repair
The pooled mitral valve re-repair rate of the studies was 36% (95% CI 0.26–0.46), ranging from 16 to 49%. Anyanwu et al. reported a systemic strategy to re-repair in all cases, and the re-repair rate was 85% (90% for degenerative disease) (24). Even mitral stenosis after MVr could be re-repaired in an experienced mitral valve reference center (19). Several factors can influence the decision to re-repair. When analyzing the mode of repair failure and valve structure, the surgeon should keep in mind that both early and long-term survival would be improved if durable re-repair could be achieved.
In studies that reported re-repair procedures in detail (15, 17, 24, 35), the most frequently used techniques include leaflet resection, ring removal/annuloplasty, edge-to-edge repair, and neochordae. Simple techniques such as edge-to-edge repair may be an effective choice (36). The technique should be selected based on individual mitral valve lesions, while the basic principles and steps of MVr outlined by Carpentier should always be adhered to.
It is not clear what kind of repair failure is suitable for re-repair with long-term durability. Our meta-analysis suggested that the percentage of technical failure might be higher in the re-repair group. This suggests that re-repair should be considered if there is a definite lesion in the previously repaired location.
Implications for Percutaneous Transcatheter Procedures
Percutaneous transcatheter procedures have been increasingly used for failed MVr in selected patients, including mitral clips (20), neochords (21), and valve-in-rings (22). However, the current literature is limited by the small number of patients and short follow-up period. There is a paucity of data on the long-term outcomes of these interventional procedures. Our meta-analysis suggested that transcatheter MVr might be chosen over transcatheter mitral valve replacement.
Limitations
Our meta-analysis has several limitations. First, there were only limited studies comparing the long-term survival of re-repair and MVR. The included studies were retrospective and observational, with relatively small sample sizes. Second, there was significant heterogeneity among the included studies regarding the patients' baseline characteristics and outcomes. Third, there was no uniform standard for selecting re-repair or MVR, and the surgical approach was selected based on the experience of the surgeon or institution. Fourth, most of the studies included in the meta-analysis focused on reintervention and survival, and few studies reported on the follow-up functional status and echocardiographic evaluation. Finally, the studies included mainly patients with degenerative mitral regurgitation. The optimal treatment for rheumatic and infective disease has yet to be studied.
Conclusions
Mitral valve re-repair should be recommended for failed MVr if technically feasible, because re-repair has improved perioperative and long-term results compared with MVR.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
ZZ and SL conceived and designed the study. ZZ, HX, and WS collected and analyzed the data and wrote the manuscript. ZZ, HX, WS, and SL reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Capital Science and Technology Program, Beijing (Grant no: Z201100005520005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.868980/full#supplementary-material
References
1. Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. (1999) 341:1–7. doi: 10.1056/NEJM199907013410101
2. Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. (1999) 83:897–902. doi: 10.1016/S0002-9149(98)01064-9
3. Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. (2009) 373:1382–94. doi: 10.1016/S0140-6736(09)60692-9
4. Lazam S, Vanoverschelde JL, Tribouilloy C, Grigioni F, Suri RM, Avierinos JF, et al. Twenty-Year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: Analysis of a large, prospective, multicenter, international registry. Circulation. (2017) 135:410–22. doi: 10.1161/CIRCULATIONAHA.116.023340
5. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JR, Gentile F, et al. ACC/AHA guideline for the management of patients with valvular heart disease: a report of the american college of Cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2020) 2020:R923. doi: 10.1161/CIR.0000000000000923
6. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43:561–632. doi: 10.1093/ejcts/ezac209
7. Castillo JG, Anyanwu AC, Fuster V, Adams DH. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: Implications for future guidelines. J Thorac Cardiovasc Surg. (2012) 144:308–12. doi: 10.1016/j.jtcvs.2011.12.054
8. Suri RM, Clavel MA, Schaff HV, Michelena HI, Huebner M, Nishimura RA, et al. Effect of recurrent mitral regurgitation following degenerative mitral valve repair: Long-Term analysis of competing outcomes. J Am Coll Cardiol. (2016) 67:488–98. doi: 10.1016/j.jacc.2015.10.098
9. Imielski B, Malaisrie SC, Pham DT, Kruse J, Andrei AC, Liu M, et al. The impact of intraoperative residual mild regurgitation after repair of degenerative mitral regurgitation. J Thorac Cardiovasc Surg. (2021) 161:1215–24. doi: 10.1016/j.jtcvs.2019.10.033
10. el AB, Perier P, Couetil JP, Carpentier A. Failures in reconstructive mitral valve surgery. J Med Liban. (1991) 39:7–11.
11. Gillinov AM, Cosgrove DM, Lytle BW, Taylor PC, Stewart RW, McCarthy PM, et al. Reoperation for failure of mitral valve repair. J Thorac Cardiovasc Surg. (1997) 113:467–73, 473–5. doi: 10.1016/S0022-5223(97)70359-3
12. Suri RM, Schaff HV, Dearani JA, Sundt TR, Daly RC, Mullany CJ, et al. Recurrent mitral regurgitation after repair: Should the mitral valve be re-repaired? J Thorac Cardiovasc Surg. (2006) 132:1390–7. doi: 10.1016/j.jtcvs.2006.07.018
13. Dumont E, Gillinov AM, Blackstone EH, Sabik JR, Svensson LG, Mihaljevic T, et al. Reoperation after mitral valve repair for degenerative disease. Ann Thorac Surg. (2007) 84: 444–50, 450. doi: 10.1016/j.athoracsur.2007.03.078
14. Zegdi R, Sleilaty G, Latremouille C, Berrebi A, Carpentier A, Deloche A, et al. Reoperation for failure of mitral valve repair in degenerative disease: a single-center experience. Ann Thorac Surg. (2008) 86:1480–4. doi: 10.1016/j.athoracsur.2008.07.020
15. Nishida H, Fukui T, Kasegawa H, Kin H, Yamazaki M, Takanashi S. Causes of repair failure for degenerative mitral valve disease and reoperation outcomes. Eur J Cardiothorac Surg. (2018) 53:1244–50. doi: 10.1093/ejcts/ezx468
16. Kilic A, Helmers MR, Han JJ, Kanade R, Acker MA, Hargrove WC, et al. Redo mitral valve surgery following prior mitral valve repair. J Card Surg. (2018) 33:772–7. doi: 10.1111/jocs.13944
17. Trumello C, Giambuzzi I, Del FB, Bargagna M, Blasio A, Ruggeri S, et al. Re-repair after previous mitral valve reconstruction: Handle with care!. Interact Cardiovasc Thorac Surg. (2020) 31:35–41. doi: 10.1093/icvts/ivaa057
18. Noack T, Kiefer P, Vivell N, Sieg F, Marin-Cuartas M, Leontyev S, et al. Annuloplasty ring dehiscence after mitral valve repair: Incidence, localization and reoperation. Eur J Cardiothorac Surg. (2020) 57:300–7. doi: 10.1093/ejcts/ezz219
19. El-Eshmawi A, Sun E, Boateng P, Pandis D, Rimsukcharoenchai C, Anyanwu A, et al. Lessons from reoperations for mitral stenosis after mitral valve repair. J Thorac Cardiovasc Surg. (2021) 161:937–46. doi: 10.1016/j.jtcvs.2020.12.022
20. Rahhab Z, Lim DS, Little SH, Taramasso M, Kuwata S, Saccocci M, et al. MitraClip after failed surgical mitral valve Repair-An international multicenter study. J Am Heart Assoc. (2021) 10:e19236. doi: 10.1161/JAHA.120.019236
21. Gerosa G, Besola L, Beiras-Fernandez A, Salizzoni S, Vairo A, D'Aleo S, et al. The neochord procedure after failed surgical mitral valve repair. Semin Thorac Cardiovasc Surg. (2021) 33:35–44. doi: 10.1053/j.semtcvs.2020.06.015
22. Simonato M, Whisenant B, Ribeiro HB, Webb JG, Kornowski R, Guerrero M, et al. Transcatheter mitral valve replacement after surgical repair or replacement: Comprehensive midterm evaluation of Valve-in-Valve and Valve-in-Ring implantation from the VIVID registry. Circulation. (2021) 143:104–16. doi: 10.1161/CIRCULATIONAHA.120.049088
23. Veerappan M, Cheekoty P, Sazzad F, Kofidis T. Mitral valve re-repair vs replacement following failed initial repair: a systematic review and meta-analysis. J Cardiothorac Surg. (2020) 15:304. doi: 10.1186/s13019-020-01344-3
24. Anyanwu AC, Itagaki S, Varghese R, Castillo J, Chikwe J, Adams DH. Re-repair of the mitral valve as a primary strategy for early and late failures of mitral valve repair. Eur J Cardiothorac Surg. (2014) 45:352–7, 357–8. doi: 10.1093/ejcts/ezt256
25. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
26. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
27. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8
28. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. Bmc Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
29. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. Bmc Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
30. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
31. Zegdi R, Sleilaty G, Khabbaz Z, Noghin M, Latrémouille C, Carpentier A, et al. Late posterior failure after mitral valve repair in degenerative disease. Eur J Cardiothorac Surg. (2008) 34:776–9. doi: 10.1016/j.ejcts.2008.05.047
32. Ma W, Shi W, Zhang W, Wu W, Ye W, Kong Y. Management of incomplete initial repair in the treatment of degenerative mitral insufficiency. Int Heart J. (2018) 59:510–7. doi: 10.1536/ihj.17-287
33. Kwedar K, McNeely C, Zajarias A, Markwell S, Vassileva CM. Outcomes of early mitral valve reoperation in the medicare population. Ann Thorac Surg. (2017) 104:1516–21. doi: 10.1016/j.athoracsur.2017.05.001
34. Anyanwu AC, Adams DH. Why do mitral valve repairs fail? J Am Soc Echocardiogr. (2009) 22:1265–8. doi: 10.1016/j.echo.2009.09.024
35. Aphram G, De Kerchove L, Mastrobuoni S, Navarra E, Solari S, Tamer S, et al. Re-repair of the failed mitral valve: Insights into aetiology and surgical management. Eur J Cardiothorac Surg. (2018) 54:774–80. doi: 10.1093/ejcts/ezy111
Keywords: mitral valve repair, failure, recurrence, reoperation, mitral valve replacement
Citation: Zhong Z, Xu H, Song W and Liu S (2022) Re-repair vs. Replacement for Failed Mitral Valve Repair: A Systemic Review and Meta-Analysis. Front. Cardiovasc. Med. 9:868980. doi: 10.3389/fcvm.2022.868980
Received: 03 February 2022; Accepted: 12 May 2022;
Published: 14 June 2022.
Edited by:
Sandro Gelsomino, Maastricht University, NetherlandsReviewed by:
Prakash P. Punjabi, Imperial College London, United KingdomPaul Philipp Heinisch, German Heart, Germany
Copyright © 2022 Zhong, Xu, Song and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Liu, bGl1c2hlbmdAZnV3YWkuY29t
 Zhaoji Zhong
Zhaoji Zhong Hang Xu
Hang Xu Sheng Liu
Sheng Liu