- 1Department of Anesthesiology, Fuwai Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 2Department of Cardiac Surgery, The 7th People's Hospital of Zhengzhou, Zhengzhou, China
Background: Previous studies have found atrial fibrillation (AF) is associated with valvular heart disease (VHD). However, whether there is a causal relationship between these two diseases or it is just a result of bias caused by confounding factors is uncertain. This study aims to examine the potential causal association between AF and VHD by using Mendelian randomization.
Methods: In order to examine the causal relationship between AF and VHD, we performed a two-sample Mendelian randomization study by collecting exposure and outcome data from genome-wide association study (GWAS) datasets. We utilized data from FinnGen project (FinnGen, 11,258 cases for VHD including rheumatic fever, 3,108 cases for non-rheumatic VHD, and 75,137 cases for participants) and European Bio-informatics Institute database (EBI, 55,114 cases for AF and 482,295 cases for participants). Inverse-variance weighted (IVW), MR-Egger, and weighted median approaches were performed to estimate the causal effect.
Results: The Mendelian randomization analysis indicated that AF increased the risk of VHD by all three MR methods [For VHD including rheumatic fever: IVW, odds ratio (OR) = 1.255; 95% confidence interval (CI), 1.191~1.322; p = 1.23 × 10−17; Weighted median, OR = 1.305, 95% CI, 1.216~1.400, p = 1.57 × 10−13; MR-Egger, OR = 1.250, 95% CI, 1.137~1.375, p = 1.69 × 10−5; For non-rheumatic VHD: IVW, OR = 1.267; 95% CI, 1.169~1.372; p = 6.73 × 10−9; Weighted median, OR = 1.400; 95% CI, 1.232~1.591; p = 2.40 × 10−7; MR-Egger, OR = 1.308; 95% CI, 1.131~1.513; p = 5.34 × 10−4]. After the one outlier SNP was removed by heterogeneity test, the results remained the same. No horizontal pleiotropic effects were observed between AF and VHD.
Conclusions: Our study provides strong evidence of a causal relationship between AF and VHD. Early intervention for AF patients may reduce the risk of developing into VHD.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. It affects about 10% of the general population (1). About 70% of AF is asymptomatic or present with palpitations, dizziness, and difficulty breathing during mild physical activity (2). AF can lower ejection fraction and produce a variety of side effects, including tachycardia, thromboembolism, and a 15 20% reduction in cardiac output due to a loss of ventricular filling. Although AF can be treated with drugs and interventional surgery, there are still some patients with AF who tend to have severe complications such as stroke and valvular heart disease (VHD), especially in those with non-valvular AF patients (3, 4).
To date, several observational studies found that significant functional VHD is common in patients with AF, accounting for about 20-27.7%, and this rate is even higher in patients with long-standing AF (5–9). Compared with isolated AF patients, AF with concomitant VHD patients had a 23-55% increased mortality (10–12). Therefore, clarifying the relationship between these two diseases may be a key strategy to reducing patient mortality.
However, due to the limitation of the observational study that it can be easily affected by confounding factors such as environment and selection bias, we cannot get an accurate conclusion. A previous mendelian randomization (MR) analysis suggested a genetic association between AF on various cardiovascular diseases such as stroke and ischemic heart disease (13). But there is no sufficient statistical power to identify the causal relationship between AF and VHD alone at conventional significance thresholds.
To address the gap, we assessed the causal role by using two-sample mendelian randomization (MR) analysis to assess whether a genetically higher risk of VHD is associated with AF. Two-sample MR is a method to assess the causal effect of an exposure on an outcome using an instrument which is defined by one or more single-nucleotide polymorphisms (SNPs), as a proxy for the exposure (14). It is important to find out the association between AF and VHD in order to add insight into the underlying etiology because AF is preventable and treatable.
Materials and Methods
Overall Study Design
We obtained the summary data from published studies, which have already been approved by institutional review committees in their respective studies. Therefore, no further sanction was required. We used two-sample MR (15, 16) to assess the causal effect between AF and VHD (Figure 1).
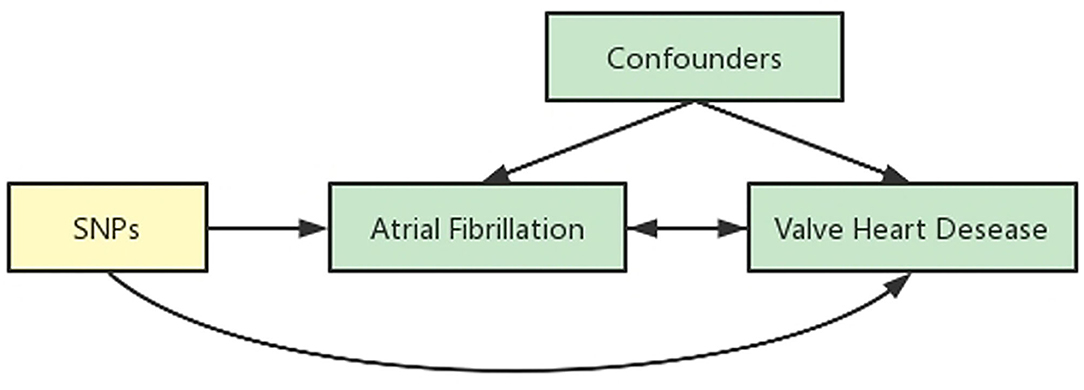
Figure 1. Schematic representation of an MR analysis. We selected SNPs which associated with AF from the European Bio-informatics Institute database and the corresponding effect for these SNPs was estimated based on the risk of VHD obtained from the FinnGen. Because of the randomization and independence of alleles at meiosis, MR is a powerfully predictive tool to assess causal relationships with on bias inherent to observational study designs.
Data Sources
Identification of SNPs Associated With AF
Summary statistics of AF were obtained from the public genome-wide association study (GWAS) that had been assembled in the European Bio-informatics Institute (EBI) database at https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST006061/. Only European ancestry populations were used in our study, which include 55,114 cases and 482,295 control participants.
Genetic variants that passed uncorrelated (r2 < 0.001) SNPs associated with the risk factor at thresholds for a genome-wide level of statistical significance (p < 5 × 10−8) were selected as instruments.
Study Outcome: VHD
Data on VHD were drawn from the GWAS summary data sources on the FennGenn database, which is available at https://gwas.mrcieu.ac.uk/datasets/finn-a-I9_VHD/ (VHD including rheumatic fever, 11,258 cases, 75,137 participants) and https://gwas.mrcieu.ac.uk/datasets/finn-a-I9_NONRHEVALV/ (Non-rheumatic VHD, 3,108 cases, 75,137 participants). As described earlier, independent variants that meet the criteria (r2 < 0.001, p < 5 × 10−8) were considered as low correlation.
Statistical Analysis
Due to the lack of individual-level GWAS data, we chose to use the recently developed method of MR analysis to evaluate whether there is a causal relationship between AF on VHD.
As a genetic method, MR can enhance inferences about the causal nature of exposure-outcome associations by reducing the likelihood of confounding and eliminating reverse causality in conventional observational studies (17). This is because the genetic alleles associated with exposure are conceptually randomly classified and therefore independent of confounding factors such as self-selected lifestyle and environmental factors, also are not affected by disease, and the time sequence is reasonable. Therefore, genotype can be used as an instrumental variable to infer the association between exposure and disease.
But the outcome can be influenced by the genetic variant through a pathway rather than the exposure alone, known as horizontal pleiotropy, which violates the assumption of MR and can bias causal estimates. To correct for this, Inverse-variance weighted (IVW), MR-Egger, and weighted median analytical approaches were used in our MR analysis. IVW is the most preferred and commonly used statistical method, but it only gives consistent estimates if all of the genetic variants in the analysis are valid instrumental variables. MR-Egger statistical method allows for horizontal pleiotropy in the included instrumental SNPs. Weighted median statistical method is complementary to the MR-Egger regression method. It can provide a valid estimate if there are more than 50% of the information comes from SNPs that are valid instrumental variables. All three methods are based on different horizontal pleiotropic models (18). The value of comparing all the results is that the consistency of the different kinds of methods makes our results more reliable (19, 20).
The statistical coding and related data of this study can be obtained from the corresponding author if you need based on reasonable request. MR analysis were conducted in R, version 4.0.3 (http://www.r-project.org) using the TwoSampleMR package (21, 22).
Results
Genetic Instrumental Variables for AF
Identified from previous GWAS database, we find that there are 77 SNPs associated with AF at the genome-wide significance level (p < 5 × 10−8), as shown in Supplementary Table 1.
Of all 77 genetic instruments, an SNP rs2106261 in zinc finger homeobox 3 (ZFHX3) gene locus has previously been reported to have a significant association with AF in European ancestry populations by affecting the myocardial tissue function, regulating myogenic and neuronal differentiation (23, 24). The associations of VHD risk for each genetic variant are shown in Figure 2 and Supplementary Figure 1. We use beta coefficient to describe the correlation coefficient (for Figure 2A, beta = 0.269; for Figure 2B, beta = 0.223).
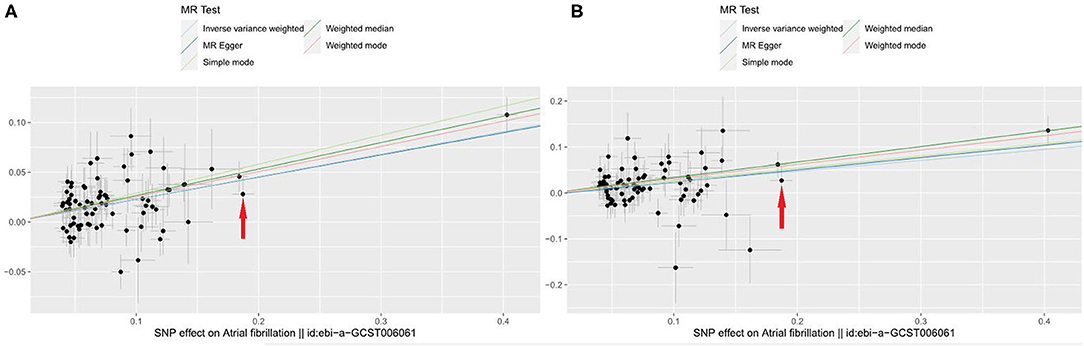
Figure 2. Scatter plot to visualize causal effect of AF on total VHD risk. (A) AF on VHD including rheumatic fever; (B) AF on non-rheumatic VHD. The slope of the straight line indicates the magnitude of the causal association. Red arrows indicates a certain SNP (rs2106261) (X-axis: beta_exposure = 0.1872; Y-axis: beta_outcome = 0.0280). IVW indicates inverse-variance weighted; and MR, Mendelian randomization.
Mendelian Randomization Analysis for VHD
Genetically predicted AF showed a consistent association with VHD including rheumatic fever under the IVW method [odds ratio (OR) = 1.255; 95% confidence interval (CI), 1.191 1.322; p = 1.23 × 10−17]. Similar results were obtained of the weighted median method and MR-Egger method (Weighted median, OR = 1.305; 95% CI, 1.216 1.400; p = 1.57 × 10−13; MR-Egger, OR = 1.250; 95% CI, 1.137 1.375; p = 1.69 × 10−5) (Figure 3A).
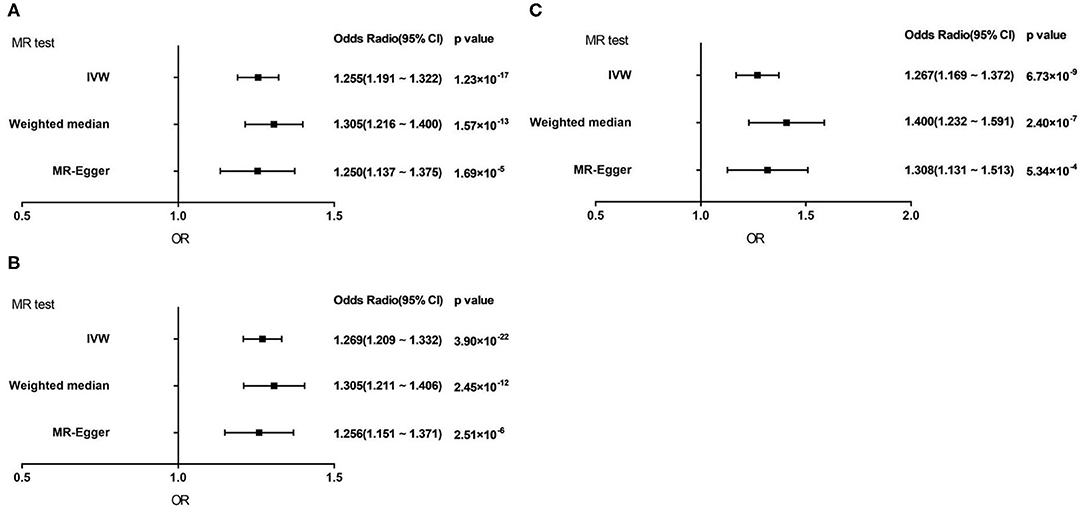
Figure 3. Forest plot to visualize causal effect of AF on total VHD risk by three methods. (A) VHD including rheumatic fever. (B) VHD including rheumatic fever with 1 SNP excluded. (C) Non-rheumatic VHD. IVW, inverse-variance weighted; MR, Mendelian randomization.
As for non-rheumatic VHD, the results demonstrated the same trends as the above studies in three statistic methods (IVW, OR = 1.267; 95% CI, 1.169 1.372; p = 6.73 × 10−9; Weighted median, OR = 1.400; 95% CI, 1.232 1.591; p = 2.40 × 10−7; MR-Egger, OR = 1.308; 95% CI, 1.131 1.513; p = 5.34 × 10−4) (Figure 3C).
Sensitivity Analysis
1. Heterogeneity Analysis
Q statistic was conducted to detect heterogeneous outcomes. We found that for VHD including rheumatic fever, there was heterogeneity among 77 SNPs (IVW: Q = 103.277, p = 0.017; MR-Egger: Q = 103.290, p = 0.020). To identify the specific SNPs responsible for the heterogeneous results, we performed the MR-PRESSO outliner test and found that the heterogeneous results were due to a certain SNP (rs34750263). Based on the three assumptions of two-sample MR analysis, we reanalyzed the association between AF and VHD including rheumatic fever by excluding this outliner SNP, leaving 76 SNPs as genetic instruments. The result showed that after excluding 1 SNPs, the Q statistic revealed no notable heterogeneity under the IVW model and MR-Egger model (IVW: Q = 86.335, p = 0.175; MR-Egger: Q = 86.246, p = 0.156).
After excluding one outliner SNP, we repeated the analysis to estimate the causal relationships between AF and VHD including rheumatic fever and the result did not change substantially (IVW, OR = 1.269; 95% CI, 1.209 1.332; p = 3.90 × 10−22; Weighted median, OR = 1.305; 95% CI, 1.211 1.406; p = 2.45 × 10−12; MR-Egger, OR = 1.256; 95% CI, 1.151 1.371; p = 2.51 × 10−6; Figure 3B). It suggested that our result is reliable and stable.
2. Horizontal Pleiotropy Analysis
To reduce the bias caused by horizontal pleiotropy, we performed the MR-Egger intercept test, and the visualized results were displayed in funnel plot (Figure 4). The result from the test did not reveal any horizontal pleiotropy in our study (for VHD including rheumatic fever: intercept = 0.0004, p = 0.923; for non-rheumatic VHD, intercept = −0.003, p = 0.605).
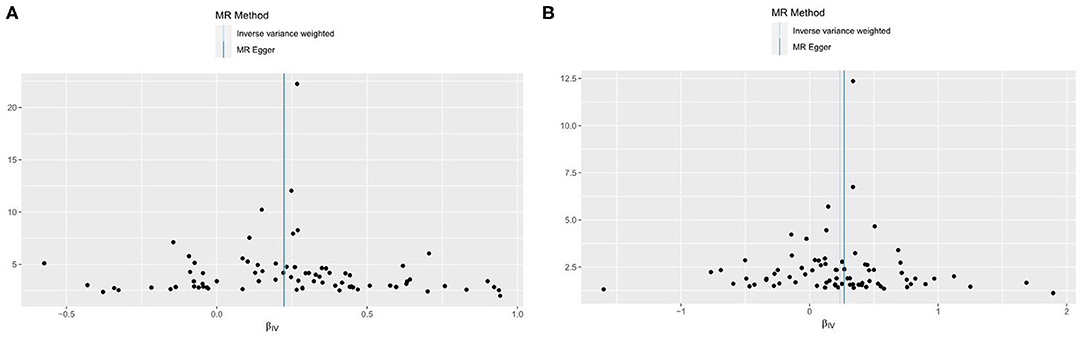
Figure 4. Funnel plots to visualize overall heterogeneity of MR estimates for the effect of AF on VHD. (A) VHD including rheumatic fever. (B) non-rheumatic VHD. IVW, inverse-variance weighted; MR, Mendelian randomization.
3. Effects of Individual Genetic Instruments in Relation to VHD
To verify the influence of each SNP on the overall causal estimate, leave-one-out analysis was performed. No substantial difference appeared in estimated causal effect when systematically removing individual SNP and repeating the MR analysis (Supplementary Figure 2). Therefore, not any single genetic instrument resulted in the estimated effects.
Discussion
Based on previous observational research, the present study systematically evaluated the association between AF and VHD by MR method, which is a natural RCT, using publicly available large-scale GWAS datasets. With 77 SNPs that were significantly associated with AF as the main instruments in the two-sample MR analysis, we found that to some extent, a genetic predisposition of people with AF can increase the incidence of both VHD including rheumatic fever and non-rheumatic VHD.
VHD is mainly manifested as valve stenosis or insufficiency, which is the main cause of impaired activity tolerance, heart failure, and death in patients (11). According to epidemiological statistics in British, at least 50% of people over the age of 65 suffered from VHD (25). VHD is mainly caused by congenital dysplasia or other pathological changes, such as degenerative diseases, infections, and trauma. Previous studies found that individuals with a diagnosis of coronary heart disease, hypertension, chronic kidney disease and AF had a higher risk of VHD, which has been reported in many large sample-size researches (25, 26). The incidence of VHD also increases with age (25). Recently, the 2021 ESC/EACTS Guidelines for the Management of VHD (27) suggested that VHD may arise as a consequence of atrial enlargement and mitral annular dilatation in patients with longstanding AF, which means that people have begun to pay attention to the mechanism of secondary valvular disease. Notably, in a study of 47 AF patients, Oren et al. (28) found that people who were diagnosed with permanent lone AF had a 6.5-fold higher likelihood of having tricuspid regurgitation (p = 0.0031) and were marginally more likely to have mitral regurgitation compared to those with paroxysmal AF. Utsunomiya et al. (29) reported that functional tricuspid regurgitation with a structurally normal tricuspid valve may occur secondary to chronic AF, which indicated that there might be a relationship between AF and VHD. These results provided a basis for our study.
However, the causal association between these two diseases is not clear yet. Previous genetic studies of VHD mainly focused on genetic risk factors involved in blood pressure or obesity, including high LDL-cholesterol and Lipoprotein(a) (30–33). However, there is no research studied the causal relationship between AF and VHD before. We therefore aimed at filling this research gap.
To systematically examine if VHD is the cause or consequence of AF in the European ancestry population, two-sample MR method was used. In our study, with 77 SNPs that were significantly associated with AF as genetic instruments, we found that 1-SD increase of AF was associated with a 30.5% increase of VHD including rheumatic fever and a 26.7% increase of non-rheumatic VHD. This may be because that the AF and VHD share the same genetic susceptibility factors.
Of the 77 SNPs, only rs2106261, in the transcription factor gene ZFHX3, had previously been reported. Lubitz et al. (34) identified an AF susceptibility gene, the transcription factor ZFHX3, which was originally identified in the Caucasian population by scanning SNPs rs2106261. This finding was subsequently replicated in other populations, such as the Chinese Han population (35) and the Japanese population (36). A recent study published by Zaw et al. (36) showed a similar result with the above researches that the A allele of the rs2106261 SNP was significantly associated with AF, after adjusting for age, sex, diabetes, hypertension, and smoking, which indicated that this SNP variant might be an independent risk marker for AF. ZFHX3 is a candidate tumor suppressor gene for prostate, breast and gastric cancer, which acts by inducing cell cycle arrest. ZFHX3 has also been found to be closely related to neuronal and myogenic differentiation in brain and heart (36–38). In cell signaling, ZFHX3 interacts with a protein that specifically inhibits signal transducer and activator of transcription 3 (STAT3). ZFHX3 can activate this protein inhibitor of activated STAT 3 (PIAS3) as well. Research shows that in AF models, tachycardia induced a decreased expression of ZFHX3, then STAT3 signaling is activated by decreasing PIAS3 activity. Therefore, the inflammatory process of atrial tissue resulting from the down-regulation of ZFHX3 may influence the occurrence of atrial arrhythmia (39). These lines of evidence provide a mechanistic basis for the genetic association between ZFHX3 and AF. Though we did not find the causal association between rs2106261 and VHD directly, they all involved in the same biological mechanisms such as inflammatory mechanisms, which may play a significant role in the causal effect of AF on VHD.
An observational study performed by Davutoglu et al. (40) reported that the pathogenesis of VHD was also associated with persistent serum inflammatory mediators that were strongly linked to the severity of valve involvement, valve scarring, subsequent valve calcification, and reduced functional status. Cho et al. (41) identified that VHD was no longer thought to be a simple passive process caused by calcium deposition that occurs with advanced age, immunological and inflammatory responses were also involved, including oxidized lipids, various cytokines, and biomineralization. From a clinical perspective, several studies have suggested that AF can lead to atrial dilation, atrial systolic dysfunction and reconstruction of atrioventricular valve ring, which may cause damage to the valve, but it would be improved if sinus rhythm was restored (42–45). However, no studies have shown whether it is affected by only one mechanism or multiple mechanisms, which needs to be further confirmed.
A chief strength of the present study is that we assessed the causal associations between AF and VHD in the same study population using the MR method. Because alleles are randomly combined and fixed at conception, these genetic variations are not affected by socioeconomic status and associated attributes, or subsequent disease. It means that our results from the MR method are more accurate than from observational studies, and represent a lifetime risk of VHD on AF. Another strength is that VHD GWAS of our study was finished just in European ancestry populations, which could reduce bias due to population stratification.
Three limitations should be acknowledged. Firstly, since no specific diseases such as mitral regurgitation were found in GWAS database, we only analyzed AF and VHD without subgroup analysis. Secondly, there is no way to obtain information about disease severity or classification because we only extract data from disease diagnosis, not from examination indicators such as cardiac ultrasound. Thirdly, it has been well accepted that the risk factors are various in different races and ethnicities (46). Therefore, the reliability of the causal associations should be validated in other races.
Conclusion
In conclusion, our results provide strong evidence of a causal relationship between AF and VHD by using MR method. Based on this result, we recommend that early treatment should be performed for AF patients to reduce the risk of VHD.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
Ethical approval was not provided for this study on human participants because in this study, we obtained the summary data from published studies, which have already been approved by institutional review committees in their respective studies. Therefore, no further sanction was required. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JG and HJ designed the research. JG and YB performed the research, analyzed the data, and wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by grants from the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (ID 2016-I2M-3-024). The funding source had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript, and the decision to submit the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledged Union_of_Researchers (WeChat Subscription) for his help on the methods of our analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.845734/full#supplementary-material
Supplementary Figure 1. Forest plot to visualize causal effect of each single SNP on total VHD risk. (A) VHD including rheumatic fever. (B) non-rheumatic VHD. MR, Mendelian randomization; VHD, Valvular heart disease.
Supplementary Figure 2. Leave-one-out plot to visualize causal effect of AF on total VHD risk when leaving one SNP out. (A) VHD including rheumatic fever. (B) non-rheumatic VHD. MR, Mendelian randomization.
Supplementary Table 1. List of genetic instruments for AF and log odds ratios of VHD risk by each instrumental SNPs (GWAS significance with p <5 × 10-8 and linkage disequilibrium threshold with r2 <0.005).
References
1. Joseph PG, Healey JS, Raina P, Connolly SJ, Ibrahim Q, Gupta R, et al. Global variations in the prevalence, treatment, and impact of atrial fibrillation in a multi-national cohort of 153 152 middle-aged individuals. Cardiovasc Res. (2021) 117:1523-31. doi: 10.1093/cvr/cvaa241
2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373-498. doi: 10.1093/eurheartj/ehab648
3. Dietz MF, Goedemans L, Vo NM, Prihadi EA, van der Bijl P, Gersh BJ, et al. Prognostic implications of significant isolated tricuspid regurgitation in patients with atrial fibrillation without left-sided heart disease or pulmonary hypertension. Am J Cardiol. (2020) 135:84-90. doi: 10.1016/j.amjcard.2020.08.034
4. Vo NM, Leung M, van Rosendael PJ, Goedemans L, van Wijngaarden SE, Prihadi EA, et al. Characteristics and prognosis of patients with nonvalvular atrial fibrillation and significant valvular heart disease referred for electrical cardioversion. Am J Cardiol. (2020) 128:84-91. doi: 10.1016/j.amjcard.2020.04.035
5. Banerjee A, Allan V, Denaxas S, Shah A, Kotecha D, Lambiase PD, et al. Subtypes of atrial fibrillation with concomitant valvular heart disease derived from electronic health records: phenotypes, population prevalence, trends and prognosis. Europace. (2019) 21:1776-84. doi: 10.1093/europace/euz220
6. Doi K, Ogawa H, Ishigami K, Ikeda S, Aono Y, Hamatani Y, et al. Impact of valvular heart disease on mortality, thromboembolic and cardiac events in japanese patients with atrial fibrillation- the fushimi AF registry. Circ J. (2020) 84:714-22. doi: 10.1253/circj.CJ-19-1158
7. Takahashi Y, Izumi C, Miyake M, Imanaka M, Kuroda M, Nishimura S, et al. Actual management and prognosis of severe isolated tricuspid regurgitation associated with atrial fibrillation without structural heart disease. Int J Cardiol. (2017) 243:251-7. doi: 10.1016/j.ijcard.2017.05.031
8. Caro Martinez C, Elvira Ruiz G, Flores Blanco PJ, Cerezo Manchado JJ, Albendin Iglesias H, Lova Navarro A, et al. Prevalence and prognostic implications of valve disease in patients with atrial fibrillation initiating direct oral anticoagulants. Revista espanola de cardiologia. (2019) 72:935-43. doi: 10.1016/j.rec.2018.08.026
9. Abe Y, Akamatsu K, Ito K, Matsumura Y, Shimeno K, Naruko T, et al. Prevalence and prognostic significance of functional mitral and tricuspid regurgitation despite preserved left ventricular ejection fraction in atrial fibrillation patients. Circ J. (2018) 82:1451-8. doi: 10.1253/circj.CJ-17-1334
10. Thomas KL, Jackson LR 2nd, Shrader P, Ansell J, Fonarow GC, et al. Prevalence, characteristics, and outcomes of valvular heart disease in patients with atrial fibrillation: insights from the ORBIT-AF (Outcomes Registry for Better Informed Treatment for Atrial Fibrillation). J Am Heart Assoc. (2017) 6:e006475. doi: 10.1161/JAHA.117.006475
11. Samaras A, Vrana E, Kartas A, Moysidis DV, Papazoglou AS, Doundoulakis I, et al. Prognostic implications of valvular heart disease in patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord. (2021) 21:453. doi: 10.1186/s12872-021-02264-3
12. Prapan N, Ratanasit N, Karaketklang K. Significant functional tricuspid regurgitation portends poor outcomes in patients with atrial fibrillation and preserved left ventricular ejection fraction. BMC Cardiovasc Disord. (2020) 20:433. doi: 10.1186/s12872-020-01716-6
13. Kwok MK, Schooling CM. Mendelian randomization study on atrial fibrillation and cardiovascular disease subtypes. Sci Rep. (2021) 11:18682. doi: 10.1038/s41598-021-98058-w
14. Tan JS, Liu NN, Guo TT, Hu S, Hua L. Genetic predisposition to COVID-19 may increase the risk of hypertension disorders in pregnancy: a two-sample Mendelian randomization study. Pregnancy Hyperten. (2021) 26:17-23. doi: 10.1016/j.preghy.2021.08.112
15. Lawlor DA. Commentary: two-sample Mendelian randomization: opportunities and challenges. Int J Epidemiol. (2016) 45:908-15. doi: 10.1093/ije/dyw127
16. Richmond RC, Hemani G, Tilling K, Davey Smith G, Relton CL. Challenges and novel approaches for investigating molecular mediation. Hum Mol Genet. (2016) 25:R149-56. doi: 10.1093/hmg/ddw197
17. Smith GD, Ebrahim S. Mendelian randomization: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1-22. doi: 10.1093/ije/dyg070
18. Tan JS, Liu NN, Guo TT, Hu S, Hua L. Genetically predicted obesity and risk of deep vein thrombosis. Thromb Res. (2021) 207:16-24. doi: 10.1016/j.thromres.2021.08.026
19. Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. (2015) 181:251-60. doi: 10.1093/aje/kwu283
20. Xu L, Borges MC, Hemani G, Lawlor DA. The role of glycaemic and lipid risk factors in mediating the effect of BMI on coronary heart disease: a two-step, two-sample Mendelian randomisation study. Diabetologia. (2017) 60:2210-20. doi: 10.1007/s00125-017-4396-y
21. Broadbent JR, Foley CN, Grant AJ, Mason AM, Staley JR, Burgess S. Mendelian randomization v0.5.0: updates to an R package for performing Mendelian randomization analyses using summarized data. Wellcome Open Res. (2020) 5:252. doi: 10.12688/wellcomeopenres.16374.2
22. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693-8. doi: 10.1038/s41588-018-0099-7
23. Benjamin EJ, Rice KM, Arking DE, Pfeufer A, van Noord C, Smith AV, et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. (2009) 41:879-81. doi: 10.1038/ng.416
24. Li C, Wang F, Yang Y, Fu F, Xu C, Shi L, et al. Significant association of SNP rs2106261 in the ZFHX3 gene with atrial fibrillation in a Chinese Han GeneID population. Hum Genet. (2011) 129:239-46. doi: 10.1007/s00439-010-0912-6
25. Taylor CJ, Ordonez-Mena JM, Jones NR, Roalfe AK, Myerson SG, Prendergast BD, et al. Survival of people with valvular heart disease in a large, English community-based cohort study. Heart. (2021) 107:1336-43. doi: 10.1136/heartjnl-2020-318823
26. Andell P, Li X, Martinsson A, Andersson C, Stagmo M, Zoller B, et al. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart. (2017) 103:1696-703. doi: 10.1136/heartjnl-2016-310894
27. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2021) 60:727–800. doi: 10.1093/ejcts/ezab557
28. Oren M, Oren O, Feldman A, Bloch L, Turgeman Y. Permanent lone atrial fibrillation and atrioventricular valve regurgitation: may the former lead to the latter? J Heart Valve Dis. (2014) 23:759-64. Available online at: http://api.jlss.vip/s/58dt1o
29. Utsunomiya H, Itabashi Y, Mihara H, Berdejo J, Kobayashi S, Siegel RJ, et al. Functional tricuspid regurgitation caused by chronic atrial fibrillation: a real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging. (2017) 10:e004897. doi: 10.1161/CIRCIMAGING.116.004897
30. Nazarzadeh M, Pinho-Gomes AC, Smith Byrne K, Canoy D, Raimondi F, Ayala Solares JR, et al. Systolic blood pressure and risk of valvular heart disease: a Mendelian randomization study. JAMA Cardiol. (2019) 4:788-95. doi: 10.1001/jamacardio.2019.2202
31. Nazarzadeh M, Pinho-Gomes AC, Bidel Z, Dehghan A, Canoy D, Hassaine A, et al. Plasma lipids and risk of aortic valve stenosis: a Mendelian randomization study. Eur Heart J. (2020) 41:3913-20. doi: 10.1093/ehjci/ehaa946.1990
32. Kaltoft M, Langsted A, Nordestgaard BG. Triglycerides and remnant cholesterol associated with risk of aortic valve stenosis: Mendelian randomization in the Copenhagen General Population Study. Eur Heart J. (2020) 41:2288-99. doi: 10.1093/eurheartj/ehaa172
33. Kaltoft M, Langsted A, Nordestgaard BG. Obesity as a causal risk factor for aortic valve stenosis. J Am Coll Cardiol. (2020) 75:163-76. doi: 10.1016/j.jacc.2019.10.050
34. Lubitz SA, Lunetta KL, Lin H, Arking DE, Trompet S, Li G, et al. Novel genetic markers associate with atrial fibrillation risk in Europeans and Japanese. J Am Coll Cardiol. (2014) 63:1200-10. doi: 10.1016/j.jacc.2013.12.015
35. Liu Y, Ni B, Lin Y, Chen XG, Fang Z, Zhao L, et al. Genetic polymorphisms in ZFHX3 are associated with atrial fibrillation in a Chinese Han population. PLoS ONE. (2014) 9:e101318. doi: 10.1371/journal.pone.0101318
36. Zaw KTT, Sato N, Ikeda S, Thu KS, Mieno MN, Arai T, et al. Association of ZFHX3 gene variation with atrial fibrillation, cerebral infarction, and lung thromboembolism: an autopsy study. J Cardiol. (2017) 70:180-4. doi: 10.1016/j.jjcc.2016.11.005
37. Tsai CT, Hsieh CS, Chang SN, Chuang EY, Juang JM, Lin LY, et al. Next-generation sequencing of nine atrial fibrillation candidate genes identified novel de novo mutations in patients with extreme trait of atrial fibrillation. J Med Genet. (2015) 52:28-36. doi: 10.1136/jmedgenet-2014-102618
38. Zhou M, Liao Y, Tu X. The role of transcription factors in atrial fibrillation. J Thorac Dis. (2015) 7:152-8. doi: 10.3978/j.issn.2072-1439.2015.01.21
39. Kao YH, Hsu JC, Chen YC, Lin YK, Lkhagva B, Chen SA, et al. ZFHX3 knockdown increases arrhythmogenesis and dysregulates calcium homeostasis in HL-1 atrial myocytes. Int J Cardiol. (2016) 210:85-92. doi: 10.1016/j.ijcard.2016.02.091
40. Davutoglu V, Celik A, Aksoy M. Contribution of selected serum inflammatory mediators to the progression of chronic rheumatic valve disease, subsequent valve calcification and NYHA functional class. J Heart Valve Dis. (2005) 14:251-6. Available online at: http://api.jlss.vip/s/6a23p1
41. Cho KI, Sakuma I, Sohn IS, Jo SH, Koh KK. Inflammatory and metabolic mechanisms underlying the calcific aortic valve disease. Atherosclerosis. (2018) 277:60-5. doi: 10.1016/j.atherosclerosis.2018.08.029
42. Muraru D, Guta AC, Ochoa-Jimenez RC, Bartos D, Aruta P, Mihaila S, et al. Functional regurgitation of atrioventricular valves and atrial fibrillation: an elusive pathophysiological link deserving further attention. J Am Soc Echocardiogr. (2020) 33:42-53. doi: 10.1016/j.echo.2019.08.016
43. Silbiger JJ. Atrial functional tricuspid regurgitation: an underappreciated cause of secondary tricuspid regurgitation. Echocardiography. (2019) 36:954-7. doi: 10.1111/echo.14327
44. Gertz ZM, Raina A, Saghy L, Zado ES, Callans DJ, Marchlinski FE, et al. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: reversal with arrhythmia control. J Am Coll Cardiol. (2011) 58:1474-81. doi: 10.1016/j.jacc.2011.06.032
45. Gertz ZM, Herrmann HC, Lim DS, Kar S, Kapadia SR, Reed GW, et al. Implications of atrial fibrillation on the mechanisms of mitral regurgitation and response to mitraclip in the COAPT trial. Circ Cardiovasc Intervent. (2021) 14:e010300. doi: 10.1161/CIRCINTERVENTIONS.120.010300
Keywords: atrial fibrillation (AF), valvular heart disease (VHD), genome-wide association study (GWAS), Mendelian randomization (MR), risk
Citation: Gao J, Bai Y and Ji H (2022) Genetically Predicted Atrial Fibrillation and Valvular Heart Disease: A Two-Sample Mendelian Randomization Study. Front. Cardiovasc. Med. 9:845734. doi: 10.3389/fcvm.2022.845734
Received: 30 December 2021; Accepted: 18 February 2022;
Published: 28 March 2022.
Edited by:
Christoph Sinning, University Medical Center Hamburg-Eppendorf, GermanyReviewed by:
Ya-Li Chen, Second Hospital of Hebei Medical University, ChinaLazaros Lataniotis, University of California, San Francisco, United States
Mirjam Keßler, Ulm University Medical Center, Germany
Copyright © 2022 Gao, Bai and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongwen Ji, MTM3MDEyMjk1NTNAMTYzLmNvbQ==
 Jie Gao
Jie Gao Yulin Bai
Yulin Bai Hongwen Ji
Hongwen Ji