
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 03 May 2022
Sec. Atherosclerosis and Vascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.868889
This article is part of the Research TopicWomen in Atherosclerosis and Vascular MedicineView all 4 articles
 Diana Thomas Manapurathe1
Diana Thomas Manapurathe1 Joseph Vaughan Moxon1,2
Joseph Vaughan Moxon1,2 Smriti Murali Krishna1,2
Smriti Murali Krishna1,2 Frank Quigley3
Frank Quigley3 Michael Bourke1,4
Michael Bourke1,4 Bernard Bourke1,4
Bernard Bourke1,4 Rhondda E. Jones2
Rhondda E. Jones2 Jonathan Golledge1,2,5*
Jonathan Golledge1,2,5*Background and Aim: The benefit of controlling cardiovascular risk factors in slowing the progression of small abdominal aortic aneurysm (AAA) is controversial. This study investigated the association of optimal blood pressure control at entry with the growth of small AAA.
Methods and Results: A total of 1,293 patients with initial AAA diameter <50 mm were followed by a median 5 (inter-quartile range, IQR, 3–7) ultrasound scans for a median of 3.6 years (IQR 1.8, 5.3). Optimal blood pressure control was defined as blood pressure ≤140/90 mmHg at recruitment. The association of optimal blood pressure control at entry with AAA growth was assessed using linear mixed effects models adjusted for established risk factors of AAA growth and factors which were unequally distributed among the blood pressure groups. Optimal blood pressure control at entry was not significantly associated with AAA growth. In the risk factor adjusted model the mean difference in AAA growth between blood pressure groups was 0.04 mm/year (95% CI −0.20, 0.13; p = 0.65). The results were similar in sensitivity analyses excluding outliers or focused on systolic or diastolic blood pressure alone.
Conclusions: This observational study suggests that optimal blood pressure control at entry is not associated with slower AAA growth.
Abdominal aortic aneurysm (AAA) is an important cause of mortality in older people (1). In many parts of the world, due to the frequent use of abdominal imaging, AAAs are identified when asymptomatic and of small diameter (2, 3). Small AAAs have a low risk of rupture, but about 50% grow to a larger size (≥55 mm), associated with a much higher risk of rupture (1, 4). There are currently no drug treatments with proven efficacy in limiting AAA growth (5).
Hypertension is an established risk factor for AAA diagnosis, however, there is lack of evidence on whether controlling blood pressure can reduce AAA growth. Hemodynamic factors, such as peak wall stress, which have been associated with AAA growth and rupture, are dependent on systemic blood pressure, suggesting that the blood pressure could be an important target to limit AAA progression (4, 6, 7). Past clinical trials testing blood pressure lowering medications have reported no effect of propranolol, perindopril, amlodipine or telmisartan on AAA growth, however, these trials were limited by small sample sizes and underpowered to identify a moderate treatment effect (8–11).
Several observational studies and meta-analyses have evaluated whether blood pressure or hypertension is associated with AAA growth (12–22). Some studies have reported hypertension was associated with an increased AAA growth rate, (12, 15, 16, 20) while others have found no association (13, 14, 17, 18, 21). These studies have used inconsistent definitions of hypertension including either history, (12, 13, 15, 17) medication prescription, (15, 20, 21) actual blood pressure measurements (13, 14, 16, 18–22) or a combination of these. This may have contributed to the inconsistent findings from these studies and it remains uncertain whether blood pressure lowering reduces AAA growth.
Current guidelines recommend maintaining blood pressure less or equal to 140/90 mmHg in people diagnosed with AAA in order to reduce the risk of cardiovascular events (23, 24). There has however been no study of whether having a blood pressure ≤140/90 mmHg is associated with slower AAA growth. The aim of this study was to assess whether people with a blood pressure measured in the out-patient clinic of ≤140/90 mmHg had slower AAA growth than those who did not meet this target.
This investigation was designed as part of an ongoing prospective cohort study that aims to identify risk factors associated with AAA diagnosis and outcome. The study commenced in 2002 and remains ongoing. Patients were recruited from vascular services in Australia, including Townsville University Hospital, the Mater Hospital Townsville, Gosford Vascular Services and The Royal Brisbane and Women's Hospital. The patients were initially identified through outpatient and surveillance programs at the respective hospitals. For inclusion in the current study, patients had to have infrarenal AAA diagnosed by a vascular specialist; at least two ultrasound scans to monitor AAA growth; an initial AAA diameter <50 mm; minimum follow up of 6 months; and the assessment of blood pressure at recruitment. These inclusion criteria were selected in order to enable testing of our hypothesis that optimal blood pressure control was associated with faster AAA growth. This study included data for a follow up period of up to 6 years. Patients with a AAA diameter ≥50 mm, symptomatic AAA patients and patients who had a AAA repair were all excluded from the study. The study was performed in accordance with the Declaration of Helsinki, and ethical approval was granted from the respective institutional Ethics Committees. Written informed consent was obtained from all participants.
Risk factors, and medications of participants were recorded at study entry by clinical interview and physical examination. Smoking status was classified as ever (including current and former smoker) or never smoker (25–27). Hypertension, diabetes and stroke were defined by a history of diagnosis or treatment for these conditions (25–27). Ischemic heart disease (IHD) was defined by a history of myocardial infarction (MI), angina, or treatment for IHD (25–27). Body mass index (BMI) was measured as previously described (28). The patient's medications were recorded at recruitment, including anti-platelets, diuretics, frusemide, angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), beta blockers (BB), calcium channel blockers (CCB), HMG-CoA reductase inhibitors (statins), fibrates, metformin and other hypoglycaemic agents.
This was measured at recruitment using a digital blood pressure monitor - Omron Intellisense (HEM – 907) according to current clinical guidelines (29). Resting blood pressure was measured at the patient's first visit. Three blood pressure measurements were recorded in the right arm at 3-min intervals and the average was used. Study investigators did not undertake any blood pressure management which was under the care of the participants' general practitioners. A sub-set of patients had repeat blood pressure measurements during follow-up as part of standard clinical care.
Maximum anterior to posterior and transverse infrarenal aortic diameters were measured by an experienced sonographer using ultrasound machines employed in the vascular laboratories at each center (Toshiba Capasee (Toshiba Medical Systems, North Ryde, New South Wales, Australia); Philips HDI 5,000 (Philips Medical Systems, Bothell, Washington, USA); GE LOGIQ 9 (GE Healthcare, Chicago, Illinois, USA); Siemens Acuson Antares™ (Siemens Healthcare, Bayswater, Victoria, Australia); Philips IU22 (Philips Medical Systems) and a standard protocol, as described previously (30–32). Aortic diameter was measured from outer wall to outer wall of the artery. The reproducibility of aortic diameter measurements was assessed in each vascular laboratory, with inter-observer reproducibility coefficients being <4 mm as previously reported (30–33).
This study analyzed the association between blood pressure at entry and AAA growth. The primary objective was to analyze the association between optimal blood pressure at entry and AAA growth. Optimal blood pressure was defined as that ≤140/90 mmHg measured at recruitment. Blood pressure higher than this was defined as sub-optimal. Sensitivity analyses investigated the association of optimal systolic blood pressure ( ≤140mmHg) and optimal diastolic blood pressure ( ≤90 mmHg) at entry with AAA growth. This blood pressure target was chosen in line with current guidelines (23, 34). Data were analyzed using the SPSS v 23 and R statistical software packages. The quantitative data were not normally distributed confirmed using the Shapiro Wilk test. Continuous data were presented as median and inter-quartile range (IQR). Nominal data are reported as count and percentages and were compared using Chi–squared tests. It has been proposed that a clinically important difference in AAA growth is attained only if growth is reduced by at least 30% (35). In a previous study, annual increase in AAA diameter was 1.62 ± 2.45 mm (32). To detect a 30% slower AAA growth rate in people with optimal blood pressure control, at a power of 90% and an alpha of 5%, a minimum of 1,168 participants were required. The study was therefore planned to include at least 1,200 participants. Random slope, random intercept linear mixed effects (LME) models were used to examine the association between AAA growth and blood pressure groups using unadjusted and multivariable models. Multivariate model 1 was adjusted for risk factors that have consistently been associated with AAA growth including sex, smoking, diabetes and initial AAA diameter (31). The second multivariate model included these risk factors and also those that were observed to be significantly unequally distributed among the blood pressure groups (main analysis focused on optimal blood pressure control: smoking, IHD, initial diameter, sex, diabetes, stroke, aspirin, BB, frusemide, diuretics, ACEI and statin; sensitivity analysis focused on optimal systolic blood pressure control: IHD, prior stroke, aspirin, BB and statin prescription; sensitivity analysis focused on optimal diastolic blood pressure control: IHD, prior stroke, aspirin, beta blockers, ACE-I, CCB, frusemide and statin prescription). Data for an individual at different follow up time is treated as individual observations in LME leading to 6,130 observations. Individual patients were treated as random effects in all models. Time, blood pressure group and the included covariates were included as fixed effects. The interaction of time and blood pressure group was used as the test statistic for all LME-based analyses (32, 36, 37). Model fit was assessed by visual inspection of the standardized residual distribution and q-q norm plots, suggesting the presence of potentially influential outliers. Sensitivity analyses excluding outliers (defined as data points lying >4 standard deviations away from the mean of all model residuals) were performed. These were calculated using the R nlme and car packages (http://www.r-project.org), as described previously (37). P-values < 0.05 were considered significant for all the analyses.
Out of the 1,293 participants, 475 (36.7%) had optimum blood pressure control and 818 (63.3%) had sub-optimal blood pressure control at recruitment. Participants with optimal blood pressure control had a significantly higher prevalence of diabetes, IHD, past smoking and past stroke and they were also significantly more likely to be prescribed aspirin, frusemide, BB, ACEI, diuretics, statins and drugs to treat diabetes (Table 1). They also had significantly larger AAAs at the time of recruitment (Table 1). A sub-set of participants (n = 304; 23.5%) had a repeat blood pressure measurement a median (IQR) of 2.0 (1.0, 3.9) years after recruitment. Participants with sub-optimal blood pressure at recruitment had significantly higher median (IQR) systolic blood pressure when assessed during follow-up than those with optimal blood pressure at recruitment (141, 130 to 154, vs. 130, 120 to 142 mmHg; p < 0.001).
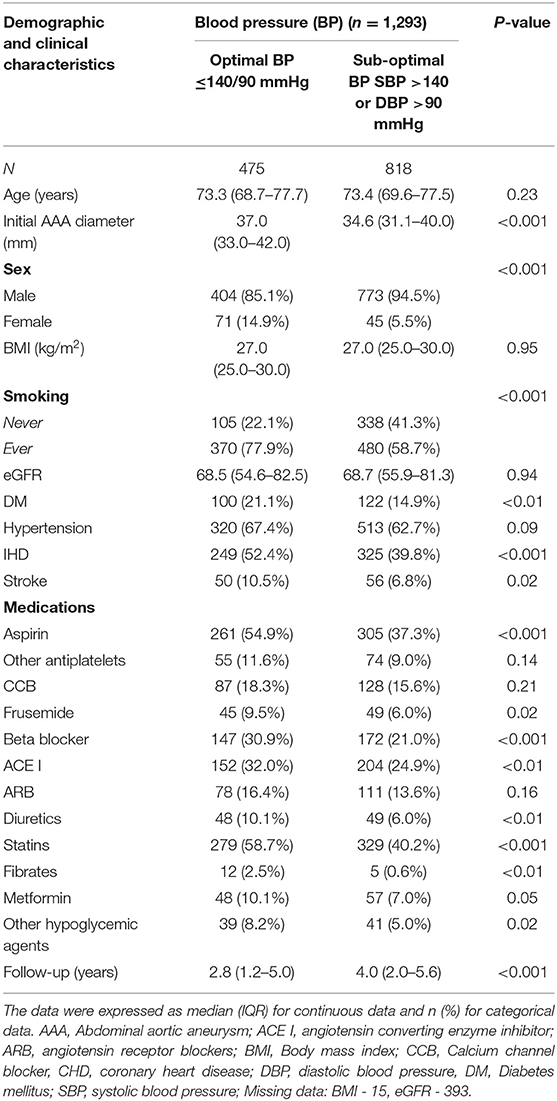
Table 1. Characteristics of the participants in relation to whether they had optimal or sub-optimal blood pressure control.
Participants were followed by a median of 5 (inter-quartile range, IQR, 3, 7) ultrasound scans for a median of 3.6 years (IQR 1.8, 5.3). The unadjusted analysis suggested that participants with sub-optimal blood pressure control had significantly slower AAA growth. The mean difference in AAA growth between blood pressure groups was 1.75 mm/year (95% CI 2.37, 1.12) p < 0.001 (Table 2, Figure 1). This association did not remain significant when adjusted for other risk factors (Model 1: The mean difference of AAA growth was −0.03 mm/year (95% CI −0.19, 0.13, p = 0.74; Model 2: The mean difference of AAA growth was −0.04 mm/year (95% CI −0.20, 0.13, p = 0.65) (Table 2, Supplementary 1 Table 1). A sensitivity analysis excluding potential outliers did not alter the findings (Table 3, Supplementary 1 Table 2).

Figure 1. Association of optimal blood pressure with abdominal aortic aneurysm growth. The graph illustrates the mean growth (95% CI) of AAA growth during follow up (years) according to optimal blood pressure in AAA patients. The red line represents patients with BP ≤140/90 mmHg and the green line represents patients with SBP >140 or DBP >90 mmHg. BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
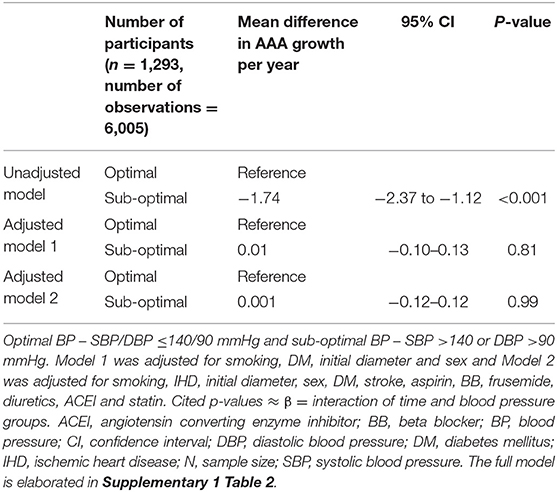
Table 3. Association between optimal blood pressure and AAA growth after removing participants with outlier measurements.
At recruitment, 503 (38.9%) participants had systolic blood pressure ≤140 mm Hg and 790 (61.6%) had systolic blood pressure >140 mmHg (Supplementary 2 Table 1). The association of systolic blood pressure groups with risk factors is shown in Supplementary 2 Table 2. AAA growth was significantly slower in patients with systolic blood pressure > than ≤140 mmHg in the unadjusted analysis. The mean difference in AAA growth was 1.53 mm/year (95% CI −2.15, −0.91; p <0.001; Supplementary 2 Table 2). This association did not remain significant when adjusted for other risk factors (Model 1: mean difference in AAA growth was −0.0003, 95% CI −0.16, 0.16, p = 1.00; Model 2: mean difference in AAA growth was −0.01, 95% CI −0.17, 0.15, p = 0.90). A sensitivity analysis excluding potential outliers did not alter the findings (Supplementary 2 Table 3). Furthermore, systolic blood pressure recorded at entry as a continuous variable was not significantly associated with AAA growth after adjusting for other risk factors (Supplementary 2 Table 4).
At recruitment, 917 (67.3%) participants had a diastolic blood pressure ≤90 mmHg and 376 (27.6%) had a diastolic blood pressure >90 mmHg. The association of diastolic blood pressure groups with risk factors is shown in Supplementary 3 Table 1. Similar to other analyses AAA growth was significantly slower in patients with diastolic blood pressure > than ≤90 mmHg in unadjusted (mean difference in AAA growth was −1.78 mm/year, 95% CI −2.45, −1.12, p = <0.001, Supplementary 3 Table 2) but not adjusted analyses (Model 1: mean difference in AAA growth was−0.14, 95% CI−0.32, 0.02, p = 0.09; Model 2: mean difference in AAA growth was −0.16, 95% CI −0.33, 0.01, p = 0.07). Removal of potentially influential outliers did not change these results (Supplementary 3 Table 3). Additional analyses using diastolic blood pressure measured at entry as a continuous variable show no significant association with AAA growth after adjusting for other risk factors (Supplementary 3 Table 4).
The findings of the current study suggest that guideline recommended control of blood pressure at entry is not associated with reduced AAA growth in patients that have small AAA. Findings were consistent in sensitivity analyses. The unadjusted analysis suggested that patients with suboptimal blood pressure at entry had reduced AAA growth. This association was however not maintained in analyses adjusted for importance confounding factors. Larger initial AAA diameter was the major predictor of faster AAA growth. However, no interaction was observed between the initial diameter and AAA growth, suggesting that blood pressure levels does not affect AAA growth depending on the initial diameter.
Several observational studies and meta-analyses have evaluated whether blood pressure or hypertension is associated with AAA growth (12–22). Overall these studies have suggested an inconsitent association of blood pressure with AAA growth, in keeping with the findings of the current study.
A number of small randomized control trials have assessed the effect of blood pressure lowering medications, including propranolol, amlodipine, perindopril and telmisartan, on AAA growth (8–11). None of these studies found that these anti-hypertensive medications slowed AAA growth. All these studies were underpowered, but a meta-analysis of these trials suggested that blood pressure lowering did not slow AAA growth or reduce requirement for AAA repair (38, 39).
Due to the small size of these prior randomized trials and the limited range of anti-hypertensive drugs tested, it remains uncertain whether blood pressure lowering reduces AAA growth. Observational studies may provide some evidence whether high blood pressure is a treatment target to limit AAA growth, although in previous such studies findings have been inconsistent (12–14, 16, 17, 20–22). A previous meta-analysis reported that hypertension was not associated with AAA growth although it is notable that the definitions of hypertension, samples sizes and methods of examining AAA growth varied which may have contributed to the inconsistency reported (40). The current study represents the largest cohort using a clear definition of optimal blood pressure control and standard method of measuring AAA diameter to investigate the association of blood pressure with AAA growth. The findings suggest that optimal blood pressure control does not slow AAA growth. It is well-established that hypertension is an important risk factors for cardiovascular events, such as MI and stroke, which are common in people with AAA and therefore controlling blood pressure in patients with small AAA remains important despite the lack of evidence on its effect on AAA growth (40, 41).
The current study has a number of strengths and limitations. In comparison to the other observational studies, the current study had a large sample size in which data were collected according to standardized protocols. This allowed for well-powered analyses. Also, a range of sensitivity and adjusted analyses were performed. The main limitation of the study was that the participants only had one blood pressure measurement performed at study entry. Only patients with clinical requirement had repeat blood pressure measurements and a separate analysis could not be performed due to the small sample size. Medical management changes were also not recorded during the study. Blood pressure was only measured in the right arm which could have resulted in underestimation of values in the presence of subclavian artery stenosis or occlusion. Even though the study included a large number of patients, there was a difference in sample sizes of the two blood pressure groups which may have influenced the power of the analyses. The current study was also not a randomized control trial; hence it remains possible that factors we did not measure including chronic airways disease and therefore could not adjust for led to residual confounding. AAA diameter was measured with ultrasound rather than computed tomography but we have previously reported excellent reproducibility of ultrasound measured AAA diameter (30–33).
In conclusion this study suggests that optimal control of blood pressure at entry is unlikely to limit growth of small AAA. This does not outweigh the benefits of blood pressure management in AAA patients to reduce cardiovascular events and other AAA complications. Further well-designed studies including patients with multiple blood pressure measurements are required for conclusive evidence.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Townsville Hospital and Health Services Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
DT was involved in project administration, data curation, conceptualization, formal analysis, investigation, methodology, validation, visualization, writing, and editing. JG was involved in project administration, conceptualization, funding acquisition, data collection, investigation, methodology, supervision, writing, and editing. JM was involved in conceptualization, formal analysis, investigation, methodology, supervision, validation, and editing. SK was involved in supervision and editing. FQ, MB, and BB were involved in data collection and data curation and editing. RJ was involved in formal analysis, methodology, and editing. All authors contributed to the article and approved the submitted version.
This study was supported by funding from the National Health and Medical Research Council (1063476 and 1180736) and the Queensland Government (Australia). JG holds a Practitioner Fellowship from the National Health and Medical Research Council (1117061) and a Senior Clinical Research Fellowship from the Queensland Government. JM was supported by an Advance Queensland Fellowship from the Queensland Government. DT was supported by a JCU (James Cook University) Postgraduate Research Scholarship and a JCU College of Medicine and Dentistry Scholarship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.868889/full#supplementary-material
1. Moxon JV, Parr A, Emeto TI, Walker P, Norman PE, Golledge J. Diagnosis and monitoring of abdominal aortic aneurysm: current status and future prospects. Curr Probl Cardiol. (2010) 35:512–48. doi: 10.1016/j.cpcardiol.2010.08.004
2. Rowbotham SE, Krishna SM, Moran CS, Golledge J. Fenofibrate and telmisartan in the management of abdominal aortic aneurysm. Curr Drug Targets. (2018) 19:1241–6. doi: 10.2174/1389450119666171227224655
3. Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. (2004) 329:1259. doi: 10.1136/bmj.38272.478438.55
4. Khosla S, Morris DR, Moxon JV, Walker PJ, Gasser TC, Golledge J. Meta-analysis of peak wall stress in ruptured, symptomatic, and intact abdominal aortic aneurysms. Br J Surg. (2014) 101:1350–7. doi: 10.1002/bjs.9578
5. Persson SE, Boman K, Wanhainen A, Carlberg B, Arnerlov C. Decreasing prevalence of abdominal aortic aneurysm and changes in cardiovascular risk factors. J Vasc Surg. (2017) 65:651–8. doi: 10.1016/j.jvs.2016.08.091
6. Soto B, Vila L, Dilmé JF, Escudero JR, Bellmunt S, Camacho M. Increased peak wall stress, but not maximum diameter, is associated with symptomatic abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. (2017) 54:706–11. doi: 10.1016/j.ejvs.2017.09.010
7. Stevens RRF, Grytsan A, Biasetti J, Roy J, Lindquist Liljeqvist M, Gasser TC. Biomechanical changes during abdominal aortic aneurysm growth. PLoS ONE. (2017) 12:e0187421. doi: 10.1371/journal.pone.0187421
8. Lindholt JS, Henneberg EW, Juul S, Fasting H. Impaired results of a randomised double blinded clinical trial of propranolol vs. placebo on the expansion rate of small abdominal aortic aneurysms. Int Angiol. (1999) 18:52–7.
9. Propanolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg. (2002) 35:72–9. doi: 10.1067/mva.2002.121308
10. Kiru G, Bicknell C, Falaschetti E, Powell J, Poulter N. An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomised placebo-controlled trial (aardvark). Health Technol Assess. (2016) 20:1–180. doi: 10.3310/hta20590
11. Golledge J, Pinchbeck J, Tomee SM, Rowbotham SE, Singh TP, Moxon JV, et al. Efficacy of telmisartan to slow growth of small abdominal aortic aneurysms: a randomized clinical trial. JAMA. (2020) 5:1–9. doi: 10.1001/jamacardio.2020.3524
12. Baba T, Ohki T, Kanaoka Y, Maeda K, Ito E, Shukuzawa K, et al. Risk factor analyses of abdominal aortic aneurysms growth in japanese patients. Ann Vasc Surg. (2019) 55:196–202. doi: 10.1016/j.avsg.2018.07.045
13. Brady AR, Thompson SG, Fowkes FGR, Greenhalgh RM, Powell JT. UK small aneurysm trial participants. abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance Circulation. (2004) 110:16–21. doi: 10.1161/01.CIR.0000133279.07468.9F
14. MacSweeney ST, Ellis M, Worrell PC, Greenhalgh RM, Powell JT. Smoking and growth rate of small abdominal aortic aneurysms. Lancet. (1994) 344:651–2. doi: 10.1016/S0140-6736(94)92087-7
15. Bhak RH, Wininger M, Johnson GR, Lederle FA, Messina LM, Ballard DJ, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA. (2015) 150:44–50. doi: 10.1001/jamasurg.2014.2025
16. Behr-Rasmussen C, Grøndal N, Bramsen MB, Thomsen MD, Lindholt JS. Mural thrombus and the progression of abdominal aortic aneurysms: a large population-based prospective cohort study. Eur J Vasc Endovasc Surg. (2014) 48:301–7. doi: 10.1016/j.ejvs.2014.05.014
17. Lindholt JS, Heickendorff L, Antonsen S, Fasting H, Henneberg EW. Natural history of abdominal aortic aneurysm with and without coexisting chronic obstructive pulmonary disease. J Vasc Surg. (1998) 28:226–33. doi: 10.1016/S0741-5214(98)70158-2
18. Schlösser FJ, Tangelder MJ, Verhagen HJ, van der Heijden GJ, Muhs BE, van der Graaf Y, et al. Growth predictors and prognosis of small abdominal aortic aneurysms. J Vasc Surg. (2008) 47:1127–33. doi: 10.1016/j.jvs.2008.01.041
19. Vammen S, Lindholt JS, Ostergaard L, Fasting H, Henneberg EW. Randomized double-blind controlled trial of roxithromycin for prevention of abdominal aortic aneurysm expansion. Br J Surg. (2001) 88:1066–72 doi: 10.1046/j.0007-1323.2001.01845.x
20. Päivänsalo MJ, Merikanto J, Jerkkola T, Savolainen MJ, Rantala AO, Kauma H, et al. Effect of hypertension and risk factors on diameters of abdominal aorta and common iliac and femoral arteries in middle-aged hypertensive and control subjects: a cross-sectional systematic study with duplex ultrasound. Atherosclerosis. (2000) 153:99–106. doi: 10.1016/S0021-9150(00)00374-9
21. Vardulaki KA, Walker NM, Day NE, Duffy SW, Ashton HA, Scott RA. Quantifying the risks of hypertension, age, sex, and smoking in patients with abdominal aortic aneurysm. Br J Surg. (2000) 87:195–200. doi: 10.1046/j.1365-2168.2000.01353.x
22. Patel AS, Mackey RH, Wildman RP, Thompson T, Matthews K, Kuller L, et al. Cardiovascular risk factors associated with enlarged diameter of the abdominal aortic and iliac arteries in healthy women. Atherosclerosis. (2005) 178:311–7. doi: 10.1016/j.atherosclerosis.2004.08.026
23. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. (2018) 67:2–77. doi: 10.1016/j.jvs.2017.10.044
24. Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Editor's choice - european society for vascular surgery (esvs) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. (2019) 57:8–93. doi: 10.1016/j.ejvs.2018.09.020
25. Golledge J, Jayalath R, Oliver L, Parr A, Schurgers L, Clancy P. Relationship between ct anthropometric measurements, adipokines and abdominal aortic calcification. Atherosclerosis. (2008) 197:428–34. doi: 10.1016/j.atherosclerosis.2007.06.027
26. Parr A, McCann M, Bradshaw B, Shahzad A, Buttner P, Golledge J. Thrombus volume is associated with cardiovascular events and aneurysm growth in patients who have abdominal aortic aneurysms. J Vasc Surg. (2011) 53:28–35. doi: 10.1016/j.jvs.2010.08.013
27. Parr A, Buttner P, Shahzad A, Golledge J. Relation of infra-renal abdominal aortic calcific deposits and cardiovascular events in patients with peripheral artery disease. Am J Cardiol. (2010) 105:895–9. doi: 10.1016/j.amjcard.2009.10.067
28. Golledge J, Cronin O, Iyer V, Bradshaw B, Moxon JV, Cunningham MA. Body mass index is inversely associated with mortality in patients with peripheral vascular disease. Atherosclerosis. (2013) 229:549–55. doi: 10.1016/j.atherosclerosis.2013.04.030
29. Gabb GM, Mangoni AA, Anderson CS, Cowley D, Dowden JS, Golledge J, et al. Guideline for the diagnosis and management of hypertension in adults - 2016. Med J Aust. (2016) 205:85–9. doi: 10.5694/mja16.00526
30. Golledge J, Karan M, Moran CS, Muller J, Clancy P, Dear AE, et al. Reduced expansion rate of abdominal aortic aneurysms in patients with diabetes may be related to aberrant monocyte–matrix interactions. Eur Heart J. (2008) 29:665–72. doi: 10.1093/eurheartj/ehm557
31. Ferguson CD, Clancy P, Bourke B, Walker PJ, Dear A, Buckenham T, et al. Association of statin prescription with small abdominal aortic aneurysm progression. Am Heart J. (2010) 159:307–13. doi: 10.1016/j.ahj.2009.11.016
32. Golledge J, Moxon J, Pinchbeck J, Anderson G, Rowbotham S, Jenkins J, et al. Association between metformin prescription and growth rates of abdominal aortic aneurysms. Br J Surg. (2017) 104:1486–93. doi: 10.1002/bjs.10587
33. Norman P, Spencer CA, Lawrence-Brown MM, Jamrozik K. C-reactive protein levels and the expansion of screen-detected abdominal aortic aneurysms in men. Circulation. (2004) 110:862–6. doi: 10.1161/01.CIR.0000138746.14425.00
34. Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the european society for vascular surgery. Eur J Vasc Endovasc Surg. (2011) 41(Suppl 1:S1–S58). doi: 10.1016/j.ejvs.2010.09.011
35. Baxter BT, Matsumura J, Curci J, McBride R, Blackwelder WC, Liu X, et al. Non-invasive treatment of abdominal aortic aneurysm clinical trial (n-ta(3)ct): Design of a phase iib, placebo-controlled, double-blind, randomized clinical trial of doxycycline for the reduction of growth of small abdominal aortic aneurysm. Contemp Clin Trials. (2016) 48:91–8. doi: 10.1016/j.cct.2016.03.008
36. Moxon JV, Jones RE, Norman PE, Clancy P, Flicker L, Almeida OP, et al. Plasma ferritin concentrations are not associated with abdominal aortic aneurysm diagnosis, size or growth. Atherosclerosis. (2016) 251:19–24. doi: 10.1016/j.atherosclerosis.2016.05.022
37. Pinheiro J, Bates D, DebRoy SS, Sarkar D. Nlme: linear and non-linear mixed effects models. R Package Version 31-110. (2013) 3:1–113.
38. Sweeting M, Thompson S, Brown L, Powell J. RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. (2012) 99:655–65. doi: 10.1002/bjs.8707
39. Golledge J, Moxon JV, Singh TP, Bown MJ, Mani K, Wanhainen A. Lack of an effective drug therapy for abdominal aortic aneurysm. J Intern Med. (2020) 288:6–22. doi: 10.1111/joim.12958
40. Takagi H, Umemoto T. Association of hypertension with abdominal aortic aneurysm expansion. Ann Vasc Surg. (2017) 39:74–89. doi: 10.1016/j.avsg.2016.04.019
Keywords: abdominal aortic aneurysm, systolic blood pressure, diastolic blood pressure, hypertension, AAA growth
Citation: Thomas Manapurathe D, Moxon JV, Krishna SM, Quigley F, Bourke M, Bourke B, Jones RE and Golledge J (2022) Cohort Study Examining the Association of Optimal Blood Pressure Control at Entry With Infrarenal Abdominal Aortic Aneurysm Growth. Front. Cardiovasc. Med. 9:868889. doi: 10.3389/fcvm.2022.868889
Received: 03 February 2022; Accepted: 24 March 2022;
Published: 03 May 2022.
Edited by:
Hiroki Aoki, Kurume University, JapanReviewed by:
Koichi Yoshimura, Yamaguchi University, JapanCopyright © 2022 Thomas Manapurathe, Moxon, Krishna, Quigley, Bourke, Bourke, Jones and Golledge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan Golledge, Sm9uYXRoYW4uR29sbGVkZ2VAamN1LmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.