- 1Shenzhen Baoan Women’s and Children’s Hospital, Jinan University, Shenzhen, China
- 2Department of Medicine and Therapeutics, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China
- 3Department of Chemical Pathology, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China
- 4Faculty of Medicine, Macau University of Science and Technology, Macau, Macau SAR, China
- 5School of Health Sciences, Caritas Institute of Higher Education, Hong Kong, Hong Kong SAR, China
Purpose: This study examined the effects of plasma renin activity (PRA), angiotensin II (Ang II) and aldosterone (PAC) concentrations as well as common polymorphisms in the β1-Adrenoceptor gene (ADRB1) and the G-protein α-Subunit (Gαs) protein gene the G protein α-Subunit 1 gene (GNAS) on the blood pressure (BP) and heart rate (HR) response to bisoprolol in Chinese patients with hypertension.
Methods: Patients with sitting clinic systolic BP (SBP) 140–169 mmHg and/or diastolic BP (DBP) 90–109 mmHg after placebo run-in were treated with open-label bisoprolol 2.5 mg daily for 6 weeks. Patients diagnosed as having primary aldosteronism or renal artery stenosis were excluded. PRA, Ang II and PAC concentrations were measured after the placebo run-in and after 6 weeks of treatment. The Ser49Gly and Arg389Gly polymorphisms in ADRB1 and the c.393C > T polymorphism in GNAS were genotyped by the TaqMan® assay.
Results: In 99 patients who completed the study, baseline PAC levels were significantly associated with baseline DBP and plasma potassium on univariate but not on multivariate linear regression analysis. PRA, Ang II, and PAC concentrations at baseline were not associated with changes in BP with bisoprolol treatment, but the values were all significantly reduced (PRA −0.141 ± 0.595 ng/mL/h, Ang II −2.390 ± 5.171 pmol/L and aldosterone −51.86 ± 119.1 pg/mL; all P < 0.05) following 6 weeks of bisoprolol treatment. There were no significant differences in BP or HR responses in patients with baseline PRA above or below the PRA cut-point of 0.65 ng/mL/h or the median value of 0.9 ng/ml/hour. There were no significant associations of the ADRB1 and GNAS polymorphisms with the clinic and ambulatory BP and HR responses to bisoprolol.
Conclusion: Baseline PRA, PAC and Ang II concentrations showed no significant association with the BP response to bisoprolol treatment, but all these parameters were reduced after 6 weeks of treatment with bisoprolol. The two common polymorphisms in ADRB1 and the c.393C > T polymorphism in GNAS had no significant association with the BP and HR response to bisoprolol in these patients.
Introduction
High blood pressure (BP) is highly prevalent worldwide, and it is the major risk factor for ischemic and haemorrhagic stroke and an important risk factor for coronary heart disease, heart failure and chronic kidney disease (1–3). According to international guidelines, BP control is only achieved in a relatively small proportion of patients (4, 5). For any particular class of antihypertensive drugs, BP responses vary among individuals due to differences in clinical characteristics (e.g., age, body mass index) (6, 7) and genetic factors (8).
The renin-angiotensin-aldosterone system (RAAS) is closely related to vasomotor control and water and salt homeostasis in the body. It plays a crucial role in developing hypertension (9). Plasma renin, angiotensin II and aldosterone directly affect the proliferation of arterial endothelial cells and participate in the pathological process of hypertensive arterial damage (10).
Beta-adrenoceptor antagonists (β-Blockers) represent an important class of cardiovascular medications for managing hypertension and reducing morbidity and mortality in patients with heart failure, angina, and after myocardial infarction (11, 12). Bisoprolol is a moderately lipophilic and highly β1-Selective adrenoceptor antagonist devoid of intrinsic sympathomimetic activity (ISA), vasodilatory effects or membrane-stabilizing properties. The antihypertensive effects of some antihypertensive drugs, including β-Blockers, may be related to an effect on the RAAS and a reduction in the sympathetic tone and baseline activity of these systems may influence the response (13, 14). It has been suggested that measuring plasma renin activity (PRA) may be helpful to predict the BP response to certain drugs (15), but other reports considered it not useful (16). A recent study suggested PRA may be useful to predict BP responses in European Americans but not in African Americans, and European Americans with PRA ≥ 0.65 ng/ml/hour showed a better response to a β-Blocker than to a diuretic (17).
β-Adrenergic receptor polymorphisms may influence responses to β-Blocker treatments (18). It has been demonstrated that the Arg16Gly and Gln27Glu β2-Adrenoceptor polymorphisms were associated with changes in triglyceride levels over time following carvedilol or metoprolol succinate treatments (19). The β1-Adrenoceptor exists in two agonist conformations – a high-affinity catecholamine conformation and a low-affinity secondary agonist conformation. The polymorphic variants may affect receptor-effector coupling and intracellular signaling from the different conformations. The two common polymorphisms, 145A > G (rs1801252, Ser49Gly) and 1165G > C (rs1801253, Arg389Gly) within the human β1-Adrenoceptor gene (ADRB1), have been reported to be associated with reduced BP or heart rate (HR) responses to β-Blockers (20–22).
The stimulatory G protein subtype Gs is a trimeric transmembrane protein that mediates the signals from β1-Adrenoceptors to adenylyl cyclase, which catalyzes the production of the second-messenger cAMP. The G protein α-Subunit 1 gene (GNAS) in chromosome 20 (20q13.2) encodes the α-Subunit that couples β1-Adrenoceptors with adenylyl cyclase. The synonymous c.393C > T (rs7121) polymorphism in this gene has been reported to be associated with autonomic nervous system (ANS) dysfunction and to modulate BP and HR responses to exercise or β-Blocker treatment (23–25). It has also been shown to be a marker for disease progression and survival in certain cancers (26).
The present study aimed to investigate the effects of baseline PRA, angiotensin II (Ang II) and plasma aldosterone (PAC) concentrations on the response to bisoprolol and the effect of bisoprolol treatment on these parameters in Chinese patients with primary hypertension. We also examined the effects of common polymorphisms in ADRB1 and GNAS on the clinic and ambulatory BP and HR responses to bisoprolol.
Materials and Methods
Patients
Patients with primary hypertension identified from outpatient clinics at the Prince of Wales Hospital, Hong Kong, were enrolled in the study. The study involving human participants was reviewed and approved by the Joint Clinical Research Ethics Committee of the Chinese University of Hong Kong and New Territories East Cluster (CUHK-NTEC) with reference number CRE-2011.616-T. The study was performed following the ethical standards in the Declaration of Helsinki and subsequent revisions. All patients signed the Informed Consent.
The inclusion and exclusion criteria have been described previously (27). Briefly, subjects with sitting clinic systolic BP (SBP) 140–169 mmHg and/or diastolic BP (DBP) 90–109 mmHg in patients without type 2 diabetes (T2D) or SBP 130–169 mmHg and/or DBP of 80–109 mmHg in those patients with T2D after placebo run-in were treated with open-label bisoprolol 2.5 mg daily for 6 weeks. Other antihypertensive treatments were discontinued except for amlodipine which was continued if necessary to achieve BPs in the defined range at the end of the run-in. Patients diagnosed as having primary aldosteronism or renal artery stenosis were excluded.
Study Design
The study participants consisted of two groups with 50 subjects in each group, according to the total duration of bisoprolol treatment. The study protocol has been described previously (27). In group A, subjects were given bisoprolol 2.5 mg once daily for 6 weeks. Venous blood samples were collected after the placebo run-in to determine PRA, Ang II and PAC. In group B, subjects were treated with bisoprolol 2.5 mg once daily for 6 weeks and then continued treatment for a total of 24 weeks with optional titration of the dose of bisoprolol by doubling the dose after 6-week intervals up to 10 mg to achieve target BP levels. Venous blood samples were collected after the placebo run-in and after 6 weeks of treatment. For both groups, clinic BP and 24-hr ABP measurements were made at baseline and at the end of 6 weeks’ treatment with bisoprolol 2.5 mg. ABP measurements were taken using a wrist-type (BPro, HealthSTATS International, Singapore) or arm-type (A&D TM-2430, Tokyo, Japan) ABP device for 24 h, and the BPs were measured at intervals automatically throughout 24 h (27). All patients in group A completed the study while one patient from group B did not complete the 24-week treatment.
Measurement of Renin-Angiotensin-Aldosterone System Biomarkers by Liquid Chromatography-Tandem Mass Spectrometry Methods
Materials
The aldosterone standard was from Toronto Research Chemical Inc. (Toronto, ON, Canada), and the aldosterone_d7 internal stand (IS) was from IsoSciences (Ambler, PA, United States). Angiotensin I (Ang I), Ang I internal standard (Ang I IS) [DR[V13C,15N]YIHPFHL], and Ang I degradation standard (DS) [DR[V13C,15N]Y[I13C,15N]HPFHL] were from Anaspec (Fremont, CA, United States). Aminoethyl benzene sulfonyl fluoride (AEBSF) hydrochloride was from ACROS Organics (Thermo Fisher Scientific, Branchburg, NJ, United States). Ammonium acetate, maleic acid, and tert-butyl methyl ether (MBTE) were from Sigma Aldrich (St Louis, MI, United States). LCMS grade acetonitrile, methanol, and water were from Thermo Fisher Scientific. Formic acid was from BDH (Radnor, PA, United States). Phosphate buffered saline (PBS) was purchased from Sigma-Aldrich (St. Louis, MO, United States).
Measurement of PAC
The quantitation of PAC was by an electrospray negative ionization LC-MS/MS after organic solvent extraction of PAC from EDTA plasma samples. Two hundred microliters of EDTA plasma and IS was added to 1.2 mL MBTE and vortex-mixed for 10 min to extract PAC. The mixture was centrifuged and frozen at −80°C for at least 1 h. The supernatant was dried at 45°C, and the dried residue was reconstituted with 120 μL of 90% methanol. The solution was washed with 1 mL hexane to reduce matrix interference. The upper hexane layer was discarded, and the lower layer containing PAC and PAC IS dried at 45°C in a centrifugal evaporator (Savant SpeedVac Concentrator from Thermo Fisher Scientific). The dried extract was reconstituted with 120 μL 10% acetonitrile and was ready for LC-MS/MS analysis.
Separation of matrix interferences from PAC and its IS was on a Waters BEH C18 column (100 × 2.1 mm, 1.7 μm) (Waters, Milford, MA, United States) by gradient programming of two mobile phase solutions (A: 1 mM ammonium acetate in water and B: acetonitrile). PAC calibrators were prepared in 1% acetonitrile solution. The Waters ACQUITY Xevo-TQ system was used to detect PAC and IS. The quantitative analysis of PAC and IS was performed by monitoring the MRM transitions: m/z 359→331 and m/z 366→338, respectively. An additional MRM of PAC at m/z 359→189 was used as a qualitative MRM. Results calculated from qualifying MRM should differ from quantifying MRM by less than 20%. Waters MassLynx version 4.1 was used for data acquisition and processing. The relative peak area response ratios of PAC to IS were plotted against the corresponding calibrator concentrations with 1/× weighting to set up a calibration curve on the Waters TargetLynx version 4.1.
The analytical measuring range of this method was 50–5200 pmol/L with a between-batch coefficient of variation (CV) <5%. There was no significant carryover or matrix interference.
Measurement of PRA
PRA measurement in EDTA plasma samples was by electrospray positive LC-MS/MS. The increase in Ang I calculated the PRA after incubation of two aliquots from the same plasma sample at 4 and 37°C for 3 h in the presence of AEBSF, a serine protease inhibitor. The generation of Ang I was automated on a liquid handler workstation (Perkin Elmer Janus, Waltham, MA, United States). DS was included in the generation cocktail to monitor the presence of endogenous peptidases that could degrade the generated Ang I. The addition of acetonitrile terminated the PRA enzymatic reaction. Plasma protein was removed after centrifugation. The supernatant was acidified with 10% formic acid, and Ang I-IS was added to each aliquot on a 96-well plate. The LC-MS/MS measurement was performed on a Waters ACQUITY Xevo-TQ system. Ang I and Ang I IS were first purified by online solid-phase extraction using Waters Oasis HLB cartridge column (3.9 × 20 mm, 15 μm). Further separation of matrix interference was on a Waters Xbridge C18 column (2.1 × 50 mm, 5 μm) by gradient programming of two mobile phase solutions (A: 0.1% formic acid in LCMS water; and B: 0.1% formic acid in 20/80 acetonitrile/methanol). Ang I, IS, and DS were detected by monitoring the MRM transitions: m/z 433→534, 437→660, and 435→653, respectively. An additional MRM of Ang I at m/z 433→619 was used as a qualitative MRM. Ang I calibrators were prepared in 50% acetonitrile. Quantitation of Ang I on the Waters MassLynx and TargetLynx managers was similar to those described for PAC. The analytical measuring range of this method was 0.07–183 ng/mL/h with a between-batch CV <8%. There was no significant carryover or matrix interference.
Measurement of Ang II
The LC-MS/MS method to measure EDTA plasma Ang II was described by Lo et al. (28).
Biochemical Assessments
Measurements of total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), fasting plasma glucose (FPG), and glycosylated hemoglobin (HbA1c) have been described previously (27). The low-density lipoprotein cholesterol (LDL-C) level was estimated using the Friedewald formula (29) or directly measured when the TG level was over 4.5 mmol/L.
Genotyping
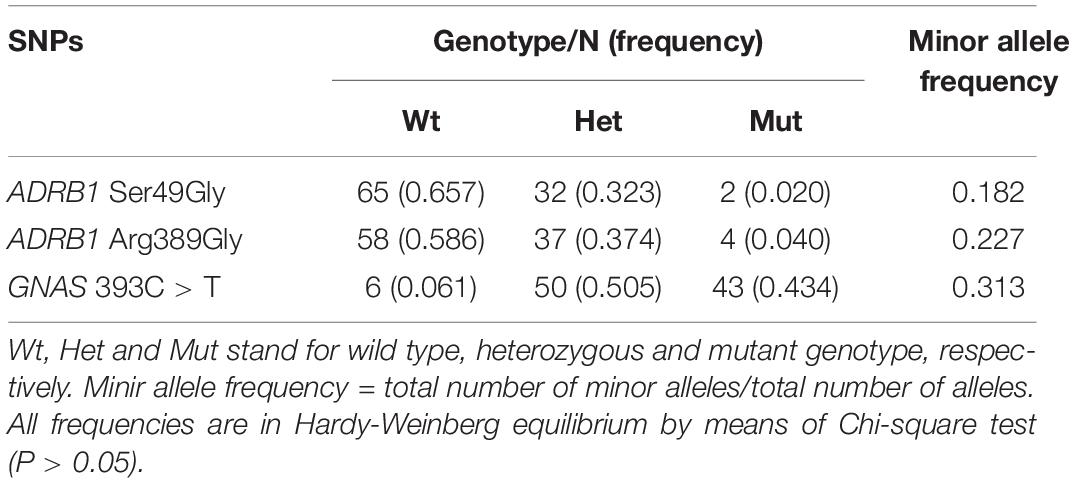
Two common polymorphisms in ADRB1 [49A > G (rs1801252), 389C > G (rs1801253)] and the 393C > T (rs7121) polymorphism in GNAS were selected in this study. DNA was extracted from peripheral whole blood samples by the phenol-chloroform method. Genetic polymorphisms in ADRB1 and GNAS were genotyped by the TaqMan® assay using the geneAmp PCR system 9700 (Applied Biosystems, Foster City, CA, United States). The polymorphisms were determined using a previously reported polymerase chain reaction (PCR) restriction fragment length polymorphism (RFLP) method (26). All polymorphisms examined in this study were in Hardy–Weinberg equilibrium (χ2 test P > 0.05), and the frequencies of the minor alleles were similar to those reported in Han Chinese in HapMap.
Power Calculation
Using the Arg389Gly (389Arg allele frequency 0.761 in Chinese Han) and Ser49Gly (49Gly frequency 0.161 in Chinese Han) polymorphisms in ADRB1 gene, a two group Chi-square test with a 0.05 two-sided significance level will have 80% power to detect a 10% difference of DBP between the reference group and the affected group after 6 weeks of treatment when sample size is 75. The present studies recruited 100 subjects which is sufficient to detect the relevant differences based on the allele frequencies.
Statistical Analysis
All statistical analyses were performed using SPSS software (Version 26, SPSS Inc., IBM Company, Chicago, IL, United States). Data were pooled from two open-label, placebo run-in pharmacogenetic studies of bisoprolol treatment. The distribution of continuous data was evaluated according to the Shapiro–Wilk test. Logistic regression analyses were applied to determine significantly independent predictors of PRA, PAC and Ang II levels. Correlations between baseline PRA, PAC, and Ang II and changes in BP and HR were assessed using Pearson correlation coefficient analysis. BP and HR responses were compared in subjects with PRA values below and ≥0.65 ng/ml/h and between subjects above and below the median value. Skewed data were log transformed. Statistical analysis on the effect of bisoprolol treatment on PRA, Ang II and PAC was performed using a paired-sample t-test or Wilcoxon signed-rank test, as appropriate. Statistical analysis on the effect of genetic polymorphisms on the BP and HR responses to bisoprolol was performed using an independent samples t-test. Data are presented as mean ± standard deviation unless otherwise specified. A P-value <0.05 was considered statistically significant.
Results
Study Population
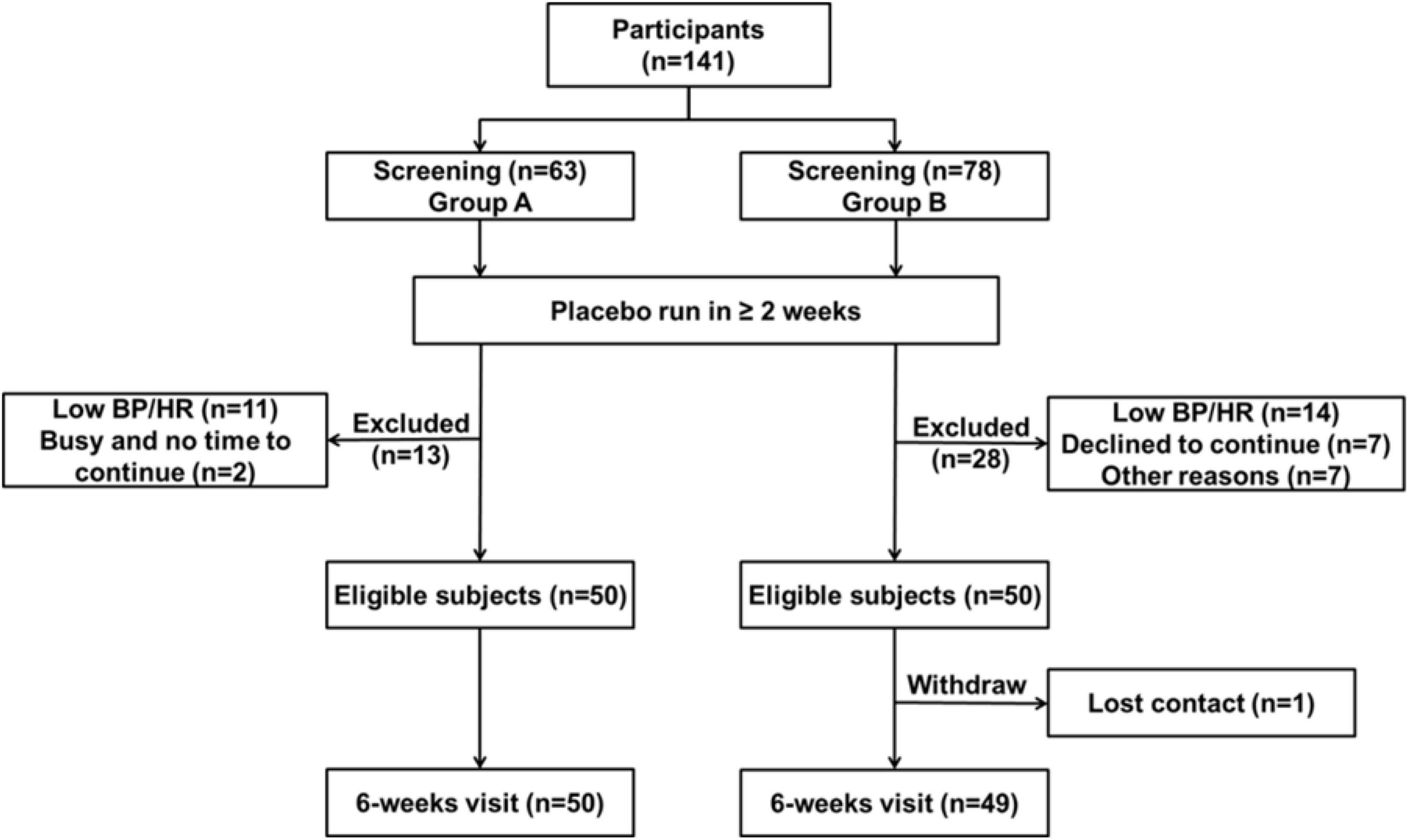
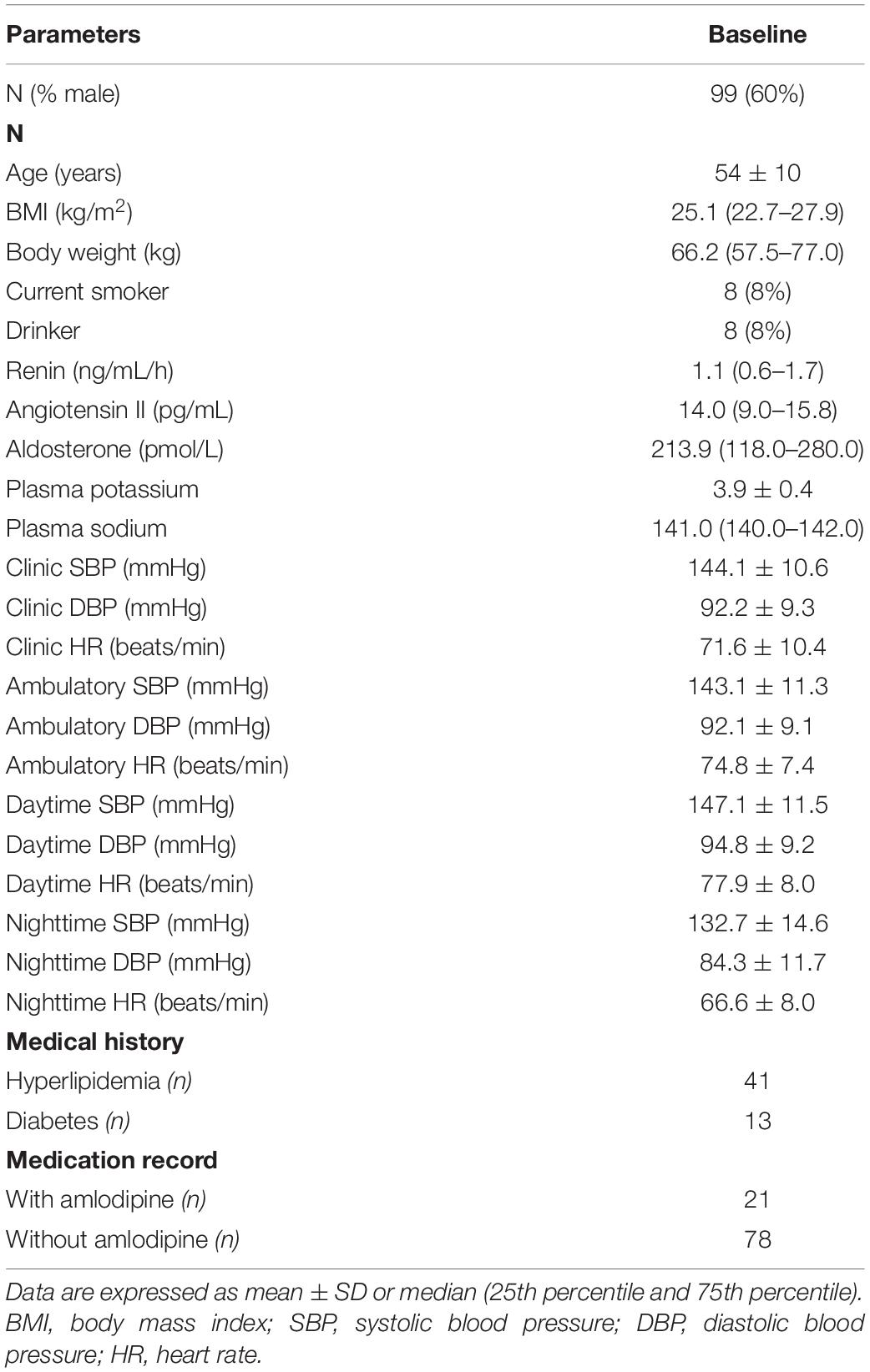
From 141 patients who underwent screening, 99 subjects of Chinese ethnicity completed the studies (Figure 1). The data from the two studies were combined in further analysis (27). The demographic and baseline characteristics and concomitant diseases of the study patients are shown in Table 1. Subjects were generally overweight by Asian standards (median body weight of 66.2 kg, 25th percentile 57.5, 75th percentile 77.0 kg), with a median BMI of 25.1 kg/m2 (25th percentile 22.7, 75th percentile 27.9 kg/m2) with a mean age of 54 ± 10 years. The mean baseline PRA, Ang II, and PAC were 1.1 ng/mL/h, 14.0 pg/mL, and 213.8 pmol/L, respectively. The baseline clinic BPs were 144.1 ± 10.6 mmHg/92.2 ± 9.3 mmHg.
Effects of Renin, Angiotensin II and Aldosterone on Blood Pressure and Heart Rate Responses to Bisoprolol and Effects of Bisoprolol Treatment on These Parameters
On univariate linear regression analysis, PAC levels were significantly associated with age (P = 0.026), baseline body weight (P = 0.040), baseline DBP (P = 0.024) and baseline plasma potassium (P = 0.027), but not sex, baseline SBP and sodium level. The multiple linear regression analysis failed to detect a significant association of any of these factors with PAC. Baseline Ang II levels were significantly associated with sex on univariate (P = 0.010) and multivariate (P = 0.008) linear regression analyses (Table 2). The PRA level was not significantly associated with any of these factors.
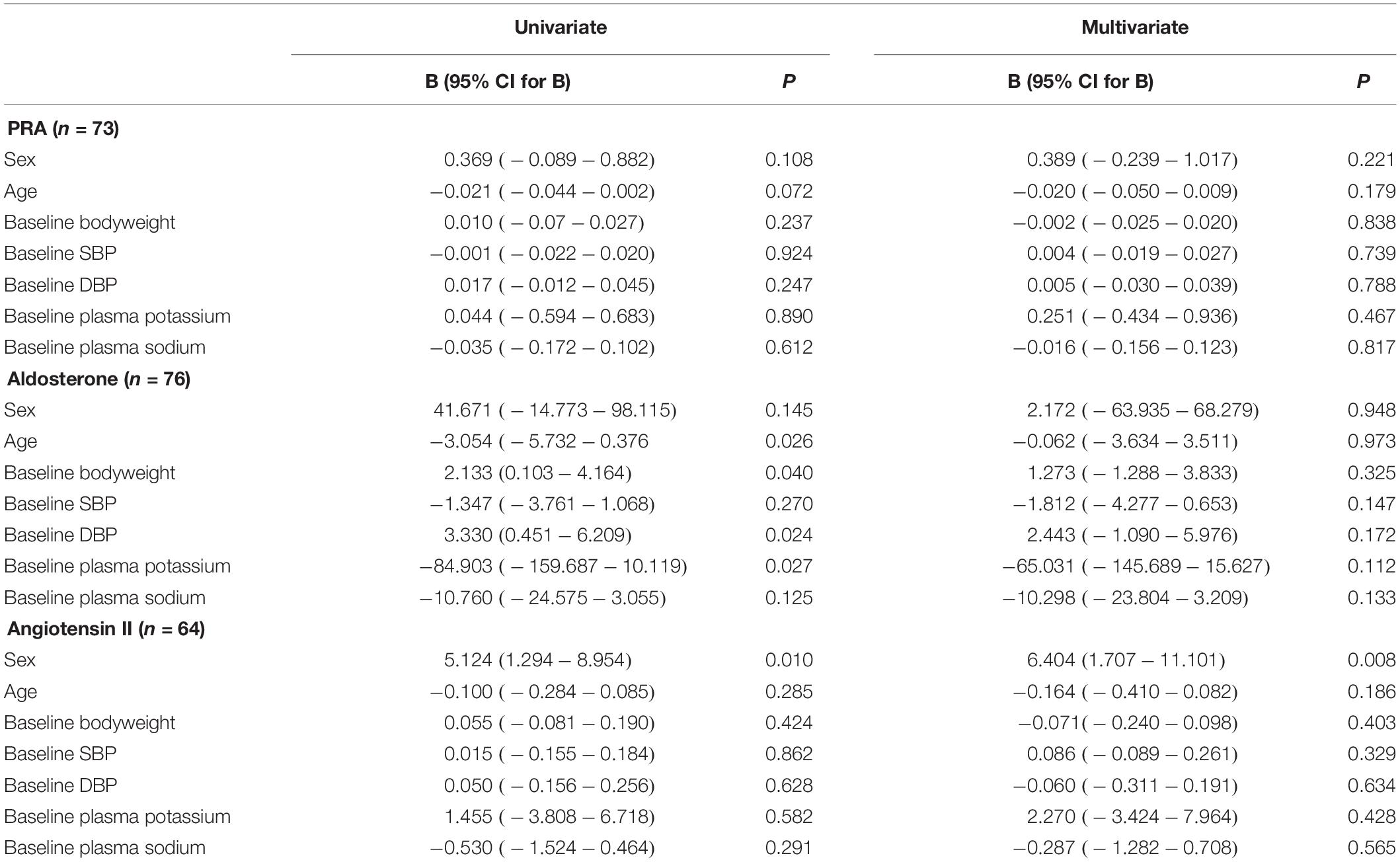
Table 2. Linear regression analysis for the factors that may influence plasma renin activity (PRA), aldosterone and angiotensin II concentrations.
After 6 weeks of bisoprolol treatment, change in clinic HR showed a weak but significant correlation with baseline PRA (P = 0.044), but not PAC and Ang II levels (Table 3). There was no significant correlation between ambulatory HR values changes or any changes in BP values with baseline PRA, PAC or Ang II levels. There was no significant difference in the BP or HR responses in subjects with PRA values below or ≥0.65 ng/ml/hour (number of patients with PRA <0.65 ng/ml/h = 27; ≥ 0.65 ng/ml/h = 46) or between subjects with PRA values above and below the median value of 0.90 (25th percentile 0.40, 75th percentile 1.69) ng/ml/hour (data not shown). These three RAAS biomarker levels were reduced significantly compared to baseline (PRA −0.141 ± 0.595 ng/mL/h, Ang II −2.390 ± 5.171 pmol/L and PAC −51.86 ± 119.1 pg/mL; all P < 0.05) following 6 weeks of bisoprolol treatment (Table 4).
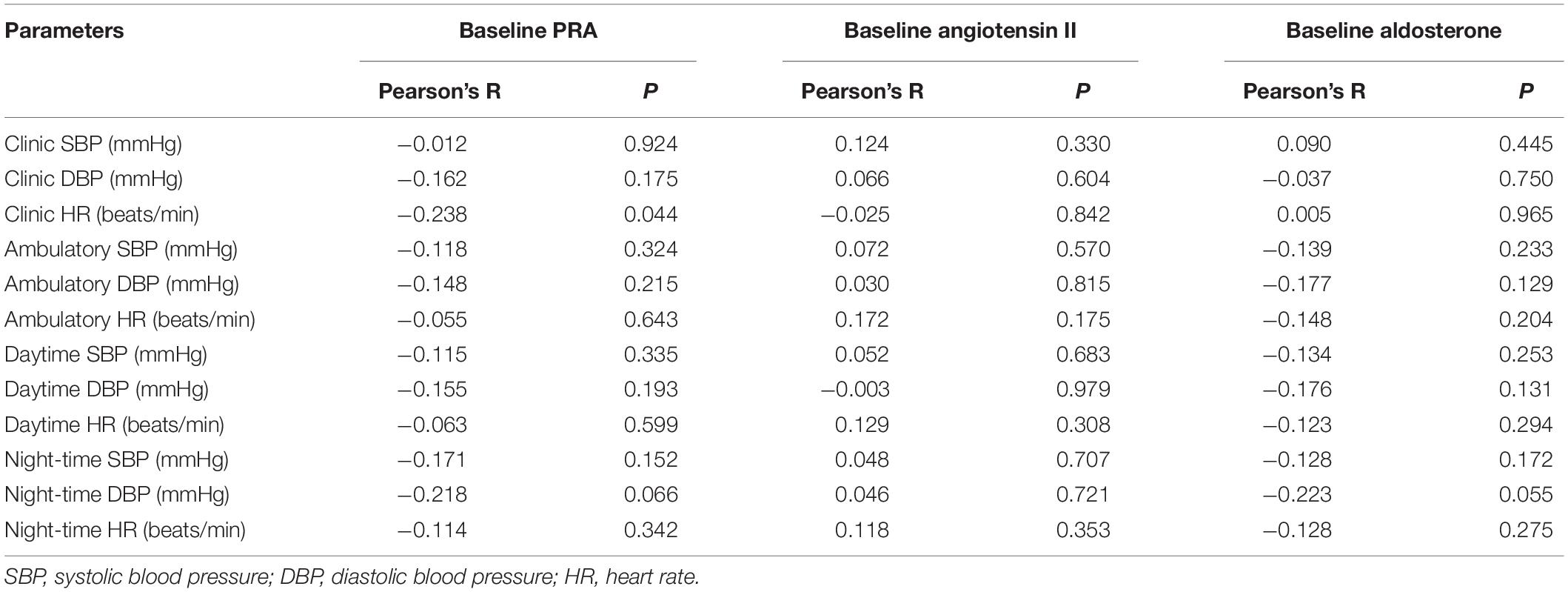
Table 3. Correlations between baseline plasma renin activity (PRA), angiotensin II and aldosterone concentrations and changes in blood pressure and heart rate after 6 weeks of treatment with bisoprolol 2.5 mg.
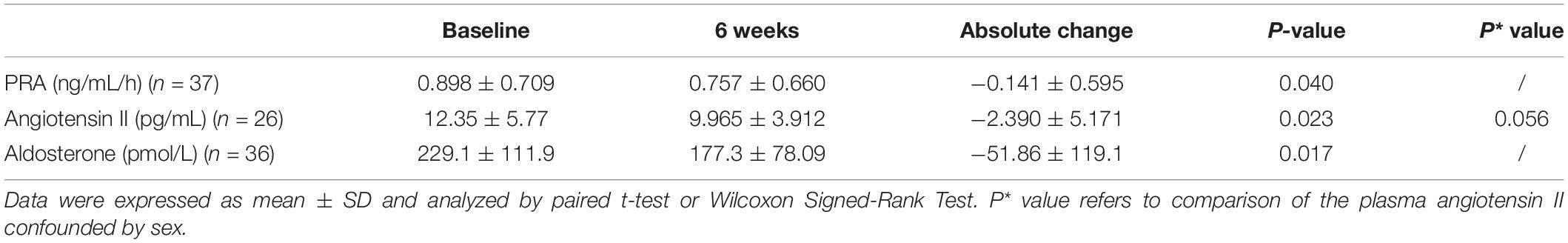
Table 4. Effect of bisoprolol on plasma renin activity (PRA), angiotensin II and aldosterone after 6 weeks’ treatment.
Effect of β1-Adrenoceptor Gene and the G Protein α-Subunit 1 Gene Polymorphisms on the Blood Pressure and Heart Rate Responses to Bisoprolol
The genotype and allele frequencies of the 3 ploymorphisms studied are shown in Table 5. There were insufficient subjects with the homozygous genotype of the minor alleles to perform statistical tests on those groups separately and they were combined with the heterozygotes as carriers of the variant allele. The small number of subjects with the homozygous variants did not have extreme baseline BP and HR values or changes in BP and HR with bisoprolol treatment. There was no significant relationship between the genotypes of the 3 polymorphisms and baseline BP or HR. After 6 weeks of treatment with bisoprolol 2.5 mg daily, there was no significant difference in the clinic and ambulatory BP reductions among ADRB1 Ser49Gly, Arg389Gly and GNS1 393T > C genotypes comparing the wild type with variant carriers (Figures 2A–C). Similarly, no significant difference was observed in the clinic and ambulatory HR according to ADRB1 Ser49Gly, Arg389Gly and GNS1 393T > C genotypes (Figures 3A–C). Moreover, there was no significant difference in the clinic and ambulatory BP or HR reductions between the 4 ADRB1 diplotype groups (Gly49Arg389/Ser49Gly389, Ser49Arg389/Ser49Arg389, Ser49Arg389/Ser49Gly389 and Ser49Arg389/Gly49Arg389) (data not shown).
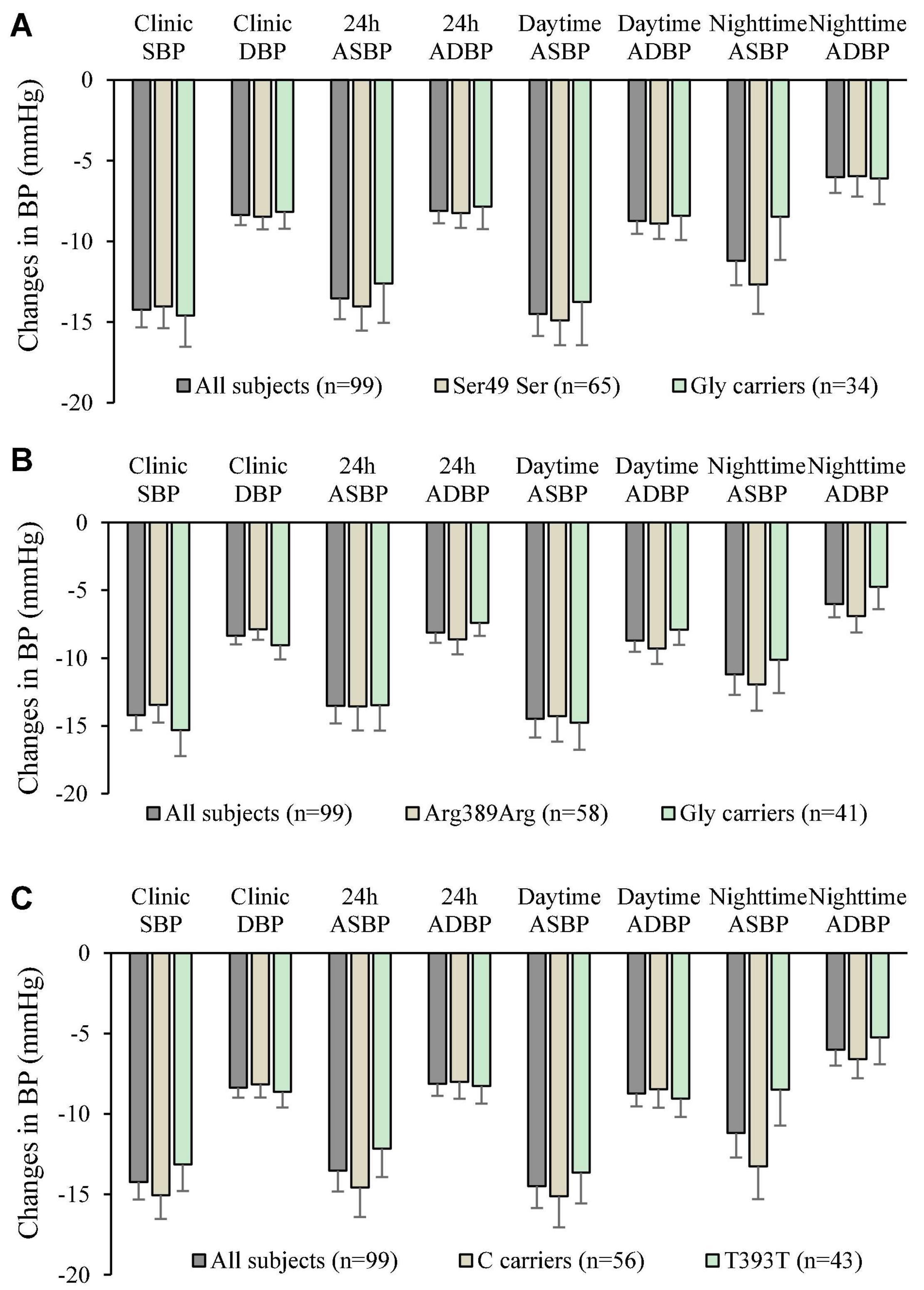
Figure 2. Changes in clinic and ambulatory blood pressure after 6 weeks treatment with bisoprolol 2.5 mg daily according to (A) ADRB1 Ser49Gly genotypes, (B) ADRB1 Arg389Gly genotypes, and (C) The G protein α-Subunit 1 gene (GNAS) 393C > T genotypes. Data are presented as mean ± SEM. There is no significant difference between groups (independent samples test was used).
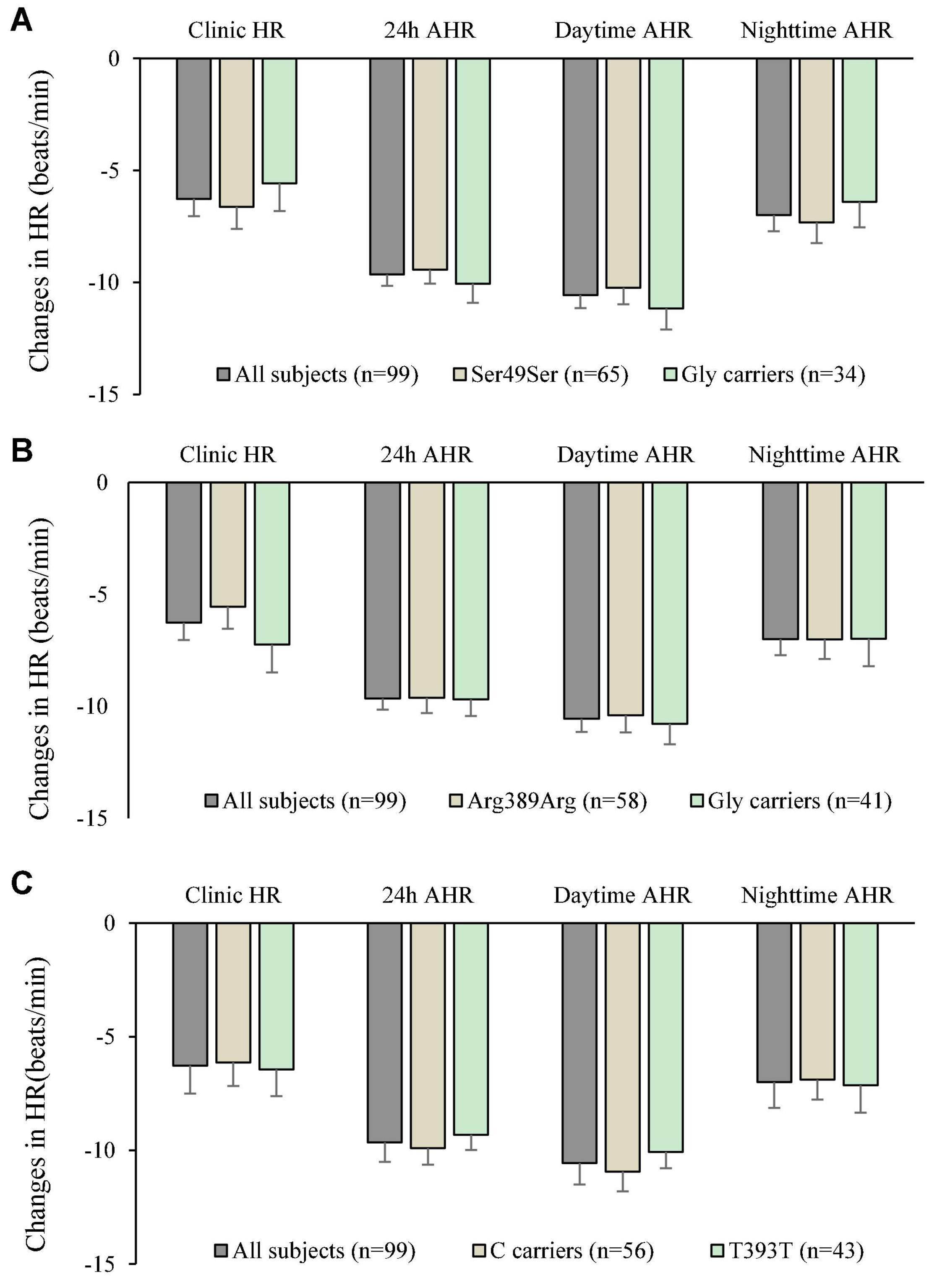
Figure 3. Changes in clinic heart rate (HR) and ambulatory heart rate (AHR) after 6 weeks treatment with bisoprolol 2.5 mg daily according to (A) ADRB1 Ser49Gly genotypes, (B) ADRB1 Arg389Gly genotypes, and (C) GNAS 393C > T genotypes. Data are presented as mean ± SEM. There is no significant difference between groups (independent samples test was used).
Discussion
Primary hypertension is the most common cardiovascular disorder and a major risk factor for cardiovascular disease mortality (30, 31). It is a polygenic disease in which the genetic components interact with physiological and environmental factors. The RAAS represents a hormonal cascade in which PRA cleaves angiotensinogen into inactive Ang I, which undergoes further cleavage by the membrane-bound metalloproteinase angiotensin-converting enzyme to produce Ang II that is active in stimulating angiotensin receptors and aldosterone synthesis. The RAAS plays an essential role in the pathogenesis of hypertension (32). The present study found that the PAC was related to baseline DBP and plasma potassium. The HR-lowering effects of bisoprolol treatment correlated significantly with baseline PRA but not with baseline PAC and Ang II levels.
It was reported that patients with higher baseline PRA would have a greater reduction in BP with RAAS-blocking drugs than patients with lower PRA (33). β-Blockers can reverse the neurohumoral effects of the sympathetic nervous system and lower PRA with potential ensuing symptomatic and prognostic benefits, and thus remain an option for the first-line antihypertensive treatment in several guidelines. In this study, bisoprolol, a β1-Selective non-vasodilatory β-Blocker, decreased PRA, PAC, and Ang II levels, which is consistent with previous studies with nebivolol, another β1-Selective but vasodilatory β-Blocker, and with other β-Blockers (14, 34, 35).
Consistent with some previous reports, we did not find a better BP response to the β-Blocker in individuals homozygous for either the ADRB1 Arg389Gly or Ser49Gly polymorphisms (36, 37). However, a recent study reported that patients with the ADRB1 homozygous form showed a better response to metoprolol compared with those harboring the heterozygous variant (38). Suonsyrja et al. (36) reported that patients with the Gly389Gly genotype tended to better BP response to bisoprolol than those with Arg389Arg genotypes and no significant difference was found. Johnson et al. (22) reported a more than 2-fold greater percentage reduction in 24 h DBP in Arg389 homozygotes than in Gly389 carriers and a nearly 3-fold greater percentage reduction in daytime DBP after metoprolol treatment in 40 hypertensive subjects, including 10 African Americans. In addition to codon 389, we evaluated the impact of codon 49 genotype and the codon 49/389 haplotype on the antihypertensive response to bisoprolol. Although the functional data for the codon 49 variant indicate that the effect of this polymorphism is primarily on receptor regulation, with the Gly49 allele undergoing greater agonist-mediated receptor down regulation in vitro, we did not find a greater antihypertensive response to bisoprolol in the Ser49 homozygotes. Johnson et al. (22) also investigated the ADRB1 haplotypes and found the subjects with the Ser49Arg389/Ser49Arg389 diplotype showed a decrease in DBP of 14.7 mm Hg versus 0.5 mm Hg in patients with the Gly49Arg389/Ser49Gly389 diplotype. However, in our study, there was no significant difference in the reduction of BP and HR between the Gly49Arg389/Ser49Gly389 diplotype, Ser49Arg389/Gly49Arg389 and the Ser49Arg389/Ser49Gly389 diplotype subjects after bisoprolol treatment. Moreover, there was a study that showed Arg389-homozygous subjects had a greater response in resting SBP after a single dose of atenolol compared to Gly389 homozygotes (39).
In white subjects, a common silent polymorphism (393C > T) involving a change in codon 131 from ATT (Ile) to ATC (Ile) in the GNAS gene was linked to hypertension and the BP response to β-Blocker therapy (24). Another study in 2,308 Japanese individuals found a marginally significant difference in the frequencies of the alleles and genotypes between the hypertensives and normotensives, which was more significant in non-heavy smokers (40). In our study, there were 8 (8%) patients who were smokers, and all of them were non-heavy smokers. However, we did not find any relationship between the GNAS 393C > T polymorphism and BP or HR changes with bisoprolol treatment in our study. Differences between the present study and previous reports may be related to the subjects’ ethnicity or the type and duration of β-Blocker therapy, or various other factors.
This study had several limitations. Firstly, the number of subjects in this study is not large enough to detect minor effects of the RAAS parameters or genotypes. Secondly, we only examined some common polymorphisms in ADRB1 and GNAS and the number of subjects homozygous for the minor alleles of each polymorphism was insufficient to assess the responses in those groups separately. Lastly, we cannot exclude a small effect of these genotypes due to the relatively small sample size. We have previously reported that plasma concentrations of bisoprolol and polymorphisms in the drug metabolizing enzymes CYP2D6 and CYP3A5 did not influence the BP and HR response to bisoprolol in these subjects.
Conclusion
Baseline values of PRA, PAC, and Ang II were not associated with the BP response to bisoprolol treatment, but bisoprolol treatment had a suppressive effect on all these parameters. The common Ser49Gly and Arg389Gly polymorphisms in the ADRB1 gene had no significant association with the reduction of the clinic and ambulatory BP and HR after bisoprolol treatment. There was no significant effect of the GNAS T393C polymorphisms on BP and HR changes with bisoprolol treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Joint Clinical Research Ethics Committee of The Chinese University of Hong Kong and New Territories East Cluster (CUHK-NTEC) with reference number CRE-2011.616-T. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WZ and SC analyzed the data and wrote this manuscript. BT and TC designed the research project. TC and WZ included the patients and followed this study. CL, AC, and CH performed the experiments. BT, AK, and CH revised this manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We wish to thank Evelyn Chau, Maybo Lin, and other research staff for helping with study procedures, Timothy H. Rainer and Miya Cheng for helping with patient recruitment, Emily Poon for helping with genotyping and other laboratory work and all the patients who participated in the study.
References
1. Reges O, Ning H, Wilkins JT, Wu CO, Tian X, Domanski MJ, et al. Association of cumulative systolic blood pressure with long-term risk of cardiovascular disease and healthy longevity: findings from the lifetime risk pooling project cohorts. Hypertension. (2021) 77:347–56. doi: 10.1161/HYPERTENSIONAHA.120.15650
2. Liu M, Zhang S, Chen X, Zhong X, Xiong Z, Yang D, et al. Association of mid- to late-life blood pressure patterns with risk of subsequent coronary heart disease and death. Front Cardiovasc Med. (2021) 8:632514. doi: 10.3389/fcvm.2021.632514
3. Hagglund O, Svensson P, Linde C, Ostergren J. Ambulatory blood pressure monitoring and blood pressure control in patients with coronary artery disease-a randomized controlled trial. Int J Cardiol Hypertens. (2021) 8:100074. doi: 10.1016/j.ijchy.2020.100074
4. Li G, Hu R, Zhang X. The impact of the 2020 international society of hypertension global hypertension practice guidelines on the prevention and control of hypertension in China. Hypertens Res. (2021) 44:1040–1. doi: 10.1038/s41440-021-00649-7
5. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 international society of hypertension global hypertension practice guidelines. J Hypertens. (2020) 38:982–1004. doi: 10.1097/HJH.0000000000002453
6. Lakatta EG, AlunniFegatelli D, Morrell CH, Fiorillo E, Orru M, Delitala A, et al. Impact of stiffer arteries on the response to antihypertensive treatment: a longitudinal study of the SardiNIA Cohort. J Am Med Dir Assoc. (2020) 21:720–5. doi: 10.1016/j.jamda.2019.11.014
7. Pal A, Martinez F, Aguila AP, Akey MA, Chatterjee R, Conserman MGE, et al. Beat-to-beat blood pressure variability in patients with obstructive sleep apnea. J Clin Sleep Med. (2021) 17:381–92. doi: 10.5664/jcsm.8866
8. Rechcinski T, Kasprzak JD. A systematic review of nonsynonymous single nucleotide polymorphisms in the renin-angiotensin-aldosterone system. Cardiol J. (2021). doi: 10.5603/CJ.a2021.0055
9. Hsu CN, Tain YL. Targeting the renin-angiotensin-aldosterone system to prevent hypertension and kidney disease of developmental origins. Int J Mol Sci. (2021) 22:2298. doi: 10.3390/ijms22052298
10. Almeida LF, Tofteng SS, Madsen K, Jensen BL. Role of the renin-angiotensin system in kidney development and programming of adult blood pressure. Clin Sci (Lond). (2020) 134:641–56. doi: 10.1042/CS20190765
11. Zhang YQ, Sun NN, Jiang XL, Xi Y. Comparative efficacy of β-blockers on mortality and cardiovascular outcomes in patients with hypertension: a systematic review and network meta-analysis. J Am Soc Hypertens. (2017) 11:394–401. doi: 10.1016/j.jash.2017.05.001
12. Ziff OJ, Samra M, Howard JP, Bromage DI, Ruschitzka F, Francis DP, et al. Beta-blocker efficacy across different cardiovascular indications: an umbrella review and meta-analytic assessment. BMC Med. (2020) 18:103. doi: 10.1186/s12916-020-01564-3
13. Cody RJ. The sympathetic nervous system and the renin-angiotensin-aldosterone system in cardiovascular disease. Am J Cardiol. (1997) 80:9J–14J. doi: 10.1016/s0002-9149(97)00832-1
14. Blumenfeld JD, Sealey JE, Mann SJ, Bragat A, Marion R, Pecker MS, et al. β-adrenergic receptor blockade as a therapeutic approach for suppressing the renin-angiotensin-aldosterone system in normotensive and hypertensive subjects. Am J Hypertens. (1999) 12:451–9. doi: 10.1016/S0895-7061(99)00005-9
15. Brown MJ. Personalised medicine for hypertension. Br Med J. (2011) 343:d4697. doi: 10.1136/bmj.d4697
16. Mancia G, Grassi G. Individualization of antihypertensive drug treatment. Diabetes Care. (2013) 36:S301–6. doi: 10.2337/dcS13-2013
17. Mehanna M, Wang Z, Gong Y, McDonough CW, Beitelshees AL, Gums JG, et al. Plasma renin activity is a predictive biomarker of blood pressure response in European but not in African Americans with uncomplicated hypertension. Am J Hypertens. (2019) 32:668–75. doi: 10.1093/ajh/hpz022
18. Thomas CD, Johnson JA. Pharmacogenetic factors affecting beta-blocker metabolism and response. Expert Opin Drug Metab Toxicol. (2020) 16:953–64. doi: 10.1080/17425255.2020.1803279
19. Vardeny O, Nicholas G, Andrei A, Buhr KA, Hermanson MP, Moran JJ, et al. beta-AR polymorphisms and glycemic and lipid parameters in hypertensive individuals receiving carvedilol or metoprolol. Am J Hypertens. (2012) 25:920–6. doi: 10.1038/ajh.2012.54
20. Liu J, Liu ZQ, Yu BN, Xu FH, Mo W, Zhou G, et al. beta1-Adrenergic receptor polymorphisms influence the response to metoprolol monotherapy in patients with essential hypertension. Clin Pharmacol Ther. (2006) 80:23–32. doi: 10.1016/j.clpt.2006.03.004
21. Sofowora GG, Dishy V, Muszkat M, Xie HG, Kim RB, Harris PA, et al. A common beta1-adrenergic receptor polymorphism (Arg389Gly) affects blood pressure response to beta-blockade. Clin Pharmacol Ther. (2003) 73:366–71. doi: 10.1016/s0009-9236(02)17734-4
22. Johnson JA, Zineh I, Puckett BJ, McGorray SP, Yarandi HN, Pauly DF. beta1-adrenergic receptor polymorphisms and antihypertensive response to metoprolol. Clin Pharmacol Ther. (2003) 74:44–52. doi: 10.1016/S0009-9236(03)00068-7
23. Nieminen T, Lehtimaki T, Laiho J, Rontu R, Niemela K, Koobi T, et al. Effects of polymorphisms in beta1-adrenoceptor and alpha-subunit of G protein on heart rate and blood pressure during exercise test. The Finnish cardiovascular study. J Appl Physiol. (2006) 100:507–11. doi: 10.1007/s11033-020-05699-7
24. Jia H, Hingorani AD, Sharma P, Hopper R, Dickerson C, Trutwein D, et al. Association of the G(s)alpha gene with essential hypertension and response to beta-blockade. Hypertension. (1999) 34:8–14. doi: 10.1161/01.hyp.34.1.8
25. Yasuda K, Matsunaga T, Moritani T, Nishikino M, Gu N, Yoshinaga M, et al. T393C polymorphism of GNAS1 associated with the autonomic nervous system in young, healthy Japanese subjects. Clin Exp Pharmacol Physiol. (2004) 31:597–601. doi: 10.1111/j.1440-1681.2004.04059.x
26. Mohlendick B, Schmid KW, Siffert W. The GNAS SNP c.393C > T (rs7121) as a marker for disease progression and survival in cancer. Pharmacogenomics. (2019) 20:553–62. doi: 10.2217/pgs-2018-0199
27. Chan SW, Chu TTW, Ho CS, Kong APS, Tomlinson B, Zeng W. Influence of CYP2D6 and CYP3A5 polymorphisms on the pharmacokinetics and pharmacodynamics of bisoprolol in hypertensive chinese patients. Front Med. (2021) 8:683498. doi: 10.3389/fmed.2021.683498
28. Lo CW-S, Tsui TK-C, Ma RC-W, Chan MH-M, Ho C-S. Quantitation of plasma angiotensin II in healthy Chinese subjects by a validated liquid chromatography-tandem mass spectrometry method. Biomed Chromatogr. (2022) e5318. doi: 10.1002/bmc.5318
29. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. (1972) 18:499–502.
30. Sakaniwa R, Tromp J, Shirai K, Yamagishi K, Tamakoshi A, Iso H. The association of conventionally medicated systolic and diastolic blood pressure level and mortality from cardiovascular disease: is the lower the better in high stroke population? Clin Res Cardiol. (2020) 109:944–8. doi: 10.1007/s00392-019-01587-8
31. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. (2020) 75:285–92. doi: 10.1161/HYPERTENSIONAHA.119.14240
32. Griffin TP, Wall D, Browne GA, Dennedy MC, O’Shea PM. Associations between glycaemic control and activation of the renin-angiotensin-aldosterone system in participants with type 2 diabetes mellitus and hypertension. Ann Clin Biochem. (2018) 55:373–84. doi: 10.1177/0004563217728964
33. Preston RA, Materson BJ, Reda DJ, Williams DW, Hamburger RJ, Cushman WC, et al. Age-race subgroup compared with renin profile as predictors of blood pressure response to antihypertensive therapy. JAMA. (1998) 280:1168–72. doi: 10.1001/jama.280.13.1168
34. Giles TD, Bakris G, Oparil S, Weber MA, Li H, Mallick M, et al. Correlations of plasma renin activity and aldosterone concentration with ambulatory blood pressure responses to nebivolol and valsartan, alone and in combination, in hypertension. J Am Soc Hypertens. (2015) 9:845–54. doi: 10.1016/j.jash.2015.08.003
35. Lezama-Martinez D, Valencia-Hernandez I, Flores-Monroy J, Martinez-Aguilar L. Combination of beta adrenergic receptor block and renin-angiotensin system inhibition diminished the angiotensin II-induced vasoconstriction and increased bradykinin-induced vasodilation in hypertension. Dose Response. (2017) 15:1559325817737932. doi: 10.1177/1559325817737932
36. Suonsyrja T, Donner K, Hannila-Handelberg T, Fodstad H, Kontula K, Hiltunen TP. Common genetic variation of beta1- and beta2-adrenergic receptor and response to four classes of antihypertensive treatment. Pharmacogenet Genomics. (2010) 20:342–5. doi: 10.1097/FPC.0b013e328338e1b8
37. Rau T, Düngen HD, Edelmann F, Waagstein F, Lainšèak M, Dimkoviæ S, et al. Impact of the β1-adrenoceptor Arg389Gly polymorphism on heart-rate responses to bisoprolol and carvedilol in heart-failure patients. Clin Pharmacol Ther. (2012) 92:21–8. doi: 10.1038/clpt.2012.18
38. Chen L, Xiao T, Chen L, Xie S, Deng M, Wu D. The association of ADRB1 and CYP2D6 polymorphisms with antihypertensive effects and analysis of their contribution to hypertension risk. Am J Med Sci. (2018) 355:235–9. doi: 10.1016/j.amjms.2017.11.002
39. Parvez B, Chopra N, Rowan S, Vaglio JC, Muhammad R, Roden DM, et al. A common β1-adrenergic receptor polymorphism predicts favorable response to rate-control therapy in atrial fibrillation. J Am Coll Cardiol. (2012) 59:49–56. doi: 10.1016/j.jacc.2011.08.061
Keywords: β1-adrenoceptor polymorphism, bisoprolol, blood pressure, renin, aldosterone, angiotensin II
Citation: Zeng W, Chu TTW, Ho CS, Lo CWS, Chan ASL, Kong APS, Tomlinson B and Chan SW (2022) Lack of Effects of Renin-Angiotensin-Aldosterone System Activity and Beta-Adrenoceptor Pathway Polymorphisms on the Response to Bisoprolol in Hypertension. Front. Cardiovasc. Med. 9:842875. doi: 10.3389/fcvm.2022.842875
Received: 24 December 2021; Accepted: 10 February 2022;
Published: 01 April 2022.
Edited by:
Maddalena Illario, University of Naples Federico II, ItalyReviewed by:
Elise Peery Gomez-Sanchez, University of Mississippi Medical Center, United StatesClaudio de Lucia, Scientific Clinical Institute Maugeri (ICS Maugeri), Italy
Copyright © 2022 Zeng, Chu, Ho, Lo, Chan, Kong, Tomlinson and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian Tomlinson, YnRvbWxpbnNvbkBtdXN0LmVkdS5tbw==; Sze Wa Chan, c3djaGFuQGNpaGUuZWR1Lmhr
 Weiwei Zeng
Weiwei Zeng Tanya T. W. Chu
Tanya T. W. Chu Chung Shun Ho
Chung Shun Ho Clara W. S. Lo3
Clara W. S. Lo3 Alice P. S. Kong
Alice P. S. Kong Brian Tomlinson
Brian Tomlinson Sze Wa Chan
Sze Wa Chan