
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 09 March 2022
Sec. Heart Valve Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.847259
This article is part of the Research TopicAdvances in the Imaging and Treatment of Valvular Heart DiseaseView all 22 articles
 Yeela Talmor-Barkan1,2
Yeela Talmor-Barkan1,2 Ran Kornowski1
Ran Kornowski1 Noam Bar2
Noam Bar2 Jeremy Ben-Shoshan3
Jeremy Ben-Shoshan3 Hanna Vaknin-Assa1
Hanna Vaknin-Assa1 Ashraf Hamdan1
Ashraf Hamdan1 Boris Kruchin1
Boris Kruchin1 Israel M. Barbash4
Israel M. Barbash4 Haim Danenberg5
Haim Danenberg5 Gidon Y. Perlman5
Gidon Y. Perlman5 Maayan Konigstein3
Maayan Konigstein3 Ariel Finkelstein3
Ariel Finkelstein3 Arie Steinvil3
Arie Steinvil3 Ilan Merdler3
Ilan Merdler3 Amit Segev4
Amit Segev4 Alon Barsheshet1
Alon Barsheshet1 Pablo Codner1*
Pablo Codner1*Background: Transcatheter heart valve (THV) selection for transcatheter aortic valve implantation (TAVI) is crucial to achieve procedural success. Borderline aortic annulus size (BAAS), which allows a choice between two consecutive valve sizes, is a common challenge during device selection. In the present study, we evaluated TAVI outcomes in patients with BAAS according to THV size selection.
Methods: We performed a retrospective study including patients with severe aortic stenosis (AS) and BAAS, measured by multi-detector computed tomography (MDCT), undergoing TAVI with self-expandable (SE) or balloon-expandable (BE) THV from the Israeli multi-center TAVI registry. The aim was to evaluate outcomes of TAVI, mainly paravalvular leak (PVL) and valve hemodynamics, in patients with BAAS (based on MDCT) according to THV sizing selection in between 2 valve sizes. In addition, to investigate the benefit of shifting between different THV types (BE and SE) to avoid valve size selection in BAAS.
Results: Out of 2,352 patients with MDCT measurements, 598 patients with BAAS as defined for at least one THV type were included in the study. In BAAS patients treated with SE-THV, larger THV selection was associated with lower rate of PVL, compared to smaller THV (45.3 vs. 64.5%; pv = 0.0038). Regarding BE-THV, larger valve selection was associated with lower post-procedural transvalvular gradients compared to smaller THV (mean gradient: 9.9 ± 3.7 vs. 12.5 ± 7.2 mmHg; p = 0.019). Of note, rates of mortality, left bundle branch block, permanent pacemaker implantation, stroke, annular rupture, and/or coronary occlusion did not differ between groups.
Conclusion: BAAS is common among patients undergoing TAVI. Selection of a larger THV in these patients is associated with lower rates of PVL and optimized THV hemodynamics with no effect on procedural complications. Additionally, shift from borderline THV to non-borderline THV modified both THV hemodynamics and post-dilatation rates.
Aortic valve stenosis (AS) is the most common valvular heart disease among elderly (1). Transcatheter aortic valve implantation (TAVI) has become an established and effective therapeutic procedure for symptomatic patients with severe AS regardless of procedural risk (2, 3), and recently is offered to a younger and lower risk population. These changes in TAVI candidates emphasize the need for optimal transcatheter heart valve (THV) implantation to achieve procedural success and prolonged durability. The selection of an appropriately sized THV is a crucial component of the TAVI procedure. Valve undersizing may lead to paravalvular leak (PVL), valve embolization and poor hemodynamics. Oversizing may result in coronary occlusion, atrioventricular block, mitral valve injury, periaortic hematoma, septal or annular rupture (4).
Multi-detector computed tomography (MDCT) is the gold standard method for pre-procedural planning and annular sizing of both balloon-expandable (BE) and self-expanding (SE) THV (5). THVs are currently available in a limited number of sizes and the manufacturer's sizing guidelines allow for a gray area with considerable overlap, where patients with borderline aortic annulus size (BAAS) may be candidates for either of the two suitable THV sizes (smaller or larger). Recently, study from the PARTNER 3 (Placement of Aortic Transcatheter Valves 3) trial demonstrated that in selected patients with annular dimensions in between 2 valve sizes, the larger THV device oversized to both the annular area and perimeter reduced PVL and optimized THV hemodynamics (6). Meanwhile, BAAS remains a common challenge during device size selection and the most effective THV selection strategy for these patients remains unclear.
The aim of the present study was to evaluate outcomes of TAVI, mainly PVL and valve hemodynamics, in patients with BAAS (based on MDCT) according to THV sizing selection in between 2 valve sizes. In addition, to investigate the benefit of shifting between different THV types (BE and SE) to avoid valve size selection in BAAS.
We performed a retrospective analysis from the Israeli multi-center TAVI registry, including patients with severe symptomatic AS and BAAS, measured by MDCT, undergoing TAVI with SE-THV (CoreValve, Evolut R and Evolut PRO) or BE-THV (Sapien XT, Sapien 3) during the years 2015–2019, at 1 of 4 tertiary centers in Israel: Rabin Medical Center, Sheba Medical Center, Tel-Aviv Sourasky Medical Center, and Hadassah Medical Center. The study was approved by the institutional review board of each of the participating centers.
Eligibility for TAVI was established after a multi-disciplinary approach as indicated by the current recommendations. The preoperative workout included MDCT scan to plan the most appropriate route of intervention and to establish the aortic size and dimensions. Aortic sizing and valve measurements were performed by the local team in each center. All centers adopted a transfemoral-first approach policy; other vascular accesses (trans-apical, trans-subclavian, etc.) were considered in cases in which the transfemoral access was not feasible. According to the local policy, TAVIs were performed under local or general anesthesia. The selection of prosthesis type and size was at the discretion of the treating physicians at each center. Pre-specified clinical and laboratory data were collected for all patients at baseline before the procedure, immediately after the procedure, during the index hospitalization, and during long-term follow-up. Collected data included medical history, electrocardiogram, echocardiography studies, MDCT measurements, laboratory tests, and clinical outcomes. Outcomes were collected according to the Valve Academic Research Consortium (VARC) 2 consensus document (7).
BAAS was defined based on THV manufacturer sizing instructions; size cut-off ±1 mm for SE-THV (62.8 ± 1 mm for valve size of 23 vs. 26; 72.3 ± 1 for 26 vs. 29; 81.7 ± 1 for 29 vs. 34 mm) and borderline range for BE-THV (330–350 mm2 for valve size of 20 vs. 23 mm; 420–440 mm2 for 23 vs. 26 mm; 530–560 mm2 for 26 vs. 29 mm) (Figure 1) (8). Patients who underwent valve in valve or valve in ring procedures were excluded from the study.
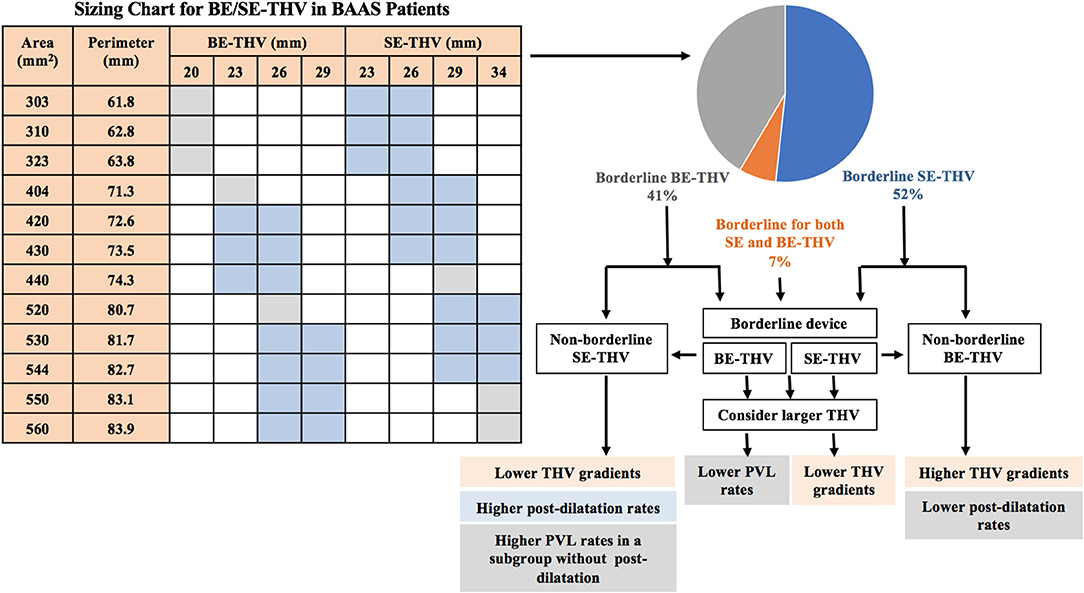
Figure 1. Valve selection in BAAS patients. BE, balloon-expandable; SE, self-expandable; THV, transcatheter heart valve; BAAS, borderline aortic annulus size; PVL, paravalvular leak.
The Evolut R SE valve is constituted by a nitinol frame mounting three porcine pericardial leaflets. The valve is repositionable, partially recapturable, and it is deliverable using a dedicated delivery system 14/16-Fr compatible depending on valve size. The Evolut PRO device represents an evolution of its predecessor and features a porcine pericardial outer wrap that contributes to reduce the risk of residual PVL. Evolut R covers a wide range of sizes and is available in 23, 26, 29, and 34 mm sizes (9); the PRO valve is available in 23, 26, and 29 mm sizes (10).
The Sapien XT/3 BE valve incorporates a cobalt chromium stent that mounts bovine pericardial leaflets. Sapien 3, has both an inner and an outer polyethylene terephthalate fabric seal to minimize the risk of paravalvular leaks. The delivery system has an active 3-dimensional coaxial positioning catheter and a 16-Fr expandable sheath (11).
Continuous variables were expressed as mean ± standard deviation and as median and interquartile range (IQR) and compared using Mann-Whitney test. Categorical variables were compared using Chi-square or Fisher's exact tests as needed. All analyses were conducted using Python version 3.5, p < 0.05 was considered statistically significant.
Out of 2,352 patients following implantation of SE-THV (CoreValve, Evolut R and Evolut PRO) or BE-THV (Sapien XT, Sapien 3) with pre-procedural MDCT measurements, 124 were excluded due to valve in valve, valve in ring, or mitral valve interventions. Additional 38 patients with BAAS and an annulus area of 330–350 mm2 who were implanted with BE-THV were excluded from the analysis since the smaller valve size of 20 mm was not implanted. Eventually, 598 patients with BAAS as defined for at least one THV type, 309 for SE-THV, 248 for BE-THV and 41 patients for both devices were included in the analysis. Of them, 367 (61.4%) patients were implanted with borderline valves, while all others were implanted with non-borderline valves due to shift from SE-THV to BE-THV, or vice versa. The SE-THV group included 93 patients implanted with smaller valves, and 150 patients implanted with larger valves. In the BE-THV group, 22 patients were implanted with smaller valves, and 102 patients with larger valves (Figure 2).
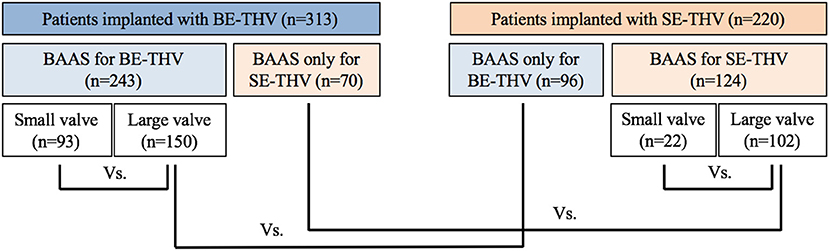
Figure 2. Flow chart for study cohort. BE, balloon-expandable; SE, self-expandable; THV, transcatheter heart valve, BAAS, borderline aortic annulus size.
In BAAS patients implanted with SE-THV, the baseline clinical characteristics of both groups (smaller and larger valves) did not differ, except for the New York Heart Association (NYHA) functional class (Table 1A). In addition, no significant differences were observed in imaging (echocardiography and MDCT) measurements (Table 2A). In BAAS patients implanted with BE-THV, differences were noted in left ventricular function (Table 2B). Other measured baseline clinical and imaging characteristics did not differ between smaller and larger valves implantation (Tables 1B, 2B).
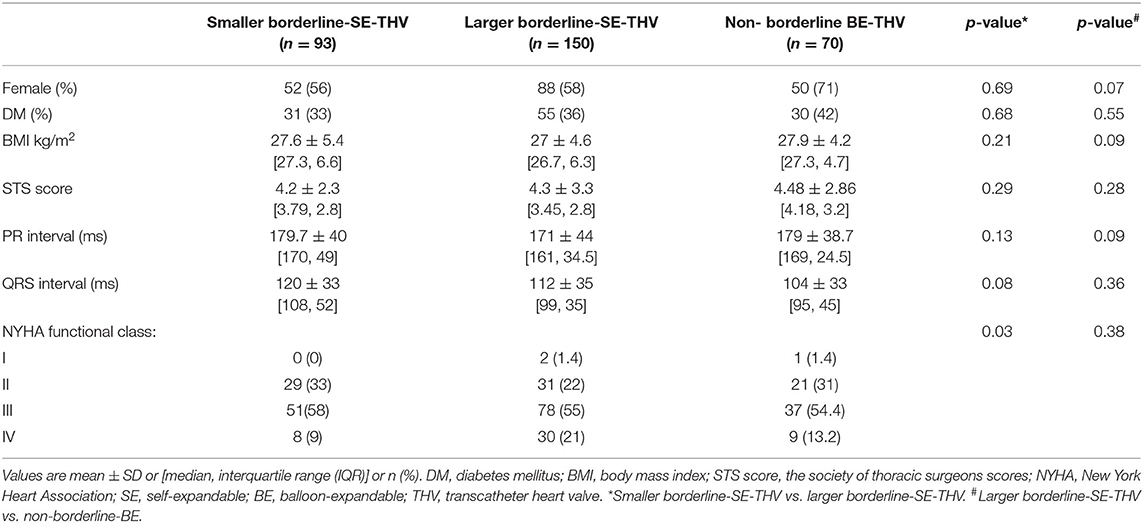
Table 1A. Baseline clinical characteristics of patients with BAAS for SE-THV, implanted with SE or non-borderline BE-THV.
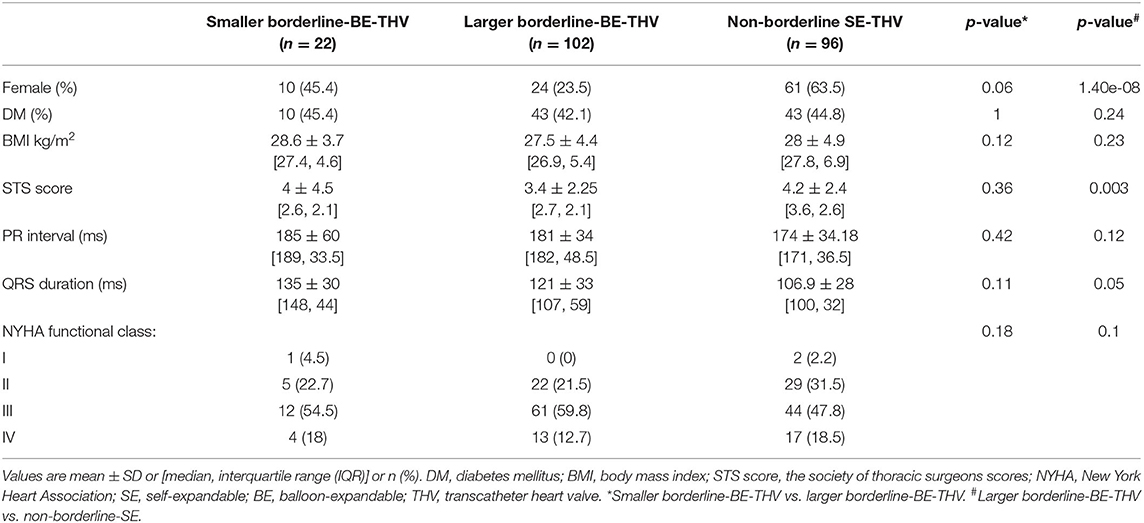
Table 1B. Baseline clinical characteristics of patients with BAAS for BE-THV, implanted with BE or non-borderline SE-THV.
Baseline clinical and imaging characteristics of patients with borderline annulus for SE devices implanted with borderline large SE-THV or non-borderline BE-THV did not differ, except for aortic valve mean pressure gradient (Table 2A). Comparison between non-borderline SE-THV implantation to large BE-borderline valves implantation in patients with borderline annulus for BE devices showed more females and higher Society of Thoracic Surgeons (STS) score in patients implanted with non-borderline SE-THV compared to large BE-borderline valves (Table 1B). In addition, in patients implanted with non-borderline SE-THV the left main (LM) and right coronary artery (RCA) heights were shorter compared with patients implanted with larger BE-borderline valves (Table 2B).
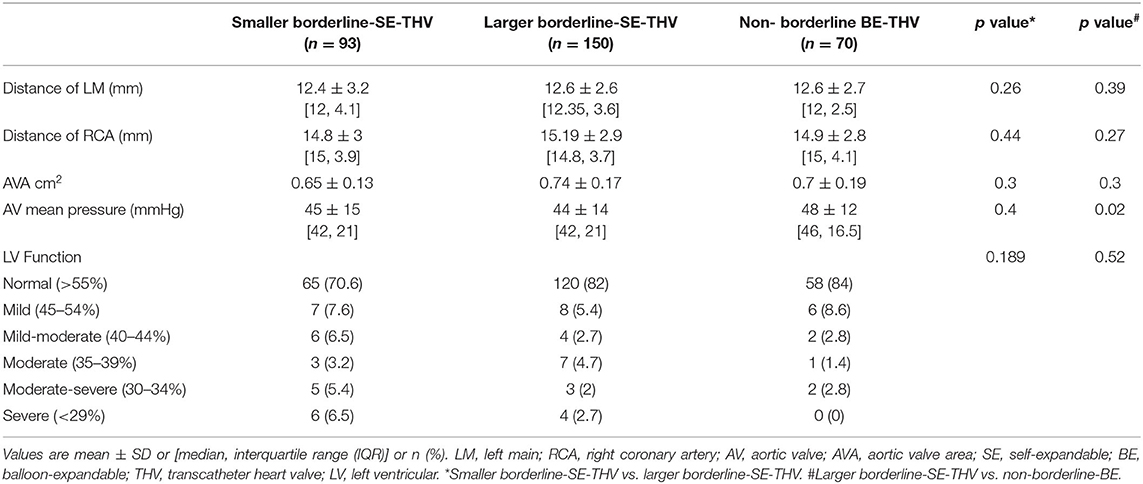
Table 2A. Baseline echocardiography and MDCT characteristics of patients with BAAS for SE-THV implanted with SE or non-borderline BE-THV.
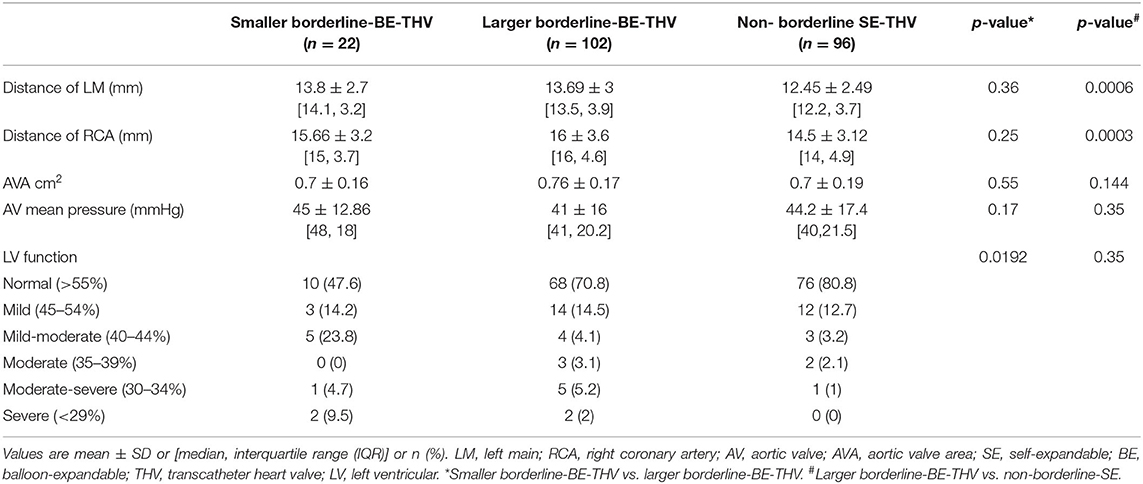
Table 2B. Baseline echocardiography and MDCT characteristics of patients with BAAS for BE-THV implanted with BE or non-borderline SE-THV.
In the present cohort, favorable outcomes were observed while using larger valves in BAAS patients. For SE-THV, selection of larger valves compared to smaller valves was accompanied with significantly lower rates of PVL measured by both echocardiography (none: 54.6 vs. 35.5%, mild: 36 vs. 54.8%, mild to moderate: 7.3 vs. 6.4%, moderate: 2 vs. 2.1%, moderate to severe: 0 vs. 1%; pv = 0.0282; Figure 3; Table 3) and angiography (none: 85.3 vs. 68.8%, mild: 13.3 vs. 27.9%, moderate: 1.3 vs. 3.2%; pv = 0.0088; Table 3) and a trend toward lower gradients across the THV (7.9 ± 5.4 vs. 10.2 ± 10.8; pv = 0.083; Figure 4; Table 3); for BE-THV, selection of larger valves compared to smaller valves resulted in better hemodynamics with lower gradients across the THV (9.9 ± 3.7 vs. 12.5 ± 7.2; pv = 0.019; Figure 4; Table 3). In BE-THV no significant differences were demonstrated in PVL rates while comparing larger to smaller valves implantation in BAAS patients (Figure 2; Table 3). Selection of larger valves (either SE or BE) in BAAS patients did not change the rate of post-dilatation as well as adverse clinical outcomes such as new left bundle branch block (LBBB), rate of new pacemaker implantation, stroke or transient ischemic attack (TIA), annular rupture, coronary occlusion, or mortality (Table 3).

Figure 3. Incidence of paravalvular leak in patients with BAAS. BE, balloon-expandable; SE, self-expandable; THV, transcatheter heart valve; BAAS, borderline aortic annulus size; PVL, paravalvular leak.
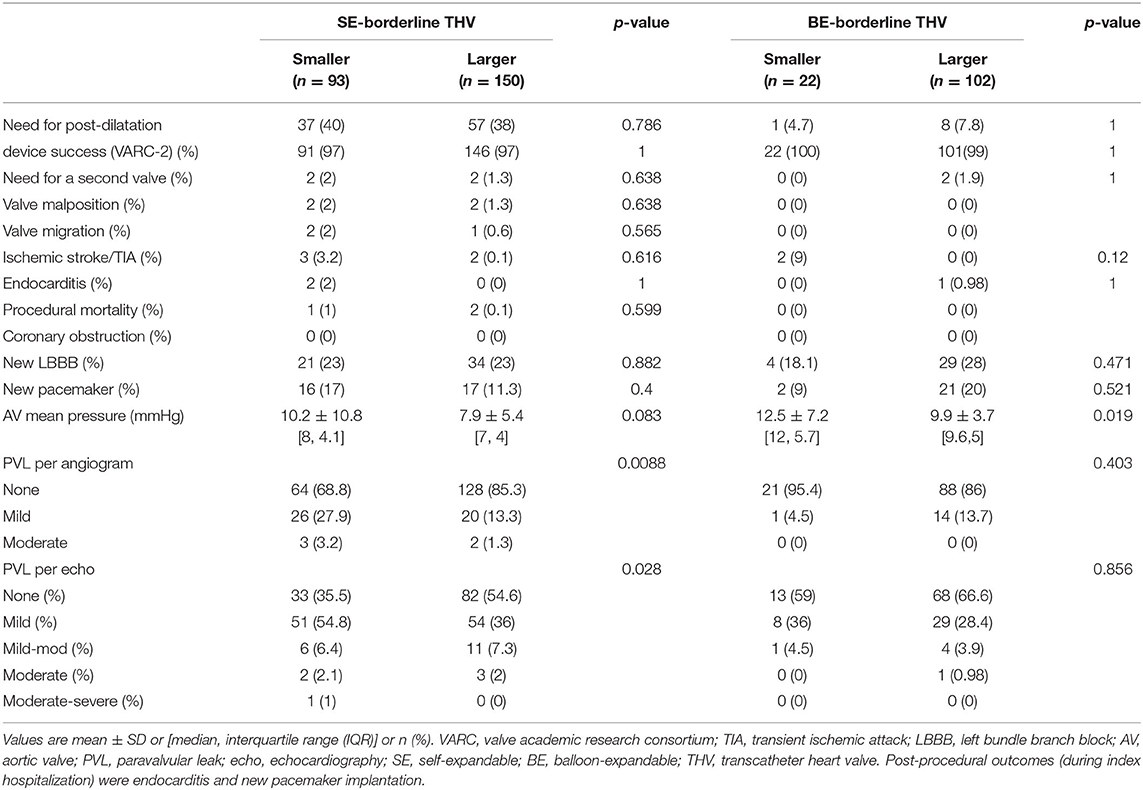
Table 3. Comparison of procedural and post-procedural outcomes between BAAS patients implanted with smaller vs. larger valves (SE or BE).
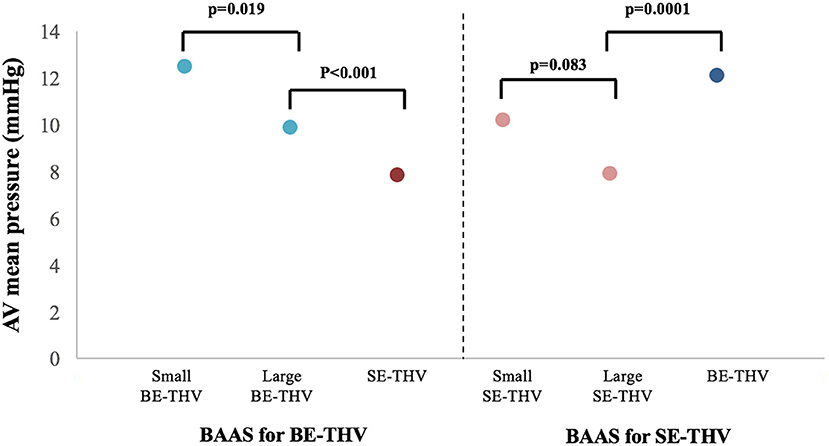
Figure 4. Transcatheter heart valve hemodynamics in patients with BAAS. AV, aortic valve; BE, balloon-expandable; SE, self-expandable; THV, transcatheter heart valve, BAAS, borderline aortic annulus size.
Shift from large borderline SE-THV to non-borderline BE-THV was associated with higher gradients across the THV (7.98.5 ± 5.46.3 vs. 12.11 ± 4.53; pv <0.0001; Figure 4; Table 4); However, lower rates of post-dilatation were observed (38 vs. 12.8%; pv = 0.0001; Table 4), but without significant differences in PVL rates (Table 4). In a subgroup of patients who didn't undergo post-dilatation the PVL rates also did not differ (Table 4). Shift from large borderline BE-THV to non-borderline SE-THV resulted in lower gradients (9.9 ± 3.7 vs. 7.8 ± 3.5, pv <0.001; Figure 4; Table 4), and increased rates of post-dilatation (7.8 vs. 35.4%, pv <0.001; Table 4) with a trend toward increased overall PVL rated per echocardiography (33 vs. 45.8%, pv = 0.08; Table 4). In a subgroup of patients who didn't undergo post-dilation the PVL rates were increased in non-borderline SE-THV compared to large borderline BE-THV (none: 35.8 vs. 72%, mild: 51.2 vs. 25.8%, mild to moderate: 10.2 vs. 3.37%, moderate: 3 vs. 0%, pv = 0.001; Table 4).
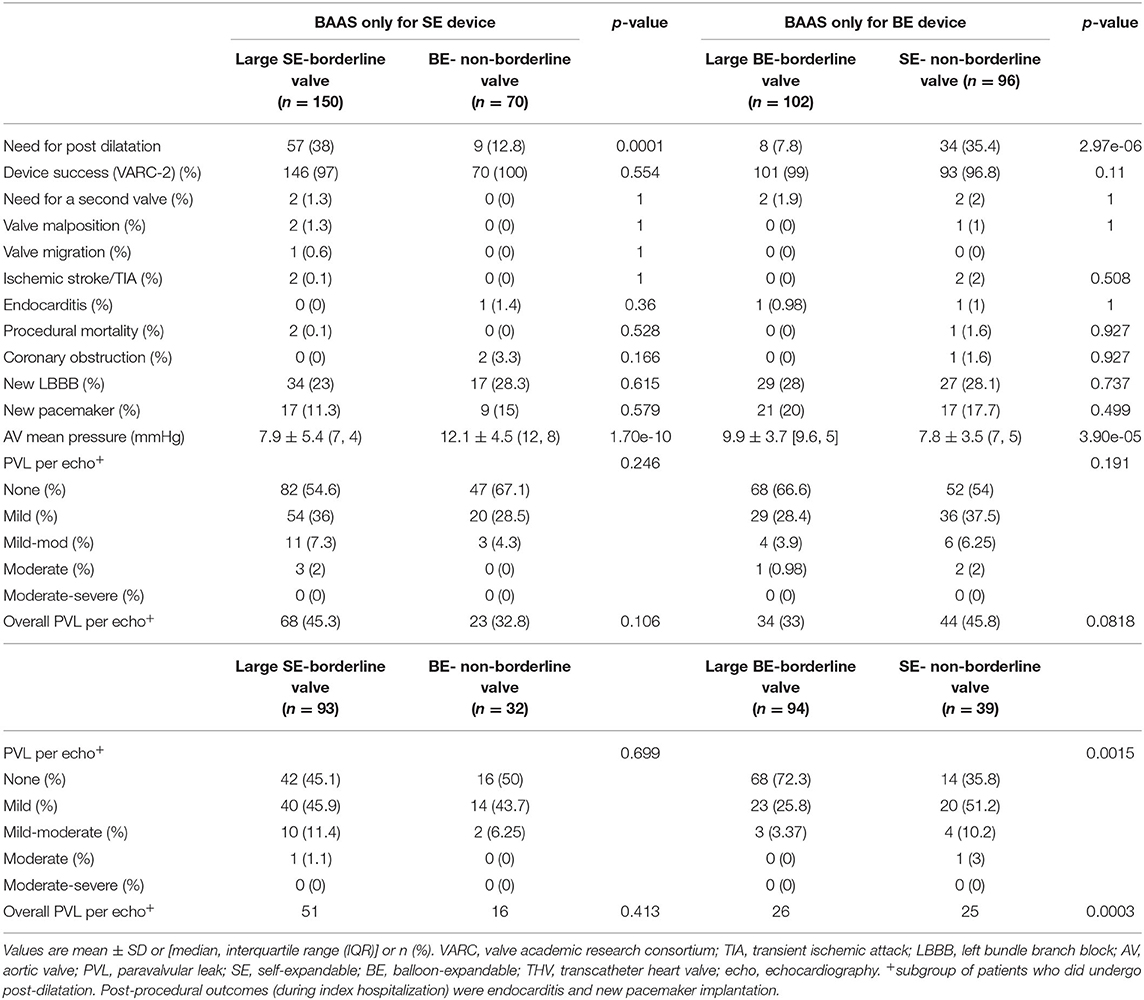
Table 4. Comparison of procedural and post-procedural outcomes between patients implanted with large borderline-SE vs. non-borderline-BE devices; or large borderline-BE vs. non-borderline-SE devices.
Borderline annulus size (annular dimensions in between 2 valve sizes) is common among patients undergoing TAVI, however, the most effective THV selection strategy for these patients remains unclear. The present study of 598 patients with severe symptomatic AS undergoing TAVI based on the ISRAELI-TAVI registry is the largest observational study to date comparing clinical outcomes according to size selection of SE-THV and BE-THV in patients with BAAS. The main findings of our study (Figure 1) are as follows:
• In patients with BAAS, the larger THV device reduced PVL rates and optimized THV hemodynamics.
• Selection of a larger valve in BAAS patients did not increase adverse clinical outcomes such as new LBBB, rate of new pacemaker implantation, stroke or TIA, annular rupture, coronary occlusion or mortality.
• Shift from borderline THV to non-borderline THV modified THV hemodynamics and post dilatation rates. Shift from borderline SE-THV to non-borderline BE-THV was associated with lower post dilatation rates, but with higher gradients. Shift from borderline BE-THV to non-borderline SE-THV led to optimized THV hemodynamics, but with increased post-dilatation rates; In addition, in a subgroup of patients in whom post-dilatation was not performed, increased PVL rates were observed.
Large size THV implantation was previously shown to be associated with favorable hemodynamics and lower PVL rate, both are important determinants of clinical outcomes in AS patients following TAVI (4, 12). In fact, evidence shows deleterious prognostic effects even with mild residual PVL after TAVI, including increased mortality (13, 14). In addition, higher post-TAVI transaortic gradients are associated with decreased THV long-term durability (15). The advantages of implanting larger, over smaller, devices were indeed reflected in our cohort of BAAS patients by reduced PVL rates and optimized THV hemodynamics. These findings are particularly important in the current era, in which younger and relatively healthier patients are being treated with TAVI and in whom the durability of the device is extremely important to minimize the need for future reintervention. Importantly, the use of a larger THV in BAAS patients was not associated with increased adverse outcomes commonly encountered with large prostheses, such as conduction disturbances, annular rupture and coronary occlusion (16, 17). Given the above results, the present study strengthens recent results from the PARTNER 3 trial (6) and advocates the selection of a large THV for BAAS patients undergoing TAVI with either SE or BE prostheses.
In our cohort of BAAS patients, 93% of cases were defined as BAAS for one device only (i.e., either BE or SE). In these patients, it is thus conceivable to apply a strategy of selecting the non-borderline device whenever possible. In fact, non-borderline devices were selected over borderline devices in 38.6% of patients in our cohort. We found that selection of non-borderline SE-THV over borderline BE-THV led to lower gradients. On the other hand, selection of non-borderline BE-THV over borderline SE-THV was associated with lower rates of post-dilatation, but at the cost of increased gradients. This trade-off between PVL and higher gradients was repeatedly described in comparative studies between BE and SE devices both in tricuspid and bicuspid AS patients (18, 19). These changes were mainly attributed to THV mechanical characteristics, such as annular/supra-annular valve position, radial forces, and the presence of outer skirt (15). Therefore, our findings point out that similar considerations taken while selecting THV type for non-BAAS patients (including calcifications, coronary height, sinus of valsalva dimensions), should be applied also in BAAS patients.
We acknowledge several limitations of our study. The main limitation is the observational nature of the study. Therefore, undocumented factors, such as sinus of valsalva diameter or calcium score, may have affected device selection. In addition, potential impact of unknown or unmeasured confounding factors on study outcomes cannot be excluded. The low number of patients implanted with smaller BE-valve may affect the significance of the results and even necessitated the exclusion of patients with annulus measurements of 330–350 mm2 from the analysis. BE-TVH over or under-sizing by over or under-inflation of the valve balloon in order to fine tune the valve dimensions was not registered and might have affected in-situ valve size. Nevertheless, the practice of over/under-inflation in the four centers was according to a known algorithm proposed by the company.
The results of the present study support, for both devices (BE and SE), the selection of larger valves for TAVI candidates with BAAS. Shifting from borderline devices to non-borderline devices resulted in significant changes in post-dilatation, PVL, and gradients across the THV. Therefore, our findings point out that the same consideration taken while selecting THV type for non-BAAS patients, should be applied in BAAS patients, and whenever a borderline device is selected the larger valve device should be recommended.
The results of the present study support, for both devices (BE and SE), the selection of larger valves for TAVI candidates with BAAS. Shifting from borderline devices to non-borderline devices resulted in significant changes in PVL and THV hemodynamics. Therefore, our findings point out that the same consideration taken while selecting THV type for non-BAAS patients, should be applied in BAAS patients, and whenever a borderline device is selected the larger valve device is recommended.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
YT-B, RK, JB-S, and PC conceived the project, designed and interpreted the results, and wrote the manuscript. NB conducted all analyses and interpreted the results. HV-A, AH, BK, HD, GP, MK, AF, ASt, IM, ASe, and AB coordinated and designed data collection and interpreted the results. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. (2006) 368:1005–11. doi: 10.1016/S0140-6736(06)69208-8
2. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380:1695–705. doi: 10.1056/NEJMoa1814052
3. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2016) 374:1609–20. doi: 10.1056/NEJMoa1514616
4. Popma JJ, Gleason TG, Yakubov SJ, Harrison JK, Forrest JK, Maini B, et al. Relationship of annular sizing using multidetector computed tomographic imaging and clinical outcomes after self-expanding CoreValve transcatheter aortic valve replacement. Circ Cardiovasc Interv. (2016) 9:3282. doi: 10.1161/CIRCINTERVENTIONS.115.003282
5. Blanke P, Weir-McCall JR, Achenbach S, Delgado V, Hausleiter J, Jilaihawi H, et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (tavi)/transcatheter aortic valve replacement (TAVR): an expert consensus document of the society of cardiovascular computed tomography. JACC Cardiovasc Imaging. (2019) 12:1–24. doi: 10.1016/j.jcmg.2018.12.003
6. Ihdayhid AR, Leipsic J, Hahn RT, Pibarot P, Thourani V, Makkar R, et al. Impact of annular oversizing on paravalvular regurgitation and valve hemodynamics: new insights from PARTNER 3. JACC Cardiovasc Interv. (2021) 14:2158–69. doi: 10.1016/j.jcin.2021.07.018
7. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. (2012) 33:2403–18. doi: 10.1093/eurheartj/ehs255
9. Grube E, Van Mieghem NM, Bleiziffer S, Modine T, Bosmans J, Manoharan G, et al. Clinical outcomes with a repositionable self-expanding transcatheter aortic valve prosthesis: the international forward study. J Am Coll Cardiol. (2017) 70:845–53. doi: 10.1016/j.jacc.2017.06.045
10. Forrest JK, Mangi AA, Popma JJ, Khabbaz K, Reardon MJ, Kleiman NS, et al. Early outcomes with the evolut PRO repositionable self-expanding transcatheter aortic valve with pericardial wrap. JACC Cardiovasc Interv. (2018) 11:160–8. doi: 10.1016/j.jcin.2017.10.014
11. Wendler O, Schymik G, Treede H, Baumgartner H, Dumonteil N, Neumann F-J, et al. 3: 1-year outcomes post-transcatheter aortic valve implantation using the latest generation of the balloon-expandable transcatheter heart valve. Eur Heart J. (2017) 38:2717–26. doi: 10.1093/eurheartj/ehx294
12. Willson AB, Webb JG, Labounty TM, Achenbach S, Moss R, Wheeler M, et al. 3-dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement: a multicenter retrospective analysis. J Am Coll Cardiol. (2012) 59:1287–94. doi: 10.1016/S0735-1097(12)60326-X
13. Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. (2012) 366:1686–95. doi: 10.1056/NEJMoa1200384
14. Kodali S, Pibarot P, Douglas PS, Williams M, Xu K, Thourani V, et al. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J. (2015) 36:449–56. doi: 10.1093/eurheartj/ehu384
15. Abdel-Wahab M, Landt M, Neumann F-J, Massberg S, Frerker C, Kurz T, et al. 5-year outcomes after TAVR with balloon-expandable versus self-expanding valves: results from the CHOICE randomized clinical trial. JACC Cardiovasc Interv. (2020) 13:1071–82. doi: 10.1016/j.jcin.2019.12.026
16. Lin S-I, Miura M, Tagliari AP, Lee Y-H, Shirai S, Puri R, et al. Intraventricular conduction disturbances after transcatheter aortic valve implantation. Interv Cardiol. (2020) 15:e11. doi: 10.15420/icr.2020.07
17. Pasic M, Unbehaun A, Buz S, Drews T, Hetzer R. Annular rupture during transcatheter aortic valve replacement: classification, pathophysiology, diagnostics, treatment approaches, and prevention. JACC Cardiovasc Interv. (2015) 8:1–9. doi: 10.1016/j.jcin.2014.07.020
18. Van Belle E, Vincent F, Labreuche J, Auffret V, Debry N, Lefèvre T, et al. Balloon-expandable versus self-expanding transcatheter aortic valve replacement: a propensity-matched comparison from the FRANCE-TAVI registry. Circulation. (2020) 141:243–59. doi: 10.1161/CIRCULATIONAHA.120.047270
19. Mangieri A, Tchetchè D, Kim W-K, Pagnesi M, Sinning J-M, Landes U, et al. Balloon versus self-expandable valve for the treatment of bicuspid aortic valve stenosis: insights from the BEAT international collaborative registrys. Circ Cardiovasc Interv. (2020) 13:e008714. doi: 10.1161/CIRCINTERVENTIONS.119.008714
Keywords: borderline aortic annulus, transcatheter aortic valve implantation, paravalvular leak, valve hemodynamics, multi-detector computed tomography
Citation: Talmor-Barkan Y, Kornowski R, Bar N, Ben-Shoshan J, Vaknin-Assa H, Hamdan A, Kruchin B, Barbash IM, Danenberg H, Perlman GY, Konigstein M, Finkelstein A, Steinvil A, Merdler I, Segev A, Barsheshet A and Codner P (2022) Impact of Valve Size on Paravalvular Leak and Valve Hemodynamics in Patients With Borderline Size Aortic Valve Annulus. Front. Cardiovasc. Med. 9:847259. doi: 10.3389/fcvm.2022.847259
Received: 01 January 2022; Accepted: 01 February 2022;
Published: 09 March 2022.
Edited by:
Ronak Rajani, Guy's and St Thomas' NHS Foundation Trust, United KingdomReviewed by:
Hiroyuki Kamiya, Asahikawa Medical University, JapanCopyright © 2022 Talmor-Barkan, Kornowski, Bar, Ben-Shoshan, Vaknin-Assa, Hamdan, Kruchin, Barbash, Danenberg, Perlman, Konigstein, Finkelstein, Steinvil, Merdler, Segev, Barsheshet and Codner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pablo Codner, ZHJwYWJsb2NvZG5lckBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.