
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 23 June 2022
Sec. Atherosclerosis and Vascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.848121
 Pil Gyu Park1
Pil Gyu Park1 Jung Yoon Pyo1
Jung Yoon Pyo1 Sung Soo Ahn1
Sung Soo Ahn1 Hyun Joon Choi2
Hyun Joon Choi2 Jason Jungsik Song1,3
Jason Jungsik Song1,3 Yong-Beom Park1,3
Yong-Beom Park1,3 Ji Hye Huh4*†
Ji Hye Huh4*† Sang-Won Lee1,3*†
Sang-Won Lee1,3*†Background: This study investigated whether the fatty liver index (FLI) could predict all-cause mortality and cerebrovascular accident (CVA) during follow-up in patients with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) without substantial liver disease.
Methods: The medical records of 75 AAV patients with AAV were retrospectively reviewed. An equation for the FLI is as follows: FLI = (e0.953×loge(triglycerides)+0.139×BMI+0.718×loge(GGT)+0.053×waistcircumference–15.745)/(1 + e0.953×loge(triglycerides)+0.139×BMI+0.718×loge(GGT)+0.053×waistcircumference–15.745) × 100. The cut-offs of the FLI were obtained using the receiver operator characteristic (ROC) curve analysis.
Results: The mean age at AAV diagnosis was 59.1 years and 42.7% were male. Eight patients (10.7%) died and 8 patients had CVA during follow-up. When the cut-offs of the FLI for all-cause mortality and CVA were set as the FLI ≥ 33.59 and the FLI ≥ 32.31, AAV patients with the FLI over each cut-off exhibited a higher risk for all-cause mortality or CVA than those without (RR 8.633 and 8.129), respectively. In addition, AAV patients with the FLI over each cut-off exhibited a significantly lower cumulative patients’ survival rate or CVA-free survival rate than those without, respectively. In the multivariable Cox analysis, only the FLI ≥ 33.59 at AAV diagnosis was an independent predictor of all-cause mortality during follow-up in AAV patients (HR 10.448).
Conclusion: The FLI at AAV diagnosis can be a potential independent predictor of all-cause mortality and CVA during follow-up in AAV patients. We suggest that physicians measure the FLI at AAV diagnosis and pay more attention to those with a high FLI value for prevention of future mortality and CVA.
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a small vessels vasculitis and primarily affects the capillaries and adjacent venules and arterioles (1). AAV is primarily categorised into three subtypes such as microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA) (2) and it is occasionally classified as myeloperoxidase (MPO)-ANCA vasculitis, proteinase 3 (PR3)-ANCA vasculitis, and ANCA-negative vasculitis (3). AAV can affect almost all organs in the body, and may inflict serious damage to the major organs, leading to death (4, 5). A recent study reported that the rate of all-cause mortality in AAV patients was 38.4 per 1,000 patient-years and the standardised mortality ratio was 2.3 (6). Another study in 2014 provided a global age-standardised mortality rate for AAV of 0.53 deaths per million inhabitants (0.62 for men and 0.46 for women) for AAV (7). We also previously reported that 1-, 5-, and 10-year cumulative patient survival rates of 96.1, 94.8, and 92.8%, respectively, in Korean patients with AAV (8). Therefore, it is believed that in addition to accurate early diagnosis and early treatment initiation, the efforts to discover new indices to predict poor outcomes, particularly all-cause mortality may improve the prognosis of AAV (9, 10).
The fatty liver index (FLI), which is calculated based on an equation composed of body mass index (BMI), waist circumference, triglyceride (TG), and gamma-glutamyl transferase (GGT), was recently introduced as a surrogate marker of non-alcoholic fatty liver disease (NAFLD), and its predictability for NAFLD was validated by imaging methods (11). NAFLD was reportedly associated with metabolic disorders including type 2 diabetes mellitus (T2DM), hypertension, cerebrovascular accident (CVA), cardiovascular disease (CVD), and chronic kidney diseases (12–15). Although this index was initially created to assess the presence and degree of hepatic steatosis in individuals, FLI recently has been also considered an emerging marker of chronic inflammation-related cardiovascular disease regarding the close association between hepatic steatosis subclinical atherosclerosis. On the other hand, to date, the predictive potential of the FLI for CVD and all-cause mortality has been reported in the various ethnic population or patients with diverse diseases (16–19). Moreover, the FLI was also reportedly associated with the risk of CVD and all-cause mortality in the general Korean population (20, 21). Therefore, it could be reasonably assumed that the FLI may predict the risk of all-cause mortality by predicting the development of various complications associated with metabolic diseases such as CVA or CVD through NAFLD-related mechanisms, induced by systemic inflammation in AAV patients. However, despite these hypotheses, no studies to date have examined whether the FLI predicts all-cause mortality or the occurrence of CVA, and CVD among AAV patients. Given the serious systemic complications and poor outcomes of AAV, it is believed that proving the clinical implications of the FLI will be of great help in selecting the best treatment option. Hence, here, we investigated whether the FLI at AAV diagnosis could predict all-cause mortality and poor outcomes of AAV during follow-up in AAV patients without substantial liver disease.
In this study, the medical records of 75 patients with AAV were selected from the Severance Hospital ANCA-associated VasculitidEs (SHAVE) observational cohort. The patients who fulfilled the inclusion criteria were retrospectively reviewed. The inclusion criteria were (i) first classified as AAV at the Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital between 2000 and 2021; (ii) met both the 2012 revised Chapel Hill Consensus Conference nomenclature of vasculitis and the 2007 European Medicine Agency algorithm for AAV and polyarteritis nodosa (1, 2); (iii) availability of complete medical records, which were sufficient to obtain clinical, laboratory, radiological and histological data at diagnosis as well as information about poor outcomes of AAV including all-cause mortality, and the occurrence of CVA and CVD during follow-up; (iv) medical records that included all four variables for calculating the FLI at diagnosis; (v) follow-up periods of longer than 3 months; (vi) absence of chronic liver diseases such as B and C viral hepatitis and liver cirrhosis or structural abnormalities on liver imaging including, computed tomography or ultrasonography (22); (vii) no right–sided heart failure which may affect liver function; (viii) absence of concurrent serious medical conditions such as malignancies, infectious diseases requiring the hospitalisation, or systemic vasculitis other than AAV; and (ix) no history of exposure to immunosuppressive drugs or prednisolone > 20 mg/day before AAV diagnosis. This study was approved by the Institutional Review Board (IRB) of Severance Hospital (Seoul, South Korea, IRB No. 4-2020-1071) and conducted according to the principles of the Declaration of Helsinki. Given the retrospective design of the study and the use of anonymised patient data, the requirement for written informed consent was waived.
Epidemiological, clinical, and laboratory data were collected (Table 1). AAV-related data included AAV subtypes, ANCA types and positivity, Birmingham vasculitis activity score version 3(BVAS), and five-factor score (FFS) (23, 24). Comorbidities such as T2DM and hypertension were evaluated using questionnaires on medical history including medication use. Along with laboratory results, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), liver-related variables, cholesterol profiles, and the FLI-related variables were measured from blood samples obtained after an overnight fast. Liver-related variables included prothrombin time (international normalised ratio, [INR]), alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), GGT and total bilirubin.
During follow-up, poor outcomes including all-cause mortality, relapse, end-stage renal disease (ESRD), CVA, and CVD were assessed. ESRD, CVA and CVD were defined based on BVAS and FFS (23, 24). The follow-up period based on all-cause mortality was defined as the period between AAV diagnosis and the last visit for surviving patients and as the period between AAV diagnosis and death for deceased patients. For patients with a poor outcome, the follow-up period based on each poor outcome was defined as the period between AAV diagnosis and the occurrence of each poor outcome. Conversely, for patients without poor outcomes, the follow-up period was defined as the period between AAV diagnosis and the last visit. The medications that were administered were also assessed.
FLI = (e0.953×loge(triglycerides)+0.139×BMI+0.718×loge(GGT)+0.053×waistcircumference–15.745)/(1 + e0.953×loge(triglycerides)+0.139×BMI+0.718×loge(GGT) + 0.053×waistcircumference–15.745) × 100 (11).
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY, United States). Continuous variables were expressed as medians with interquartile ranges, whereas categorical variables were expressed as numbers (percentages). Significant differences between the two categorical variables were analysed using the chi-square and Fisher’s exact tests. The Mann-Whitney U test was used to compare significant differences between two continuous variables. Significant differences among more than three continuous variables were investigated using the Kruskal-Wallis test. We conducted the ROC curve analysis with all-cause mortality as a state variable and the FLI as a test variable in the statistical analysis and obtained the sensitivity and specificity of each value of the FLI. We set the FLI of which the sum of sensitivity and the specificity was highest among these FLI values as the optimal cut-off for all-cause mortality. The relative risk (RR) of the cut-off for the high AAV activity was analysed using contingency tables and the chi-square test. The cumulative survival rates were compared between the two groups was analysed using the Kaplan-Meier survival analysis with the log-rank test. The multivariable Cox hazards model using variables with statistical significance on the univariable Cox hazard model was conducted to appropriately obtain the hazard ratios (HRs) during the considerable follow-up period. Statistical significance was set P < 0.05.
The values for the detailed variables are summarised in Table 1. The mean age at AAV diagnosis was 59.1 years and 32 (42.7%) patients were male. Of the total, 43 patients were classified as MPA, 17 as GPA, and 15 as EGPA. Twenty-five patients (33.3%) had T2DM, while 27 patients (36.0%) had hypertension. The median prothrombin time (INR), ALP, AST, ALT, and total bilirubin were all within normal reference ranges. The median TG, BMI, GGT, waist circumference, and the FLI were 126.0 mg/dL, 22.2 kg/m2, 36.0 IU/L, 85.3 cm, and 32.9, respectively. During follow-up, eight patients (10.7%) died of any cause, while 24 patients experienced relapse after remission. Twenty, eight, and nine patients exhibited ESRD, CVA, and CVD, respectively. The follow-up duration based on all-cause mortality in all patients was 34.1 months. For surviving and deceased patients, they were 39.8 and 6.7 months, respectively.
When using the ROC curve to evaluate the predictive ability of the FLI for five poor outcomes of AAV, and with readjustment for statistical significance to P < 0.1 based on clinical judgment, the FLI was turned out to be associated with all-cause mortality (AUC, 0.675, 95% confidence interval CI 0.499, 0.859, P = 0.099) and CVA (AUC, 0.694, 95% CI 0.500, 0.888, P = 0.074) (Figures 1A,C). When the optimal cut-off of the FLI for all-cause mortality was set as the FLI ≥ 33.59, the sensitivity and specificity were 87.5 and 55.2%, respectively. All-cause mortality was identified more frequently in AAV patients with the FLI ≥ 33.59 than in those with the FLI < 33.59 (18.9 vs. 2.6%, P = 0.022). Furthermore, AAV patients with the FLI ≥ 33.59 exhibited a significantly higher risk for all-cause mortality than those with the FLI < 33.59 (RR 8.633) (Figure 1B). When the optimal cut-off of the FLI for CVA occurrence was set as the FLI ≥ 32.31, the sensitivity was 87.5% and the specificity was 53.7%. CVA occurrence was identified more frequently in AAV patients with the FLI ≥ 32.31 than those with the FLI < 33.59 (18.4 vs. 2.7%, P = 0.027). Furthermore, AAV patients with the FLI ≥ 32.31 exhibited a significantly higher risk for CVA occurrence than those with the FLI < 32.31 (RR 8.129) (Figure 1D). However, CVD, which was considered to be theoretically associated with NAFLD, and ESRD, which showed a significant difference (Table 2), was not predicted by the FLI (Figures 1E,F).
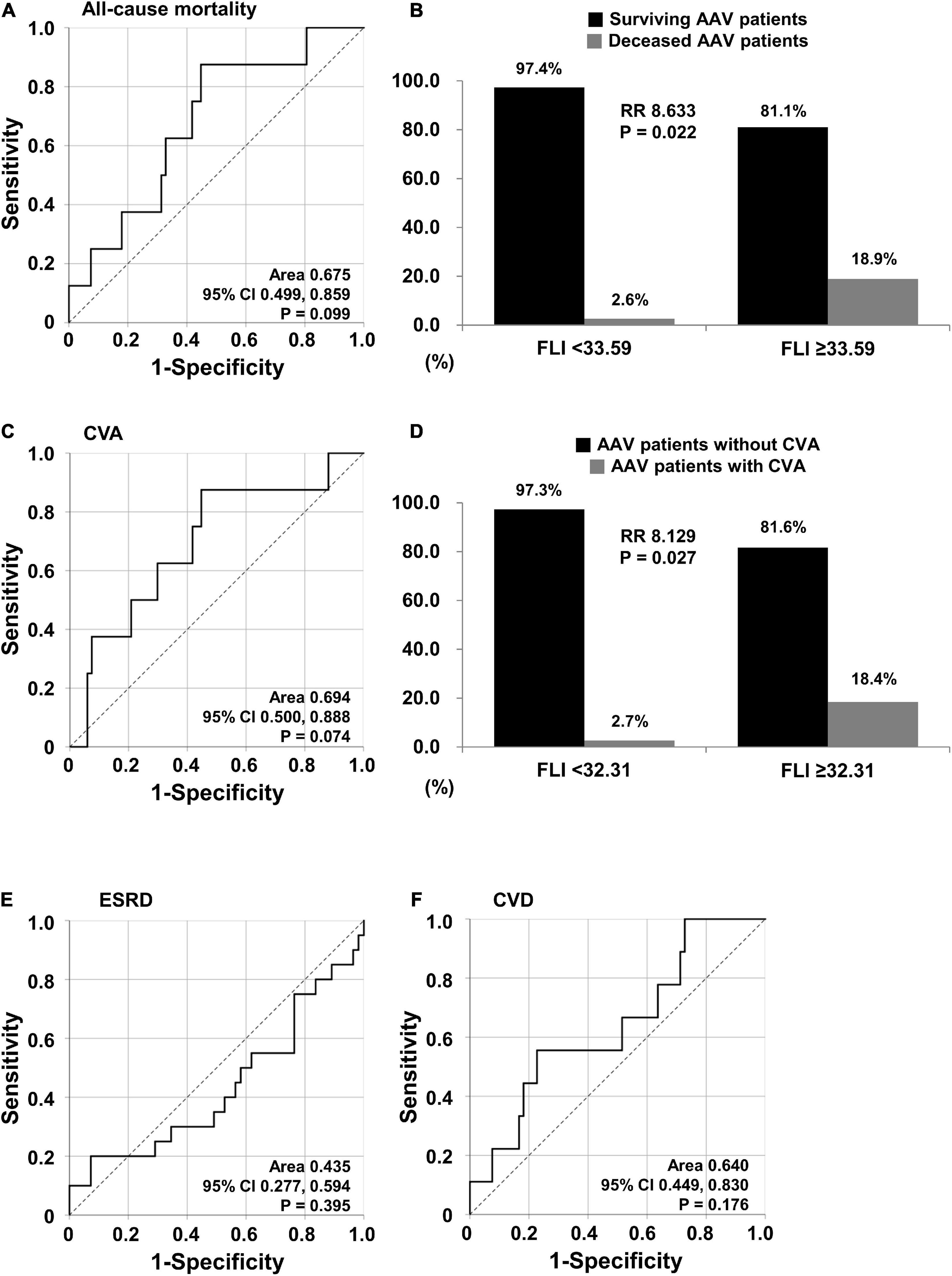
Figure 1. Cut-offs of the FLI for all-cause mortality and CVA. (A) Identification of the cut-off of the FLI for all-cause mortality using the ROC curve. (B) RR of all-cause mortality according to the cut-off of the FLI. (C) Identification of the cut-off of the FLI for CVA using the ROC curve. (D) RR of CVA based on the cut-off of the FLI. (E,F) Identification of the cut-offs of the FLI for ESRD and CVD. FLI, fatty liver index; RR, relative risk; CVA, cerebrovascular accident; ROC, receiver operator characteristic; ESRD, end-stage renal disease; CVD, cardiovascular disease.
As for variables at AAV diagnosis, deceased AAV patients were older (70.9 vs. 58.7 years, P = 0.032) and exhibited a higher value of BVAS (19.0 vs. 14.0, P = 0.046) and FFS (3.0 vs. 1.0, P = 0.028) than surviving AAV patients. There were no statistically significant intergroup differences in liver-related variables. The FLI values were higher in deceased AAV patients than that in surviving AAV patients; however, the difference was not statistically significant (P = 0.099). As for variables during follow-up, deceased AAV patients had higher frequencies of ESRD (75.0 vs. 20.9%, P = 0.004) and CVA (37.5 vs. 7.5%, P = 0.035) than surviving AAV patients. However, the frequency of CVD did not differ between the two groups. Overall, the follow-up period for poor outcomes in deceased AAV patients was shorter than that in surviving AAV patients. Among the therapeutic modalities, only the number of plasma exchanges performed was significantly higher in the deceased AAV patients (Table 2).
As for variables at AAV diagnosis, AAV patients with CVA exhibited higher BVAS than those without CVA (18.5 vs. 14.0, P = 0.026), but not FFS. AAV patients with CVA exhibited the lower levels of serum albumin (2.5 vs. 3.5 g/dL, P = 0.004), and high density lipoprotein (HDL)-cholesterol (21.0 vs. 47.0 mg/dL), and higher levels of GGT (94.0 vs. 31.0 IU/L, P = 0.018), ESR (93.5 vs. 61.0 mm/hr, P = 0.036) and CRP (110.7 vs. 10.5 mg/L, P = 0.047) than in those without CVA. There were no statistically significant intergroup differences in liver-related variables. The FLI was higher in AAV patients with CVA than in those without CVA but the difference was not statistically significant (P = 0.074). As for variables during follow-up, AAV patients with CVA exhibited a higher frequency of all-cause mortality than those without CVA (37.5 vs. 7.5%, P = 0.035) (Supplementary Table 1).
Thus far, the analysis may not reflect the actual clinical situation because the follow-up period was not included. Therefore, we re-evaluated the predictive ability of the FLI for all-cause mortality and CVA using Kaplan Meier survival analysis including follow-up. AAV patients with the FLI ≥ 33.59 exhibited a significantly lower cumulative patients’ survival rate than those with the FLI < 33.59 (P = 0.016). In addition, AAV patients with the FLI ≥ 32.31 also exhibited a significantly lower cumulative CVA-free survival rate than those with the FLI < 32.31 (P = 0.026) (Figure 2).
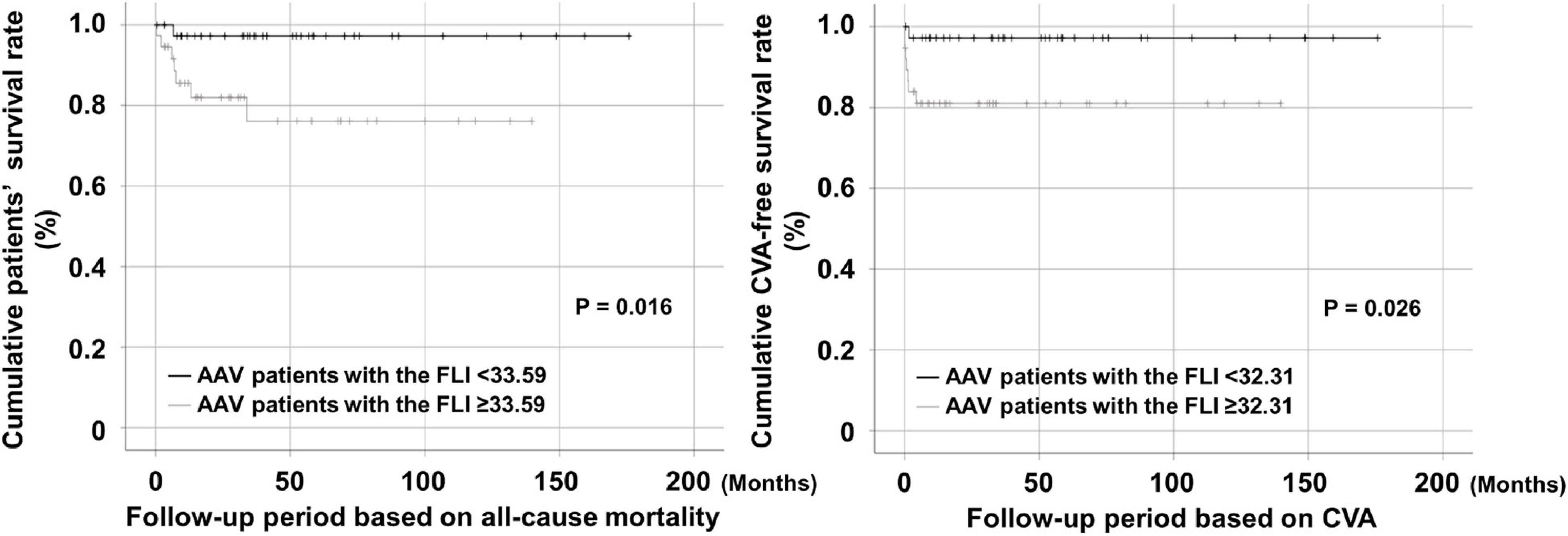
Figure 2. Comparison of cumulative survival rates. AAV patients with the FLI ≥ 33.59 and that ≥ 32.31 at diagnosis exhibited significantly lower cumulative patients’ and CVA-free survival rates than those without, respectively. AAV, ANCA-associated vasculitis; ANCA, antineutrophil cytoplasmic antibody; FLI, fatty liver index; CVA, cerebrovascular accident.
In the univariable analysis, age (HR 1.079), FFS (HR 2.460), total bilirubin (HR 20.799), and the FLI ≥ 33.59 (HR 8.532) at AAV diagnosis were significantly associated with all-cause mortality. In the multivariable analysis, only the FLI ≥ 33.59 at AAV diagnosis was turned out to be an independent predictor of all-cause mortality during follow-up in AAV patients (HR 10.448, 95% CI 1.109, 98.398, P = 0.040) (Table 3).
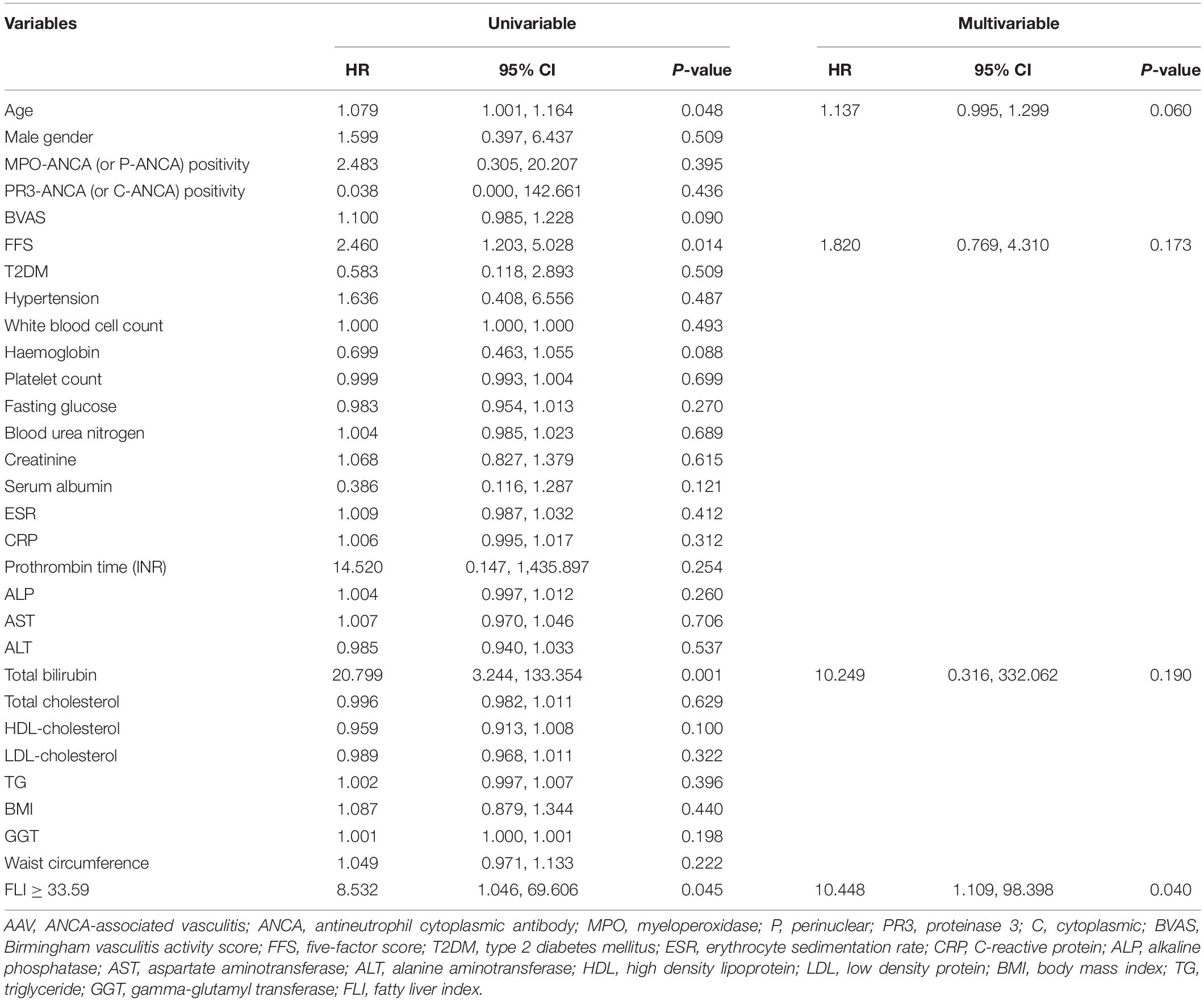
Table 3. Cox hazards model analyses of variables at AAV diagnosis for all-cause mortality during follow-up in AAV patients.
In this study, we investigated the clinical significance of the FLI in AAV patients and obtained several interesting results. First, the FLI was associated with all-cause mortality and CVA in the ROC curve, and thus each optimal cut-off of the FLI for predicting all-cause mortality or CVA could be obtained. Second, when the cut-offs for all-cause mortality and CVA were set at 33.59 and 32.31, the RRs were 8.633 and 8.129, respectively. Third, deceased AAV patients had higher frequencies of ESRD (75.0 vs. 20.9%, P = 0.004) and CVA (37.5 vs. 7.5%, P = 0.035) than surviving AAV patients, whereas, AAV patients with CVA had a higher frequency of all-cause mortality than those without CVA (37.5 vs. 7.5%, P = 0.035). Fourth, AAV patients with the FLI ≥ 33.59 exhibited a significantly lower cumulative patients’ survival rate than those with the FLI < 33.59 (P = 0.016). In addition, AAV patients with the FLI ≥ 32.31 also exhibited a significantly lower cumulative CVA-free survival rate than those with the FLI < 32.31 (P = 0.026). Therefore, the FLI can predict all-cause mortality and CVA in AAV patients, suggesting the possible relationship between all-cause mortality and CVA.
The FLI is known to reflect the presence of NAFLD. In addition, NAFLD is reportedly closely related to current T2DM and insulin resistance (IR) and can predict the occurrence of CVA or CVD, the main causes of all-cause mortality after a certain period of time (12–15). On the other hand, liver involvement in AAV patients is very rare, and the liver-related indices are not included in the BVAS form for measuring AAV activity (23). We considered the potential FLI involvement in predicting all-cause mortality and CVA in AAV patients and hypothesised its underlying mechanism. First, we evaluated whether AAV has a direct influence on NAFLD development. Since systemic inflammation can induce IR, which in turn exacerbates systemic inflammation, a vicious cycle is formed. Moreover, such a vicious cycle between systemic inflammation and IR could, in turn, promote and accelerate the development of NAFLD (25). Furthermore, among inflammation patterns, persistent low-grade inflammation may be more closely associated with IR and NAFLD than high-grade inflammation (26) (Figure 3).
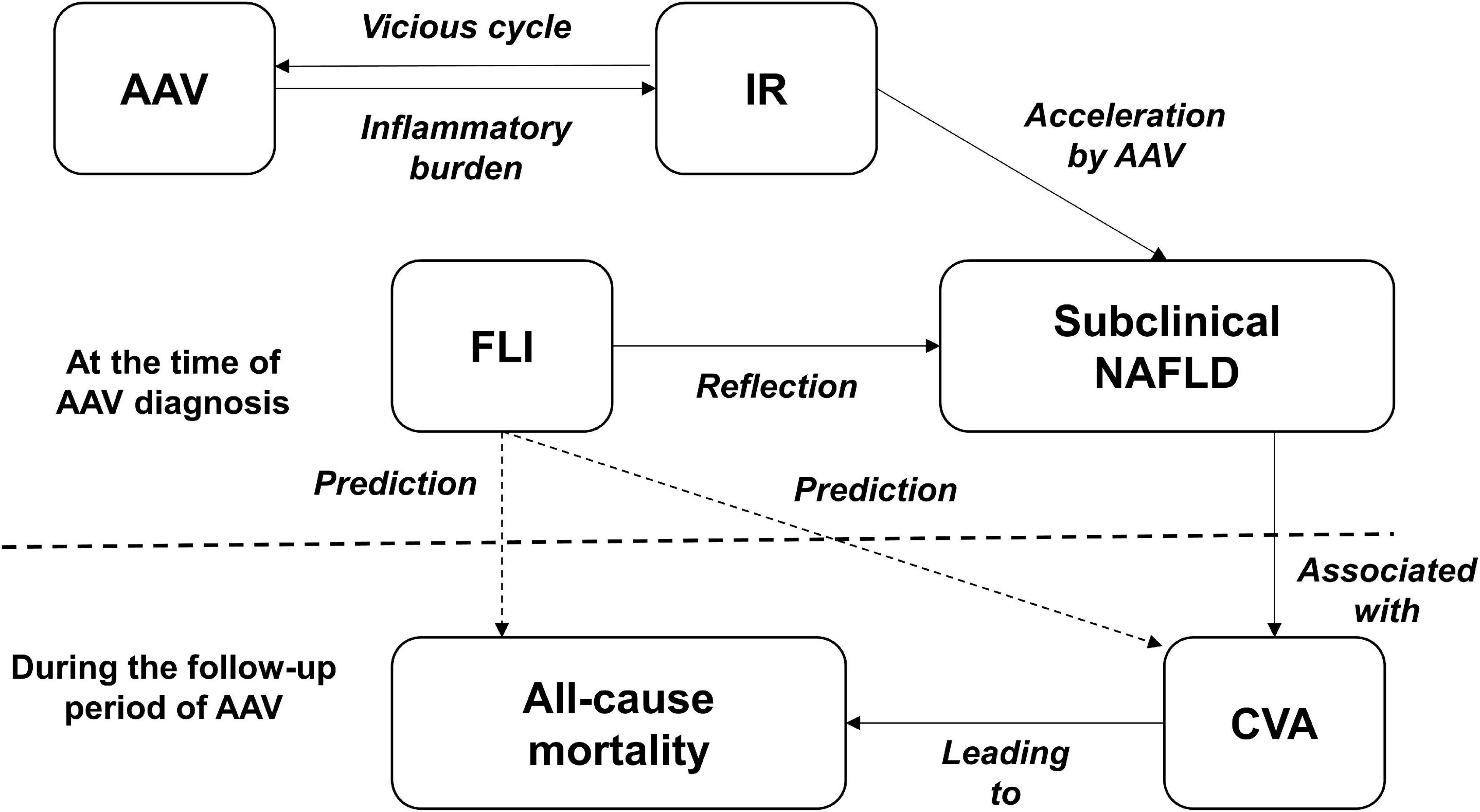
Figure 3. A hypothesis of the mechanism. AAV, ANCA-associated vasculitis; ANCA, antineutrophil cytoplasmic antibody; IR, insulin resistance; FLI, fatty liver index; NAFLD, non-alcoholic fatty liver disease; CVA, cerebrovascular accident.
Second, we evaluated whether subclinical NAFLD is relevant to poor outcomes in patients with AAV. Although the most exact method of diagnosing NAFLD is liver biopsy, it is invasive and has limitations in actual clinical use. Ultrasonography is the most common tool used to assess the presence of NAFLD. However, it also has several limitations for the detection of milder degrees of steatosis (<33% fat in hepatocytes) (27, 28). Therefore, Bedogni et al. developed a simple scoring system called the FLI as a predictor of hepatic steatosis (11), which has been well validated in Korean populations and other ethnic groups (20, 21). The value of the FLI is expressed from 0 to 100, and when it is less than 30, NAFLD can be ruled out. The FLI ≥ 60 suggests a high likelihood of NAFLD or clinical NAFLD, whereas a value of FLI ≥ 30 but < 60 indicates an intermediate likelihood of NAFLD (29). In this study, the cut-off values of FLI were 33.59 and 32.31 for all-cause mortality and CVA, respectively. These results indicate that the presence of NAFLD might be a predictor of CVA and mortality in AAV patients, similar to the general population. Therefore, AAV patients with high FLI value need to monitor and lower its’ value by lifestyle modifications consisting of calorie restriction diet, regular exercise, and weight reduction which are traditional management of NAFLD for their prognosis.
In principle, AAV patients with abnormal liver-related variables were excluded from this study for two reasons. First, chronic liver diseases may distract the interpretation of the FLI’s ability to reflect subclinical NAFLD, which can promote progression and CVA and possible death. Second, the association between liver-related variables and AAV is weak because AAV rarely provokes progressive liver involvement or autoimmune hepatic diseases (30). A strict exclusion criteria applied in this study were as follows: ALT > 40 IU/L, AST > 40 IU/L, ALP > 115 IU/L, total bilirubin > 1.2 mg/dL, prothrombin time (INR) > 1.16, GGT > 54 IU/L, platelet count < 150,000/mm3, and serum albumin < 3.5 mg/dL (22). Of these variables, platelet count and serum albumin level were not included because they may be affected by the inflammatory burden in AAV patients. As GGT is one of the parameters of an equation used to calculate the FLI, it was not included in the exclusion criteria. Of the 75 patients with AAV, 11 with an ALT > 40 IU/L, one with an AST > 40 IU/L, nine with an ALP > 115 IU/L, two with a total bilirubin > 1.2 mg/dL, and seven with a prothrombin time (INR) > 1.16 were excluded. Remaining 45 AAV patients with normal liver-related variables were included and re-evaluated. The identification of the cut-offs of the FLI for predicting all-cause mortality and CVA was attempted using the ROC curve, but no cut-offs with values of P < 0.1 were obtained (AUC, 0.635; 95% CI, 0.286–0.984; P = 0.439 for all-cause mortality and AUC, 0.779; 95% CI, 0.636–0.922; P = 0.186 for CVA) (Supplementary Figure 1).
Alanine aminotransferase is a parameter that is closely related to liver damage. Thus, Of the 75 AAV patients, 11 patients with an ALT > 40 IU/L were excluded. With remaining 64 AAV patients, the efficacy of the FLI was re-evaluated. First, the ROC curve was used to obtain the FLI cut-off value for predicting all-cause mortality (re-adjusted statistical significance to P < 0.1). The FLI was associated with all-cause mortality (AUC, 0.714; 95% CI, 0.539–0.890; P = 0.051). When the optimal cut-off of FLI for all-cause mortality was set as ≥ 33.59, the sensitivity and specificity were 87.5 and 60.7%, respectively. All-cause mortality was identified more frequently in AAV patients with the FLI ≥ 33.59 than in those with the FLI < 33.59 (24.1 vs. 2.9%; P = 0.019). Furthermore, AAV patients with the FLI ≥ 33.59 exhibited a significantly higher risk for all-cause mortality than those with the FLI < 33.59 (RR, 10.818) (Supplementary Figure 2). However, significant cut-offs of the FLI for predicting CVA (P = 0.105) and CVD (P = 0.180) were not obtained using the ROC curve analysis (Supplementary Figure 3). AAV patients with the FLI ≥ 33.59 exhibited a significantly lower cumulative patients’ survival rate than those with the FLI < 33.59 (P = 0.007) (Supplementary Figure 4). Therefore, the FLI reflecting NAFLD at the AAV diagnosis could predict all-cause mortality during follow-up in AAV patients regardless of ALT levels. However, the FLI at diagnosis could predict the occurrence of CVA in all AAV patients but not in those with normal ALT levels.
For variables other than ALT which is closely related to liver damage, the systemic inflammation due to AAV could subsequently increase their serum level and should be considered when analysing the result. As for AST, FIB-5, for which AST is a parameter located in the numerator of the equation, was proven to significantly predict ESRD in patients with MPA and GPA without substantial liver diseases (31). In addition, AST levels may be elevated in cardiovascular diseases including myocarditis and ischaemic heart disease in AAV patients (32). ALP reportedly predicts CVD occurrence and serious complications of chronic kidney disease (33). Total bilirubin is reported to have an anti-inflammatory mechanism and can be elevated to subside inflammation (34). In addition, prothrombin time is considered to be linked to inflammatory indices (35). Meanwhile, ALT is strongly associated with NAFLD, and its elevation is largely attributed to NAFLD severity (36).
The primary advantage of this study was that we demonstrated for the first time that the FLI could predict all-cause mortality in patients with AAV. We could generate a hypothesis that NAFLD occurrence may be increased through the axis of systemic inflammation and IR in patients with AAV, which does not directly affect liver. Also, instead of providing the absolute FLI cut-off value, we provided a method for obtaining the cut-off value of the FLI at diagnosis for predicting all-cause mortality during follow-up in AAV patients, which may enable the application of this concept to AAV patients with different ethnic and geographical backgrounds. In particular, despite the small number of study subjects, our study has additional three advantages: First, the SHAVE cohort from which the study subjects were collected is the cohort containing the largest number of AAV patients in Korea. Second, the same three rheumatologists made new diagnoses of AAV or participated in the reclassification of AAV in existing patients. Third, since the same three rheumatologists applied the same classification criteria for AAV, the diagnosis concordance rate was high.
This study has several limitations. Because GGT is not necessarily included in routine tests for AAV diagnosis, the number of AAV patients with GGT results included in this study was insufficient to clarify whether our results could be applied to all patients with AAV. In addition, this study did not reflect the dynamic change through the evaluation of NAFLD at every visit because of the limitations of the retrospective study design. In addition, liver biopsy and measurements of biomarkers related to liver fibrosis or steatohepatitis were not performed to evaluate the exact degree of NAFLD, that is, the severity of liver fibrosis or steatohepatitis. Therefore, we could not determine the association between severe form of NAFLD and health outcome in our patients. Although glucocorticoids and immunosuppressive drugs could be controlled through the drug utilisation review system, we could not strictly control the drug-related occurrence of hepatitis or NAFLD because we did not obtain complete information about general or herbal medications. On the other hand, if we showed the status of the circulating inflammatory load including hs-CRP and fibrinogen, we could have more concretely proved the hypothesis that inflammatory burden might induce IR and accelerate the occurrence of subclinical NAFLD which is associated with all-cause mortality. However, because this study was conducted retrospectively, we could not demonstrate it directly. Future prospective studies with larger numbers of AAV patients that include transient elastography along with FLI assessments will solve these limitations, validate our results, and will further suggest the clinical implications of the FLI in AAV patients.
The FLI, a validated surrogate marker of NAFLD, at AAV diagnosis can be a potential independent predictor of all-cause mortality and the occurrence of CVA during follow-up in patients with AAV. This finding suggests that the presence of NAFLD may contribute to poor outcomes in these patients. Moreover, we demonstrated that FLI is a simple and useful marker for the classification of prognosis in patients with AAV. Given the higher prevalence of all-cause mortality and CVA in AAV patients, we suggest that physicians measure the FLI when AAV is diagnosed and pay more attention to those with NAFLD or a high FLI value for prevention of future mortality and CVA.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of Severance Hospital (Seoul, South Korea, IRB No. 4-2020-1071). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
PP and HC carried out the statistical analysis. PP, JH, and S-WL wrote the first draft of the manuscript. JP, SA, HC, JS, and Y-BP collected the data. JH and S-WL responsible for the conception, funding, design of the study, the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors corrected and approved the revisions and final version of the manuscript, contributed to the article, and approved the submitted version.
This research was supported by a faculty research grant from Yonsei University College of Medicine (6-2019-0184) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, and funded by the Ministry of Health and Welfare, South Korea (HI14C1324).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Byung-Wan Lee for his advice on the endocrinological interpretation of the results.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.848121/full#supplementary-material
1. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised international Chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. (2013) 65:1–11. doi: 10.1002/art.37715
2. Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. (2007) 66:222–7. doi: 10.1136/ard.2006.054593
3. Cornec D, Cornec-Le Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis – clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol. (2016) 12:570–9. doi: 10.1038/nrrheum.2016.123
4. Millet A, Pederzoli-Ribeil M, Guillevin L, Witko-Sarsat V, Mouthon L. Antineutrophil cytoplasmic antibody-associated vasculitides: is it time to split up the group? Ann Rheum Dis. (2013) 72:1273–9. doi: 10.1136/annrheumdis-2013-203255
5. Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, et al. ANCA-associated vasculitis. Nat Rev Dis Primers. (2020) 6:71. doi: 10.1038/s41572-020-0204-y
6. Wallace ZS, Fu X, Harkness T, Stone JH, Zhang Y, Choi H. All-cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA type. Rheumatology (Oxford). (2020) 59:2308–15. doi: 10.1093/rheumatology/kez589
7. Scherlinger M, Mertz P, Sagez F, Meyer A, Felten R, Chatelus E, et al. Worldwide trends in all-cause mortality of auto-immune systemic diseases between 2001 and 2014. Autoimmun Rev. (2020) 19:102531. doi: 10.1016/j.autrev.2020.102531
8. Choi CB, Park YB, Lee SW. Antineutrophil cytoplasmic antibody-associated vasculitis in Korea: a narrative review. Yonsei Med J. (2019) 60:10–21. doi: 10.3349/ymj.2019.60.1.10
9. Kim MK, Choi H, Kim JY, Song JJ, Park YB, Lee SW. Multivariable index for assessing the activity and predicting all-cause mortality in antineutrophil cytoplasmic antibody-associated vasculitis. J Clin Lab Anal. (2020) 34:e23022. doi: 10.1002/jcla.23022
10. Do H, Song JJ, Park YB, Lee SW. Novel mortality-predicting index at diagnosis can effectively predict all-cause mortality in patients with antineutrophil cytoplasmic antibody-associated vasculitis. J Clin Lab Anal. (2021) 35:e23885. doi: 10.1002/jcla.23885
11. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33. doi: 10.1186/1471-230X-6-33
12. Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care. (2018) 41:372–82. doi: 10.2337/dc17-1902
13. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. (2015) 313:2263–73. doi: 10.1001/jama.2015.5370
14. Alkagiet S, Papagiannis A, Tziomalos K. Associations between nonalcoholic fatty liver disease and ischemic stroke. World J Hepatol. (2018) 10:474–8. doi: 10.4254/wjh.v10.i7.474
15. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. (2015) 62:S47–64. doi: 10.1016/j.jhep.2014.12.012
16. Ould Setti M, Voutilainen A, Tuomainen TP. Renal hyperfiltration, fatty liver index, and the hazards of all-cause and cardiovascular mortality in Finnish men. Epidemiol Health. (2021) 43:e2021001. doi: 10.4178/epih.e2021001
17. Calori G, Lattuada G, Ragogna F, Garancini MP, Crosignani P, Villa M, et al. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology. (2011) 54:145–52. doi: 10.1002/hep.24356
18. Lerchbaum E, Pilz S, Grammer TB, Boehm BO, Stojakovic T, Obermayer-Pietsch B, et al. The fatty liver index is associated with increased mortality in subjects referred to coronary angiography. Nutr Metab Cardiovasc Dis. (2013) 23:1231–8. doi: 10.1016/j.numecd.2013.02.004
19. Barré T, Protopopescu C, Bani-Sadr F, Piroth L, Rojas Rojas T, Salmon-Ceron D, et al. Elevated Fatty liver index as a risk factor for all-cause mortality in human immunodeficiency virus-hepatitis C virus-coinfected patients (ANRS CO13 HEPAVIH Cohort study). Hepatology. (2020) 71:1182–97. doi: 10.1002/hep.30914
20. Kim JH, Moon JS, Byun SJ, Lee JH, Kang DR, Sung KC, et al. Fatty liver index and development of cardiovascular disease in Koreans without pre-existing myocardial infarction and ischemic stroke: a large population-based study. Cardiovasc Diabetol. (2020) 19:51. doi: 10.1186/s12933-020-01025-4
21. Lee CH, Han KD, Kim DH, Kwak MS. The repeatedly elevated fatty liver index is associated with increased mortality: a population-based cohort study. Front Endocrinol (Lausanne). (2021) 12:638615. doi: 10.3389/fendo.2021.638615
22. Lee SW, Park HJ, Kim BK, Han KH, Lee SK, Kim SU, et al. Leflunomide increases the risk of silent liver fibrosis in patients with rheumatoid arthritis receiving methotrexate. Arthritis Res Ther. (2012) 14:R232. doi: 10.1186/ar4075
23. Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al. Modification and validation of the Birmingham vasculitis activity score (version 3). Ann Rheum Dis. (2009) 68:1827–32. doi: 10.1136/ard.2008.101279
24. Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Toumelin PL, et al. The five-factor score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French vasculitis study group (FVSG) cohort. Medicine (Baltimore). (2011) 90:19–27. doi: 10.1097/MD.0b013e318205a4c6
25. Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. (2017) 16:203. doi: 10.1186/s12944-017-0572-9
26. Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. (2015) 2015:508409. doi: 10.1155/2015/508409
27. Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed). (1986) 292:13–5. doi: 10.1136/bmj.292.6512.13
28. Osawa H, Mori Y. Sonographic diagnosis of fatty liver using a histogram technique that compares liver and renal cortical echo amplitudes. J Clin Ultrasound. (1996) 24:25–9. doi: 10.1002/(SICI)1097-0096(199601)24:13.0.CO;2-N
29. Chen LW, Huang PR, Chien CH, Lin CL, Chien RN. A community-based study on the application of fatty liver index in screening subjects with nonalcoholic fatty liver disease. J Formos Med Assoc. (2020) 119:173–81. doi: 10.1016/j.jfma.2019.03.016
30. Willeke P, Schlüter B, Limani A, Becker H, Schotte H. Liver involvement in ANCA-associated vasculitis. Clin Rheumatol. (2016) 35:387–94. doi: 10.1007/s10067-015-2882-5
31. Kwon HC, Song JJ, Park YB, Lee SW. Fibrosis-5 predicts end-stage renal disease in patients with microscopic polyangiitis and granulomatosis with polyangiitis without substantial liver diseases. Clin Exp Med. (2021) 21:399–406. doi: 10.1007/s10238-021-00691-2
32. Weng SF, Kai J, Guha IN, Qureshi N. The value of aspartate aminotransferase and alanine aminotransferase in cardiovascular disease risk assessment. Open Heart. (2015) 2:e000272. doi: 10.1136/openhrt-2015-000272
33. Haarhaus M, Gilham D, Kulikowski E, Magnusson P, Kalantar-Zadeh K. Pharmacologic epigenetic modulators of alkaline phosphatase in chronic kidney disease. Curr Opin Nephrol Hypertens. (2020) 29:4–15. doi: 10.1097/MNH.0000000000000570
34. Tran DT, Jeong YY, Kim JM, Bae HB, Son SK, Kwak SH. The anti-inflammatory role of bilirubin on “two-hit” sepsis animal model. Int J Mol Sci. (2020) 21:8650. doi: 10.3390/ijms21228650
35. Papageorgiou C, Synetos A, Tampakis K, Anninos H, Kontogiannis C, Kapelouzou A, et al. Activated clotting time as a marker of inflammation in hospitalized patients. Clin Appl Thromb Hemost. (2020) 26:1076029620929090. doi: 10.1177/1076029620929090
Keywords: antineutrophil cytoplasmic antibody, vasculitis, fatty liver index, mortality, cerebrovascular accident
Citation: Park PG, Pyo JY, Ahn SS, Choi HJ, Song JJ, Park Y-B, Huh JH and Lee S-W (2022) Fatty Liver Index Independently Predicts All-Cause Mortality in Patients With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis but No Substantial Liver Disease. Front. Cardiovasc. Med. 9:848121. doi: 10.3389/fcvm.2022.848121
Received: 23 February 2022; Accepted: 03 June 2022;
Published: 23 June 2022.
Edited by:
Xuebin Qin, Tulane University, United StatesReviewed by:
Bishuang Cai, Icahn School of Medicine at Mount Sinai, United StatesCopyright © 2022 Park, Pyo, Ahn, Choi, Song, Park, Huh and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji Hye Huh, cG5nMTIxMkBoYW5tYWlsLm5ldA==; Sang-Won Lee, c2FuZ3dvbmxlZUB5dWhzLmFj
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.