- 1Department of Cardiology, The Heart Center Rigshospitalet, Copenhagen, Denmark
- 2Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
- 3Department of Forensic Genetics, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
- 4Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
- 5Cardiovascular Research Unit, University of Southern Denmark, Svendborg, Denmark
Introduction: Increased left ventricular mass (LVM) is one of the most powerful predictors of adverse cardiovascular events. Clinical evaluation requires reliable, accurate and reproducible echocardiographic LVM-quantification to manage patients. For this purpose, we have developed a novel two-dimensional (2D) method based on adding the mean wall thickness to the left ventricular volume acquired by the biplane method of disks, which has recently been validated in humans using cardiac magnetic resonance as reference value. We assessed the hypothesis that the novel method has better accuracy than conventional one-dimensional (1D) methods, when compared to necropsy LVM in pigs.
Materials and Methods: Echocardiography was performed during anesthesia in 34 Danish Landrace pigs, weight 47–59 kg. All pigs were euthanized, cardiac necropsy was performed and the left ventricle was trimmed and weighed for necropsy LVM. Trans-thoracic echocardiography was applied for parasternal images. Transdiaphragmal echocardiography was applied for the apical images, which are otherwise difficult to obtain in pigs. We compared the conventional 1D- and 2D-methods and the novel 2D-method to the LVM from cardiac necropsy.
Results: Necropsy LVM was 132 ± 11 g (mean ± SD). The novel method had better accuracy than other methods (mean difference ± 95% limits of agreement; coefficients of variation; standard error of the estimate, Pearson's correlation). Novel (−1 ± 20 g; 8%; 11 g; r = 0.70), Devereux (+26 ± 37 g; 15%; 33 g; r = 0.52), Area-Length (+27 ± 34 g; 13 %; 33 g; r = 0.63), Truncated Ellipsoid (+10 ± 30 g; 12%; 19 g; r = 0.63), biplane endo-/epicardial tracing (−3 ± 2 g; 10%; 14 g; r = 0.57). No proportional bias in linear regression was detected for any method, when compared to necropsy LVM.
Conclusion: We confirm high accuracy of the novel 2D-based method compared to conventional 1D/2D-methods.
Introduction
Increased left ventricular mass (LVM) is a well-known strong and independent predictor of adverse cardiovascular events and sudden death (1–4). The natural adaptive mechanisms of almost every cardiac condition are reflected in the degree of left ventricular (LV) hypertrophy. Increased LVM is associated with LV fibrosis (5) and reduction in LVM by blood pressure management (6) or valvular surgery (7) is associated with better outcome (6). For this reason, LVM has the potential to be used as a prognostic marker to detect clinical deterioration and may facilitate decision making for clinicians. Unfortunately, recommended conventional one-dimensional (1D) linear echocardiographic methods are less accurate and not suited for individual usage (8). Consequently, LVM quantification is often not performed or ignored and not included in the individual clinical decision-making.
We have recently presented a novel two-dimensional (2D) method based on adding the mean wall thickness to the left ventricular volume acquired by the biplane method of disks (9). The method is validated in humans using cardiac magnetic resonance (CMR) as gold standard. Furthermore, it is simpler but still as accurate as three-dimensional (3D) echocardiography and compared to the other 1D/2D/3D-methods it performs better regardless of LV geometry. The novel 2D-based method also demonstrated better reproducibility compared to the other methods, which is necessary for detection of small differences that may indicate early signs of deterioration.
Our aim was to assess whether the novel method has better accuracy than conventional 1D methods, when compared to necropsy LVM in pigs.
Materials and Methods
Study Population
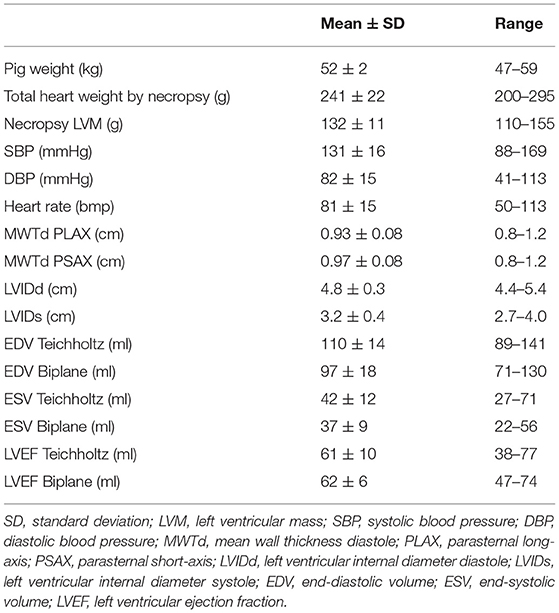
We included thirty-four female Danish Landrace pigs, weight range 47–59 kg. All pigs were part of a project investigating arrhythmias during myocardial infarction and echocardiography was performed as part of the protocol (10, 11). The experiments were performed under the animal license number (2015-15-0201-00613) authorized by the Danish Animal Inspectorate in accordance with EU legislations for animal protection and care.
Procedure
The pigs were premedicated, intubated and ventilated and anesthesia was maintained with continuous propofol infusion of 12.5 mg/h/kg (Propolipid 10 mg/ml, Fresenius Kabi AB, Uppsala, Sweden) and fentanyl 5 μg/h/kg (Fentanyl-Hameln 50 μg/ml, Hameln, Germany). Echocardiography was performed at baseline after placing a pulmonary artery catheter but before any other intervention. As part of the initial protocols, myocardial infarction was induced by balloon occlusion of the left anterior descending (LAD) artery just after the take-off of the first diagonal (D1) branch for 60–120 min and electrophysiological outcomes were studied. Pigs were euthanized at the end of the procedure by inducing ventricular fibrillation (VF) via burst pacing (50 Hz, 3 s, 7 V output) and cardiac necropsy was performed minutes after.
Echocardiographic Acquisition and Analysis
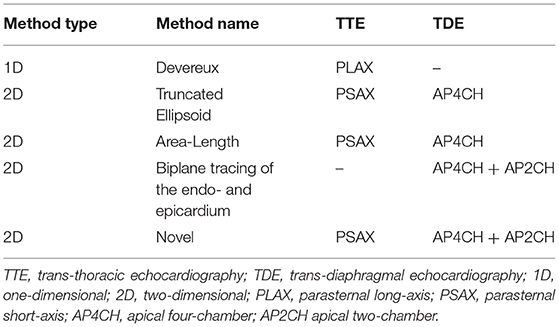
Echocardiographic examinations were performed using an iE33 Echocardiography System scanner (Philips Medical Systems Nederlands B.V., Best, The Netherlands) with the pigs in supine position. The protocol for echocardiographic assessment included four views; the parasternal long-axis view (PLAX), the parasternal short-axis view (PSAX), the apical four-chamber view (A4CH) and the apical two-chamber view (A2CH). PLAX and PSAX were acquired by trans-thoracic echocardiography (TTE) using the S5-1-xMATRIX array transducer (1–5 MHz). A4CH and A2CH were acquired by trans-diaphragmal echocardiography (TDE) (Figure 1) through a small midline incision distal of the sternal xiphoid (12). The TDE approach was necessary to achieve appropriate images as the heart is aligned differently in the thorax of pigs compared to humans (13). For the apical views we used the Pediatric X7-2-xMATRIX array transducer (2–7 MHz) and because of limited space in the acoustic window we applied electronic rotation function iRotate, instead of physical rotation of the probe. All images were transferred as Digital Imaging and Communications in Medicine (DICOM) files to a local workstation and analyzed using the software EchoPAC Version: 203 Revision: 66.0 (GE Healthcare Vingmed Ultrasound, Horten, Norway). End-diastole was defined as the first frame with closure of the mitral valve. End-systole was defined as the frame with the smallest LV volume. End-diastolic volume (EDV), end-systolic volume (ESV) and left ventricular ejection fraction (LVEF) was evaluated by the 1D Teichholtz method (14) and by the 2D biplane methods of disks (8). We evaluated five different methods for LVM quantification, all presented in Table 1. All LVM-measurements were made at end-diastole. The electrocardiogram (ECG) was applied as reference in PLAX to find the corresponding frame in PSAX, where the mitral valve is not fully visible. Three of the methods; Devereux (15), Truncated Ellipsoid (TE) (16) and Area-Length (A-L) (17) are well-recognized and described in the current echocardiographic guidelines (8). For the biplane tracing of the endo- and epicardium we traced both the endo- and the epicardium, subtracted the inner volume (tracing of the endocardium) with the outer volume (tracing of the epicardium) to achieve the myocardial volume. The novel method (Figure 2) recently described (9) is validated in humans using CMR. The method adds the LV wall thickness from PSAX to EDV acquired by tracing of the endocardium in the biplane model, i.e., no tracing of the epicardium is necessary. The myocardial density/gravity of 1.05 g/ml was applied to convert myocardial volume to LVM. All measurements were performed by one reader (CK). Intra-reader analysis was performed by the same reader (CK) on same recordings and compared to the baseline measurements. Inter-reader analysis was performed by another reader (SS) on the same recordings and compared to the baseline measurements. For intra/inter-reader variability we only included pigs who had feasible measurements for all methods.

Figure 1. Trans-thoracic and trans-diaphragmal echocardiographic approach (12).
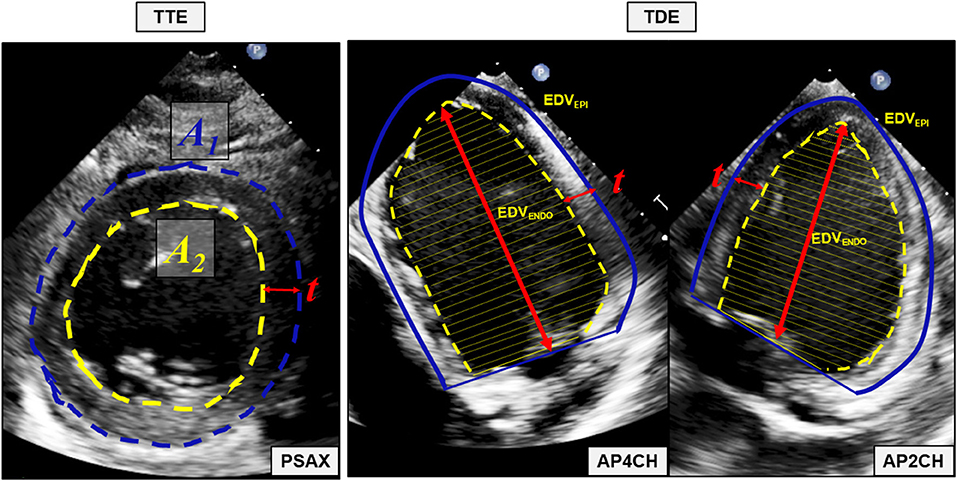
Figure 2. The novel 2D-based echocardiographic method for quantification of left ventricular mass. Mean wall thickness (t) is calculated by tracing of the endocardium (A2) and the epicardium (A1) in the parasternal short axis (PSAX) view. The left ventricular volume defined by the endocardium (EDVENDO) is acquired using the biplane methods of disks with tracings of the endocardium in the apical four chamber (AP4CH) and apical two-chamber (AP2CH) view. Mean wall thickness (t) is added to each unique disk and a new volume, the left ventricular volume defined by the defined by the epicardium (EDVEPI) is quantified. Myocardial volume is calculated by subtracting EDVENDO from EDVEPI and left ventricular mass is quantified by multiplying the myocardial volume with the myocardial density/gravity of 1.05 g/ml. TTE transthoracic echocardiography, TDE, transdiaphragmatic echocardiography; PSAX, parasternal short axis view; A1, Area defined by the epicardium; A2, Area defined by the endocardium; t, mean wall thickness; AP4CH, apical four chamber view; EDVENDO, the left ventricular volume defined by the endocardium; EDVEPI, the left ventricular volume defined by the epicardium; AP2CH, apical two-chamber view.
Necropsy
At the end of the experiment, ~10 min after VF was induced, a midline sternotomy was performed, the pericardium was removed and the heart including both ventricles and atria was explanted. If present, epicardial fat (which in general is scarce in young, lean pigs) and soft tissue was removed, and total heart weight measured. For measuring LVM the free wall of the right ventricle as well as valves, atria, papillary muscles and blood clots were removed. We used a commonly available digital scale (Wedo Electronic Precision Scale Optimo 1000, Werner Dorsch GmbH, Dieburg, Germany).
Statistics
Statistical data analysis was performed in SPSS v25.0 (IBM Corp. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY). LVM-quantifications were performed in Windows Excel 2010 (Microsoft Office Professional Plus). Continuous variables expressed as mean and standard deviation (SD) and categorical values expressed as frequencies and percentage. Correlation was evaluated using Pearson's r. The accuracy was evaluated by paired t-test and presented as mean difference (bias) and 95% limits of agreement (LOA). The variations were expressed as the standard error of the estimate (SEE) and as coefficients of variation (CV) in percent and we adjusted the CV according to the anatomical LVM from necropsy. In the same manner we plotted the differences according to necropsy LVM. We decided to use this approach because we considered the necropsy LVM as the true value and not a method for comparison. Proportional bias was evaluated by linear regression with the necropsy LVM as independent variable and mean difference as dependent variable. Intra- and inter-reader variability was expressed as bias, LOA, SEE and CV compared to the baseline measurements. P-values <0.05 were considered statistically significant.
Results
The baseline characteristics for the pigs are presented in Table 2. The total mass of the hearts by necropsy ranged from 200 to 295 g and the necropsy LVM ranged from 110 to 155 g. The hearts were relatively uniform in geometry and no pig had significant valve disease. Induced infarcts affected 15–20% of LV by visual inspection. LVEF by the biplane model of disks was 62 ± 6% (mean ± SD) and ranged from 47 to 74%.
Figure 3 demonstrated the mean difference between the quantified LVM and the necropsy LVM (y-axis) according to the necropsy LVM (x-axis) (left panel) and correlation for LVM quantified by echocardiography (y-axis) according to necropsy LVM (x-axis) (right panel) for the various methods for the whole population. The novel model presented the lowest mean difference and the smallest variation than any of the other methods; CV was 8% compared to 10–13% for the other 2D-methods and 15% for the cube formula by Devereux. No method presented significant proportional bias in linear regression. The novel method had the best correlation to necropsy LVM; Pearson's r = 0.70, p < 0.001 followed by A-L and TE, both r = 0.63, p < 0.001. The results for all pigs (n = 34) are also presented in Table 3A.
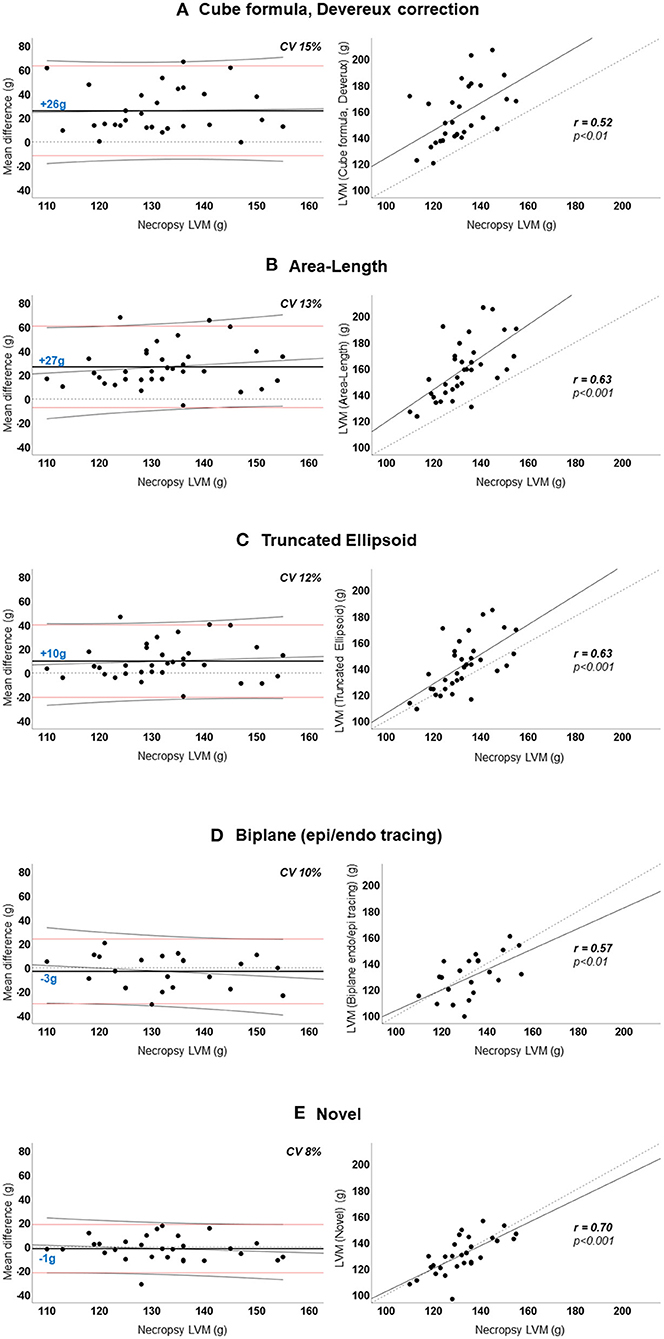
Figure 3. (A–E) Agreement between echocardiographic left ventricular mass and necropsy left ventricular mass. Left panel: Agreement between mean difference (echocardiographic-LVM – necropsy-LVM) and necropsy-LVM. Horizontal dotted black line indicates 0 (no difference). Horizontal solid black line and blue number is the mean difference; positive value indicates overestimation by echocardiography. Horizontal red lines are the 95% limits of agreement. Diagonal thin black line is the regression line with 95% confidence interval visualizing the proportional bias. Right panel: Linear regression curves of echocardiographic-LVM and necropsy-LVM with Pearson's correlation coefficient (r). Diagonal dotted line is the reference line. LVM, left ventricular mass; CV, coefficient of variation.
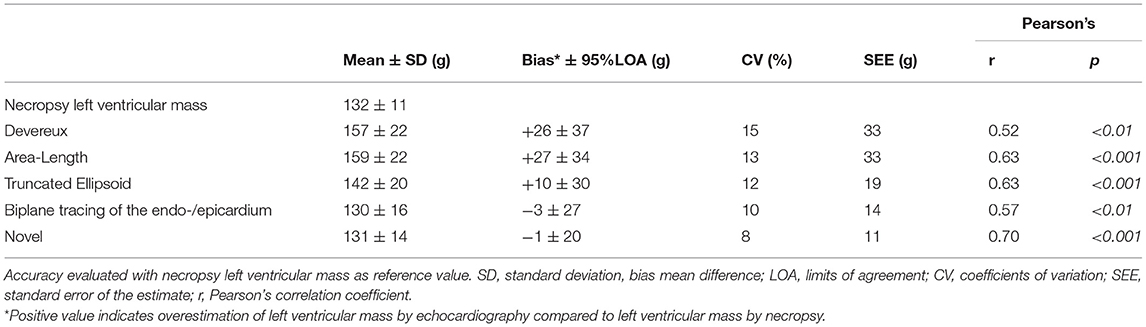
Table 3A. Accuracy of various methods for left ventricular mass quantification among all pigs (n = 34).
We performed a subgroup analysis of the pigs where it was possible to quantify LVM by all echocardiographic methods (n = 21). The results for this subgroup are presented in Table 3B. The percentual difference between echocardiographic LVM and necropsy LVM was plotted in the y-axis for each pig (Figure 4). The novel method was the most accurate method for 11 (52%) of the pigs, followed by TE and biplane tracing of the endo-/epicardium, both 4 (19%) of the pigs, respectively. Devereux was the least accurate method for 10 (48%) pigs followed by A-L for 7 (33%) of the pigs.
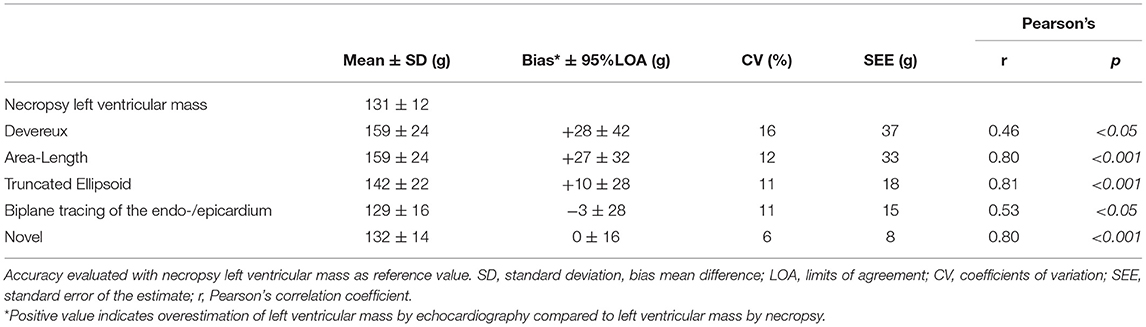
Table 3B. Accuracy of various methods for left ventricular mass quantification among the pigs with 100% feasible measurements (n = 21).
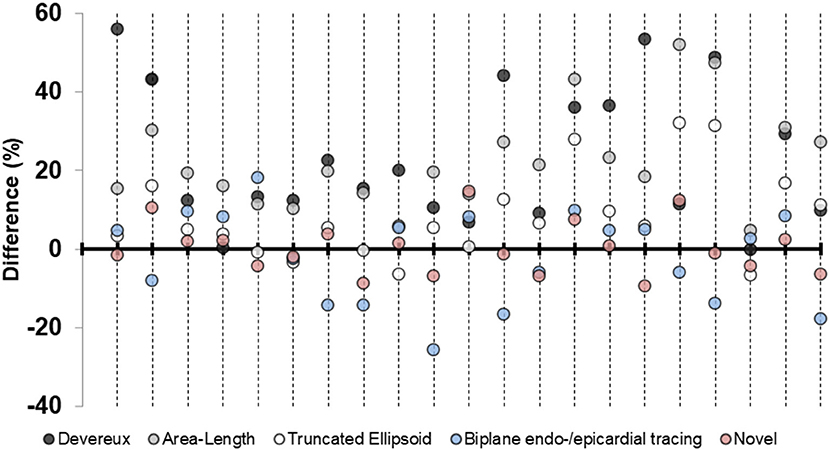
Figure 4. Percentage differences in left ventricular mass plotted for each pig. Differences in percent for the five echocardiographic methods for left ventricular mass-quantification for the subgroup of pigs (n = 21) where all measures were available. Positive value indicates overestimation by echocardiography compared to necropsy left ventricular mass. Each longitudinal line represents one pig.
Intra- and inter-reader variability is presented in Table 4 and we observe similar reproducibility as compared to the other 1D- and 2D-methods.
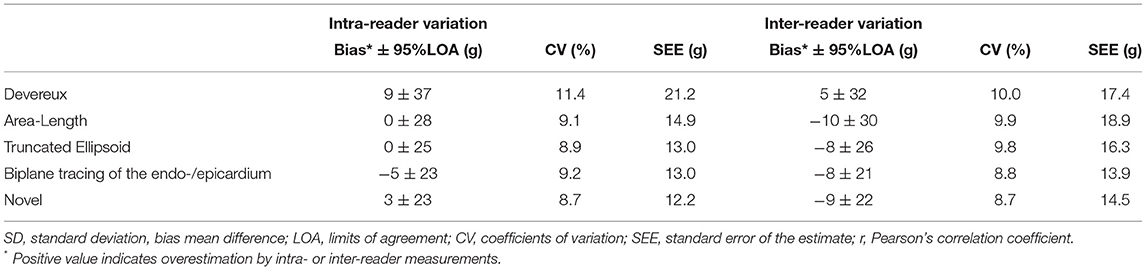
Table 4. Precision (reproducibility) of the various methods for left ventricular mass quantification among the pigs where all measures were available (n = 21).
Discussion
We demonstrate the accuracy of various echocardiographic methods for LVM-quantification in a porcine model with necropsy LVM as reference for LVM. The novelty of our approach is the study design combining necropsy validation with a novel echocardiographic method and improved imaging tools. The most important findings are the following:
1) The novel method for LVM-quantification is characterized by higher accuracy to LVM by necropsy and at least as good reproducibility as for the other 1D- and 2D-methods.
2) The conventional linear method by Devereux, which is recommended by current guidelines (8), overestimates LVM and demonstrates lower accuracy compared to the other methods.
3) We highlight an alternative way to increase echocardiographic apical image quality in animal models, by applying a trans-diaphragmatic approach and by applying the function iRotate since correct physical rotation of the probe may be difficult.
Comparison to Previous Necropsy Validation Studies
Most studies with necropsy comparisons are published several decades ago and are naturally based on M-mode without 2D-guiding, or by less refined 2D echocardiographic technology. In 1979 Wyatt et al. (17) demonstrated better correlation and lower mean errors deploying 2D methods compared to linear methods and Salcedo et al. (18) demonstrated improved accuracy using 2D-methods. In 1986 Schiller et al. (19) confirmed high correlation and low SEE using 2D-methods. Two other studies by Woythaler et al. (20) in 1983 and Park et al. (21) in 1996 could not illustrate any improvement in 2D-methods compared to linear methods. On the contrary, some studies demonstrate excellent accuracy of linear methods among subjects with normal LV geometry (22, 23) and some studies unsatisfactory accuracy despite normal LV geometry (24). A direct comparison to these studies may be misleading compared to current imaging technology and improved post-processing software. A recent study by Miyashita et al. (25) validating LVM to necropsy values in pigs, demonstrates overestimation of LVM using linear methods as 2D-guided M-mode, in particular among the pigs with ischemic heart disease and LV dilatation. The same study also demonstrates a much narrower LOA using 2D-methods, which is in line with our results. The strength of our study compared to previous necropsy validation studies is that we have examined a variety of different methods on the same population, which makes it easier to compare the methods with each other instead of one-by-one in different populations.
Aspects of Necropsy Validation as Reference for Left Ventricular Mass
What is the true value of LVM and how can it be measured? Previous studies have applied angiography, direct linear measures or echocardiography to validate LVM against cardiac autopsy in human models (15, 16, 20–24, 26–28) or cardiac necropsy in animal models (17–19, 29). Our validation methodology was to explant the heart of the pig immediately postmortem and to isolate the LV without papillary muscles and without any preservation. There are several aspects to consider when validating to the anatomical LVM by autopsy/necropsy. These are:
1) The time between measurement and actual autopsy/necropsy. Validation studies to human autopsy are usually limited by increased time duration between measurement and actual autopsy, whereas animal studies are usually validated at the same day, or at least preserved at the same day as the measurement by echocardiography was made. Human studies report durations of up to 454 days (28) between echocardiography and autopsy. As we performed necropsy immediately postmortem and at the same day as echocardiographic assessment, we were less limited by potential changes of the LV that may occur over time.
2) The preservation methodology. Formaldehyde or the aqueous solution of formaldehyde, also known as formalin, have often been applied as preservation methodology. This approach is reported to reduce LVM and volume without significant impact on myocardial density (30–32), whereas some studies report increased LVM after preservation (33). To overcome this potential limitation, several studies report adjustment of shrinkage by adding 5% to autopsy/necropsy LVM (23) or adjustment of increase by reducing autopsy/necropsy LVM by 2–3% (33). However, no adjustment at all (16, 24) is also reported. We did not use any preservation methodology and did not apply any adjustment to the weighed necropsy LVM.
3) The autopsy/necropsy methodology. Most studies report similar autopsy/necropsy methodology with removal of atria, right ventricle, epicardial fat and valves inspired by Geiser and Bove (16). In contrast to previous reports, we removed the papillary muscles from the myocardium. We claim that this approach is most truthful since the papillary muscles are excluded when LVM is measured by both echocardiography and CMR (9). We washed the hearts and removed visual blood clots and although we were very thorough, there may still be small clots left in the trabeculae or inside the vessels of the heart. The significance of this small amount of blood is probably negligible.
4) Inhibition of the cardiac cycle in diastole or systole. Cardiac arrest and eventually cardiac death was initiated by induced VF and consequently, most hearts were inhibited contracted, i.e., in systole. Inhibition in systole or diastole has impact on the volumes of the heart but should not affect the LVM significantly (16). This may even have minimized the effect of potential blood left in the vessels of the heart.
5) Edema or fibrosis affecting the density/gravity of the LV. Edema may be present in the ischemic region of the LV but also to some degree in adjacent and remote areas (34). During myocardial infarction, the density of the myocardium increases during initial edema and remains slightly higher after transition to fibrotic tissue compared to healthy myocardium (32). The pigs in our study were part of a protocol where myocardial infarction was induced by balloon occlusion of the LAD, which may have affected the density of the myocardium. Infarct sizes were determined to be within 15–20% of the LV myocardial mass.
Necropsy Compared to Gold Standard Cardiac Magnetic Resonance
Necropsy validation has gradually been replaced by imaging modalities such as CMR, which is now considered the gold standard or reference method for left ventricular mass (35, 36). Excellent agreement between LVM by necropsy and by CMR-segmentation has been reported (37–40). Studies using CMR as reference method report overestimation of LVM by linear methods (41, 42) and better agreement for the 2D-methods (43). We Kristensen et al. (9) have demonstrated similar overestimation for the 1D-methods and better agreement for 2D-methods, especially the novel method. Also, reproducibility from the whole cohort and accuracy of the patients with normal geometry were very similar to the accuracy for the pigs in this cohort, which all had normal geometry as well.
The Porcine and the Human Heart
The porcine heart is aligned differently in the thoracic cage compared to the human heart (13). In humans, the upright orientation of the body and the location of the heart in the thoracic cage, gives the heart a “trapezoidal shape” with its apex pointing downward to the left with an oblique angle to the long axis of the body. Since pigs walk on four legs and have differences in the shape of the thoracic cage, the heart is “Valentines heart” shaped with its apex pointing forward and perpendicular to the long axis of the body. In supine position, the heart will orientate with its axis pointing more downwards and toward the diaphragm. This facilitates parasternal images acquisition through transthoracic acquisition in pigs. However, correctly aligned apical views become more difficult to acquire. To be able to place the transducer as close to the apex of the heart as possible, we made a small incision below the sternum and placed the probe under the diaphragm for transdiaphragmatic acquisition, which improved the image quality significantly (12). Because of limited space within the incision and consequently difficulties in performing physical rotation of the probe, we also applied the iRotate application for optimal 4CH- and 2CH-view. We recommend this echocardiographic approach among research animals who are euthanized at the study day. Other potentially differences that have been described between the human and porcine hearts are a more conical morphology of the LV and the heart, coarser papillary muscles and thicker LV wall compared to humans (13). The wall thickness range in our cohort was 0.8–1.2 cm, which would be mildly abnormal if translated to human references (8).
Limitations
The pigs in this cohort did not have any structural or congenital heart disease and the geometrical pattern of the LV was considered normal and uniform for all pigs. The results from this study may not be applicable to deviating LV geometries. All pigs were subjected to acute myocardial infarction affecting 15–20% of the LV. Affected myocardium might have had higher weight due to edema. As echocardiography was performed before myocardial infarction, this could have resulted in a systematic error. We were not able to perform 3D echocardiography due to technical limitations such as, difficulties in pausing the respirator, reverberations and artifacts disturbing the images. As the purpose of this study was to validate echocardiographic method on pigs with the intention of usage on humans, geometrical differences between porcine and human hearts must be kept in mind.
Conclusions
We demonstrate necropsy validation in a porcine model of a recently presented 2D-based echocardiographic method for LVM-quantification. We confirm high accuracy of the novel 2D-based echocardiographic method compared to the other conventional 1D/2D echocardiographic methods.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the Danish Animal Inspectorate in accordance with EU legalisations for animal protection and care (animal license number 2015-15-0201-00613).
Author Contributions
CK, SS, JT-H, and RM planned the study. CK, SS, and AL conceived the study. CK performed the echocardiographic examinations, the data analysis, and wrote the manuscript. SS contributed to the inter-observer analysis. TJ and CH provided critical feedback. All authors discussed the results and contributed to the manuscript.
Funding
This work was funded by the Novo Nordisk Foundation Tandem Programme (TJ and JT-H), registered under Grant No. 31364.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med. (1990) 322:1561–6. doi: 10.1056/NEJM199005313222203
2. Casale PN, Devereux RB, Milner M, Zullo G, Harshfield GA, Pickering TG, et al. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med. (1986) 105:173–8. doi: 10.7326/0003-4819-105-2-173
3. Barbieri A, Bursi F, Mantovani F, Valenti C, Quaglia M, Berti E, et al. Prognostic impact of left ventricular mass severity according to the classification proposed by the American Society of Echocardiography/European Association of Echocardiography. J Am Soc Echocardiogr. (2011) 24:1383–91. doi: 10.1016/j.echo.2011.08.012
4. Narayanan K, Reinier K, Teodorescu C, Uy-Evanado A, Aleong R, Chugh H, et al. Left ventricular diameter and risk stratification for sudden cardiac death. J Am Heart Assoc. (2014) 3:e001193. doi: 10.1161/JAHA.114.001193
5. Charles CJ, Lee P, Li RR, Yeung T, Ibraham Mazlan SM, Tay ZW, et al. A porcine model of heart failure with preserved ejection fraction: magnetic resonance imaging and metabolic energetics. ESC Hear Fail. (2020) 7:92–102. doi: 10.1002/ehf2.12536
6. Koren MJ, Ulin RJ, Koren AT, Laragh JH, Devereux RB. Left ventricular mass change during treatment and outcome in patients with essential hypertension. Am J Hypertens. (2002) 15:1021–8. doi: 10.1016/S0895-7061(02)03061-3
7. Guenzinger R, Wildhirt SM, Voegele K, Wagner I, Schwaiger M, Bauernschmitt R, et al. Comparison of magnetic resonance imaging and transthoracic echocardiography for the identification of LV mass and volume regression indices 6 months after mitral valve repair. J Card Surg. (2008) 23:126–32. doi: 10.1111/j.1540-8191.2007.00558.x
8. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
9. Kristensen CB, Myhr KA, Grund FF, Vejlstrup N, Hassager C, Mogelvang R. Quantification of left ventricular mass using two-dimensional transthoracic echocardiography - a novel method with high accuracy and reproducibility. Research Square [Preprint]. (2021). doi: 10.21203/rs.3.rs-1139410/v1
10. Sattler SM, Lubberding AF, Kristensen CB, Møgelvang R, Blanche P, Fink-Jensen A, et al. Effect of the antipsychotic drug haloperidol on arrhythmias during acute myocardial infarction in a porcine model. Int J Cardiol Hear Vasc. (2019) 26:100455. doi: 10.1016/j.ijcha.2019.100455
11. Lubberding AF, Sattler SM, Grunnet M, Sørensen US, Tfelt-Hansen J, Jespersen T. Arrhythmia development during inhibition of small-conductance calcium-activated potassium channels in acute myocardial infarction in a porcine model. Europace. (2019) 21:1584–93. doi: 10.1093/europace/euz223
12. Citerni C, Kirchhoff J, Olsen LH, Sattler SM, Gentilini F, Forni M, et al. Characterization of atrial and ventricular structural remodeling in a porcine model of atrial fibrillation induced by atrial tachypacing. Front Vet Sci. (2020) 7:179. doi: 10.3389/fvets.2020.00179
13. Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat. (1998) 193:105–19. doi: 10.1046/j.1469-7580.1998.19310105.x
14. Teichholz LE, Kreulen T, Herman M V, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. (1976) 37:7–11. doi: 10.1016/0002-9149(76)90491-4
15. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. (1977) 55:613–8. doi: 10.1161/01.CIR.55.4.613
16. Geiser EA, Bove KE. Calculation of left ventricular mass and relative wall thickness. Arch Pathol. (1974) 97:13–21.
17. Wyatt HL, Heng MK, Meerbaum S, Hestenes JD, Cobo JM, Davidson RM, et al. Cross-sectional echocardiography. I. Analysis of mathematic models for quantifying mass of the left ventricle in dogs. Circulation. (1979) 60:1104–13. doi: 10.1161/01.CIR.60.5.1104
18. Salcedo EE, Gockowski K, Tarazi RC. Left ventricular mass and wall thickness in hypertension. Comparison of M mode and two dimensional echocardiography in two experimental models. Am J Cardiol. (1979) 44:936–40. doi: 10.1016/0002-9149(79)90225-X
19. Schiller NB, Skioldebrand CG, Schiller EJ, Mavroudis CC, Silverman NH, Rahimtoola SH, et al. Canine left ventricular mass estimation by two-dimensional echocardiography. Circulation. (1983) 68:210–6. doi: 10.1161/01.CIR.68.1.210
20. Woythaler JN, Singer SL, Kwan OL, Meltzer RS, Reubner B, Bommer W, et al. Accuracy of echocardiography versus electrocardiography in detecting left ventricular hypertrophy: comparison with postmortem mass measurements. J Am Coll Cardiol. (1983) 2:305–11. doi: 10.1016/S0735-1097(83)80167-3
21. Park SH, Shub C, Nobrega TP, Bailey KR, Seward JB. Two-dimensional echocardiographic calculation of left ventricular mass as recommended by the American Society of Echocardiography: correlation with autopsy and M-mode echocardiography. J Am Soc Echocardiogr. (1996) 9:119–28. doi: 10.1016/S0894-7317(96)90019-X
22. Reichek N, Helak J, Plappert T, Sutton MS, Weber KT. Anatomic validation of left ventricular mass estimates from clinical two-dimensional echocardiography: initial results. Circulation. (1983) 67:348–52. doi: 10.1161/01.CIR.67.2.348
23. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. (1986) 57:450–8. doi: 10.1016/0002-9149(86)90771-X
24. Bachenberg TC, Shub C, Hauck AJ, Edwards WD. Can anatomical left ventricular mass be estimated reliably by m-mode echocardiography? A clinicopathological study of ninety-three patients. Echocardiography. (1991) 8:9–15. doi: 10.1111/j.1540-8175.1991.tb01399.x
25. Miyashita S, Hammoudi N, Watanabe S, Bikou O, Yamada K, Aguero J, et al. Echocardiographic left ventricular mass estimation: two-dimensional area-length method is superior to M-mode linear method in swine models of cardiac diseases. J Cardiovasc Transl Res. (2020) 13:648–58. doi: 10.1007/s12265-019-09937-7
26. Troy BL, Pombo J, Rackley CE. Measurement of left ventricular wall thickness and mass by echocardiography. Circulation. (1972) 45:602–11.
27. Daniels SR, Meyer RA, Liang Y, Bove KE. Echocardiographically determined left ventricular mass index in normal children, adolescents and young adults. J Am Coll Cardiol. (1988) 12:703–8. doi: 10.1016/S0735-1097(88)80060-3
28. Gopal AS, Schnellbaecher MJ, Shen Z, Akinboboye OO, Sapin PM, King DL. Freehand three-dimensional echocardiography for measurement of left ventricular mass: in vivo anatomic validation using explanted human hearts. J Am Coll Cardiol. (1997) 30:802–10. doi: 10.1016/S0735-1097(97)00198-8
29. Schmidt MA, Freidlin RZ, Ohazama CJ, Jones M, Laurienzo JM, Brenneman CL, et al. Anatomic validation of a novel method for left ventricular volume and mass measurements with use of real-time 3-dimensional echocardiography. J Am Soc Echocardiogr. (2001) 14:1–10. doi: 10.1067/mje.2001.108132
30. Fox CH, Johnson FB, Whiting J, Roller PP. Formaldehyde fixation. J Histochem Cytochem. (1985) 33:845–53. doi: 10.1177/33.8.3894502
31. Leonard KC, Worden N, Boettcher ML, Dickinson E, Hartstone-Rose A. Effects of freezing and short-term fixation on muscle mass, volume, and density. Anat Rec. (2021) 305:199–208. doi: 10.1002/ar.24639
32. Ford WR, Menon V, Bhambhani A, Liyanage R, Khan MI, Jugdutt BI. Changes in myocardial density during postinfarction healing: effect on estimation of in vivo left ventricular mass by echocardiographic imaging. Can J Physiol Pharmacol. (1997) 75:1075–82. doi: 10.1139/y97-132
33. Kennedy JW, Reichenbach DD, Baxley WA, Dodge HT. Left ventricular mass. A comparison of angiocardiographic measurements with autopsy weight. Am J Cardiol. (1967) 19:221–3. doi: 10.1016/0002-9149(67)90536-X
34. Pahlm US, Ubachs JFA, Heiberg E, Engblom H, Erlinge D, Götberg M, et al. Regional wall function before and after acute myocardial infarction; an experimental study in pigs. BMC Cardiovasc Disord. (2014) 14:118. doi: 10.1186/1471-2261-14-118
35. Myerson SG, Bellenger NG, Pennell DJ. Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension. (2002) 39:750–5. doi: 10.1161/hy0302.104674
36. Alfakih K, Bloomer T, Bainbridge S, Bainbridge G, Ridgway J, Williams G, et al. A comparison of left ventricular mass between two-dimensional echocardiography, using fundamental and tissue harmonic imaging, and cardiac MRI in patients with hypertension. Eur J Radiol. (2004) 52:103–9. doi: 10.1016/j.ejrad.2003.09.015
37. François CJ, Fieno DS, Shors SM, Finn JP. Left ventricular mass: manual and automatic segmentation of true FISP and FLASH cine MR images in dogs and pigs. Radiology. (2004) 230:389–95. doi: 10.1148/radiol.2302020761
38. Simprini LA, Goyal P, Codella N, Fieno DS, Afroz A, Mullally J, et al. Geometry-independent inclusion of basalmyocardium yields improved cardiacmagnetic resonance agreement with echocardiography and necropsy quantified left-ventricular mass. J Hypertens. (2013) 31:2069–76. doi: 10.1097/HJH.0b013e328362d935
39. Codella NCF, Lee HY, Fieno DS, Chen DW, Hurtado-Rua S, Kochar M, et al. Improved left ventricular mass quantification with partial voxel interpolation in vivo and necropsy validation of a novel cardiac MRI segmentation algorithm. Circ Cardiovasc Imaging. (2012) 5:137–46. doi: 10.1161/CIRCIMAGING.111.966754
40. Farber NJ, Reddy ST, Doyle M, Rayarao G, Thompson D V, Olson P, et al. Ex vivo cardiovascular magnetic resonance measurements of right and left ventricular mass compared with direct mass measurement in excised hearts after transplantation: a first human SSFP comparison. J Cardiovasc Magn Reson. (2014) 16:74. doi: 10.1186/s12968-014-0074-0
41. Stewart GA, Foster J, Cowan M, Rooney E, McDonagh T, Dargie HJ, et al. Echocardiography overestimates left ventricular mass in hemodialysis patients relative to magnetic resonance imaging. Kidney Int. (1999) 56:2248–53. doi: 10.1046/j.1523-1755.1999.00786.x
42. Bellenger NG, Marcus NJ, Davies C, Yacoub M, Banner NR, Pennell DJ. Left ventricular function and mass after orthotopic heart transplantation: a comparison of cardiovascular magnetic resonance with echocardiography. J Heart Lung Transplant. (2000) 19:444–52. doi: 10.1016/S1053-2498(00)00079-6
43. Takeuchi M, Nishikage T, Mor-Avi V, Sugeng L, Weinert L, Nakai H, et al. Measurement of left ventricular mass by real-time three-dimensional echocardiography: validation against magnetic resonance and comparison with two-dimensional and m-mode measurements. J Am Soc Echocardiogr. (2008) 21:1001–5. doi: 10.1016/j.echo.2008.07.008
Keywords: left ventricular mass, echocardiography, left ventricular hypertrophy, necropsy, animal model
Citation: Kristensen CB, Sattler SM, Lubberding AF, Tfelt-Hansen J, Jespersen T, Hassager C and Mogelvang R (2022) Necropsy Validation of a Novel Method for Left Ventricular Mass Quantification in Porcine Transthoracic and Transdiaphragmal Echocardiography. Front. Cardiovasc. Med. 9:868603. doi: 10.3389/fcvm.2022.868603
Received: 03 February 2022; Accepted: 31 March 2022;
Published: 03 May 2022.
Edited by:
Leonid Goubergrits, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Christoph Sinning, University Medical Center Hamburg-Eppendorf, GermanyYthan Goldberg, Albert Einstein College of Medicine, United States
Copyright © 2022 Kristensen, Sattler, Lubberding, Tfelt-Hansen, Jespersen, Hassager and Mogelvang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charlotte Burup Kristensen, Y2hhcmxvdHRlLmJ1cnVwLmtyaXN0ZW5zZW5AcmVnaW9uaC5kaw==
 Charlotte Burup Kristensen
Charlotte Burup Kristensen Stefan Michael Sattler
Stefan Michael Sattler Anniek Frederike Lubberding
Anniek Frederike Lubberding Jacob Tfelt-Hansen
Jacob Tfelt-Hansen Thomas Jespersen
Thomas Jespersen Christian Hassager1,4
Christian Hassager1,4