- 1Department of Cardiology and Institute of Vascular Medicine, Peking University Third Hospital; Key Laboratory of Cardiovascular Molecular Biology and Regulatory Peptides, Ministry of Health; Key Laboratory of Molecular Cardiovascular Science, Ministry of Education, Beijing Key Laboratory of Cardiovascular Receptors Research, Beijing, China
- 2Healthcare Department, National Center of Gerontology, Beijing Hospital, Beijing, China
- 3Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, China
Background: Nicorandil is recommended as a second-line treatment for stable angina; however, randomized-controlled trials to evaluate the benefit of nicorandil for patients with chronic total occlusion (CTO) are lacking.
Objective: To determine whether nicorandil can improve left ventricular (LV) myocardial strain in patients with CTO.
Methods: Patients with CTO were included and randomized to the nicorandil group (n = 31) and the control group (n = 30). Nicorandil was given orally at 15 mg/day for 3 months in the nicorandil group. Three-dimensional speckle-tracking echocardiography and the Seattle Angina Questionnaire (SAQ) survey were performed at baseline and at 3 months. The primary study endpoint was the LV global area strain (GAS) at 3 months.
Results: The nicorandil and the control groups were well-matched at baseline, including the mean GAS and SAQ scores. At 3 months, GAS in the nicorandil group was significantly higher than that in the control group (−23.7 ± 6.3% vs. −20.3 ± 5.6%, respectively; p = 0.033). There were no significant differences in LV global longitudinal strain, global circumferential strain, global radial strain, LV ejection fraction, LV end-diastolic volume, and LV end-systolic volume at 3 months between the two groups. At 3 months, the SAQ scores for angina stability, angina frequency, and treatment satisfaction in the nicorandil group were significantly higher than those in the control group.
Conclusion: Nicorandil treatment can improve GAS and angina symptoms in patients with CTO.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier: NCT05087797.
Introduction
Coronary chronic total occlusion (CTO) refers to a coronary artery with a complete obstruction resulting in thrombolysis in myocardial infarction grade 0 flows for over 3 months (1). The prevalence of CTO ranges between 30% and 50% among patients with coronary artery disease (CAD) (1). Although collaterals are capable of maintaining myocardial viability, they are insufficient to sustain myocardial perfusion during exercise, which can lead to angina (2). Furthermore, CTO is associated with a higher mortality rate (3, 4).
Observational studies and registries showed that successful CTO percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) was associated with improvements in angina pectoris symptoms, left ventricular (LV) function, quality of life, and survival (5–8). However, randomized-controlled trials (RCTs) failed to show improvements in LV function and prognosis compared with optimal medical therapy (OMT) (6). Moreover, the success rate in CTO PCI was lower than that in non-CTO PCI, and the complication rate was higher than that in non-CTO PCI (9). OMT remains the cornerstone for the management of patients with CTO. There are few studies focusing on medical therapy in patients with CTO and stable angina. Therefore, these patients are usually managed with medical therapy for stable angina. To prevent angina and ischemia, beta-blockers, calcium channel blockers, and nitrates are the primary agents (10). Nicorandil, an adenosine triphosphate (ATP)-sensitive potassium ATP channel opener with nitrate-like effects, is recommended as a second-line treatment for chronic coronary syndrome by The European Society of Cardiology (10). However, RCTs to evaluate the benefit of nicorandil for patients with CTO are lacking. In the past decade, deformation imaging has developed rapidly, and three-dimensional-speckle tracking echocardiography (3D-STE) is becoming a valuable method in the comprehensive assessment of myocardial function (11). 3D-STE can detect subclinical LV dysfunction using strain parameters in CAD patients without LV regional wall motion abnormality (11). Strain parameters derived from 3D-STE comprise global longitudinal strain (GLS), global circumferential strain (GCS), global radial strain (GRS), and global area strain (GAS). Among these parameters, GAS is ideal to detect severe coronary stenosis (12, 13). This study aimed to determine whether nicorandil can improve LV myocardial strain in patients with CTO.
Materials and Methods
Patients
This was a single-center, open-label, RCT performed at Peking University Third Hospital, and the trial was registered at https://clinicaltrials.gov (NCT05087797). Patients with CTO identified on coronary angiography between January 2018 and November 2019 were screened for inclusion in this study. The inclusion criteria were: age between 18 and 80 years and one or more CTO vessels (≥ 2 mm in diameter). The exclusion criteria were: successful PCI for all CTO lesions; ST segment elevation myocardial infarction during the past 3 months; planned CABG within 3 months; valvular heart disease or congenital heart disease; cardiomyopathy; heart failure with New York Heart Association class ≥ III; persistent atrial fibrillation or complete left bundle branch block; and severe liver, kidney, or lung disease. Participants were randomized according to an IBM SPSS version 23.0 (IBM Corp., Armonk, NY, USA)-generated randomization schedule, with a 1:1 allocation to the nicorandil group or the control group. All patients in this study received standard treatment for CAD, and patients in the nicorandil group were also given nicorandil (Tohoku Nipro Pharmaceutical Corporation, Tokyo, Japan) 5 mg orally thrice per day for 3 months.
Coronary Angiographic Assessment
Chronic total occlusion was defined as coronary artery occlusion for over 3 months. The duration was estimated on the basis of a history of an ischemic event potentially related to the coronary occlusion, or previous coronary angiography confirming the occluded vessel. Non-CTO PCI was performed, if needed. Coronary angiograms were reviewed by one specialist who was blinded to the group allocation. Collateral channels and their Rentrop classification were analyzed.
3D Echocardiography and Speckle-Tracking Analysis
All patients underwent 3D echocardiography before randomization (baseline) and 3 months after treatment, with a Vivid 95 (GE Healthcare, Wauwatosa, WI, USA) system and a 4 V-D transducer (GE Healthcare). Patients were placed in the left lateral position, and 3D images of the LV were obtained from the apical window. Multibeat-3D model was used to obtain the full-volume dataset from four consecutive cardiac cycles. The frame rate was ≥ 25 Hz to perform the speckle-tracking analysis. Images were recorded digitally and analyzed offline using the 4D Auto LVQ package (EchoPAC V.110.1.3; GE Healthcare). The LV endocardial border was detected automatically and was adjusted manually, if needed. LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were calculated, and 3D LV ejection fraction (LVEF) was provided. Subsequently, automatic tracing of the epicardial border was performed to identify the region of interest required for speckle-tracking analysis. The epicardial tracing was also manually adjusted to include the entire LV wall. Next, the strain parameters were displayed as the GLS, GCS, GRS, and GAS of the LV. Echocardiographic analyses were performed by a single experienced cardiologist who was blinded to the patients' clinical and study data.
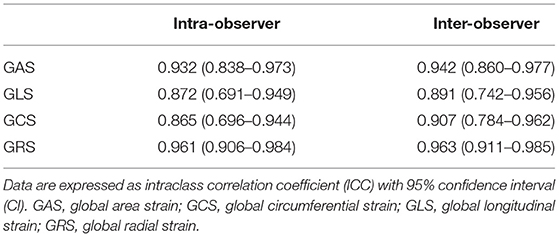
Intra- and inter-observer reproducibility for the 3D echocardiographic parameters were assessed in 20 randomly selected patients. To test intra-observer reproducibility, echocardiographic analyses were performed twice by the same cardiologist 1 week apart. To test inter-observer reproducibility, echocardiographic analyses were performed again by a second cardiologist.
Seattle Angina Questionnaire
The 19-item SAQ survey was conducted at baseline and again at 3 months. This questionnaire measures health status across five domains: physical limitation, angina stability, angina frequency, treatment satisfaction, and quality of life. All domain scores range from 0 to 100, with higher scores indicating fewer symptoms and better health status (14).
Study Endpoints
The primary study endpoint was GAS at 3 months. The secondary study endpoints were GLS, GCS, GRS, LVEF, LVEDV, and LVESV at 3 months, and health status measured by SAQ at 3 months.
Sample Size Estimation and Statistical Analysis
According to previous studies, GAS for the nicorandil group and the control group were estimated to be −18 ± 4% and −15 ± 4%, respectively (12, 13). Therefore, in this study, each group required 31 samples for an alpha level of 0.05 and 80% power using a two-sided test, allowing for 15% attrition.
SPSS 23.0 (IBM Corp.) was used for the statistical analysis. The Kolmogorov–Smirnov method was used to evaluate the normality of the continuous data. Continuous variables with normal distribution were expressed as mean ± standard deviation, and comparisons between the two groups were performed using Student's t-test. Continuous variables with non-normal distribution were expressed as median (25th, 75th percentiles), and comparisons between the two groups were performed using the Mann–Whitney U test. Categorical variables were expressed as numbers (percentages), and comparisons between the two groups were performed using the Chi-square test. The intra-group correlation coefficient was used to test the reproducibility of the 3D echocardiographic parameters between examiners and within examiners. An intra-group correlation coefficient > 0.75 indicated good repeatability. Pearson correlation was used to identify the correlations between strain parameters and SAQ scores.
Results
Study Groups
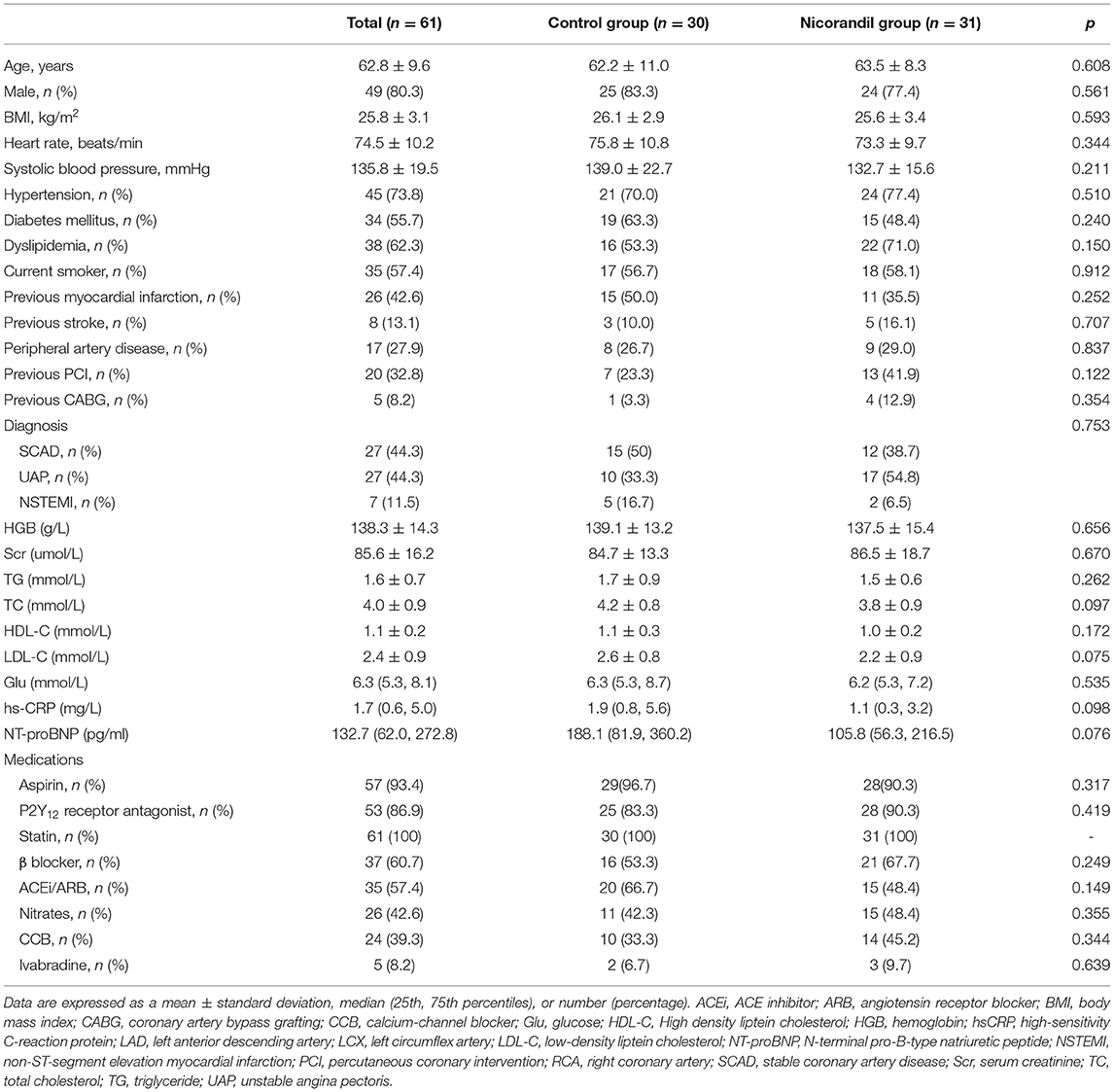
During the study period, 90 consecutive patients with CTO were screened for participation. Of all eligible patients, 68 patients were randomized to the nicorandil group or the control group. Seven patients withdrew after randomization. Data for a final 61 patients (31 patients in the treatment group and 30 patients in the control group) were analyzed (Figure 1). The mean age was 62.8 ± 9.6 years, and 49 (80.3%) patients were men. Most patients were on aspirin, P2Y12 receptor antagonist, and statin treatment. A total of 37 (60.7%) patients were given β-blockers, 26 (42.6%) were given nitrates, 24 (39.3%) were given calcium-channel blockers, and 5 (8.2%) were given ivabradine. Participants in the treatment group and the control group were well-matched in terms of their clinical characteristics (Table 1).
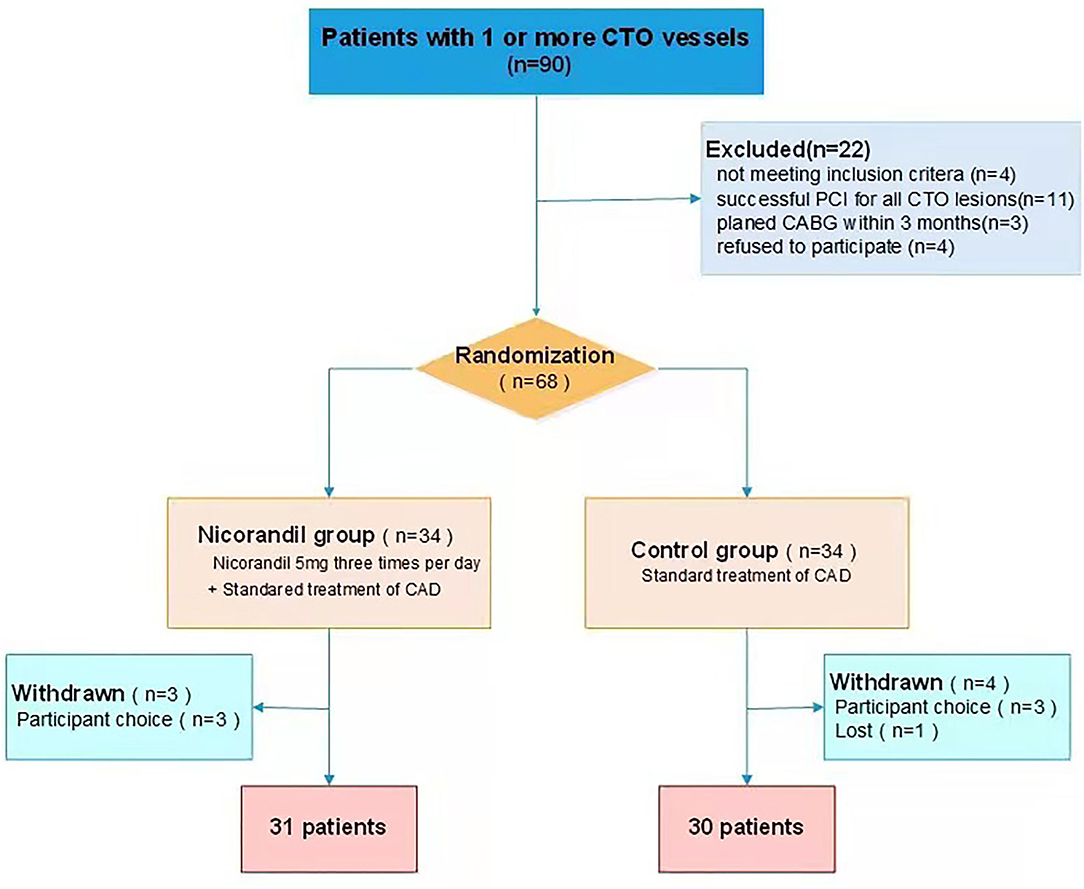
Figure 1. Patient flow chart. CABG, coronary artery bypass grafting; CTO, chronic total occlusion; PCI, percutaneous coronary intervention.
Coronary Angiography and Procedural Data
Among the patients included in this study, 53 (86.9%) patients had a single CTO vessel, and 8 (13.1%) patients had two CTO vessels. The right coronary artery was the most common location of occlusions, followed by the left circumflex artery and left anterior descending artery. CTO PCI was attempted in 23 (37.7%) patients. A total of 38 (62.3%) patients had Rentrop 2 or Rentrop 3 collateral channels, and 32 (52.5%) patients underwent non-CTO PCI. Coronary angiography and procedural data were comparable between the treatment group and the control group (Table 2).
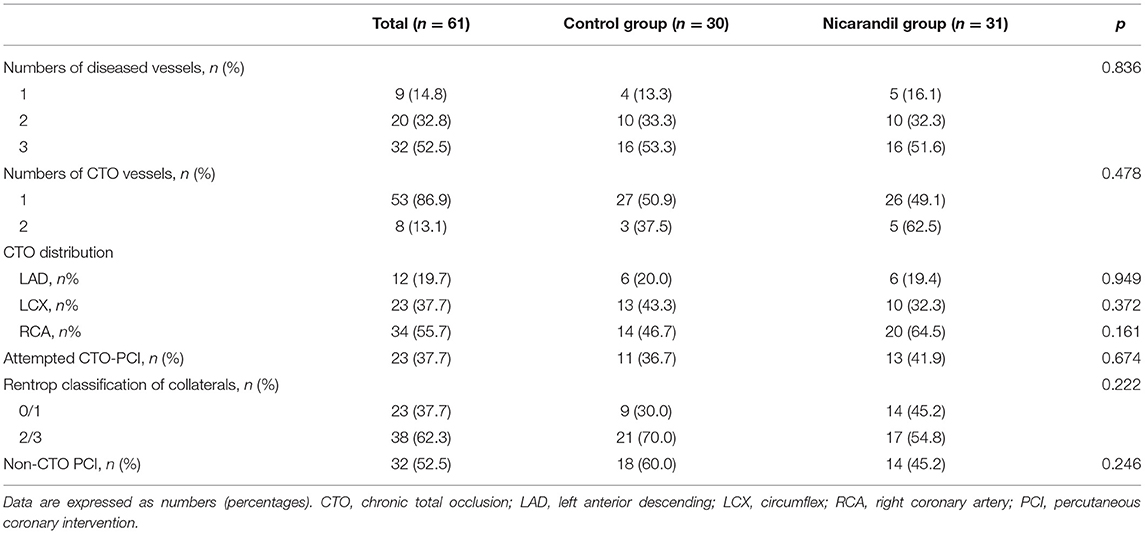
Table 2. Baseline angiographic and procedural characteristics of the nicorandil group and the control group.
Echocardiographic Data
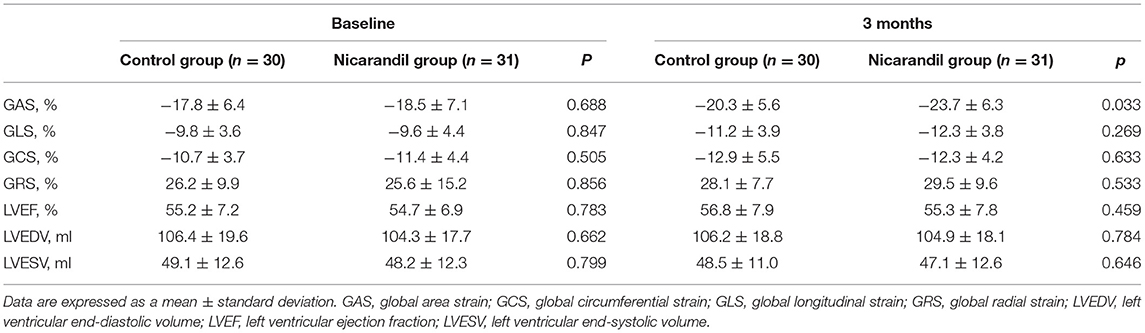
There were no significant differences in baseline echocardiographic data between the two groups. At 3 months, GAS in the nicorandil group was significantly higher than that in the control group (−23.7 ± 6.3% vs. −20.3 ± 5.6%, respectively; p = 0.033; Table 3, Figure 2). There were no significant differences in GLS, GCS, GRS, LVEF, LVEDV, and LVESV at 3 months between the two groups (Table 3). The intra- and inter-observer reproducibility for the 3D echocardiographic parameters were good (Table 4).
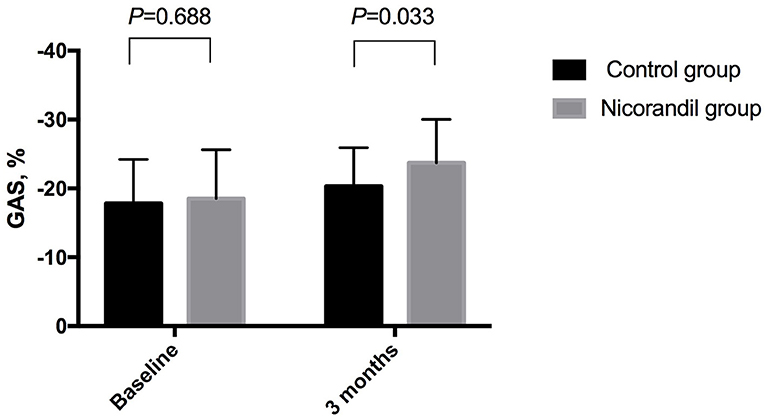
Figure 2. Comparison of GAS between the nicorandil group and the control group. GAS, global area strain.
SAQ Scores
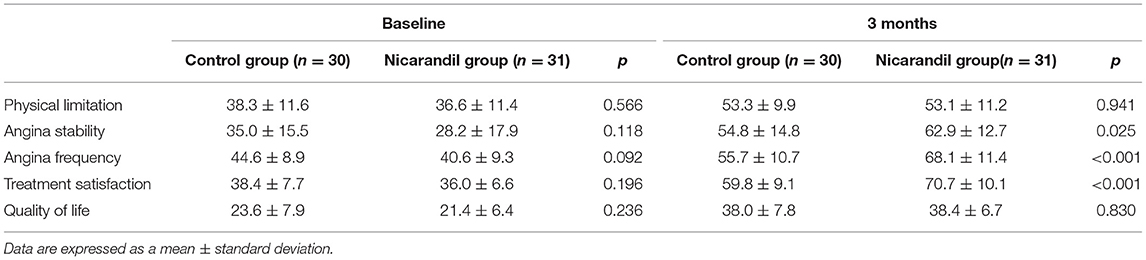
Seattle angina questionnaire scores for all five domains were not significantly different between the nicorandil group and the control group at baseline. At 3 months, SAQ scores for angina stability, angina frequency, and treatment satisfaction were significantly higher in the nicorandil group than in the control group, while the scores for physical limitation and quality of life were not significantly different between the two groups (Table 5, Figure 3).
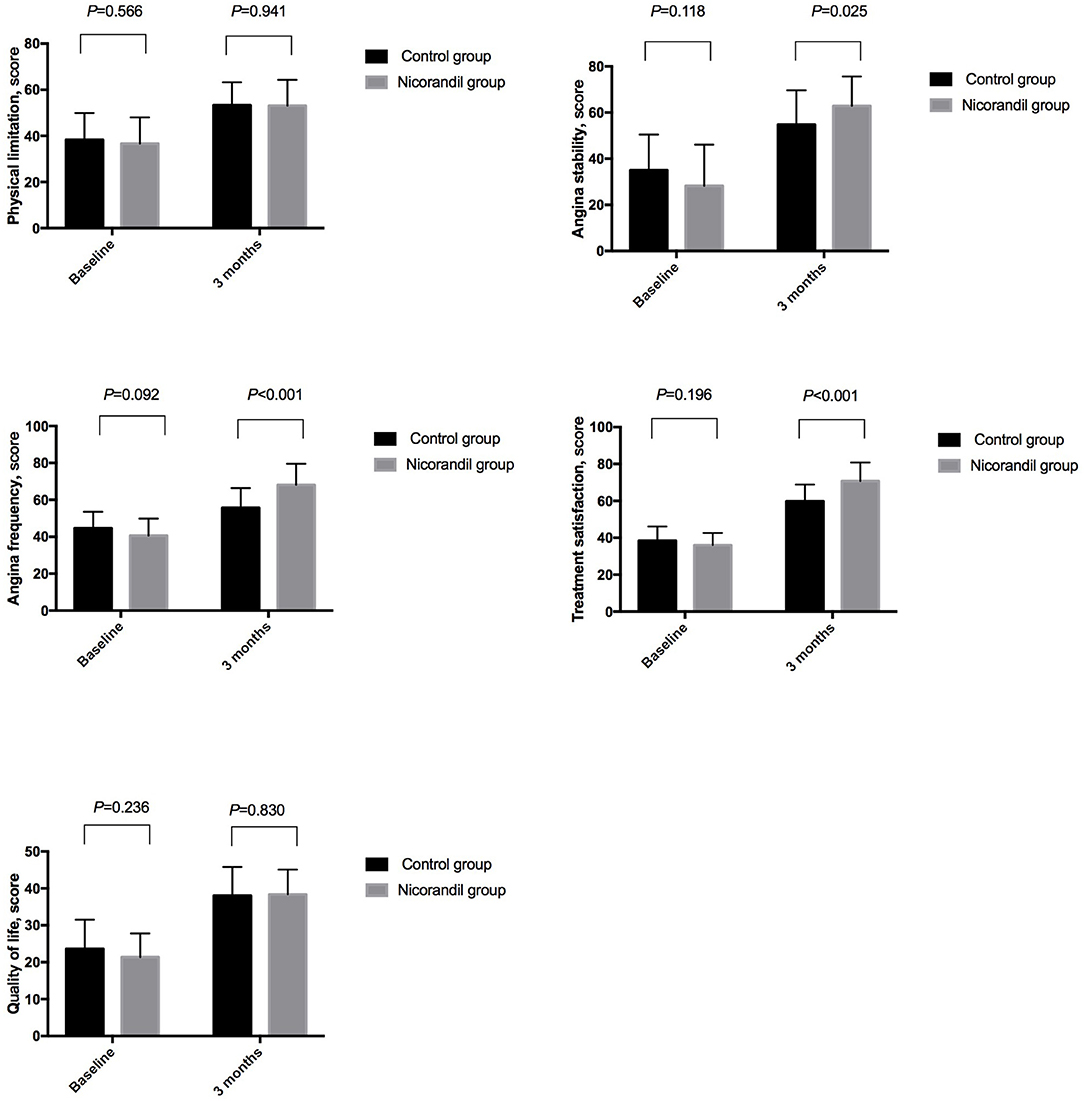
Figure 3. Comparison of seattle angina questionnaire scores between the nicorandil group and the control group.
Relationship Between Strain Parameters and SAQ Scores
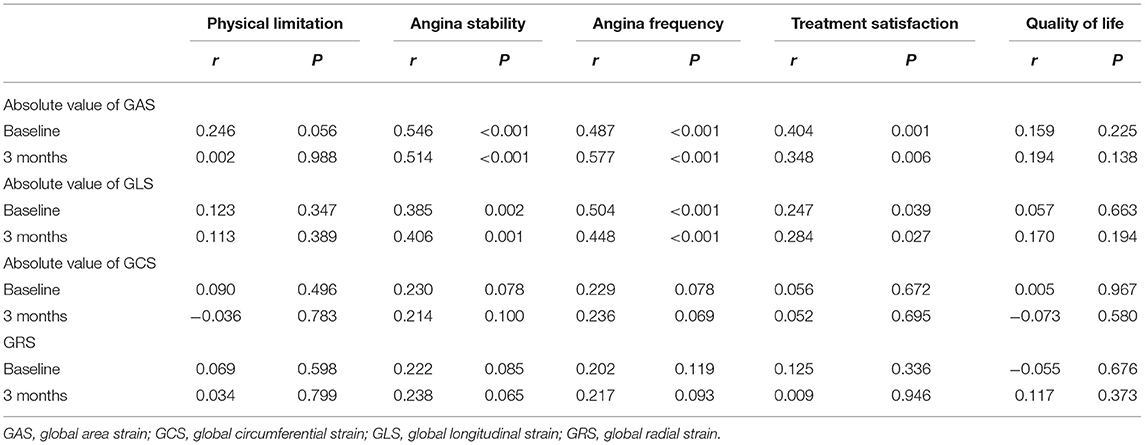
The absolute value of GAS was positively correlated with angina stability, angina frequency, and treatment satisfaction both at baseline and at 3 months. The absolute value of GLS was also positively correlated with angina stability, angina frequency, and treatment satisfaction both at baseline and at 3 months. However, GAS and GLS were not significantly correlated with a physical limitation or quality of life. GCS and GRS showed no significant relationship with SAQ scores (Table 6).
Discussion
This study has shown that nicorandil treatment as an adjunct to standard therapy improved GAS in patients with CTO. Nicorandil treatment also improved angina symptoms, as assessed by the SAQ. This study discusses their integration [(i.e., the key findings)] into the current understanding of the problem and how this advances and the current views speculate on the future direction of the research and freely postulate theories that could be tested in the future.
The potential benefits of CTO revascularization are still controversial. Although a number of observational studies have suggested that successful CTO PCI or CABG is associated with better cardiac function and long-term clinical outcomes (5–8), RCTs comparing CTO PCI plus OMT vs. OMT alone failed to show a reduction in clinical events or an improvement in LV function by CTO PCI (15–18). Thus, medications are fundamental in these patients.
Like nitrates, nicorandil dilates epicardial coronary arteries and increases coronary collateral blood flow via nitric oxide-mediated signaling pathways (19). In addition, nicorandil dilates the microvasculature to improve cardiac perfusion by activating potassium ATP channels (19). Nicorandil also protects cardiomyocytes by opening the mitochondrial potassium ATP channels in ischemia-reperfusion conditions (20). In patients with ST-segment elevation myocardial infarction, intracoronary or intravenous administration of nicorandil during primary PCI improved myocardial microcirculation and reduced infarct size (21–23). In the placebo-controlled Impact Of Nicorandil in Angina (IONA) trial (24), compared with placebo, nicorandil given in addition to standard anti-anginal treatment significantly decreased the composite endpoint (coronary heart disease death, non-fatal myocardial infarction, or unplanned hospital admission for cardiac chest pain) by 17% in patients with stable angina. In the Japanese Coronary Artery Disease (JCAD) study (25), nicorandil improved all-cause mortality by 35% in patients with CAD. An RCT by Jiang et al. (26) showed that nicorandil therapy reduced ischemic episodes on 24-h Holter monitoring. However, RCTs evaluating the effect of nicorandil in patients with CTO are lacking. Our study used 3D-STE to detect myocardial ischemia, and we found that in patients with coronary CTO, nicorandil therapy for 3 months was associated with a significant improvement in GAS.
Two-dimensional echocardiography is commonly used to detect regional wall-motion abnormalities (RWMA) and decreased LVEF in CAD patients. However, the interpretation of RWMA and the measurement of LVEF are strongly dependent on the skills of the operator. Moreover, changes in RWMA and LVEF may not be present even in patients with CTO. STE enables the objective assessment of global and regional LV myocardial deformation. Myocardial ischemia could lead to changes in LV myocardial deformation that may not be detectable with RWMA and LVEF (27). Recently, 3D-STE has emerged and can provide a comprehensive assessment of all LV segments. Longitudinal, circumferential, and radial strains, which reflect myocardial deformation in the three directions, can be calculated simultaneously using 3D-STE (12, 13). 3D-STE can also provide area strain values, which is a novel strain parameter. Area strain reflects the decrease in endocardial surface area during LV contraction and is related to longitudinal and circumferential myocardial shortening. Studies have shown that GAS has a better diagnostic value for severe coronary stenosis than that provided by other strain parameters. In the study by Li et al., (12), the area under the curve for GLS, GRS, GCS, and GAS was 0.896, 0.866, 0.797, and 0.909, respectively, for the identification of severe stenosis in suspected patients without LV-RWMA. In a study by Dogdus et al. (13), GAS was best correlated with Gensini score, and a GAS value of > −21% had 97.2% sensitivity and 88.1% specificity to detect critical CAD. In our study, the absolute value of GAS and GLS were positively correlated with SAQ scores, but GCS and GRS were not significantly correlated with SAQ scores, indicating that both GAS and GLS are sensitive for detecting myocardial ischemia. However, only GAS was significantly improved by nicorandil treatment. This could be because GAS reflects both longitudinal and circumferential components of myocardial deformation, and is more sensitive for detecting myocardial ischemia than the other strain parameters.
The 19-item SAQ has been used extensively in clinical studies and in clinical practice to assess angina-related health status. SAQ scores are independently associated with the risks of subsequent death, hospitalization, myocardial infarction, and increased health care costs. Furthermore, SAQ has been recommended as a standard outcome measure for patients with CAD by the International Consortium for Health Outcomes Measurement (28). In our study, angina stability and treatment satisfaction improved significantly, and angina frequency decreased significantly, with nicorandil treatment. However, the scores for physical limitation and quality of life were not significantly different between the two groups. This might be because physical limitation and quality of life were also influenced by several factors other than myocardial ischemia (29, 30).
Limitations
This was a single-center open-label study with small sample size. Thus, the risk of bias must be acknowledged. The follow-up time was short, and the long-term effect of nicorandil requires further investigation. Additionally, patients with heart failure (New York Heart Association class III or IV) were excluded, and most patients included in this study had preserved LVEF. The protective effect of nicorandil could not be extended to all CTO patients. Furthermore, we did not assess myocardial ischemia by stress tests in patients with CTO because we aimed to determine the effect of nicorandil on the improvement of LV myocardial strain in patients with CTO.
Conclusion
Nicorandil treatment as an adjunct to standard therapy can improve LV myocardial strain and angina symptoms in patients with CTO. Therefore, nicorandil is beneficial for CTO patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Review Board of Peking University Third Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SC: data collection and writing the article. CM: patients' following. XF: doing echocardiography. MC: designing the study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Sciences Foundation of China (82070272, to MC) and Natural Science Foundation of Beijing Municipality (7202225, to MC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Koelbl CO, Nedeljkovic ZS, Jacobs AK. Coronary chronic total occlusion (CTO): a review. Rev Cardiovasc Med. (2018) 19:33–9. doi: 10.31083/j.rcm.2018.01.896
2. Keulards DCJ, Vlaar PJ, Wijnbergen I, Pijls NHJ, Teeuwen K. Coronary physiology before and after chronic total occlusion treatment: what does it tell us? Neth Heart J. (2021) 29:22–9. doi: 10.1007/s12471-020-01470-6
3. Damluji AA, Pomenti SF, Ramireddy A, Al-Damluji MS, Alfonso CE, Schob AH, et al. Influence of total coronary occlusion on clinical outcomes (from the bypass angioplasty revascularization investigation 2 diabetes trial). Am J Cardiol. (2016) 117:1031–8. doi: 10.1016/j.amjcard.2015.12.047
4. Chi WK, Gong M, Bazoukis G, Yan BP, Letsas KP, Liu T, et al. International Health Informatics Study (IHIS) network. Impact of coronary artery chronic total occlusion on arrhythmic and mortality outcomes: a systematic review and meta-analysis. JACC Clin Electrophysiol. (2018) 4:1214–23. doi: 10.1016/j.jacep.2018.06.011
5. Christakopoulos GE, Christopoulos G, Carlino M, Jeroudi OM, Roesle M, Rangan BV, et al. Meta-analysis of clinical outcomes of patients who underwent percutaneous coronary interventions for chronic total occlusions. Am J Cardiol. (2015) 115:1367–75. doi: 10.1016/j.amjcard.2015.02.038
6. Khan AA, Khalid MF, Ayub MT, Murtaza G, Sardar R, White CJ, et al. Outcomes of percutaneous coronary intervention versus optimal medical treatment for chronic total occlusion: a comprehensive meta-analysis. Curr Probl Cardiol. (2021) 46:100695. doi: 10.1016/j.cpcardiol.2020.100695
7. Galassi AR, Boukhris M, Toma A, Elhadj Z, Laroussi L, Gaemperli O, et al. Percutaneous coronary intervention of chronic total occlusions in patients with low left ventricular ejection fraction. JACC Cardiovasc Interv. (2017) 10:2158–70. doi: 10.1016/j.jcin.2017.06.058
8. Megaly M, Saad M, Tajti P, Burke MN, Chavez I, Gössl M, et al. Meta-analysis of the impact of successful chronic total occlusion percutaneous coronary intervention on left ventricular systolic function and reverse remodeling. J Interv Cardiol. (2018) 31:562–71. doi: 10.1111/joic.12538
9. Tajti P, Brilakis ES. Chronic total occlusion percutaneous coronary intervention: evidence and controversies. J Am Heart Assoc. (2018) 7:e006732. doi: 10.1161/JAHA.117.006732
10. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41:407–77. doi: 10.1093/eurheartj/ehz425
11. Voigt JU, Cvijic M. 2- and 3-dimensional myocardial strain in cardiac health and disease. JACC Cardiovasc Imaging. (2019) 12:1849–63. doi: 10.1016/j.jcmg.2019.01.044
12. Li L, Zhang PY, Ran H, Dong J, Fang LL. Ding QS. Evaluation of left ventricular myocardial mechanics by three-dimensional speckle tracking echocardiography in the patients with different graded coronary artery stenosis. Int J Cardiovasc Imaging. (2017) 33:1513–20. doi: 10.1007/s10554-017-1147-6
13. Dogdus M, Simsek E, Cinar CS. 3D-speckle tracking echocardiography for assessment of coronary artery disease severity in stable angina pectoris. Echocardiography. (2019) 36:320–7. doi: 10.1111/echo.14214
14. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, et al. Development and evaluation of the seattle angina questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. (1995) 25:333–41. doi: 10.1016/0735-1097(94)00397-9
15. Werner GS, Martin-Yuste V, Hildick-Smith D, Boudou N, Sianos G, Gelev V, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. (2018) 39:2484–93. doi: 10.1093/eurheartj/ehy220
16. Lee SW, Lee PH, Ahn JM, Park DW, Yun SC, Han S, et al. Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic total occlusion. Circulation. (2019) 139:1674–83. doi: 10.1161/CIRCULATIONAHA.118.031313
17. Henriques JP, Hoebers LP, Råmunddal T, Laanmets P, Eriksen E, Bax M, et al. EXPLORE Trial Investigators. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: the EXPLORE trial. J Am Coll Cardiol. (2016) 68:1622–32. doi: 10.1016/j.jacc.2016.07.744
18. Mashayekhi K, Nührenberg TG, Toma A, Gick M, Ferenc M, Hochholzer W, et al. A randomized trial to assess regional left ventricular function after stent implantation in chronic total occlusion: the REVASC trial. JACC Cardiovasc Interv. (2018) 11:1982–91. doi: 10.1016/j.jcin.2018.05.041
19. Tarkin JM, Kaski JC. Nicorandil and Long-acting nitrates: vasodilator therapies for the management of chronic stable angina pectoris. Eur Cardiol. (2018) 13:23–8. doi: 10.15420/ecr.2018.9.2
20. Jiang X, Wu D, Jiang Z, Ling W, Qian G. Protective effect of nicorandil on cardiac microvascular injury: role of mitochondrial integrity. Oxid Med Cell Longev. (2021) 2021:4665632. doi: 10.1155/2021/4665632
21. Qi Q, Niu J, Chen T, Yin H, Wang T, Jiang Z. Intracoronary nicorandil and the prevention of the no-reflow phenomenon during primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Med Sci Monit. (2018) 24:2767–76. doi: 10.12659/MSM.906815
22. Feng C, Han B, Liu Y, Wang L, Niu D, Lou M, et al. Effect of nicorandil administration on myocardial microcirculation during primary percutaneous coronary intervention in patients with acute myocardial infarction. Postepy Kardiol Interwencyjnej. (2018) 14:26–31. doi: 10.5114/aic.2018.74352
23. Yamada K, Isobe S, Ishii H, Yokouchi K, Iwata H, Sawada K, et al. Impacts of nicorandil on infarct myocardium in comparison with nitrate: assessed by cardiac magnetic resonance imaging. Heart Vessels. (2016) 31:1430–7. doi: 10.1007/s00380-015-0752-3
24. IONA Study Group. Effect of nicorandil on coronary events in patients with stable angina: the impact of nicorandil in angina (IONA) randomised trial. Lancet. (2002) 359:1269–75. doi: 10.1016/S0140-6736(02)08265-X
25. Horinaka S, Yabe A, Yagi H, Ishimitsu T, Yamazaki T, Suzuki S, et al. JCAD Study Investigators. Effects of nicorandil on cardiovascular events in patients with coronary artery disease in the Japanese Coronary Artery Disease (JCAD) study. Circ J. (2010) 74:503–9. doi: 10.1253/circj.CJ-09-0649
26. Jiang J, Li Y, Zhou Y, Li X, Li H, Tang B, et al. Oral nicorandil reduces ischemic attacks in patients with stable angina: a prospective, multicenter, open-label, randomized, controlled study. Int J Cardiol. (2016) 224:183–7. doi: 10.1016/j.ijcard.2016.08.305
27. Yang B, Daimon M, Ishii K, Kawata T, Miyazaki S, Hirose K, et al. Prediction of coronary artery stenosis at rest in patients with normal left ventricular wall motion. Segmental analyses using strain imaging diastolic index. Int Heart J. (2013) 54:266–72. doi: 10.1536/ihj.54.266
28. McNamara RL, Spatz ES, Kelley TA, Stowell CJ, Beltrame J, Heidenreich P, et al. Standardized outcome measurement for patients with coronary artery disease: consensus from the international consortium for health outcomes measurement (ICHOM). J Am Heart Assoc. (2015) 4:e001767. doi: 10.1161/JAHA.115.001767
29. Joussain C, Joubert J, Laroche D, D'Antono B, Juneau M. Gremeaux V. Barriers to physical activity in coronary artery disease patients: development and validation of a new scale. Ann Phys Rehabil Med. (2017) 60:289–98. doi: 10.1016/j.rehab.2017.01.002
Keywords: chronic total occlus, nicorandil, speckle tracking echocardiograph, global area strain, coronary heart disease
Citation: Chen S, Ma C, Feng X and Cui M (2022) Nicorandil Improves Left Ventricular Myocardial Strain in Patients With Coronary Chronic Total Occlusion. Front. Cardiovasc. Med. 9:864223. doi: 10.3389/fcvm.2022.864223
Received: 28 January 2022; Accepted: 17 March 2022;
Published: 12 May 2022.
Edited by:
Erhan Tenekecioglu, University of Health Sciences, TurkeyReviewed by:
Kevser Gülcihan BalcI, Ankara City Hospital, TurkeyIbrahim Kocayigit, Sakarya University, Turkey
Copyright © 2022 Chen, Ma, Feng and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Cui, bWluZ2N1aUBiam11LmVkdS5jbg==
 Shaomin Chen
Shaomin Chen Chen Ma2,3
Chen Ma2,3 Ming Cui
Ming Cui