
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 30 March 2022
Sec. Cardiac Rhythmology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.841020
This article is part of the Research Topic Optimal Management of Atrial Fibrillation: Recent advances View all 11 articles
Background: This study aimed to investigate the prevalence and factors associated with the initiation of oral anticoagulation among patients with acute ischemic stroke (AIS) and concurrent atrial fibrillation (AF) at discharge in China.
Methods: We continuously included hospitalized patients with AIS with an AF diagnosis registered in the computer-based Online Database of Acute Stroke Patients for Stroke Management Quality Evaluation (CASE II) from January 2016 to December 2020 and divided them into a and non-anticoagulant groups according to the medications at discharge. Binary logistic regression was used to determine the factors associated with the prescription of anticoagulants in patients with AF.
Results: A total of 16,162 patients were enrolled. The mean age was 77 ± 9 years, 8,596 (53.2%) were males, and the median baseline National Institute of Health Stroke Scale score was 5 (2–12). Of the 14,838 patients without contraindications of antithrombotic therapy, 6,335 (42.7%) patients were initiated with anticoagulation treatment at discharge. Prior history of hemorrhagic stroke (OR 0.647, p < 0.001) and gastrointestinal bleeding (OR 0.607, p = 0.003) were associated with a lower rate of anticoagulation at discharge. Patients with any intracranial hemorrhage (OR 0.268, p < 0.001), gastrointestinal bleeding (OR 0.353, p < 0.001), or pneumonia during hospitalization (OR 0.601, p < 0.001) were less likely to receive anticoagulants at discharge. Among 7,807 patients with previously diagnosed AF and high risk of stroke (CHA2DS2-VASc ≥2), only 1,585 (20.3%) had been receiving anticoagulation treatment prior to the onset of stroke. However, the mean international normalized ratio (INR) was 1.5 on the first test during hospitalization in patients receiving warfarin. Patients complicated with a previous history of ischemic stroke/transient ischemic attack (TIA; OR 2.303, p < 0.001) and peripheral artery disease (OR 1.456, p = 0.003) were more common to start anticoagulants.
Conclusions: Less than half of patients with AIS and concurrent AF initiated guideline-recommended oral anticoagulation at discharge, while only 20% of patients with previously diagnosed AF with a high risk of stroke had been using anticoagulants prior to the onset of stroke, which highlights a large care gap in hospitalized stroke patients and the importance of AF management.
Atrial fibrillation (AF) increases the risk of ischemic stroke from thromboembolism, and oral anticoagulation (OAC) is recommended for preventing ischemic strokes (1, 2). Patients who receive OAC according to guideline recommendations have a better prognosis compared with those whose treatment deviated from guideline recommendations. Meanwhile, a lower rate of OAC was found to be associated with increased incidence of ischemic stroke and all-cause mortality (3). However, AF undertreatment is still a long-established healthcare concern. Multiple studies investigating the prevalence of AF undertreatment among patients with stroke have reported that the proportion of patients who did not receive OAC treatment varied worldwide, ranging from approximately 20 to 60%, which highlighted a large care gap and an opportunity to improve AF management (3–7). In Asia, the proportion of insufficient treatment is generally higher (6, 8, 9), but there is still a lack of large data about the undertreatment of anticoagulant therapy in hospitalized patients with acute ischemic stroke (AIS) with AF. Moreover, it remains unclear what factors affect an individual's risk of undertreatment. Thus, this study aimed to investigate the point prevalence and factors associated with the initiation of anticoagulation among hospitalized patients with AIS and concurrent AF in China.
This study came from the Computer-based Online Database of Acute Stroke Patients for Stroke Management Quality Evaluation (CASE-II, NCT04487340), a multicenter prospective registry. Initiated in 2016, CASE-II was designed to examine the current status of stroke care in China to help develop strategies to improve stroke care. The medical documents during hospitalization of consecutive patients with stroke were collected through a special electronic data capture system. Briefly, original hospital records of patients were saved as images or portable document formats. Specific software pre-processed the above materials and sent them to multiple Optical Character Recognition (10) engines to build documents with recognized text, which were subsequently re-segmented and synthesized. Required data was extracted from the post-processed text, and the cross-check of each case was carried out by a quality control team. Only the de-identified documents were preserved in a safe information database and monitored by an independent contract research organization throughout the study period.
The CASE-II has consecutively recruited patients with stroke who were diagnosed with AIS, transient ischemic attack (TIA), hemorrhagic stroke, or subarachnoid hemorrhage and admitted within 7 days of symptom onset in China. Because patient information in the CASE-II was de-identified and anonymized before being released to the researchers, the informed consent requirement was waived by Institutional Review Board. For the present analysis, patients with AIS with concurrent AF aged ≥18 years were enrolled in the CASE-II between January 2016 and December 2020. AIS was diagnosed according to the World Health Organization criteria (11) and confirmed by computed tomography or magnetic resonance imaging. AF was defined as atrial arrhythmia with irregular R-R intervals and no clear repetitive P waves, and diagnosed with an electrocardiograph, 24- or 48-h Holter, or telemonitoring with recording and automated rhythm detection. Patients with incomplete information on anticoagulation at discharge or who died during hospitalization were excluded.
We collected the following patient information during hospitalization: demographics (age, sex); blood pressure at admission, baseline National Institutes of Health Stroke Scale (NIHSS) score, and baseline CHA2DS2-VASc score; medical history and medication history; reperfusion therapy (intravenous thrombosis with recombinant tissue-type plasminogen activator, mechanical thrombectomy with stent retrievers, and/or thromboaspiration, balloon angioplasty, stenting, or intra-arterial thrombolysis); laboratory tests at admission; medications usage at discharge; and reasons for non-treatment were documented in patient records, which include medical contraindications, refusal against medical advice, or transfer to another hospital.
Clinical characteristics were summarized by computing the mean [standard deviation (SD)] or median [interquartile range (IQR)]. Differences between the two groups were estimated by the t-test or Mann-Whitney U test if they were continuous variables. Categorical variables were summarized by proportion (n), and differences between the two groups were estimated by the Pearson χ2 test. To avoid overfitting, we ran logistic regression with a lasso regularization to select variables associated with the use of anticoagulation. Lambda parameters, which minimize the 10-fold cross-validation prediction error rate, were determined automatically using the function cv. glmnet (12). Function fixed Lasso Inf was used to compute p-values and confidence intervals for the lasso estimate. When analyzing the factors associated with prior use of anticoagulation in previously diagnosed patients with AF with a high risk of stroke, clinical information prior to the index stroke was included in the multivariate analysis. All comparisons were two-sided, with statistical significance defined as p < 0.05. All statistical analysis was performed using SPSS, Version 24.0 (IBM, Armonk, New York), and R software, Version 4.1.0 (R Foundation, Vienna, Austria).
A total of 16,162 patients with AIS and concurrent AF were included for analysis in this study (Figure 1). Mean age was 77 ± 9 years, 8,596 (53.2%) were males, and median baseline NIHSS score was 5 (2–12). There were 14,838 patients having no clear documentation of antithrombotic contraindications at discharge. Among them, 5,895 patients (39.7%) received antiplatelets, 6,061 (40.8%) were on OAC, 274 (1.8%) were on both antiplatelet and OAC, while 2,608 patients (17.6%) did not receive any antithrombotic agents (Table 1). Initiation of anticoagulation at discharge was increased from 30.1% (52 of 173) in 2016 to 49.6% (2,266 of 4,573) in 2020. From Figure 2, we could observe an increased use of direct oral anticoagulants (DOACs) [from 8.7% (15 of 173) to 30.5% (1,397 of 4,573)] and a slight decline of warfarin usage [from 21.4% (37 of 173) to 19.0% (869 of 4573)].
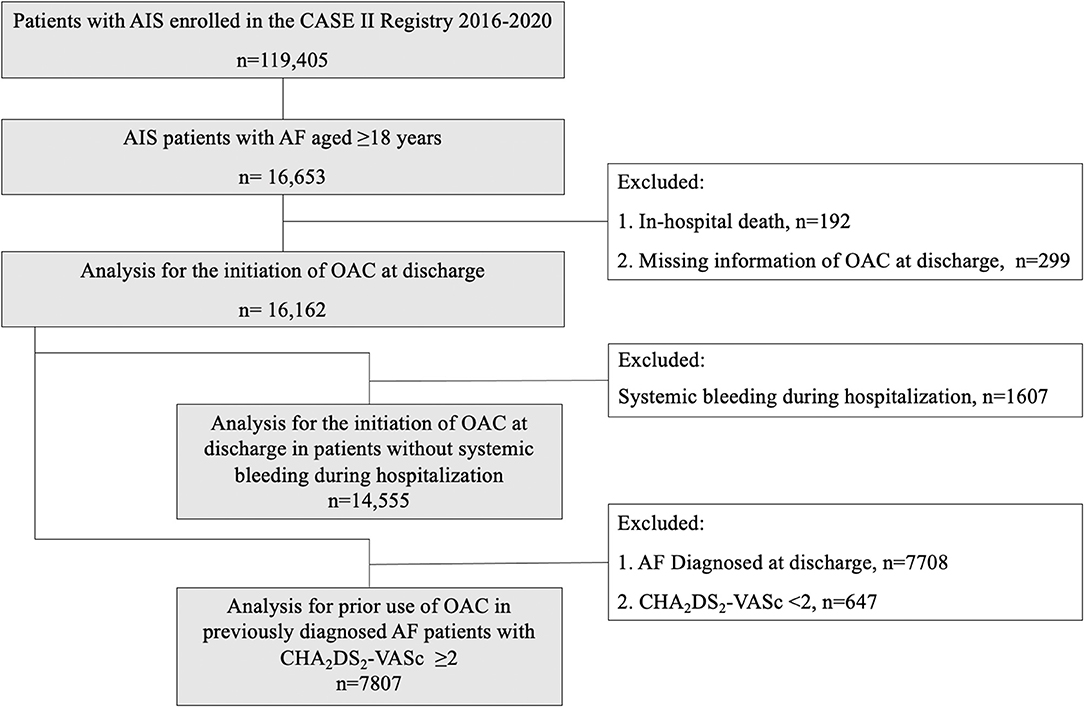
Figure 1. Flow chart of study population identification. AIS indicates acute ischemic stroke; AF, atrial fibrillation; CASE II, Computer-based Online Database of Acute Stroke Patients for Stroke Management Quality Evaluation; OAC, oral anticoagulation.
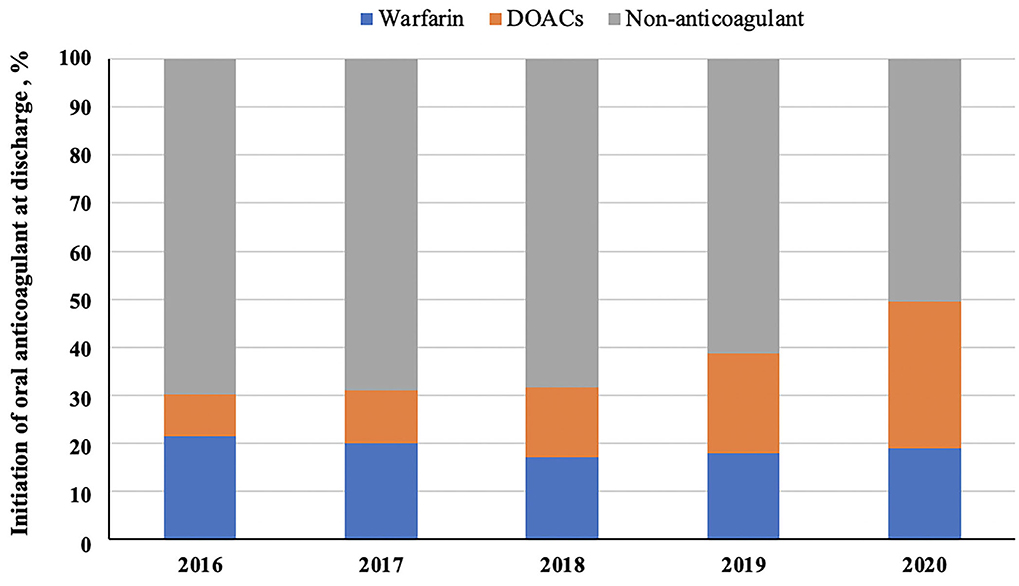
Figure 2. The trends of oral anticoagulant use at discharge. DOACs indicate direct oral anticoagulants.
As shown in Table 2, patients with the initiation of anticoagulants at discharge were younger (OR 0.973, 95% CI 0.970–0.977), had lower systolic blood pressure (OR 0.998, 95% CI 0.996–0.999), and baseline NIHSS score (OR 0.943, 95% CI 0.937–0.947) than patients who were not on OAC treatment. The prior anticoagulants usage (OR 3.408, 95% CI 3.065–3.789), complication with deep vein thrombosis (OR 1.711, 95% CI 1.446–2.020), and longer hospital stay (OR 1.044, 95% CI 1.039–1.050) were all associated with increased odds of receiving anticoagulation. The previous medical history associated with lower frequency of anticoagulation at discharge includes ischemic stroke/TIA (OR 0.890, 95% CI 0.826–0.965), hemorrhagic stroke (OR 0.647, 95% CI 0.527–0.795), and gastrointestinal bleeding (OR 0.607, 95% CI 0.463–0.804). In addition, patients with any intracranial hemorrhage (OR 0.268, 95% CI 0.232–0.309), gastrointestinal bleeding (OR 0.353, 95% CI 0.263–0.475), pneumonia during hospitalization (OR 0.601, 95% CI 0.553–0.652), or combined with renal insufficiency (OR 0.841, 95% CI 0.752–0.952) were less likely to receive anticoagulants at discharge.
Additional sensitivity analyses were performed to determine the factors associated with anticoagulant therapy at discharge in patients without systemic bleeding during hospitalization, and the findings were generally comparable with that in the primary analysis (Table 3).
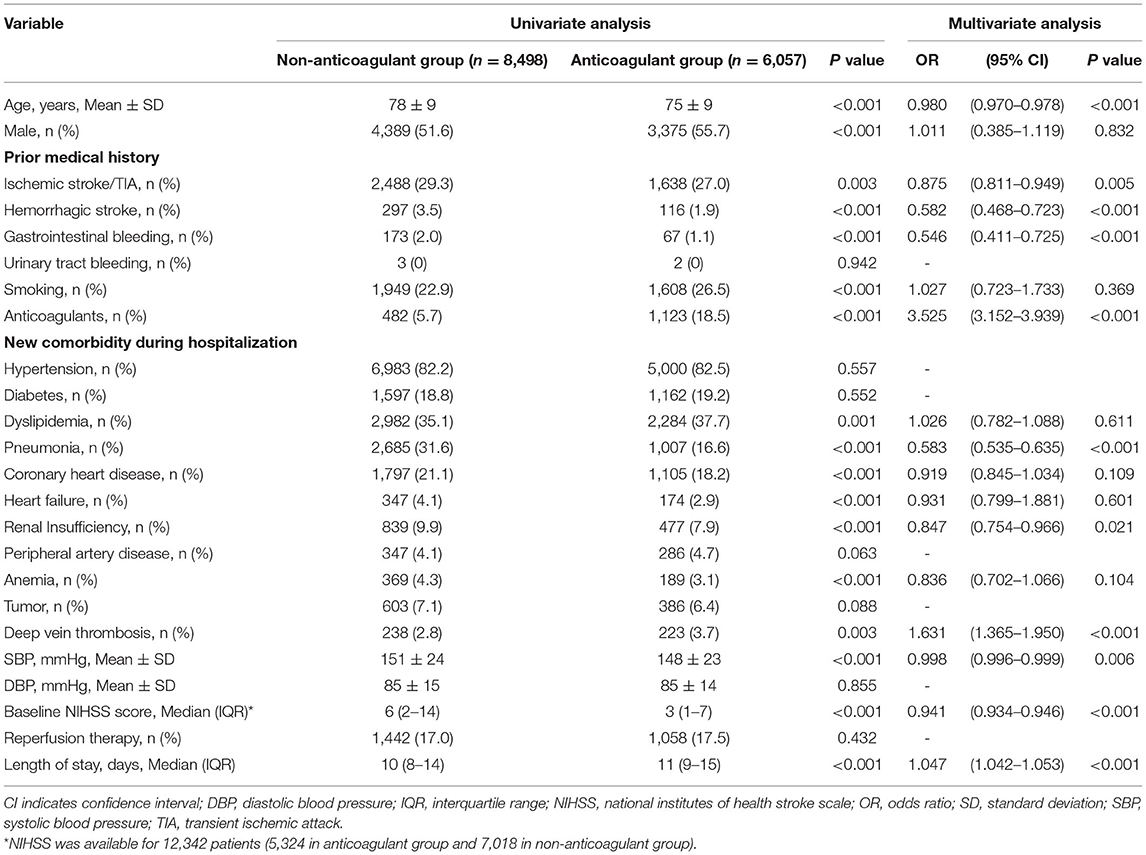
Table 3. Univariate and multivariate analysis for the initiation of anticoagulation at discharge in patients without systemic bleeding during hospitalization.
Among 7,807 patients with previously diagnosed AF and high risk of stroke (CHA2DS2-VASc ≥2), 1,585 patients (20.3%) had been receiving anticoagulation prior to the onset of stroke (17.9% anticoagulants only, and 2.4% were on both antiplatelets and anticoagulants). As seen in Table 4, patients who had been receiving anticoagulation had lower baseline NIHSS scores than those who are not. However, the mean international normalized ratio (INR) was 1.5 on the first test during hospitalization in patients receiving warfarin. Previously diagnosed patients with AF with a high risk of stroke who used anticoagulation were younger (OR 0.962, 95% CI 0.955–0.968), had a higher rate of ischemic stroke/TIA (OR 2.303, 95% CI 2.069–2.563), and peripheral artery disease (OR 1.456, 95% CI 1.176–1.795), and a lower rate of hemorrhagic stroke (OR 0.353, 95% CI 0.264–0.474) and hypertension (OR 0.634, 95% CI 0.562–0.717) compared with patients who do not use anticoagulants. Interestingly, patients who underwent anticoagulation before onset also had a higher rate of medication with antihypertensive, hypoglycemic agents, and statins.
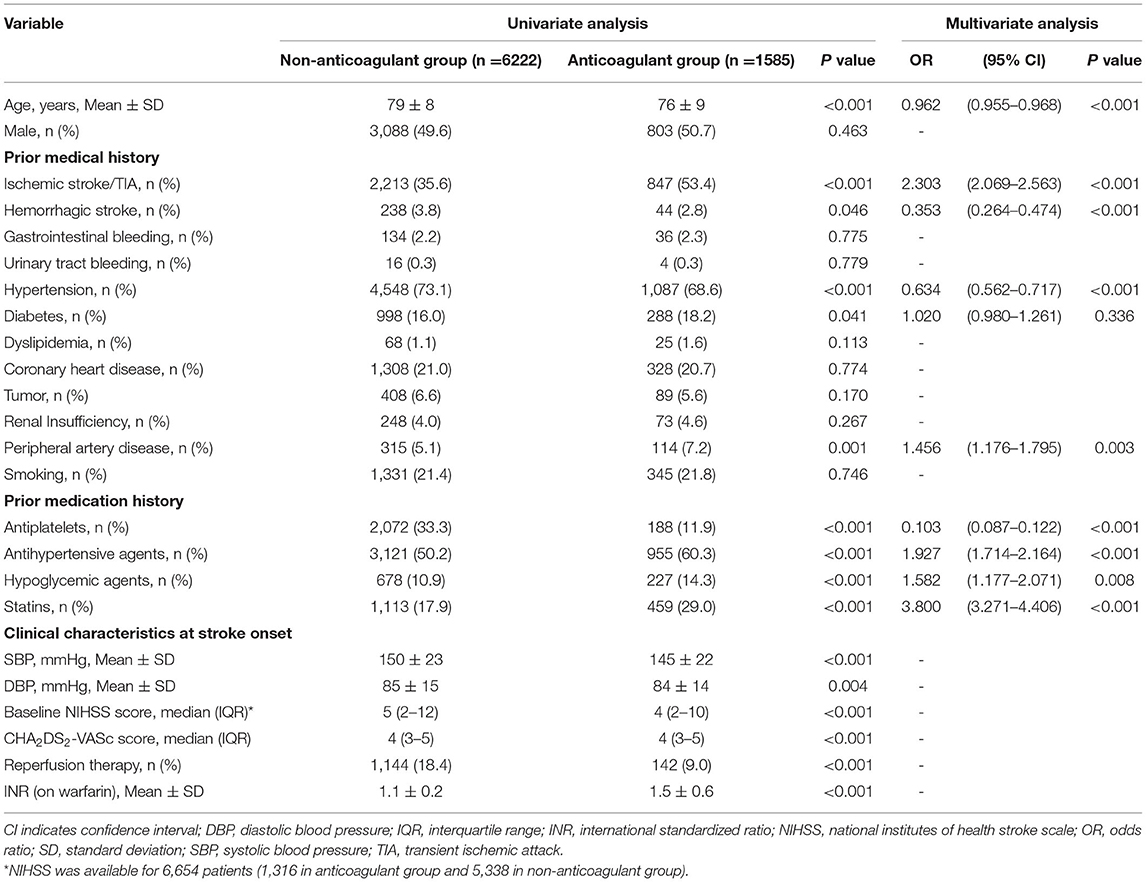
Table 4. Univariate and multivariate analysis for prior use of anticoagulation in patients with previously diagnosed atrial fibrillation (AF) with high risk of stroke.
In this large prospective multicenter registry of stroke patients in China, <50% of patients with AIS with concurrent AF received guideline-recommended anticoagulation at discharge, which highlights a large care gap between guideline and practice. Prior hemorrhagic diseases and the presence of pneumonia during hospitalization were related to a lower rate of anticoagulant therapy. Furthermore, among our patients who were having a pre-index diagnosis of AF with a high risk of stroke and final ischemic event, only 20% received guideline-recommended OAC treatment, indicating the importance of AF management.
Guidelines from the European Society of Cardiology, European Heart Rhythm Association, and Heart and Stroke Foundation Canadian Stroke Best Practice Committees all highlight that weighing the risk of recurrence and bleeding is the core strategy to initiate anticoagulation after ischemic stroke (13–15). Several factors need to be considered in support of early or delayed anticoagulation. For example, early initiation of anticoagulation can be considered for young patients with lower NIHSS scores and well-controlled blood pressure, while delayed anticoagulation could be considered for ongoing intracerebral hemorrhage and gastrointestinal bleeding or combined renal insufficiency. Our study has similar findings.
Notably, we found several factors that are not recommended in the guidelines, which may explain the low proportion of anticoagulation in the real world. Prior hemorrhagic diseases, including intracerebral and gastrointestinal hemorrhage, were related to the absence of anticoagulants in this study. Increased undertreatment among patients with past bleeding events has been shown in previous studies (16, 17), indicating an excessive concern about the bleeding risk of patients. Waldo et al. found that perceived or actual bleeding risk was a significant predictor for warfarin undertreatment (18). Patient and family education on the benefits and risks associated with using anticoagulants should be made available and widely adopted by health care professionals. In the FibStroke study done in Finland, Palomäki et al. also found that patients with high HAS-BLED scores (HAS-BLED ≥3) were at an increased risk of OAC undertreatment than those with HAS-BLED <3 (17). Especially in the Asian population, a high rate of non-adherence to guidelines was found (6, 8, 9), which may be due to the higher rate of major bleeding, including intracranial hemorrhage in Asians than Caucasians (19–21). However, despite the fear of OAC-related bleeding complications in Asian patients, individualized assessment of bleeding risk is still needed (22, 23). Moreover, a high bleeding risk itself should not inevitably result in the decision not to use anticoagulants. Stroke and bleeding risk factors overlap, and patients at high risk of bleeding often have a high risk of ischemic stroke (24). To prevent bleeding while on treatment with anticoagulants, dynamic risk assessment to minimize the modifiable risk factors is of great importance (25).
The recommended general approach on the target timing of initiation of anticoagulation after stroke is as follows: 1 day or the same day after a TIA, 3 days after a mild stroke, 6 days after a moderate stroke, and 12–14 days after a severe stroke (26). In our study, the length of hospital stay was about 10 days in the non-anticoagulant therapy group without systemic bleeding during hospitalization, with their baseline NIHSS score only at 6 (indicating a mild-to-moderate stroke). It was also found that longer hospital stay was associated with increased odds of receiving anticoagulation. Therefore, these results revealed that there was a significant delay in the initiation of anticoagulation in the current clinical practice, which needs to be improved, potentially, by the education of clinicians.
Indeed, an increasing number of studies are focusing on the early prevention of hospitalized patients with AIS with concurrent AF. Data from observational studies and randomized controlled trials suggest that early recurrence of AIS in patients with concurrent AF ranges from 0.5 to 1.3% per day during the first two weeks (27, 28). In addition, early initiation of anticoagulation is particularly important for those who have a high risk of recurrences (29–31), such as those with high NIHSS scores and those with symptoms of atrial enlargement and atrial thrombus. Nowadays, small randomized trials (32) have reported that early initiation (1–5 days) of OAC treatment in patients with mild-to-moderate stroke or small-to-medium sized infarcts (less than a third of the affected arterial territory) could lead to both low frequency of symptomatic and asymptomatic intracranial hemorrhage and low rate of recurrent ischemic stroke. Furthermore, four randomized controlled trials [ELAN (NCT03148457; Switzerland), OPTIMAS (EudraCT, 2018-003859-38; UK), TIMING (NCT02961348; Sweden), and START (NCT03021928; USA)] which plan to recruit ~9,000 participants are underway, with methods that either use safer DOACs to initiate anticoagulation earlier or selecting the initiation time of OAC based on the risk judgment from the severity or imaging features of stroke (33). The results of these trials will help to persuade the clinicians to initiate early anticoagulation, improving the secondary prevention of stroke.
Guidelines recommend OAC for all patients with a high risk of stroke (CHA2DS2-VASc ≥ 2) unless contraindicated (34, 35). However, in this study, we found that only 20.3% of patients with AF with a high risk of stroke used anticoagulants prior to the onset of stroke, far lower than the current global anticoagulation rate of 42–60% (4, 36–38). The characteristics of patients receiving OAC before the index stroke include young age, fewer history of hypertension, and intracerebral hemorrhage. This phenomenon still reflects the excessive fear of anticoagulation-related bleeding in old patients and prior hemorrhage. It is worth noting that patients with higher compliance of medication for risk factors were more likely to accept anticoagulants, highlighting the importance of patient education for primary prevention.
For patients with AF taking warfarin, careful dosing and consistent INR monitoring are important. This is due to how warfarin efficacy depends on therapeutic INR control (INR range 2.0–3.0; if the presence of mechanical valve range is 2.5–3.5) and declines when the INR falls lower than 2.0 (14). It is also important to note that the mean INR in patients with prior warfarin use was only 1.5, which does not meet the targets, which could be a direct cause of the current stroke. Moreover, INR also influences the decision of acute treatment, as the rate of reperfusion therapy was significantly decreased in patients using anticoagulants prior to the stroke. In view of this, DOACs may be a better choice as treatment compared to warfarin. Overall, optimizing the anticoagulant strategy could be another direction to improve AF management in the future.
Notably, the presence of pneumonia during hospitalization was associated with reduced use of anticoagulants at discharge. This result is hard to explain as no research has shown that pneumonia increases the risk of anticoagulation. Stroke-associated pneumonia (SAP) was reported to be associated with poor prognosis (39), preventing clinicians from initiating anticoagulation in patients. It is unclear whether prevention and improved management of SAP for patients with AIS could increase the use of anticoagulants during hospitalization. Strengthening the prevention and management of complications during the acute phase may lead to improving the anticoagulation rate.
As large cohort research that used patient data from a prospectively constructed database with predefined variables, this study is prone to bias despite how the comprehensive data collection allowed us to take a closer look at specific comorbidities, such as systemic hemorrhage, anemia, and tumor. Another limitation was the lack of information on stroke etiology, size of stroke lesions, and outcomes of patients as data were not available in the complete database. Also, we included data from regional stroke units and both tertiary and secondary hospitals, indicating that our findings could be generalizable. Since patient records were documented by the treating physician, contraindications were sought out as thoroughly as possible in patients who did not receive anticoagulants. However, 11.5% of patients in this study were unclear for the deferring reason, which may underestimate the proportion of anticoagulation contraindications. Finally, the present study was a cross-sectional study. Hence, there is a need for further longitudinal studies to determine the differences made in survival/mortality or stroke recurrence due to undertreatment of AF. Despite these limitations, our study presents a realistic view of the situation where a clinician is placed when deciding whether to use anticoagulant therapy in a given patient. In addition, provides new directions for research and quality improvement targeting anticoagulation.
Less than half of patients with AIS and AF received guideline-recommended anticoagulation at discharge, while only one in five patients with AF with a high risk of stroke had been using anticoagulants prior to the onset of stroke, which highlights a large care gap in hospitalized patients with stroke. To improve AF management in China, greater efforts must be made to educate both clinicians and patients to increase the rate of anticoagulation and optimize the anticoagulant strategy, including strengthening the health education of patients, improving the comprehensive acute stroke care quality, and enhancing the anticoagulant treatment experience of clinicians.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XG and HC performed the drafting and critical revision of the article and tables. ML had full access to all the data in the study, takes responsibility for the integrity of the data and the accuracy of the data analysis, performed conceptualization, drafting, and critical revision of the article.
The remaining authors were involved in the critical revision of the article. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (81971101, 82171276) and the Science Technology Department of Zhejiang Province (2018C04011).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. (2014) 130:e199–267. doi: 10.1161/CIR.0000000000000041
2. Lip G, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: past, present and future. Comparing the guidelines and practical decision-making. Thromb Haemost. (2017) 117:1230–9. doi: 10.1160/TH16-11-0876
3. Gebreyohannes EA, Bhagavathula AS, Tegegn HG. Poor outcomes associated with antithrombotic undertreatment in patients with atrial fibrillation attending Gondar University Hospital: a retrospective cohort study. Thromb J. (2018) 16:22. doi: 10.1186/s12959-018-0177-1
4. Aronis KN, Thigpen JL, Tripodis Y, Dillon C, Forster K, Henault L, et al. Paroxysmal atrial fibrillation and the hazards of under-treatment. Int J Cardiol. (2016) 202:214–20. doi: 10.1016/j.ijcard.2015.09.006
5. Brandes A, Overgaard M, Plauborg L, Dehlendorff C, Lyck F, Peulicke J, et al. Guideline adherence of antithrombotic treatment initiated by general practitioners in patients with nonvalvular atrial fibrillation: a Danish survey. Clin Cardiol. (2013) 36:427–32. doi: 10.1002/clc.22133
6. Chen Y, Huang QF, Sheng CS, Zhang W, Shao S, Wang D, et al. Detection rate and treatment gap for atrial fibrillation identified through screening in community health centers in China (AF-CATCH): a prospective multicenter study. PLoS Med. (2020) 17:e1003146. doi: 10.1371/journal.pmed.1003146
7. Diaz J, Koza E, Chaudhary D, Shahjouei S, Naved MMA, Malik MT, et al. Adherence to anticoagulant guideline for atrial fibrillation: a large care gap among stroke patients in a rural population. J Neurol Sci. (2021) 424:117410. doi: 10.1016/j.jns.2021.117410
8. Oh S, Goto S, Accetta G, Angchaisuksiri P, Camm AJ, Cools F, et al. Vitamin K antagonist control in patients with atrial fibrillation in Asia compared with other regions of the world: real-world data from the GARFIELD-AF registry. Int J Cardiol. (2016) 223:543–7. doi: 10.1016/j.ijcard.2016.08.236
9. Huisman MV, Ma CS, Diener HC, Dubner SJ, Halperin JL, Rothman KJ, et al. Antithrombotic therapy use in patients with atrial fibrillation before the era of non-vitamin K antagonist oral anticoagulants: the Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF) Phase I cohort. Europace. (2016) 18:1308–18. doi: 10.1093/europace/euw073
10. Li X, Hu G, Teng X, Xie G. Building structured personal health records from photographs of printed medical records. AMIA Annu Symp Proc. (2015) 2015:833–42.
11. Stroke−1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. (1989) 20:1407–31. doi: 10.1161/01.STR.20.10.1407
12. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. (2010) 33:1–22. doi: 10.18637/jss.v033.i01
13. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. (2016) 37:2893–962. doi: 10.1093/eurheartj/ehw210
14. Wein T, Lindsay MP, Cote R, Foley N, Berlingieri J, Bhogal S, et al. Canadian stroke best practice recommendations: secondary prevention of stroke, sixth edition practice guidelines, update 2017. Int J Stroke. (2018) 13:420–43. doi: 10.1177/1747493017743062
15. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace. (2018) 20:1231–42. doi: 10.1093/europace/euy054
16. Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS ONE. (2013) 8:e63479. doi: 10.1371/journal.pone.0063479
17. Palomaki A, Mustonen P, Hartikainen JE, Nuotio I, Kiviniemi T, Ylitalo A, et al. Underuse of anticoagulation in stroke patients with atrial fibrillation–the FibStroke Study. Eur J Neurol. (2016) 23:133–9. doi: 10.1111/ene.12820
18. Waldo AL, Becker RC, Tapson VF, Colgan KJ, NABOR Steering Committee. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol. (2005) 46:1729–36. doi: 10.1016/j.jacc.2005.06.077
19. Lip GY, Wang KL, Chiang CE. Non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. (2015) 180:246–54. doi: 10.1016/j.ijcard.2014.11.182
20. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. (2007) 50:309–15. doi: 10.1016/j.jacc.2007.01.098
21. Lopes RD, Guimaraes PO, Kolls BJ, Wojdyla DM, Bushnell CD, Hanna M, et al. Intracranial hemorrhage in patients with atrial fibrillation receiving anticoagulation therapy. Blood. (2017) 129:2980–7. doi: 10.1182/blood-2016-08-731638
22. Hijazi Z, Oldgren J, Lindback J, Alexander JH, Connolly SJ, Eikelboom JW, et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet. (2016) 387:2302–11. doi: 10.1016/S0140-6736(16)00741-8
23. O'Brien EC, Simon DN, Thomas LE, Hylek EM, Gersh BJ, Ansell JE, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. (2015) 36:3258–64. doi: 10.1093/eurheartj/ehv476
24. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. [2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS]. Kardiol Pol. (2016) 74:1359–469. doi: 10.5603/KP.2016.0172
25. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehab648
26. Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. (2013) 34:2094–106. doi: 10.1093/eurheartj/eht134
27. Hart RG, Coull BM, Hart D. Early recurrent embolism associated with nonvalvular atrial fibrillation: a retrospective study. Stroke. (1983) 14:688–93. doi: 10.1161/01.STR.14.5.688
28. Paciaroni M, Agnelli G, Micheli S, Caso V. Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke: a meta-analysis of randomized controlled trials. Stroke. (2007) 38:423–30. doi: 10.1161/01.STR.0000254600.92975.1f
29. Paciaroni M, Agnelli G, Falocci N, Caso V, Becattini C, Marcheselli S, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF study. Stroke. (2015) 46:2175–82. doi: 10.1161/STROKEAHA.115.008891
30. Paciaroni M, Agnelli G, Falocci N, Caso V, Becattini C, Marcheselli S, et al. Prognostic value of trans-thoracic echocardiography in patients with acute stroke and atrial fibrillation: findings from the RAF study. J Neurol. (2016) 263:231–7. doi: 10.1007/s00415-015-7957-3
31. Paciaroni M, Agnelli G, Caso V, Tsivgoulis G, Furie KL, Tadi P, et al. Prediction of early recurrent thromboembolic event and major bleeding in patients with acute stroke and atrial fibrillation by a risk stratification schema: the ALESSA score study. Stroke. (2017) 48:726–32. doi: 10.1161/STROKEAHA.116.015770
32. Hong KS, Kwon SU, Lee SH, Lee JS, Kim YJ, Song TJ, et al. Rivaroxaban vs warfarin sodium in the ultra-early period after atrial fibrillation-related mild ischemic stroke: a randomized clinical trial. JAMA Neurol. (2017) 74:1206–15. doi: 10.1001/jamaneurol.2017.2161
33. Seiffge DJ, Werring DJ, Paciaroni M, Dawson J, Warach S, Milling TJ, et al. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol. (2019) 18:117–26. doi: 10.1016/S1474-4422(18)30356-9
34. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. (2012) 33:2719–47. doi: 10.1093/eurheartj/ehs253
35. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2019) 74:104–32. doi: 10.1016/j.jacc.2019.01.011
36. Mazurek M, Shantsila E, Lane DA, Wolff A, Proietti M, Lip GYH. Secondary versus primary stroke prevention in atrial fibrillation: insights from the darlington atrial fibrillation registry. Stroke. (2017) 48:2198–205. doi: 10.1161/STROKEAHA.116.016146
37. Mikkelsen AP, Hansen ML, Olesen JB, Hvidtfeldt MW, Karasoy D, Husted S, et al. Substantial differences in initiation of oral anticoagulant therapy and clinical outcome among non-valvular atrial fibrillation patients treated in inpatient and outpatient settings. Europace. (2016) 18:492–500. doi: 10.1093/europace/euv242
38. Meinel TR, Branca M, De Marchis GM, Nedeltchev K, Kahles T, Bonati L, et al. Prior anticoagulation in patients with ischemic stroke and atrial fibrillation. Ann Neurol. (2021) 89:42–53. doi: 10.1002/ana.25917
Keywords: acute ischemic stroke, atrial fibrillation, anticoagulant therapy, cerebral infarction, undertreatment
Citation: Gong X, Chen H, Wang J, Zhong W, Chen L, Yan S and Lou M (2022) Undertreatment of Anticoagulant Therapy in Hospitalized Acute Ischemic Stroke Patients With Atrial Fibrillation. Front. Cardiovasc. Med. 9:841020. doi: 10.3389/fcvm.2022.841020
Received: 21 December 2021; Accepted: 07 March 2022;
Published: 30 March 2022.
Edited by:
Shaojie Chen, Cardioangiological Center Bethanien (CCB), GermanyReviewed by:
Eiichiro Nagata, Tokai University Isehara Hospital, JapanCopyright © 2022 Gong, Chen, Wang, Zhong, Chen, Yan and Lou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Lou, bG91bWluZ3hjQHZpcC5zaW5hLmNvbQ==; bG05OUB6anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.