
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 26 April 2022
Sec. Cardiovascular Imaging
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.857360
This article is part of the Research TopicAdvances in Cardiac Imaging and Heart Failure ManagementView all 42 articles
 Jen-Yuan Kuo1,2,3
Jen-Yuan Kuo1,2,3 Xuanyi Jin4,5
Xuanyi Jin4,5 Jing-Yi Sun6
Jing-Yi Sun6 Sheng-Hsiung Chang1,3
Sheng-Hsiung Chang1,3 Po-Ching Chi7
Po-Ching Chi7 Kuo-Tzu Sung1,2,3
Kuo-Tzu Sung1,2,3 Greta S. P. Mok8
Greta S. P. Mok8 Chun-Ho Yun2,3,9
Chun-Ho Yun2,3,9 Shun-Chuan Chang10
Shun-Chuan Chang10 Fa-Po Chung11,12
Fa-Po Chung11,12 Ching-Hsiang Yu2
Ching-Hsiang Yu2 Tung-Hsin Wu5*
Tung-Hsin Wu5* Chung-Lieh Hung1,2,13*
Chung-Lieh Hung1,2,13* Hung-I Yeh1,2,3
Hung-I Yeh1,2,3 Carolyn S. P. Lam4,5
Carolyn S. P. Lam4,5Background: Heart failure with preserved ejection fraction (HFpEF) and atrial fibrillation (AF) commonly coexist with overlapping pathophysiology like left atrial (LA) remodeling, which might differ given different underlying mechanisms.
Objectives: We sought to investigate the different patterns of LA wall remodeling in AF vs. HFpEF.
Methods: We compared LA wall characteristics including wall volume (LAWV), wall thickness (LAWT), and wall thickness heterogeneity (LAWT[SD]) and LA structure, function among the controls (without AF or HFpEF, n = 115), HFpEF alone (n = 59), AF alone (n = 37), and HFpEF+AF (n = 38) groups using multi-detector computed tomography and echocardiography.
Results: LA wall remodeling was most predominant and peak atrial longitudinal strain (PALS) was worst in HFpEF+AF patients as compared to the rest. Despite lower E/e' (9.8 ± 3.8 vs. 13.4 ± 6.4) yet comparable LA volume, LAWT and PALS in AF alone vs. HFpEF alone, LAWV [12.6 (11.6–15.3) vs. 12.0 (10.2–13.7); p = 0.01] and LAWT(SD) [0.68 (0.61–0.71) vs. 0.60 (0.56–0.65); p < 0.001] were significantly greater in AF alone vs. HFpEF alone even after multi-variate adjustment and propensity matching. After excluding the HFpEF+AF group, both LAWV and LAWT [SD] provided incremental values when added to PALS or LAVi (all p for net reclassification improvement <0.05) in discriminating AF alone, with LAWT[SD] yielding the largest C-statistic (0.78, 95% CI: 0.70–0.86) among all LA wall indices.
Conclusions: Despite a similar extent of LA enlargement and dysfunction in HFpEF vs. AF alone, larger LAWV and LAWT [SD] can distinguish AF from HFpEF alone, suggesting the distinct underlying pathophysiological mechanism of LA remodeling in AF vs. HFpEF.
Heart failure with preserved ejection fraction (HFpEF) and atrial fibrillation (AF) are frequently coexisting conditions that are growing in prevalence and share common predisposing factors such as older age, hypertension, and obesity (1–3). It remains a diagnostic dilemma to discriminate HFpEF from AF due to convergent clinical features (e.g., breathlessness, effort intolerance). Yet, to accurately distinguish one condition from the other is important since their treatments may differ, partly due to their potentially distinct underlying pathophysiology.
Beyond sharing predisposing factors, left atrial (LA) remodeling is also a key characteristic that is common to both HFpEF and AF. In HFpEF, LA remodeling is thought to be secondary to an increase in LA pressure and potential intrinsic LA myopathy; however, in AF, LA remodeling is thought to be triggered by ischemia, inflammation, or dilation and perpetuated by tachycardia-induced remodeling (4, 5). The common result in both conditions is an enlarged, dysfunctional left atrium, which is difficult to differentiate by conventional imaging modalities. Compared to these traditional methods, more advanced imaging techniques such as multi-detector computed tomography (MDCT) with higher spatial resolution or echocardiographic strain imaging are known to provide additional information about LA structure and function beyond conventional imaging modalities, which may provide novel insights into LA remodeling and distinct patterns of LA remodeling in AF vs. HFpEF (6).
Therefore, we aimed to compare the LA structure, function, and wall characteristics among four groups of patients, namely the controls without HFpEF or AF, patients with HFpEF alone, patients with AF alone, and patients with both HFpEF and AF, by using a combination of MDCT and comprehensive echocardiography.
Patients who were referred to the MacKay Memorial Hospital (Taipei, Taiwan) from a cardiovascular imaging core laboratory for clinical evaluation of ischemic heart disease or AF ablation between January 2009 and December 2014 were retrospectively identified through a medical record review. HFpEF was defined as prior HF hospitalization and a left ventricular ejection fraction (LVEF) of >50%. Adjudication of HF hospitalization was defined by two cardiologists with typical symptoms (dyspnea, breathlessness or ankle swelling) and signs of pulmonary congestion or edema by Chest X-ray. These were followed by natriuretic peptide cutoffs (BNP: 100 pg/ml, NT-proBNP: 300 pg/ml) proposed by 2021 ESC HF guideline to exclude those without acute HF. AF was ascertained by a history of paroxysmal or persistent AF or those referred for evaluation of catheter ablation for AF. Diagnosis of AF was made by standard 12-lead electrocardiogram (ECG), or ECG tracing of ≥30 s showing AF rhythm from 24-h Holter monitoring. Days of diagnosed paroxysmal AF in our AF patients was 181.1 ± 71.2 days. Each patient underwent comprehensive clinical evaluation, laboratory tests, Holter monitoring, dual-source cardiac MDCT scans, and comprehensive echocardiography. Imaging (including echocardiography and MDCT) protocol was conducted during sinus rhythm prior to AF ablation (if ablation therapy was delivered). Patients with significant valvular heart disease, severe pulmonary hypertension, known cardiomyopathy, permanent AF, and those with pacemaker implantation were excluded. Thus, a total of 249 patients were included and divided into four groups, namely the HFpEF alone (n = 59), AF alone (n = 37), HFpEF+AF (n = 38, both HFpEF and AF), and controls (n = 115 with neither HFpEF nor AF) groups (Figure 1B). The study protocol was approved by the Institutional Review Board of MacKay Memorial Hospital (19MMHIS213e).

Figure 1. Five major steps in the LA mapping workflow for LA wall indices in the current study: (1) LA delineation, (2) inner boundary segmentation, (3) outer boundary segmentation, (4) wall mass calculation, (5) three-dimensional (3D) projection map, and display of the LAWT (SD). The LAWT in each dataset was expressed by different colors as a visual projection map together with four PVs orifices. Nos. 1, 2, 3, and 4 represent the right superior, right inferior, left superior, and left inferior PVs, respectively (A). Cardiac CT images from 3 representative individuals from the Control, HFpEF, and AF groups (B). LA, left atrial; LAWT, left atrial wall thickness; LAWT (SD), left atrial wall thickness heterogeneity; PV, pulmonary vein; MDCT, multi-detector computed tomography; HFpEF, heart failure with preserved ejection fraction; AF, atrial fibrillation.
Each patient underwent cardiac MDCT using a dual-source, high-resolution CT system (Siemens Medical Systems, Forchheim, Germany). The step-by-step protocols for scanning, image acquisition, reconstruction, and subsequent LA wall characteristics analysis using our validated LA wall-mapping software have been previously published (Supplementary Materials) (6). In brief, the LA wall-mapping application enables the automatic isocenter setting of the LA chamber, LA inner-surface, and outer-boundary segmentation, and facilitates delineation after manual delineation of the pulmonary veins. The LA wall volume (LAWV) was derived by integrating the total number of voxels of the LA tissue, which yielded the total LA volume contained within the outer boundaries, minus the chamber volume within the inner surface of LA. Wall thickness was defined as the shortest distance between the outer boundaries and the inner surfaces, with the representative LA wall thickness (LAWT) of any individual calculated as the average distance within the entire LA region. LAWT heterogeneity was assessed as the variations [expressed as standard deviation (SD); thus, LAWT(SD)] in the LAWT within the entire LA region measured by the LA wall-mapping program (Figure 1A). Finally, the LA volume (LAV) was calculated by integrating the number of voxels contained within the inner surfaces, with the LAV further indexed (LAVi) to the individual's body surface area.
Each patient underwent a complete transthoracic echocardiography exam within 30 days of MCDT, using a Vivid 7 system (GE Healthcare, Chicago, IL, USA), equipped with a 2- to 4-MHz transducer (M4S). All echocardiographic image acquisition and measurements were performed on the basis of recent guidelines recommended by the American Society of Echocardiography (7). All echocardiographic measurements, including two-dimensional speckle-tracking echocardiographic analysis, were performed off-line using EchoPAC version 6.1.2 (GE Vingmed, Horten, Norway). The protocol for the same was previously described in detail (8). LV end-diastolic volume (EDV) and LVEF were measured using the biplane Simpson method. Pulse wave Doppler was applied to record the trans-mitral inflow early (E) and late diastolic (A) filling velocities, deceleration time (DT), and isovolumic relaxation time. Peak tricuspid regurgitation (TRV) velocity was obtained using continuous wave Doppler. Peak mitral annular systolic (TDI-s′) and early diastolic (TDI-e′) velocities were obtained using Tissue Doppler imaging and were averaged from the septal and lateral mitral annular sides, respectively. The ratio of mitral inflow E over A (E/A) and the average E/e' ratio (E/e') were calculated accordingly, with E/e' used for estimating the LV filling condition. The LV global longitudinal strain (LVGLS) was averaged from the peak longitudinal strain across 18 LV segments obtained from the LV apical 4-, 2-, and 3-chamber views. Global peak atrial longitudinal strain (PALS) and global longitudinal LA strain rate during the reservoir (SRs), conduit (SRe), and booster (SRa) phases were averaged from all LA segments of the apical 4- and 2-chamber views, respectively.
A priori sample size calculation, based on the LAWT differences, was performed using the Power and Sample Size software version 08 (NCSS, LLC, Kaysville, UT, USA). All data were expressed as means ± standard deviation (SD) or medians ±inter-quartile range (IQR) for continuous variables and percentage or frequency for categorical variables. Comparative analysis for parametric continuous variables was performed using a one-way analysis of variance or an independent t-test and for non-parametric continuous variables using the Kruskal–Wallis test or a Mann-Whitney U test. Categorical variables were compared using either the Fisher's exact test or the χ2 test with a Yates correction, as appropriate. Furthermore, we compared LA wall characteristics in control vs. HFpEF alone and control vs. AF alone after stratified based on median value of MDCT-based LAVi (<40 vs. ≥40 ml/m2), respectively. Associations of various LA wall indices with clinical co-variates were determined by forward stepwise selection including age, sex, body mass index (BMI), lipid profile, medical history, estimated glomerular filtration rate (eGFR) calculated by MDRD equation, high sensitivity C-reactive protein (hs-CRP), and E/e'. To further elaborate the LA wall features in patients with isolated AF, C-statistic was used to examine the performance of LA wall indices in distinguishing AF from HFpEF and controls after excluding the HFpEF+AF group. Moreover, comparisons were made again after 1:1 matching (controls and HFpEF vs. AF) for key clinical co-variates. Net reclassification index was used to assess the incremental value of LA wall indices in re-classifying AF in addition to LAVi and PALS.
The linear correlations between the LA wall indices and echocardiographic parameters were determined using the Pearson correlation coefficient (r) and maximal information criteria (MIC). The differences in absolute values between the MIC and the squared Pearson correlation (r2) were used to determine the non-linear correlation (i.e., MIC-r2 ≥ 0.1). Subsequently, the cluster model was constructed to assess the similarity between LA wall indices and echocardiographic parameters, and the details of constructing this cluster model can be found in the Supplementary Material. All analyses were performed using STATA 14.0 software (Stata Corp, College Station, TX, USA). All p-values were two-tailed, with p < 0.05 considered statistically significant.
Table 1 summarizes the baseline clinical, laboratory, and echocardiographic characteristics of all patients. Patients with HFpEF+AF demonstrated the highest comorbidity burdens, highest BNP levels, worst renal function based on the eGFR, the most advanced cardiac remodeling and worst LV, LA function as compared to the rest of patients. Despite similar age and BMI, patients with AF alone had a higher burden of hypertension and diabetes as compared to control participants. Whereas, patients with HFpEF alone were more likely to be older, female, and present with more comorbidities, including hypertension, diabetes, and coronary artery disease, and higher BMI as compared to control participants. Similarly, patients with HFpEF alone were more likely to be older, female, and present with diabetes and higher B-type natriuretic peptide (BNP) level as compared to the patients with AF alone.
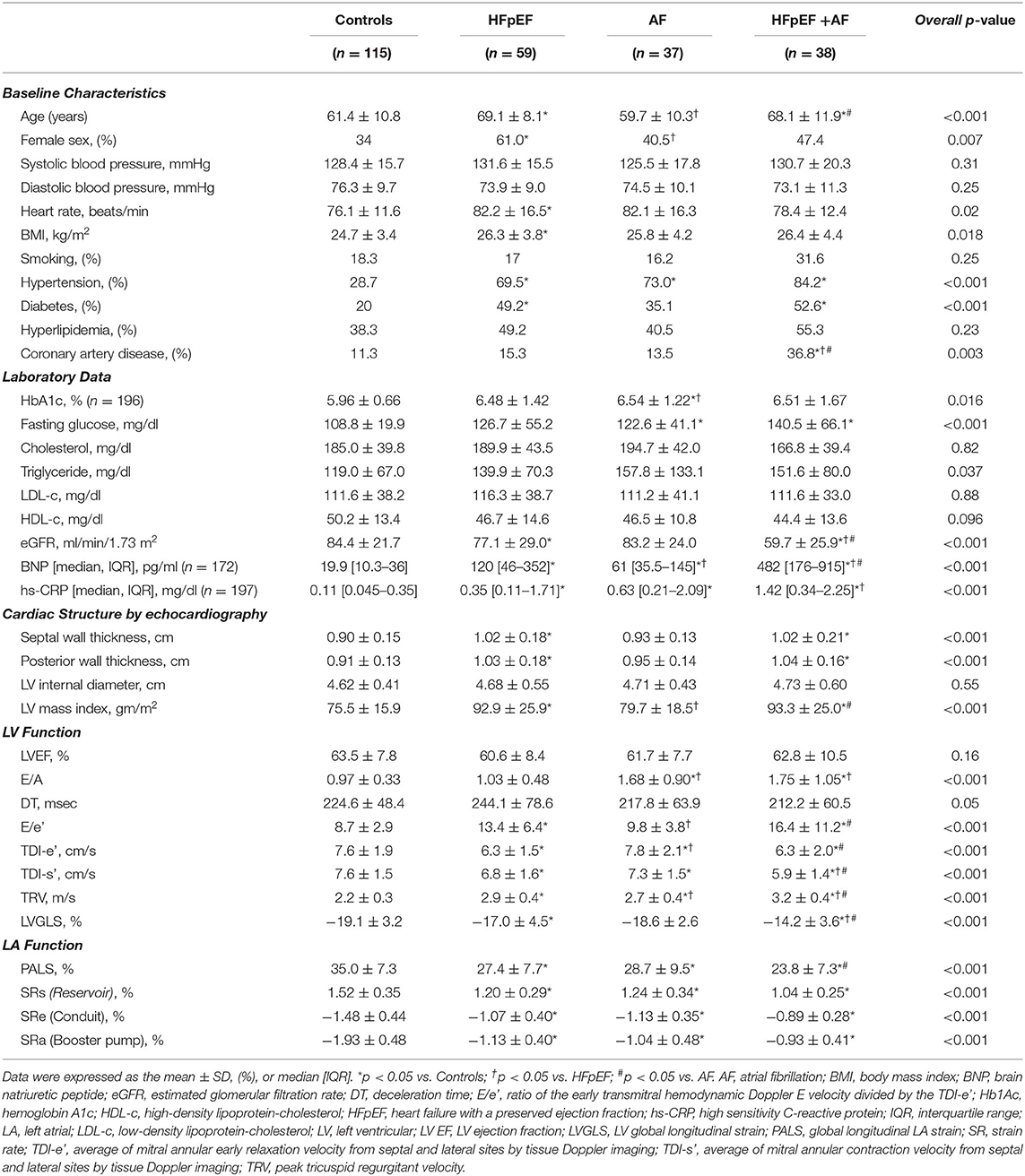
Table 1. Baseline characteristics and conventional echocardiographic parameters of the study subjects.
Patients with HFpEF alone and HFpEF+AF showed greater LV mass index as compared to both control and patients with AF alone; whereas LV mass index was similar between control and patients with AF alone (Table 1). As expected, LV function evaluated by LVGLS or TDI-s' was worse in patients with HFpEF+AF as compared to the rest of the patients, despite similar LVEF among the four groups. Although LVGLS or TDI-s' was worse in patients with HFpEF alone, but similar in patients with AF alone as compared to control patients. Notably, LV diastolic dysfunction presented with reduced TDI-e', increased E/e' ratio and higher pulmonary arterial pressure estimated by TRV, was more advanced in the patients with HFpEF alone and HFpEF+AF as compared to the patients with AF alone.
Table 2 shows the LA wall characteristics among the four groups. Reproducibility of the all LA wall indices in 30 random study participants are presented in Supplementary Table 1. All LA wall indices [LAWV, 15.4 (13.5~18.2) ml; LAWT, 2.10 (2.00~2.27) mm; LAWT (SD), 0.64 (0.57~0.71) and LAVi [62.3 (48.6~81.0) ml/m2] was largest, and PALS (23.8 ± 7.3 %) as well as phasic LA strain rate components (Table 2) were worst in patients with HFpEF+AF group, indicating the most advanced LA remodeling and LA global dysfunction in this group. In contrast, all LA wall indices [LAWV, 10.7 (9.5~12.1) ml; LAWT, 1.91 (1.81~2.02) mm; LAWT (SD), 0.58 (0.55~0.61) and LAVi [LAVi, 33.6 (27.3~39.5) ml/m2] were smallest, PALS (35 ± 7.3%) as well as LA phasic strain rate components were largest in control patients (Table 2). The LAVi (LAVi: 44.4 [37.2–52.1] vs. 45.8 [34.6~51.4] ml/m2; p = 0.46 in HFpEF vs. AF alone), all LA global phasic function (PALS, SRs, SRe, and SRa; Table 1) and LAWT [2.02 (1.91–2.12) vs. 2.06 (1.99–2.22) mm; p > 0.05 in HFpEF vs. AF alone] were similar in the patients with HFpEF alone vs. AF alone. Despite many similarities of LAVi, LAWT and global LA function, the LAWV and LAWT (SD) were significantly larger in patients with AF alone as compared to patients with HFpEF alone [LAWV:12.6 (11.6–15.3) vs. 12.0 (10.2–13.7) ml, p = 0.01; and LAWT(SD): 0.68 (0.61–0.71) vs. 0.60 (0.56–0.65), p <0.001, respectively]. Notably, LAWT(SD) was substantially greater in patients with AF alone than in patients with HFpEF alone even after adjusting for key clinical co-variates and E/e' (Table 2) or after 1:1 propensity matching based on key baseline co-variates in control patients and patients with HFpEF alone vs. patients with AF alone (Supplementary Table 2) (Table 3).
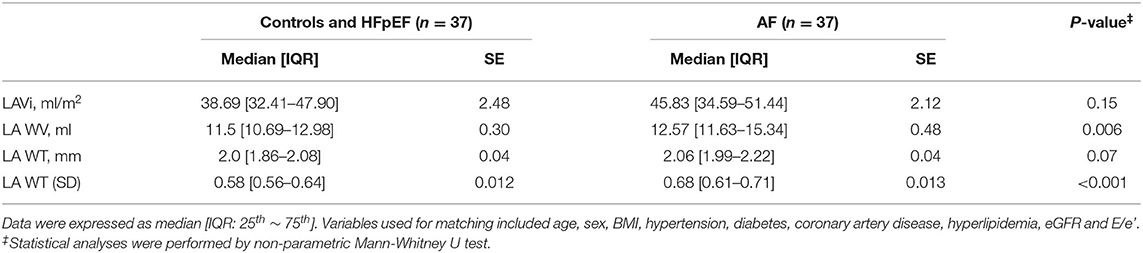
Table 3. Comparisons of atrial structure and LA wall indices of the study subjects by MDCT after matching.
By forward stepwise selection, LAVi enlargement was independently associated with greater BMI and higher E/e' (all p <0.05), and LAWV enlargement was independently associated with greater BMI and lower eGFR (all p < 0.05). Increase of LAWT was independently associated with older age and presence of hypertension (all p <0.05). Furthermore, greater LAWT(SD) was independently associated with the male sex, higher hs-CRP level, and lower high-density lipoprotein-cholesterol level (all p <0.05).
All LA wall indices were negatively associated with PALS (Figure 2B, beta-coefficients for LAVi, LAWV, LAWT, and LAWT(SD) were −0.43, −0.40, −0.36, and −0.21, respectively; all p ≤ 001), indicating the association between the increase of LA wall indices and worsening global LA function (Figure 2B). All correlations were non-linear, as indicated by MIC-r2 > 0.1 (Figure 3, Supplementary Figure 1; details in Supplementary Materials). Variable clustering and similarity assessment (Figure 3) showed that LAWV had significant proximity with LAVi; whereas LAWT(SD) tightly coupled with LAWT, did not show significant proximity with any echocardiographic parameters in the dendrogram (Supplementary Figure 2), suggesting the potential value of these measures as novel LA metrics depicting LA remodeling.
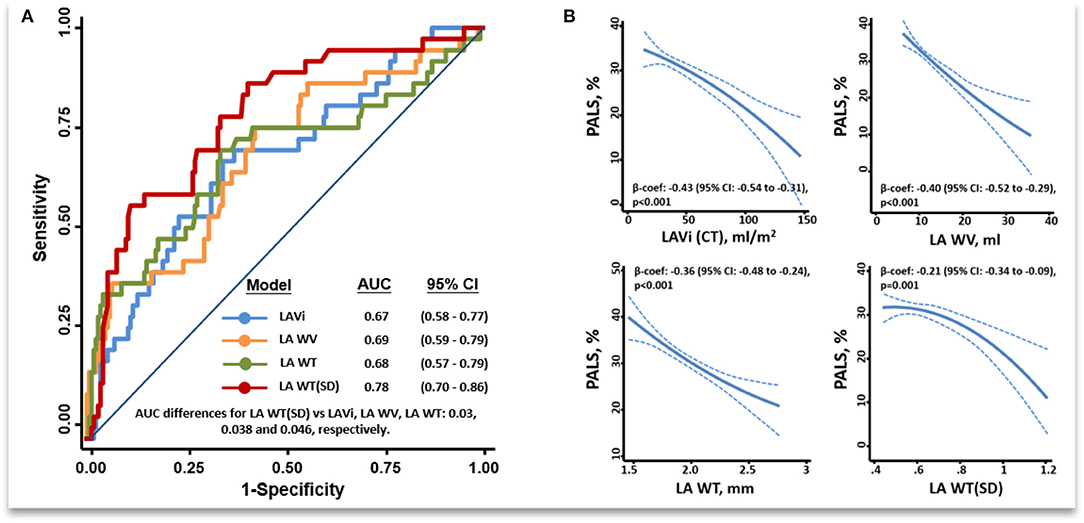
Figure 2. ROC among all LA wall indices in discriminating isolated AF from control and HFpEF after excluding patients with both HFpEF and AF (final n = 211) (A). Fitting curves showing inverse associations between a greater unfavorable remodeling of the various MDCT LA wall indices and PALS (B). ROC, receiver operating characteristic curve; PALS, Peak atrial longitudinal strain; other abbreviations as Figure 1.
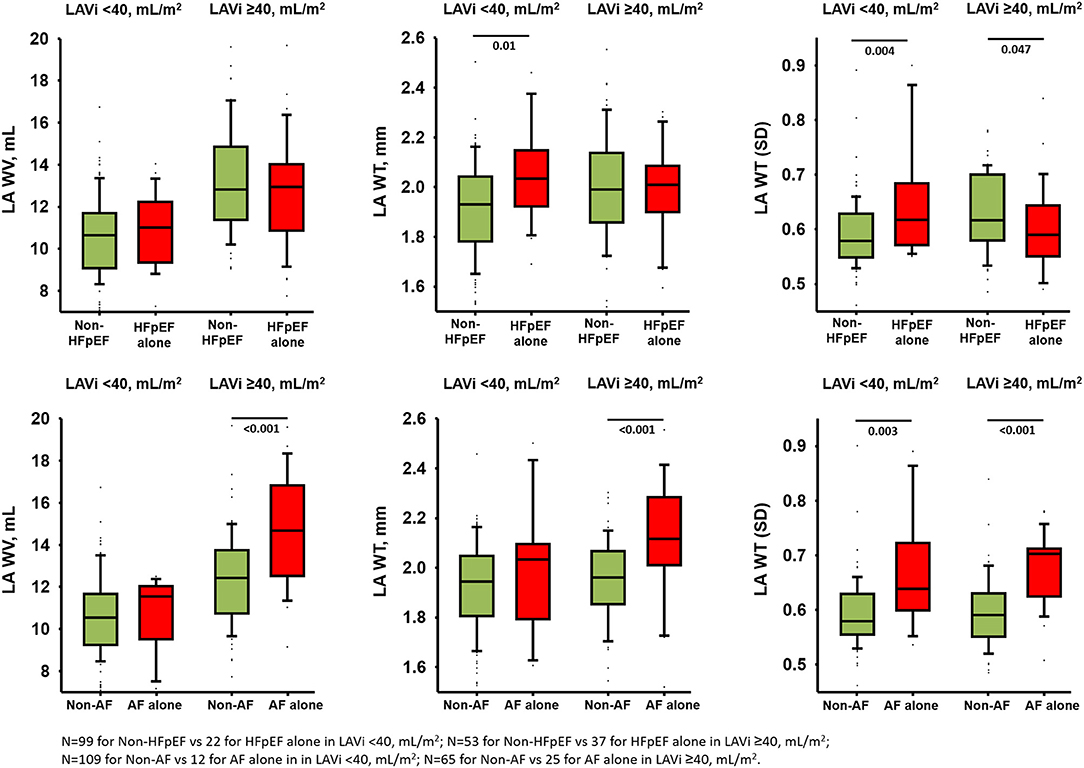
Figure 3. Comparisons of LA wall indices in isolated HFpEF and AF in smaller and larger indexed LA volume after excluding patients with both HFpEF and AF (total n = 211).
After excluding patients with HFpEF+AF (final n = 211), the LAWT(SD) demonstrated the highest discriminatory ability for distinguishing isolated AF from isolated HFpEF [optimal cutoff: 0.60, C-statistic: 0.78 (95% CI: 0.70–0.86); sensitivity: 93.8%, specificity: 68.2%] among all LA wall indices (Figure 2A). Besides, isolated AF [adjusted Coef: 0.08 (95% CI: 0.05–0.11), p <0.001] rather than isolated HFpEF was independently associated with larger LAWT (SD) after correcting baseline co-variates and E/e'. Remarkably, the trend of differences in LAWT (SD) comparing HFpEF vs. non-HFpEF was flipped after stratified by LAVi; whereas such trend was consistent when comparing AF vs. non-AF after stratified by LAVi, suggesting “diffuse” vs. “patchy” LA wall thickening in HFpEF vs. AF during LA enlargement (Figure 3). Finally, LAWV and LAWT (SD) significantly re-classify isolated AF from control and isolated HFpEF when added to LAVi [continuous net reclassification improvement (NRI): 56.9% (p = 0.002) and 71.2% (p <0.001)] and PALS [NRI: 42.2% (p = 0.02] and 72.7% (p <0.001), respectively] (Supplementary Table 3).
To the best of our knowledge, this is the first study to compare LA wall characteristics and function using MDCT method in addition to echocardiography among controls, patients with HFpEF alone, AF alone, and both HFpEF and AF. Overall, increase of each LA wall index was related to worse LA function assessed by strain, with each LA wall index showing distinctive associations with corresponding clinical risk factors. Despite similar LAVi, LAWT, and PALS, patients with AF alone had significantly larger LAWV and LAWT (SD) as compared to the patients with HFpEF alone, even after LA afterload (E/e') taken into account. Furthermore, LAWT (SD) showed the largest C-statistic value in discriminating isolated AF from isolated HFpEF patients and control. Besides, LAWT (SD) did not show significant proximity to any echocardiographic parameter assessing LA, LV function by cluster analysis, suggesting that LAWT (SD) is likely to reflect a novel dimension of LA remodeling.
Although LA remodeling has shown to be a hallmark feature in both HFpEF and AF, no study has explored the distinct LA remodeling patterns in comparison of HFpEF with AF particularly in terms of the LA wall characteristics. Particularly, no studies described LA wall characteristics in patients with HFpEF, while a few studies reported LA wall characteristics in patients with AF with discrepant findings. Nakamura et al. found that the LA wall was thicker in patients with AF than in controls, which was in line with our findings (9). In the same study, patients with paroxysmal AF showed thicker LA wall than those with persistent AF (9). Conversely, Imada et al. found no differences in the LA wall thickness between patients with paroxysmal and those with persistent AF (9, 10). Although the durations and different types AF (i.e., paroxysmal vs. persistent) among the different cohorts might explain the discrepancy in LA wall thickening vs. thinning in patients with AF, but, it is often impossible to pinpoint the exact date of onset of AF in individual patient. On the other hand, these findings indicate that LA wall thickening is a dynamical process with AF progression. We postulated that distinct changes of LA wall may occur with a transition from either AF alone or HFpEF alone to concomitant HFpEF and AF in addition to changes in LA enlargement and dysfunction, which require further validation in future studies.
Similar to patients with AF alone, patients with HFpEF alone also presented with a larger LA wall volume, thicker LA wall, and worse LA global function than the controls. Importantly, despite a similar extent of LA enlargement/dysfunction and a lower LA afterload (i.e., lower E/e', lower BNP), patients with AF alone still manifested significantly larger LAWV and LAWT (SD) than patients with HFpEF alone after multiple adjustments (Table 2), indicating LA afterload might not be the single predominant pathological determinant driving greater wall thickness heterogeneity in isolated AF. These findings support the concept of distinct LA remodeling exist in HFpEF vs. AF. Patients with HFpEF are likely more characterized by “diffuse” LA wall thickening secondary to chronic LA hypertension resulting in increased LA stiffness. Whereas, patients with AF are more characterized by “patchy” LA wall thickening with greater LAWT (SD) irrespective of extent of LA enlargement. These “patchy” LA wall thickening consisting of continuous fibrotic insulations of myo-bundles may further exaggerate LA wall heterogeneity itself and mechanistically contribute to microanatomic re-entry substrates whereby harboring or maintaining AF and set up a vicious cycle of “AF begets AF” (11–13). Nakatani et al. found that LA wall thickness heterogeneity did not differ among patients with paroxysmal and persistent AF, suggesting that “patchy” LA wall thickening as a consistent LA remodeling pattern of AF irrespective of the AF type (14). Notably, these findings are novel since no overlap was found between parameter of LAWT(SD) in current study and other LA indices using comprehensive echocardiography measures. Taken collectively, these unique yet different features of LA wall remodeling support the concept of “diffuse” vs. “patchy” pattern of LA wall thickening between HFpEF vs. AF alone. Besides, when patients with AF are prone to develop concomitant HFpEF, the LA wall will inevitably ensue “diffused” pattern superimposed “patchy” wall thickening during LA size expansion.
We found that each LA wall index was distinctively associated with corresponding clinical covariates such as aging, obesity, metabolic or renal dysfunction, and LA afterload (E/e'), which heterogeneously promote and amplify specific distinguishable LA wall remodeling patterns (15, 16). We hypothesized that LA wall thickening accompanied by atrial volume expansion, atrial myocyte hypertrophy and interstitial fibrosis is a heterogeneous process resulted from aging, obesity, fluid retention and LA afterload, and further contribute to LA afterload increase itself. During these processes, a distinct LA remodeling pattern with differential LA wall characteristics may occur in parallel with predisposition to HFpEF vs. AF driven by certain gender (male vs. female) or metabolic (such as HDL level) effects together with overlapping risk factors (Figure 4) (11, 13–17). Patients with both AF and HFpEF may present with the worst LA function from both pathological LA remodeling features as compared to the other groups (12), with LA wall changes comprising both diffuse and patchy thickening.
This was a non-randomized, single-center retrospective study with a relatively small number of patients in the HFpEF+AF group as compared to the rest groups. We acknowledged the potential technical limitations of MDCT in discriminating specific, regional anatomical landmarks (for example, the crista terminalis or cava) with averaged LAWT or LAWT (SD) which may indicate a substrate in the pathogenesis of atrial myopathy in AF. Finally, the current study could not establish the definitive mechanism of LA remodeling and the pathological causal relationship between LA wall characteristics and the clinical risk factors in HFpEF and AF. Nonetheless, this is the very first study using MDCT and echocardiography to provide novel insights into LA remodeling in both HFpEF and AF. Finally, as we sought to identify progressive and potentially distinctive LA remodeling patterns in patients manifesting HFpEF or AF when compared to those without (controls), rigorous matching for baseline characters among controls and HFpEF/AF patients were not performed. Our findings warrant further study in other larger prospective cohorts.
Despite a similar extent of LA enlargement and dysfunction in AF and HFpEF, a larger LAWV and greater LAWT (SD) distinguish AF from HFpEF, suggesting differential mechanisms underlying driving distinct LA remodeling patterns in AF vs. HFpEF.
The datasets presented in this article are not readily available because of regulations from local Institutional Review Board. Requests to access the datasets should be directed to the corresponding author.
The study protocol was approved by the Institutional Review Board of MacKay Memorial Hospital (19MMHIS213e) as a retrospective research design, written informed consent from patients/participants was waived in this study.
J-YK: collected data, analyzed data, and wrote the paper. C-LH and T-HW: conceived, designed the study, and edited the paper. XJ and J-YS: performed the statistical analysis. C-HY and F-PC: acquisition of data, analysis, and interpretation of data. S-HC, P-CC, K-TS, GM, S-CC, and C-HY: conceptual frame work. S-HC, P-CC, K-TS, GM, S-CC, CL, and C-HY: reviewed paper. H-IY: have given final approval of the version to be published. All authors have read and approved the final manuscript.
This research was supported by the Ministry of Science and Technology (Taiwan) (grants NSC-101-2314-B-195-020, NSC103-2314-B-010-005-MY3, 103-2314-B-195-001-MY3, 101-2314-B-195-020 –MY1, MOST 103-2314-B-195-006-MY3, and NSC102-2314-B-002-046-MY3, 106-2314-B-195-008 -MY2, 108-2314-B-195-018-MY2, MOST 108-2314-B-195-018-MY2, MOST 109-2314-B-715-008, and MOST 110-2314-B-715-009-MY1), MacKay Memorial Hospital (10271, 10248, 10220, 10253, 10375, 10358, and E-102003), and the Taiwan Foundation for Geriatric Emergency and Critical Care.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank P-CC for his assistance with CT data collection and J-YS for imaging analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.857360/full#supplementary-material
1. Cikes M, Claggett B, Shah AM, Desai AS, Lewis EF, Shah SJ, et al. Atrial fibrillation in heart failure with preserved ejection fraction: the TOPCAT trial. JACC Heart Fail. (2018) 6:689–97. doi: 10.1016/j.jchf.2018.05.005
2. Kotecha D, Lam CSP, Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. (2016) 68:2217–28. doi: 10.1016/j.jacc.2016.08.048
3. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. (2006) 355:251–9. doi: 10.1056/NEJMoa052256
4. Reddy YNV, Obokata M, Egbe A, Yang JH, Pislaru S, Lin G, et al. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail. (2019) 21:891–900. doi: 10.1002/ejhf.1464
5. Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, Lloyd-Jones DM, et al. Evaluating the atrial myopathy underlying atrial fibrillation: identifying the arrhythmogenic and thrombogenic substrate. Circulation. (2015) 132:278–91. doi: 10.1161/CIRCULATIONAHA.115.016795
6. Sun JY, Yun CH, Mok GSP, Liu YH, Hung CL, Wu TH, et al. Left atrium wall-mapping application for wall thickness visualisation. Sci Rep. (2018) 8:1–3. doi: 10.1038/s41598-018-22089-z
7. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–70. doi: 10.1093/ehjci/jev014
8. Hung CL, Gonçalves A, Lai YJ, Lai YH, Sung KT, Lo CI, et al. Light to moderate habitual alcohol consumption is associated with subclinical ventricular and left atrial mechanical dysfunction in an asymptomatic population: dose-response and propensity analysis. J Am Soc Echocardiogr. (2016) 29:1043–51. doi: 10.1016/j.echo.2016.07.014
9. Nakamura K, Funabashi N, Uehara M, Ueda M, Murayama T, Takaoka H, et al. Left atrial wall thickness in paroxysmal atrial fibrllation by multislice-CT is initial marker of structural remodeling and predictor of transition from paroxysmal to chronic form. Int J Cardiol. (2011) 148:139–47. doi: 10.1016/j.ijcard.2009.10.032
10. Imada M, Funabashi N, Asano M, Uehara M, Ueda M, Komuro I. Anatomical remodeling of left atria in subjects with chronic and paroxysmal atrial fibrillation evaluated by multislice computed tomography. Int J Cardiol. (2007) 119:384–8. doi: 10.1016/j.ijcard.2006.07.162
11. Chris J. Watson, Nadezhda Glezeva, Stephen Horgan, Joe Gallagher, Dermot Phelan, Ken McDonald, Michael Tolan, John Baugh, Patrick Collier, Mark Ledwidge. Atrial tissue pro-fibrotic M2 macrophage marker CD163+, gene expression of procollagen and B-type natriuretic peptide. J Am Heart Assoc. (2020) 9:e013416. doi: 10.1161/JAHA.119.013416
12. Packer M, Lam CS, Lund LH, Redfield MM. Interdependence of atrial fibrillation and heart failure with a preserved ejection fraction reflects a common underlying atrial and ventricular myopathy. Circulation. (2020) 141:4–6. doi: 10.1161/CIRCULATIONAHA.119.042996
13. Hansen BJ, Zhao J, Csepe TA, Moore BT Li N, Jayne LA, Kalyanasundaram A, et al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J. (2015) 36:2390–401. doi: 10.1093/eurheartj/ehv233
14. Nakatani Y, Sakamoto T, Yamaguchi Y, Tsujino Y, Kataoka N, Kinugawa K. Heterogeneity in the left atrial wall thickness contributes to atrial fibrillation recurrence after catheter ablation. Heart Vessels. (2018) 33:1549–58. doi: 10.1007/s00380-018-1200-y
15. Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, et al. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. (2008) 117:1255–60. doi: 10.1161/CIRCULATIONAHA.107.744466
16. Magnussen C, Niiranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, Njølstad I, et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation. (2017) 136:1588–97. doi: 10.1161/CIRCULATIONAHA.117.028981
Keywords: atrial fibrillation, heart failure with preserved ejection fraction, strain, multi-detector computed tomography, left atrial remodeling, left atrial wall
Citation: Kuo J-Y, Jin X, Sun J-Y, Chang S-H, Chi P-C, Sung K-T, Mok GSP, Yun C-H, Chang S-C, Chung F-P, Yu C-H, Wu T-H, Hung C-L, Yeh H-I and Lam CSP (2022) Insights on Distinct Left Atrial Remodeling Between Atrial Fibrillation and Heart Failure With Preserved Ejection Fraction. Front. Cardiovasc. Med. 9:857360. doi: 10.3389/fcvm.2022.857360
Received: 18 January 2022; Accepted: 24 March 2022;
Published: 26 April 2022.
Edited by:
Giulia Elena Mandoli, University of Siena, ItalyReviewed by:
Annagrazia Cecere, University of Padua, ItalyCopyright © 2022 Kuo, Jin, Sun, Chang, Chi, Sung, Mok, Yun, Chang, Chung, Yu, Wu, Hung, Yeh and Lam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tung-Hsin Wu, dHVuZ0B5bS5lZHUudHc=; Chung-Lieh Hung, am90YXJvMzc5MUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.