
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med., 07 April 2022
Sec. Cardiac Rhythmology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.865036
Background: Conflicting findings of the association between serum uric acid (SUA) and atrial fibrillation (AF) have been reported in both men and women. The sex-specific associations between SUA and the risk of AF are unclear, although hyperuricemia is independently associated with the risk of AF. We performed this meta-analysis to assess the sex-specific effect of SUA on the risk of AF.
Methods: The PubMed, EMBASE, and Cochrane Library databases were searched up to October 3, 2021, for studies that reported sex-specific associations of SUA levels with AF. Linear relationships were assessed by the generalized least squares trend estimation. This study was registered with PROSPERO (42020193013).
Results: Ten eligible studies with 814,804 participants (415,779 men and 399,025 women) were identified. In the category analysis, high SUA was associated with an increased risk of AF in both men (OR: 1.42; 95% CI, 1.18–1.71, I2 = 34%) and women (OR: 2.02; 95% CI, 1.29–3.16, I2 = 70%). In the dose-response analysis, for each 60 μmol/L (1 mg/dL) increase in the SUA level, the risk of AF increased by 15% (OR: 1.15; 95% CI, 1.07–1.25, I2 = 74%) in men and 35% (OR: 1.35; 95% CI, 1.18–1.53, I2 = 73%) in women. There was a borderline difference in the impact of SUA on the risk of AF between men and women (P for interaction = 0.05). A significant linear relationship between SUA and the risk of AF was observed in men (P for non-linearity = 0.91) and women (P for non-linearity = 0.92).
Conclusions: This study suggested that there was a significant linear relationship between SUA and the risk of AF among men and women, with a higher risk estimate for women. Additional trials are required to assess the effect of reduced SUA therapy on AF incidence.
Systematic Review Registration: https:www.crd.york.ac.uk/PROSPERO/, identifier: CRD 42020193013.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and it is associated with an increased risk of stroke, thromboembolic events, heart failure and other comorbidities. Due to its high rate of disability and mortality, AF has become a worldwide health problem (1). It is well known that sex is an independent risk factor for AF development. Accumulating evidence indicates that AF affects men and women differently in all aspects, including clinical presentation, prognosis, and outcomes (2, 3). Some studies have suggested that women with AF had more comorbidities, were more often at the highest risk for stroke, and were more often symptomatic than men (4–6). Recently, a meta-analysis of 30 studies involving more than four million participants showed that the risk of cardiovascular disease and death associated with AF was twice as high in women compared with men (7).
The specific pathophysiological mechanisms of AF are not yet fully understood but are considered complex and multifactorial (8). Neurohormone activation, oxidative stress upregulation and immune activation jointly promote the occurrence of AF (9–11). Serum uric acid (SUA), as the final purine metabolite catalyzed by xanthine oxidoreductase (XO), reflects the degree of oxidative stress in vivo (12). Over the past two decades, numerous epidemiological studies have shown that elevated SUA levels are closely related to various diseases, including hypertension (13), coronary heart disease (14), cardiovascular disease (15, 16), obesity (17), diabetes (18), metabolic syndrome (19), liver dysfunction (20), and chronic kidney disease (21). Moreover, a variety of studies have demonstrated that a high SUA level is significantly associated with AF and may be a potential risk factor for AF (22–28).
SUA levels are affected by sex, and SUA levels in men are higher than those in women in general, although their SUA levels increase after menopause. More recently, several observational studies have evaluated sex-specific associations of SUA with incident AF, but the results were contradictory (29–38). For instance, the Scottish Heart Health Extended Cohort (SHHEC) study proposed that elevated SUA was associated more strongly with AF in women than in men (37), while the Atherosclerosis Risk in Communities (ARIC) study demonstrated that an independent association with SUA was only observed in women after adjusting for other cardiovascular risk factors (29). Considering that the potential sex-specific correlation between elevated SUA levels and AF would provide new insights into the etiology of AF, studies that have probed this issue and synthesized the conflicting evidence are definitely needed (39). However, no such studies have been carried out, and all previous meta-analyses only focused on the relationship between SUA and AF, rather than the sex-specific association (26–28).
Therefore, we performed a meta-analysis to evaluate whether there are sex-related differences in the association of SUA levels with the risk of AF.
We registered the protocol in PROSPERO (International prospective register of systematic reviews https:www.crd.york.ac.uk/PROSPERO/-registration number-CRD 42020193013). This meta-analysis was performed in accordance with the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (Supplementary Table S1) (40).
Database search, literature selection, quality assessment, and data extraction were carried out independently by two authors (J-H. X and W. S). Any disagreements were settled through discussions. The PubMed, EMBASE, and Cochrane Library databases were searched up to October 3, 2021 for all related studies. No restrictions were imposed on the language of the publications. The following MeSH words were used in our search strategies: (“uric acid” OR “urate” OR “hyperuricemia” OR “gout”) AND (“atrial fibrillation” OR “atrial flutter” OR “atrial tachycardias”).
The inclusion criteria were as follows: (1) reported sex-specific association of SUA levels with AF or reported for a specific sex; (2) risk estimates [risk ratio (RR), hazard ratio (HR), or odds ratio (OR)] and corresponding 95% CIs of the association between SUA and AF were reported. In addition, studies were excluded if they met the following conditions: (1) reviews, meta-analyses, congress abstracts, practice guidelines, patents, cases, editorials, replies, or comments; (2) the required data were not extractable even after contacting the corresponding authors for further information.
The data were collected into a predesigned standard spreadsheet. The following data were abstracted from each included study: study characteristics (first author's name, publication year, location, and study design), patient characteristics (type of population, sample size, age, percentage of men, follow-up duration and AF detection), and outcomes (number of events, adjusted OR/RRs and the corresponding 95% CI, and adjustments).
We used the Joanna Briggs Institute critical appraisal checklist to evaluate the quality of the cross-sectional studies (41). The Newcastle–Ottawa Scale (NOS) was applied to assess the quality of the case-control and cohort studies (42). The NOS scoring criteria included three aspects: (1) the selection of the subjects; (2) the comparability of the subjects; and (3) the clinical outcome or exposure. The total NOS score was 9, with a score of 7 or more considered high quality.
To examine the relationships between SUA level and the risk of AF among men and women, we pooled the ORs and the corresponding 95% CIs by using the inverse-variance method. The RRs and HRs were considered equivalent to ORs. For the category analysis, both patients with highest vs. lowest and hyperuricemia vs. normal was analyzed. Hyperuricemia was defined as a SUA level > 7 mg/dl in three studies (22, 25, 31) for men and >5.7 mg/dl in two studies (22, 31) for women.
We performed dose-response analysis and calculated study-specific slopes (linear trends) by using the method proposed by Greenland and Longnecker (43). Additionally, 95% CIs were calculated from the natural logs of the ORs and CIs across the SUA categories. In addition, we assessed the potential non-linear association between SUA and the risk of AF by using restricted cubic splines with 3 knots (44, 45). Heterogeneity among the included studies was evaluated by the χ2 test (with a P-value < 0.10 considered significant) and I2 test (25%, 50%, and 75% represent low, moderate and high heterogeneity, respectively) (46). ORs and the corresponding 95% CIs were pooled by using the random-effects model, considering the potential heterogeneity across studies.
Subgroup and meta-regression analyses were used to identify potential sources of heterogeneity among the analyses. Studies were stratified by the mean age of the participants; region; NOS scores; study design; sample size; population; and adjustments for important risk factors, including age, body mass index (BMI), smoking, history of hypertension, and diabetes. We performed a sensitivity analysis by omitting 1 study at a time to assess the robustness of the conclusions. The possibility of publication bias was assessed by funnel plots, Egger's test (47), and Begg's test (48). All analyses were conducted by using Stata 14.0 (Stata Corp LP, College Station, TX, USA) and Review Manager (RevMan) version 5.3 (The Cochrane Collaboration 2014; Nordic Cochrane Centre Copenhagen, Denmark). A p-value < 0.05 was considered significant.
The literature screening process is shown in Figure 1. The initial database search identified 632 records, and after screening the titles and abstracts, 31 articles remained for full text screening. Among the 31 articles selected for full-text review, we included 10 studies (29–38) and excluded 21 for the following reasons: (1) the studies did not report the target exposure (e.g., allopurinol, gout attack, etc., n = 5); (2) the studies did not report sex-specific outcomes (n = 10); (3) editorials or review articles (n = 5); and (4) univariate analysis (n = 1). The reasons for exclusion are listed in Supplementary Table S2.
The basic characteristics of all included studies are summarized in Table 1. Overall, 10 studies were included (29–38), with 814,804 participants (415,779 men and 399,025 women). The populations included in the studies ranged from 400 to 353,613 for the sample size, 4–22.6 years for follow-up, and 38–64 years for the mean or median age. Two studies were designed as cross-sectional (33, 34), 3 were retrospective cohorts (32, 36, 38), and 5 were prospective cohorts (29–31, 35, 37). Most studies were well adjusted for potential confounders. Six reports were from Asia (China = 3, Japan = 2, and Korean = 1) (32–36, 38), 3 from Europe (Italy = 1, Scotland = 1 and Norway = 1) (30, 31, 37), and 1 from the USA (29). In accordance with the Joanna Briggs Institute Critical Appraisal Checklist, 2 cross-sectional studies met all nine criteria (33, 34). which meant that these articles applied rigorous methodology (Supplementary Table S3). Based on the NOS, the quality of all of the included reports was high (29–32, 35–38), with a score range of 7–9 (Supplementary Table S4).
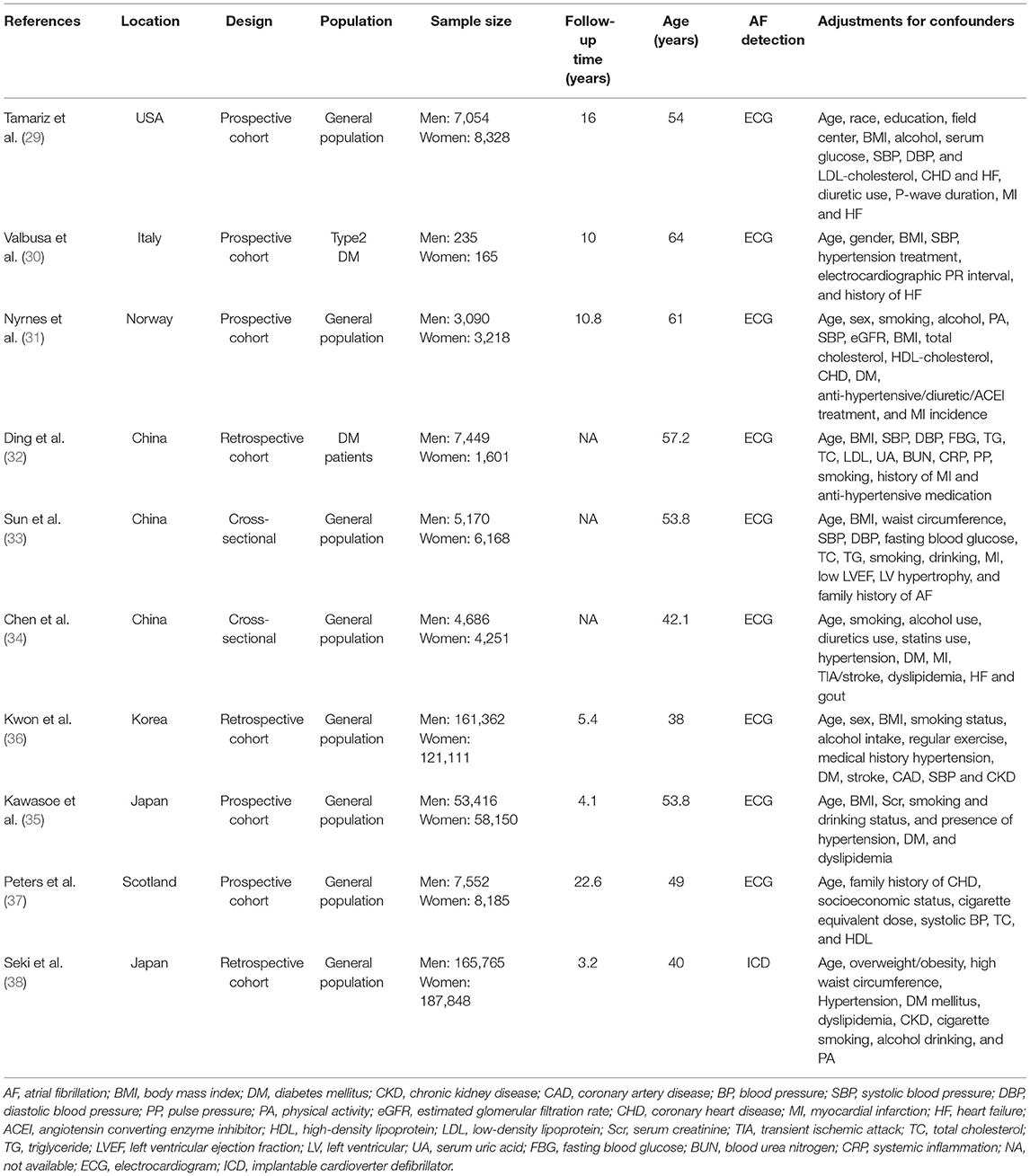
Table 1. Basic characteristics of the articles included in the meta-analysis of serum uric acid and risk of atrial fibrillation in men and women.
Six studies (31, 33–36, 38) with 393,489 men/380,746 women reported the association as a category variable between the SUA levels and AF. In the analyses of highest-vs.-lowest, an increased risk of AF with higher SUA levels was found (OR: 1.42; 95% CI, 1.18–1.71) in men, with low evidence of heterogeneity (I2 = 34%, Figure 2A). The heterogeneity was reduced to 0% when Sun et al. (33) was excluded, with a similar result (OR: 1.33; 95% CI, 1.15–1.53, I2 = 0%).
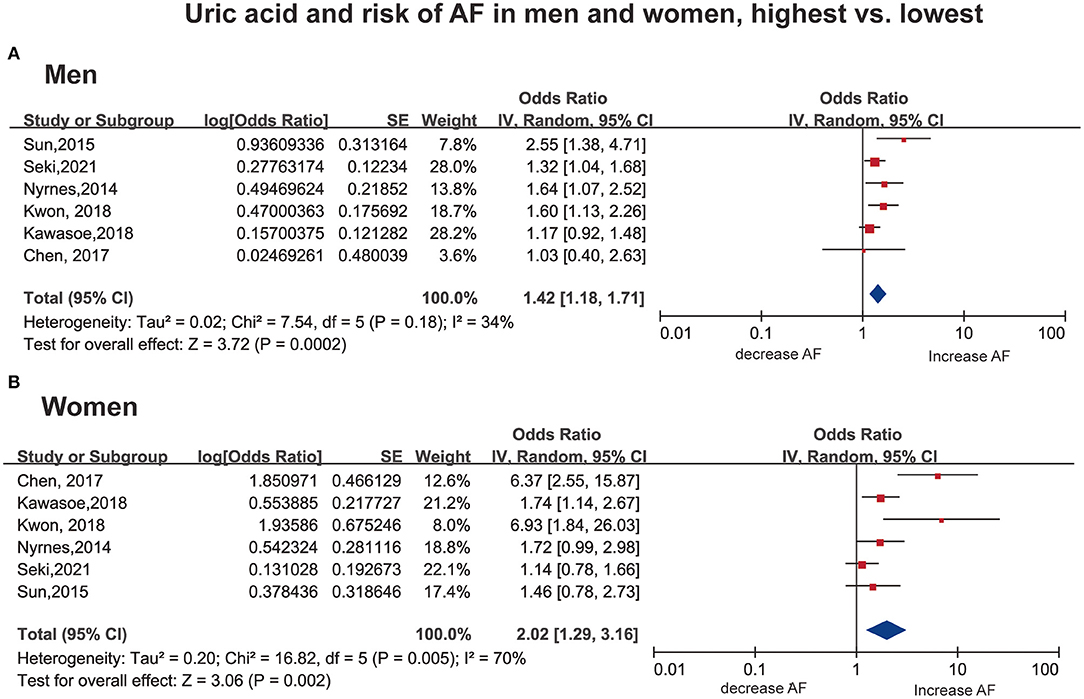
Figure 2. Forest plot for the risk of AF with the highest vs. the lowest serum uric acid among men (A) and women (B). AF, atrial fibrillation SE, standard error.
In women, a consistent result was observed (OR: 2.02; 95% CI, 1.29–3.16), with high heterogeneity (I2 = 70%, Figure 2B). Notably, two studies (34, 36) showed an OR over 6, which is much larger than the others. The results remained significant with no evidence of heterogeneity (OR: 1.44; 95% CI, 1.14–1.82, I2 = 0%) when excluding the above studies (34, 36).
Only three studies (33, 34, 36) examined the gender-specific association between hyperuricemia vs. the normal SUA and AF. The results showed that patients with hyperuricemia were associated with increased risk of AF in men (OR: 1.71; 95% CI, 1.16–2.54, I2 = 31%) but not in women (OR: 2.93; 95% CI, 0.69–12.38, I2 = 85%) (Supplementary Figure S1).
A total of 10 articles (29–38) with 415,779 men and 211,177 women were included in the dose-response analysis. Ten articles (29–38) with 415,779 participants reported an association between the SUA levels and AF in men. The results showed that each 60 μmol/L (1 mg/dL) increment in SUA increased the risk of AF by 15% (OR: 1.15; 95% CI, 1.07-1.25) in men, with significant heterogeneity (I2 = 74%; Figure 3A). There was no evidence of a non-linear association between the SUA levels and the AF risk (P for non-linearity = 0.91; Figure 4A).
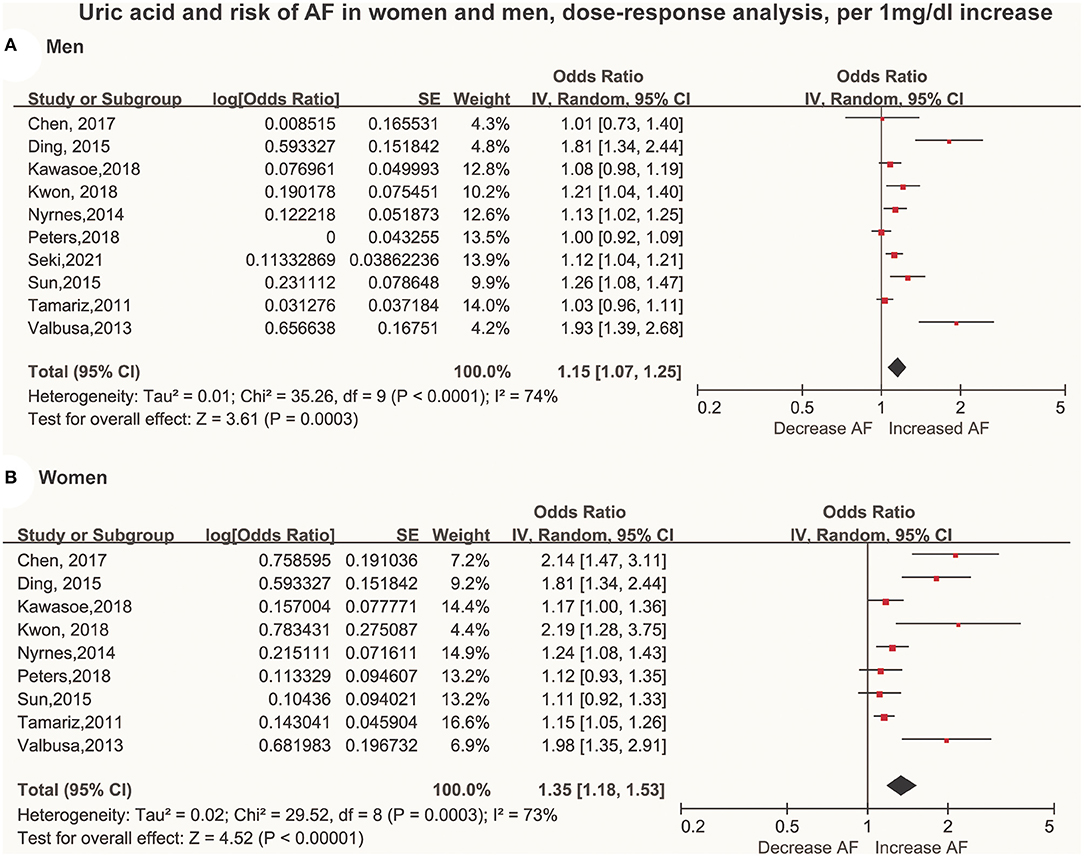
Figure 3. Forest plot of the dose-response association between serum uric acid levels and AF among men (A) and women (B). Serum uric acid was analyzed as per a 60 μmol/L (1 mg/dL) increase. AF, atrial fibrillation SE, standard error.
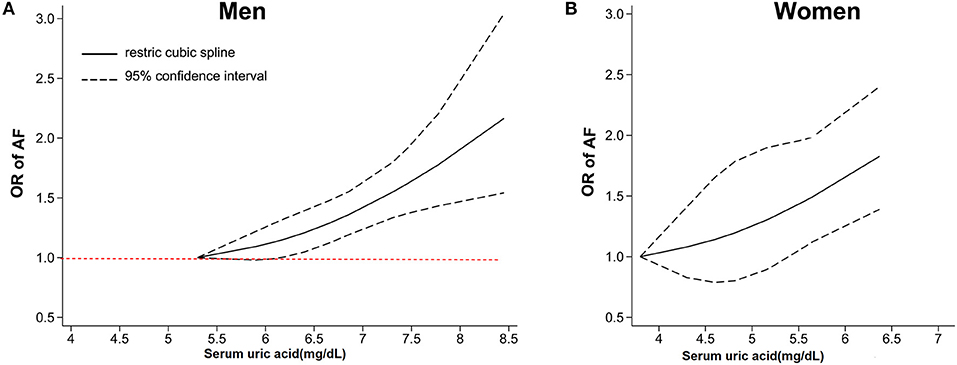
Figure 4. Dose-response analysis between serum uric acid levels and the risk of AF among men (A) and women (B). The dose-response association was fitted by the restricted cubic splines model, and the solid and dashed lines represent the estimated odds ratio and the 95% confidence interval, respectively. AF, atrial fibrillation OR, odds ratio.
Nine studies (29–37) with 211,177 participants reported an association between the SUA levels and AF in women. The results showed that each 60 μmol/L (1 mg/dL) increment in SUA increased the risk of AF by 35% (OR: 1.35; 95% CI, 1.18–1.53) in women, with high heterogeneity (I2 = 73%; Figure 3B). There was no evidence of a non-linear association between the SUA levels and the AF risk (P for non-linearity = 0.92; Figure 4B).
There was a borderline significant difference in the impact of SUA [per 60 μmol/L (1 mg/dL) increase] on the risk of AF between men and women (P for interaction = 0.05).
As shown in Supplementary Figure S2, possible publication bias was detected by funnel plots, Egger's and Begg's tests. Thus, we used a trim-and-fill method to adjust for publication bias. However, the results did not change after applying the trim-and-fill test, indicating that the impact of this bias was likely insignificant and that our results were credible (Supplementary Figure S3).
We conducted a subgroup analysis by patient characteristics, such as age, region, and confounding factors. As shown in Table 2, a positive association between SUA and the risk of AF among men and women persisted in almost all subgroup analyses defined by sex, region, sample size, study quality and adjustment for clinical confounding, and there was little evidence of heterogeneity among any of these subgroups with meta-regression analyses. There was a stronger association between SUA and the risk of AF among reports with diabetic populations, whether in men (P heterogeneity <0.001) or women (P heterogeneity = 0.001). Sensitivity analyses performed by omitting each study did not change the conclusion, which supported the robustness of our results (Data not shown). Further sensitivity analysis performed by removing the only study (38) that used an implantable cardioverter-defibrillator (ICD) to detect AF showed similar results (Supplementary Table S5).
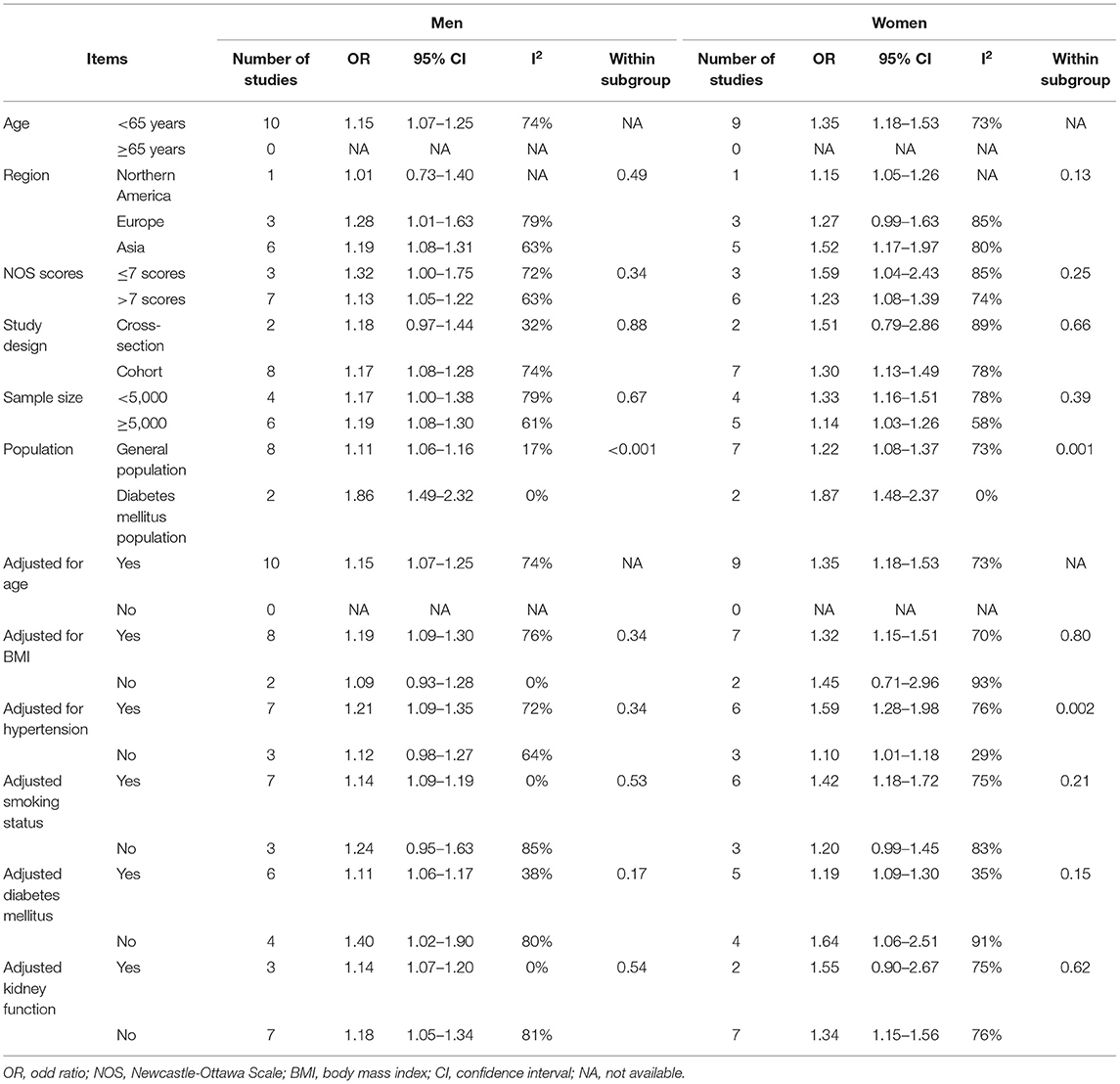
Table 2. Subgroup analysis of serum uric acid and risk of atrial fibrillation in men and women, per 1 mg/dL increment.
Based on 10 eligible studies with 814,804 participants (415,779 men and 399,025 women), we further investigated the sex-specific association and found a positive linear dose-response relationship between SUA and AF in both men and women. For each 60 μmol/L (1 mg/dL) increase in SUA levels, the risk of AF increased by 15% in men and 35% in women. There was a borderline significant sex difference in the impact of SUA on the risk of AF, with the risk estimates being higher in women.
Two studies (30, 32) with diabetic patients were included in the analysis. In a subgroup of the population, we found that there was a stronger association between SUA and the risk of AF among the reports with diabetic populations, whether in men or women. Consistent with this trend, a study that included 245 patients showed that hyperuricemia increased the risk of AF by 341% in type 2 diabetes (24). Moreover, numerous studies have reported that SUA has a potential connection with diabetes (49). Insulin resistance in diabetic patients can increase SUA reabsorption. Similarly, diabetic nephropathy leads to a decrease in SUA excretion through the kidney (50–53). Other studies have shown that high levels of SUA are a risk factor for vascular disease in type 2 diabetes and insulin resistance (54). Therefore, the relationship between SUA and AF may be amplified in diabetic patients.
SUA predicts the development of hypertension (55), which is a well-known risk factor for AF. Evidence from a double-blinded, placebo-controlled study of prehypertensive adolescents showed that lowering SUA had a positive effect on blood pressure reduction (56). In our results, although adjustments for hypertension did not influence the positive associations between SUA and AF, an unexpectedly larger magnitude risk estimate was seen in women after adjustment for hypertension. To date, we have no logical explanation for these unexpected results. More studies will likely be needed to assess the effect of hypertension on the association between SUA and AF.
Interestingly, the category analysis of hyperuricemia vs. normal SUA showed patients with hyperuricemia were associated with increased risk of AF in men but not in women. No statistically significant difference among women (P = 0.14, Supplementary Figure S1) may due to the limited data.
A sex-specific association between SUA and health was suggested by previous studies, such as stroke (57), left ventricular hypertrophy (58), metabolic syndrome (59), and coronary artery disease (60, 61). For example, the results from the First National Health and Nutrition Examination Survey (FNHANES) showed significant higher ischemic heart disease mortality, total cardiovascular mortality, and all-cause mortality in women with increasing SUA levels (62). In the context of AF, the results were also inconsistent. In several longitudinal studies, a significant association between high SUA and new-onset AF was observed in women but not in men (31, 35). However, other cohorts showed contrary results, with independent associations being significant in men but not in women (33, 38). In the present study, we showed that a high SUA was associated with a risk of AF in both men and women. However, there was a borderline significant sex difference in the impact of SUA on the risk of AF, with risk estimates being higher in women. This trend was consistent in non-linear analysis, showing a likelihood of a steeper increase in women with increasing SUA. Moreover, an analysis based on a Chronic Condition Data Warehouse showed that the reduced risk estimate of incident AF with allopurinol use was more prominent in women (63), which reinforced our results in the context of sex differences. The mechanism underlying SUA and AF has been discussed previously (28). The mechanism underlying different sex-based patterns remains unclear and may be due to differences in sex hormones. In general, estrogen is well known to have a heart-protecting effect, such as reducing the number of uric acid transporters in the kidney. However, it has been reported that estrogen can induce increased expression of urate transporters in female patients (64, 65). SUA enters cardiomyocytes through urate transporters, which increases the expression of kvl.5 protein, thus shortening the action potential duration and leading to the occurrence and progression of AF (66, 67). In addition, variations in SUA levels between men and women are thought to be linked to increased renal urate reabsorption in response to testosterone (68). Moreover, in certain large cohorts, an association between low levels of circulating testosterone and incident AF has been discovered (69, 70). Decreased testosterone levels have been shown in animal models to promote proarrhythmic alterations in calcium management, which might increase the risk of AF (71, 72). Further studies are warranted to elucidate the underlying biological mechanisms of sex-specific patterns.
The occurrence of AF increases with age. SUA levels in postmenopausal women are increased due to metabolic changes caused by menopause (73), which might contribute to the higher incidence of AF in older women. In contrast, in men, flat or slightly declining SUA levels are observed with aging (74). This age-related change in SUA might influence the sex differences associated with the AF risk. However, in the present study, the mean age of the women ranged from 38 to 57 years in the majority of studies, and the mean age of all patients in the included studies was under 65 years. The subgroup of elderly individuals was not available, which precluded us from further assessing the sex difference stratified by elderly individuals. Notably, a trend of sex difference persisted in the SUA-associated AF after adjustment for age (OR = 1.35 for women and OR = 1.15 for men), although with a non-significant P-value (0.1). Therefore, our results do not seem to support the hypothesis that increasing age accounts for the sex-based difference in the context of SUA and AF.
Considering the proven association between high SUA and an increased risk of AF, SUA-lowering therapy may be a therapeutic target for reducing the risk of AF in the future. A retrospective, single-center study of 603 patients with heart failure showed that the use of allopurinol reduced the risk of AF by 13% (75). In a retrospective cohort study including 9,244 elderly patients, Singh et al. reported that patients receiving allopurinol had a lower incidence of AF and that longer usage of allopurinol was associated with a lower risk of AF, heart failure mortality and myocardial infarction (63). Consistently, a recent meta-analysis suggested that allopurinol treatment of hyperuricemia was associated with improved endothelial function, which indicated that allopurinol might have a cardioprotective effect in addition to lowering the SUA levels (76), especially for those with cardiovascular diseases, such as diabetes. However, febuxostat might not be ideal because it increases the risk of cardiac and all-cause death compared with allopurinol (77). Thus, the Food and Drug Administration has recently issued a safety alert for febuxostat, which increases the risk of cardiovascular events in patients (78). Considering that merely reducing serum urate with a drug such as febuxostat has not been proven to benefit cardiac health, the benefits of allopurinol in lowering the incidence of AF may arise from a mechanism of action other than lowering urate. Moreover, we also found that the cutoff value of SUA was higher in women than in men for the association with AF. Additional studies are required to confirm this sex difference, and trials are also needed to clarify the optimal SUA levels and the safety of various SUA-lowering drugs for the future prevention of AF.
As we know, SUA level was regulated by kidney function. Evidence from recent studies showed renal dysfunction could impair the excretion of uric acid and contribute to a higher prevalence of hyperuricemia in the elderly. We performed a subgroup analysis stratified by adjustment for renal function. A positive association between SUA and the risk of AF among men and women persisted and there was little evidence of heterogeneity. Therefore, the association between SUA and risk of AF might be independent of kidney function.
Our results appear to agree with previous meta-analyses (26–28), which suggested that high SUA is associated with an increased prevalence and risk of AF. More importantly, our meta-analysis extended these studies and had three important strengths. First, to the best of our knowledge, this is the first dose-response meta-analysis that explored the sex-specific associations of SUA with the risk of AF. Second, all of the included studies adjusted for clinical confounding factors, indicating that our results were relatively stable. Third, the strong associations between SUA and the risk of AF among men and women persisted in all subgroup analyses and were reliable in the sensitivity analyses, suggesting that elevated SUA may be an independent risk factor for AF in both men and women.
However, there are several limitations to the present systematic review and meta-analysis that need to be discussed. First, all of the included studies were observational studies. Hence, additional randomized, controlled trials are warranted to properly validate the associations of the SUA levels with the risk of AF in both men and women. Second, a high degree of heterogeneity was observed in our results. This was not surprising because of variations in the characteristics of the study populations and study designs. Third, although the trim-and-fill method was applied to address the problem of publication bias, such a test could have limited power in the setting of relatively few studies (79). Thus, the risk estimates may be overstated due to potential studies with null results.
The findings of this study suggest that there is a significant linear relationship between SUA and the risk of AF among men and women, with a higher risk estimate in women. Additional trials are required to assess the effect of lowering SUA therapy on the AF incidence.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
XL and KM were responsible for the entire project and revised the draft. JX and WS performed the study selection, data extraction, statistical analysis, and interpreting the data. WS drafted the first version of the manuscript. All authors participated in the interpretation of the results and prepared the final version of the manuscript.
This work was supported by the National Natural Science Foundation of China (PY, 81760050 and 81760048; XL, 82100347), China Postdoctoral Science Foundation (XL, 2021M703724), the Jiangxi Provincial Natural Science Foundation for Youth Scientific Research (PY, 20192ACBL21037), and the Jiangxi Provincial Key Laboratory of Cardiovascular Diseases of Traditional Chinese Medicine (20212BCD42010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We acknowledge the help from Zhiqing Chen (The Second Affiliated Hospital of Nanchang University).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.865036/full#supplementary-material
AF, atrial fibrillation; SUA, serum uric acid; XO, xanthine oxidoreductase; OR, odds ratio; RR, risk ratio; HR, hazard ratio; NOS, Newcastle-Ottawa Scale; BMI, body mass index.
1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. (2014) 129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119
2. Dagres N, Nieuwlaat R, Vardas PE, Andresen D, Lévy S, Cobbe S, et al. Gender-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Heart Survey on Atrial Fibrillation. J Am Coll Cardiol. (2007) 49:572–7. doi: 10.1016/j.jacc.2006.10.047
3. Lip GY, Laroche C, Boriani G, Cimaglia P, Dan GA, Santini M, et al. Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Observational Research Programme Pilot survey on Atrial Fibrillation. Europace. (2015) 17:24–31. doi: 10.1093/europace/euu155
4. Chao TF, Liu CJ, Chen SJ, Wang KL, Lin YJ, Chang SL, et al. Atrial fibrillation and the risk of ischemic stroke: does it still matter in patients with a CHA2DS2-VASc score of 0 or 1? Stroke. (2012) 43:2551–5. doi: 10.1161/STROKEAHA.112.667865
5. Friberg J, Scharling H, Gadsbøll N, Truelsen T, Jensen GB. Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (The Copenhagen City Heart Study). Am J Cardiol. (2004) 94:889–94. doi: 10.1016/j.amjcard.2004.06.023
6. Humphries KH, Kerr CR, Connolly SJ, Klein G, Boone JA, Green M, et al. New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation. (2001) 103:2365–70. doi: 10.1161/01.CIR.103.19.2365
7. Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ. (2016) 532:h7013. doi: 10.1136/bmj.h7013
8. Marcus GM, Smith LM, Ordovas K, Scheinman MM, Kim AM, Badhwar N, et al. Intracardiac and extracardiac markers of inflammation during atrial fibrillation. Heart Rhythm. (2010) 7:149–54. doi: 10.1016/j.hrthm.2009.10.004
9. Friedrichs K, Klinke A, Baldus S. Inflammatory pathways underlying atrial fibrillation. Trends Mol Med. (2011) 17:556–63. doi: 10.1016/j.molmed.2011.05.007
10. Tisdale JE, Borzak S, Sabbah HN, Shimoyama H, Goldstein S. Hemodynamic and neurohormonal predictors and consequences of the development of atrial fibrillation in dogs with chronic heart failure. J Card Fail. (2006) 12:747–51. doi: 10.1016/j.cardfail.2006.08.005
11. Youn JY, Zhang J, Zhang Y, Chen H, Liu D, Ping P, et al. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J Mol Cell Cardiol. (2013) 62:72–9. doi: 10.1016/j.yjmcc.2013.04.019
12. Kirça M, Oguz N, Çetin A, Uzuner F, Yeşilkaya A. Uric acid stimulates proliferative pathways in vascular smooth muscle cells through the activation of p38 MAPK, p44/42 MAPK and PDGFRβ. J Recept Signal Transduct Res. (2017) 37:167–73. doi: 10.1080/10799893.2016.1203941
13. Ali N, Mahmood S, Islam F, Rahman S, Haque T, Islam S, et al. Relationship between serum uric acid and hypertension: a cross-sectional study in Bangladeshi adults. Sci Rep. (2019) 9:9061. doi: 10.1038/s41598-019-45680-4
14. Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res. (2010) 62:170–80. doi: 10.1002/acr.20065
15. Richette P, Perez-Ruiz F, Doherty M, Jansen TL, Nuki G, Pascual E, et al. Improving cardiovascular and renal outcomes in gout: what should we target? Nat Rev Rheumatol. (2014) 10:654–61. doi: 10.1038/nrrheum.2014.124
16. Selvaraj S, Claggett BL, Pfeffer MA, Desai AS, Mc Causland FR, McGrath MM, et al. Serum uric acid, influence of sacubitril-valsartan, and cardiovascular outcomes in heart failure with preserved ejection fraction: PARAGON-HF. Eur J Heart Fail. (2020) 22:2093–101. doi: 10.1002/ejhf.1984
17. Ali N, Perveen R, Rahman S, Mahmood S, Rahman S, Islam S, et al. Prevalence of hyperuricemia and the relationship between serum uric acid and obesity: a study on Bangladeshi adults. PLoS ONE. (2018) 13:e0206850. doi: 10.1371/journal.pone.0206850
18. Haque T, Rahman S, Islam S, Molla NH, Ali N. Assessment of the relationship between serum uric acid and glucose levels in healthy, prediabetic and diabetic individuals. Diabetol Metab Syndr. (2019) 11:49. doi: 10.1186/s13098-019-0446-6
19. Ali N, Miah R, Hasan M, Barman Z, Mou AD, Hafsa JM, et al. Association between serum uric acid and metabolic syndrome: a cross-sectional study in Bangladeshi adults. Sci Rep. (2020) 10:7841. doi: 10.1038/s41598-020-64884-7
20. Molla NH, Kathak RR, Sumon AH, Barman Z, Mou AD, Hasan A, et al. Assessment of the relationship between serum uric acid levels and liver enzymes activity in Bangladeshi adults. Sci Rep. (2021) 11:20114. doi: 10.1038/s41598-021-99623-z
21. Kuma A, Mafune K, Uchino B, Ochiai Y, Enta K, Kato A. Development of chronic kidney disease influenced by serum urate and body mass index based on young-to-middle-aged Japanese men: a propensity score-matched cohort study. BMJ Open. (2022) 12:e049540. doi: 10.1136/bmjopen-2021-049540
22. Li S, Cheng J, Cui L, Gurol ME, Bhatt DL, Fonarow GC, et al. Cohort study of repeated measurements of serum urate and risk of incident atrial fibrillation. J Am Heart Assoc. (2019) 8:e012020. doi: 10.1161/JAHA.119.012020
23. Kuwabara M, Niwa K, Nishihara S, Nishi Y, Takahashi O, Kario K, et al. Hyperuricemia is an independent competing risk factor for atrial fibrillation. Int J Cardiol. (2017) 231:137–142. doi: 10.1016/j.ijcard.2016.11.268
24. Mantovani A, Rigolon R, Civettini A, Bolzan B, Morani G, Bonapace S, et al. Hyperuricemia is associated with an increased prevalence of paroxysmal atrial fibrillation in patients with type 2 diabetes referred for clinically indicated 24-h Holter monitoring. J Endocrinol Invest. (2018) 41:223–31. doi: 10.1007/s40618-017-0729-4
25. Liu T, Zhang X, Korantzopoulos P, Wang S, Li G. Uric acid levels and atrial fibrillation in hypertensive patients. Intern Med. (2011) 50:799–803. doi: 10.2169/internalmedicine.50.4587
26. Tamariz L, Hernandez F, Bush A, Palacio A, Hare JM. Association between serum uric acid and atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. (2014) 11:1102–8. doi: 10.1016/j.hrthm.2014.04.003
27. Zhang CH, Huang DS, Shen D, Zhang LW, Ma YJ, Wang YM, et al. Association between serum uric acid levels and atrial fibrillation risk. Cell Physiol Biochem. (2016) 38:1589–95. doi: 10.1159/000443099
28. Zhang J, Zheng R, Li H, Guo J. Serum uric acid and incident atrial fibrillation: a systematic review and dose-response meta-analysis. Clin Exp Pharmacol Physiol. (2020) 47:1774–82. doi: 10.1111/1440-1681.13374
29. Tamariz L, Agarwal S, Soliman EZ, Chamberlain AM, Prineas R, Folsom AR, et al. Association of serum uric acid with incident atrial fibrillation (from the atherosclerosis risk in communities [ARIC] study). Am J Cardiol. (2011) 108:1272–6. doi: 10.1016/j.amjcard.2011.06.043
30. Valbusa F, Bertolini L, Bonapace S, Zenari L, Zoppini G, Arcaro G, et al. Relation of elevated serum uric acid levels to incidence of atrial fibrillation in patients with type 2 diabetes mellitus. Am J Cardiol. (2013) 112:499–504. doi: 10.1016/j.amjcard.2013.04.012
31. Nyrnes A, Toft I, Njølstad I, Mathiesen EB, Wilsgaard T, Hansen JB, et al. Uric acid is associated with future atrial fibrillation: an 11-year follow-up of 6308 men and women–the Tromso Study. Europace. (2014) 16:320–6. doi: 10.1093/europace/eut260
32. Ding X, Zheng X, Xing A, Wang D, Qi S, Wu Y, et al. High risk factors of atrial fibrillation in type 2 diabetes: results from the Chinese Kailuan study. QJM. (2015) 108:885–90. doi: 10.1093/qjmed/hcv051
33. Sun GZ, Guo L, Wang J, Ye N, Wang XZ, Sun YX. Association between hyperuricemia and atrial fibrillation in rural China: a cross-sectional study. BMC Cardiovasc Disord. (2015) 15:98. doi: 10.1186/s12872-015-0089-y
34. Chen Y, Xia Y, Han X, Yang Y, Yin X, Qiu J, et al. Association between serum uric acid and atrial fibrillation: a cross-sectional community-based study in China. BMJ Open. (2017) 7:e019037. doi: 10.1136/bmjopen-2017-019037
35. Kawasoe S, Kubozono T, Yoshifuku S, Ojima S, Miyata M, Miyahara H, et al. Uric acid level and new-onset atrial fibrillation in the Japanese general population-longitudinal study. Circ J. (2018) 83:156–63. doi: 10.1253/circj.CJ-18-0508
36. Kwon CH, Lee SH, Lee JY, Ryu S, Sung KC. Uric acid and risk of atrial fibrillation in the Korean general population. Circ J. (2018) 82:2728–35. doi: 10.1253/circj.CJ-18-0748
37. Peters SAE, Woodward M. Established and novel risk factors for atrial fibrillation in women compared with men. Heart. (2019) 105:226–34. doi: 10.1136/heartjnl-2018-313630
38. Seki H, Kaneko H, Morita H, Itoh H, Morita K, Matsuoka S, et al. Relation of serum uric acid and cardiovascular events in young adults aged 20-49 years. Am J Cardiol. (2021) 152:150–7. doi: 10.1016/j.amjcard.2021.05.007
39. Gorenek B, Pelliccia A, Benjamin EJ, Boriani G, Crijns HJ, Fogel RI, et al. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). Europace. (2017) 19:190–225. doi: 10.1093/europace/eux163
40. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
41. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: systematic reviews of etiology and risk. In: Aromataris, E Munn, Z editors. JBI Manual for Evidence Synthesis. JBI. (2020) 248–61. doi: 10.46658/JBIMES-20-08
42. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
43. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
44. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. (1989) 8:551–61. doi: 10.1002/sim.4780080504
45. Xu C, Sar D. The robust error meta-regression method for dose-response meta-analysis. Int J Evid Based Healthc. (2017) 16:138. doi: 10.1097/XEB.0000000000000132
46. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Handbook for Systematic Reviews of Interventions Version 6.3. Available online at: www.training.cochrane.org/handbook (accessed February 2022).
47. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
48. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
49. Conen D, Wietlisbach V, Bovet P, Shamlaye C, Riesen W, Paccaud F, et al. Prevalence of hyperuricemia and relation of serum uric acid with cardiovascular risk factors in a developing country. BMC Public Health. (2004) 4:9. doi: 10.1186/1471-2458-4-9
50. Jalal DI, Maahs DM, Hovind P, Nakagawa T. Uric acid as a mediator of diabetic nephropathy. Semin Nephrol. (2011) 31:459–65. doi: 10.1016/j.semnephrol.2011.08.011
51. Mauer M, Doria A. Uric acid and risk of diabetic kidney disease. J Nephrol. (2020) 33:995–9. doi: 10.1007/s40620-020-00796-z
52. Mauer M, Doria A. Uric acid and diabetic nephropathy risk. Contrib Nephrol. (2018) 192:103–9. doi: 10.1159/000484284
53. Bjornstad P, Lanaspa MA, Ishimoto T, Kosugi T, Kume S, Jalal D, et al. Fructose and uric acid in diabetic nephropathy. Diabetologia. (2015) 58:1993–2002. doi: 10.1007/s00125-015-3650-4
54. Fiorentino TV, Sesti F, Succurro E, Pedace E, Andreozzi F, Sciacqua A, et al. Higher serum levels of uric acid are associated with a reduced insulin clearance in non-diabetic individuals. Acta Diabetol. (2018) 55:835–42. doi: 10.1007/s00592-018-1153-8
55. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. (2008) 359:1811–21. doi: 10.1056/NEJMra0800885
56. Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension. (2012) 60:1148–56. doi: 10.1161/HYPERTENSIONAHA.112.196980
57. Li J, Muraki I, Imano H, Cui R, Yamagishi K, Umesawa M, et al. Serum uric acid and risk of stroke and its types: the Circulatory Risk in Communities Study (CIRCS). Hypertens Res. (2020) 43:313–21. doi: 10.1038/s41440-019-0385-5
58. Matsumura K, Ohtsubo T, Oniki H, Fujii K, Iida M. Gender-related association of serum uric acid and left ventricular hypertrophy in hypertension. Circ J. (2006) 70:885–8. doi: 10.1253/circj.70.885
59. Krzystek-Korpacka M, Patryn E, Kustrzeba-Wojcicka I, Chrzanowska J, Gamian A, Noczynska A. Gender-specific association of serum uric acid with metabolic syndrome and its components in juvenile obesity. Clin Chem Lab Med. (2011) 49:129–36. doi: 10.1515/CCLM.2011.011
60. Spence JD, Pilote L. Importance of sex and gender in atherosclerosis and cardiovascular disease. Atherosclerosis. (2015) 241:208–10. doi: 10.1016/j.atherosclerosis.2015.04.806
61. Tuttle KR, Short RA, Johnson RJ. Sex differences in uric acid and risk factors for coronary artery disease. Am J Cardiol. (2001) 87:1411–4. doi: 10.1016/S0002-9149(01)01566-1
62. Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. (2000) 283:2404–10. doi: 10.1001/jama.283.18.2404
63. Singh JA, Yu S. Allopurinol and the risk of atrial fibrillation in the elderly: a study using Medicare data. Ann Rheum Dis. (2017) 76:72–8. doi: 10.1136/annrheumdis-2015-209008
64. Zeng M, Chen B, Qing Y, Xie W, Dang W, Zhao M, et al. Estrogen receptor β signaling induces autophagy and downregulates Glut9 expression. Nucleosides Nucleotides Nucleic Acids. (2014) 33:455–65. doi: 10.1080/15257770.2014.885045
65. Takiue Y, Hosoyamada M, Kimura M, Saito H. The effect of female hormones upon urate transport systems in the mouse kidney. Nucleosides Nucleotides Nucleic Acids. (2011) 30:113–9. doi: 10.1080/15257770.2010.551645
66. Maharani N, Ting YK, Cheng J, Hasegawa A, Kurata Y, Li P, et al. Molecular mechanisms underlying urate-induced enhancement of Kv1.5 channel expression in HL-1 atrial myocytes. Circ J. (2015) 79:2659–68. doi: 10.1253/circj.CJ-15-0416
67. Taufiq F, Maharani N, Li P, Kurata Y, Ikeda N, Kuwabara M, et al. Uric acid-induced enhancements of Kv1.5 protein expression and channel activity via the Akt-HSF1-Hsp70 pathway in HL-1 atrial myocytes. Circ J. (2019) 83:718–26. doi: 10.1253/circj.CJ-18-1088
68. Hosoyamada M, Takiue Y, Shibasaki T, Saito H. The effect of testosterone upon the urate reabsorptive transport system in mouse kidney. Nucleosides Nucleotides Nucleic Acids. (2010) 29:574–9. doi: 10.1080/15257770.2010.494651
69. Lai J, Zhou D, Xia S, Shang Y, Want L, Zheng L, et al. Reduced testosterone levels in males with lone atrial fibrillation. Clin Cardiol. (2009) 32:43–6. doi: 10.1002/clc.20423
70. Magnani JW, Moser CB, Murabito JM, Sullivan LM, Wang N, Ellinor PT, et al. Association of sex hormones, aging, and atrial fibrillation in men: the Framingham Heart Study. Circ Arrhythm Electrophysiol. (2014) 7:307–12. doi: 10.1161/CIRCEP.113.001322
71. Tsuneda T, Yamashita T, Kato T, Sekiguchi A, Sagara K, Sawada H, et al. Deficiency of testosterone associates with the substrate of atrial fibrillation in the rat model. J Cardiovasc Electrophysiol. (2009) 20:1055–60. doi: 10.1111/j.1540-8167.2009.01474.x
72. Zhang Y, Wang HM, Wang YZ, Zhang YY, Jin XX, Zhao Y, et al. Increment of late sodium currents in the left atrial myocytes and its potential contribution to increased susceptibility of atrial fibrillation in castrated male mice. Heart Rhythm. (2017) 14:1073–80. doi: 10.1016/j.hrthm.2017.01.046
73. Wingrove CS, Walton C, Stevenson JC. The effect of menopause on serum uric acid levels in non-obese healthy women. Metabolism. (1998) 47:435–8. doi: 10.1016/S0026-0495(98)90056-7
74. Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. (1999) 131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003
75. Hernandez FE, Tamariz L, Hare J. Allopurinol decreases the incidence of atrial fibrillation in heart failure patients. J Am College Cardiol. (2013) 61:E409. doi: 10.1016/S0735-1097(13)60409-X
76. Kanbay M, Siriopol D, Nistor I, Elcioglu OC, Telci O, Takir M, et al. Effects of allopurinol on endothelial dysfunction: a meta-analysis. Am J Nephrol. (2014) 39:348–56. doi: 10.1159/000360609
77. White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. (2018) 378:1200–1210. doi: 10.1056/NEJMoa1710895
78. FDA, Drug Safety Communication,. FDA Adds Boxed Warning for Increased Risk of Death With Gout Medicine Uloric (Febuxostat). US Food and Drug Administration. Available online at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-increased-risk-death-gout-medicine-uloric-febuxostat (accessed February 21, 2019).
Keywords: atrial fibrillation, serum uric acid, sex difference, meta-analysis, hyperuricemia
Citation: Xiong J, Shao W, Yu P, Ma J, Liu M, Huang S, Liu X and Mei K (2022) Hyperuricemia Is Associated With the Risk of Atrial Fibrillation Independent of Sex: A Dose-Response Meta-Analysis. Front. Cardiovasc. Med. 9:865036. doi: 10.3389/fcvm.2022.865036
Received: 29 January 2022; Accepted: 18 March 2022;
Published: 07 April 2022.
Edited by:
Atsushi Tanaka, Saga University, JapanReviewed by:
Yutang Wang, Federation University Australia, AustraliaCopyright © 2022 Xiong, Shao, Yu, Ma, Liu, Huang, Liu and Mei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Liu, bGl1eDU4N0BtYWlsLnN5c3UuZWR1LmNu
Kaibo Mei, bWtib19sb3ZlQHFxLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.