
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 01 March 2022
Sec. Cardiovascular Epidemiology and Prevention
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.840585
This article is part of the Research Topic Insights in Cardiovascular Epidemiology and Prevention: 2022 View all 6 articles
Background: Left atrial enlargement (LAE) is associated with cardiovascular events. Machine learning for ECG parameters to predict LAE has been performed in middle- and old-aged individuals but has not been performed in young adults.
Methods: In a sample of 2,206 male adults aged 17–43 years, three machine learning classifiers, multilayer perceptron (MLP), logistic regression (LR), and support vector machine (SVM) for 26 ECG features with or without 6 biological features (age, body height, body weight, waist circumference, and systolic and diastolic blood pressure) were compared with the P wave duration of lead II, the traditional ECG criterion for LAE. The definition of LAE is based on an echocardiographic left atrial dimension > 4 cm in the parasternal long axis window.
Results: The greatest area under the receiver operating characteristic curve is present in machine learning of the SVM for ECG only (77.87%) and of the MLP for all biological and ECG features (81.01%), both of which are superior to the P wave duration (62.19%). If the sensitivity is fixed to 70–75%, the specificity of the SVM for ECG only is up to 72.4%, and that of the MLP for all biological and ECG features is increased to 81.1%, both of which are higher than 48.8% by the P wave duration.
Conclusions: This study suggests that machine learning is a reliable method for ECG and biological features to predict LAE in young adults. The proposed MLP, LR, and SVM methods provide early detection of LAE in young adults and are helpful to take preventive action on cardiovascular diseases.
Machine learning, an artificial intelligence (AI)-based computational statistic, has been broadly applied to clinical practice in medicine to assess disease risk and diagnosis (1–12). For instance, Lin et al. (12) used the support vector machine (SVM) classifier for some ECG features training successfully to identify echocardiographic left ventricular hypertrophy, and the performance of SVM was superior to the conventional ECG voltage criteria. In the modern age, the impact of machine learning is tremendously growing in medicine and has become a cost-effective and practical tool for physicians.
Left atrial enlargement (LAE) is related to high blood volume status (i.e., mitral regurgitation and elite endurance athletes) (13, 14) and elevated left ventricular (LV) diastolic pressure (i.e., obesity, hypertension, and great LV mass) (15–17). LAE is a precursor of left atrial dysfunction and has been associated with incident atrial fibrillation, ischemic stroke, and cardiovascular events in middle- and old-aged individuals (18–21). The prevalence of LAE is increased with aging, (18) and, in young adults, LAE is usually observed in those undergoing rigorous physical training, particularly accumulated lifetime training >3,600 h (22–24). A prior coronary artery disease risk development in young adults (CARDIA) study also revealed that the presence of LAE at a young age is a risk factor in incident cardiovascular events occurring in midlife (25). Therefore, early detection of LAE is vital to prevent the development of cardiovascular diseases and related sequelae.
The P wave duration in lead II ≥ 120 milliseconds is currently the most commonly used ECG criterion for the general population to screen for the presence of echocardiographic LAE, which is mainly defined as a diastolic left atrium dimension >4 cm in the parasternal long axis window (26). The P wave duration was also a predictor of atrial fibrillation, cardiovascular death, and early vascular aging (27, 28). Over the past 5 years, there were only some hospital-based studies utilizing machine learning for ECG features to detect the presence of LAE, in which the area under the curve (AUC) of the receiver operating characteristic curve (ROC) varied much from 0.62 to 0.98 (29–31). However, there were no previous reports performed in the general population. The aim of the study was to investigate the performance of machine learning for ECG features to identify LAE in a military cohort of young male adults.
A population of 2,268 military males aged 17–43 years were obtained from the cardiorespiratory health in eastern armed forces study (CHIEF Heart Study) for the machine learning experiment (32–35). All the participants received the annual health examination for their demographic, anthropometric, and hemodynamic measurements in the Hualien Armed Forces General Hospital of Taiwan from 2016 to 2021. Anthropometric parameters, including body height, weight, and waist circumference, of each participant were measured in the standing position. The hemodynamic parameter for blood pressure of each participant was measured one time over the right upper arm in a sitting position after at least 15 min of rest by an automatic oscillometric monitor (PARAMA TECH FT-201, Fukuoka, Japan). In addition, all the participants received 12-lead ECG and echocardiography to assess their cardiac structure and function during the same period. Sixty-two participants were excluded for a lack of relevant data (n = 36) or were unwilling to sign informed consent (n = 26), leaving a sample of 2,206 males for analysis.
A 12-lead ECG was performed for each participant (Schiller AG CARDIOVIT MS-2015, Baar, Switzerland). If the quality of the ECG report was not interpretable (i.e., baseline wandering), a new ECG would be repeated by the technician. The analysis for the ECG parameters, such as the heart rate and P-QRS-T wave duration or interval, was performed by the software in the ECG machine and interpreted by a board-certified cardiologist.
Transthoracic echocardiography using a 1–5 MHz transducer (iE33; Philips Medical Systems, Andover, MA, USA) was performed following the ECG procedure at the Hualien-Armed Forces General Hospital. Measurements of left atrial dimensions were based on the recommendations of the American Society of Echocardiography (36). LAE was defined as the left atrial diameter in the image of 2-D or M-mode > 4 cm, which was calculated from the posterior aortic wall to the posterior left atrial wall for men in the parasternal long-axis view at the end-ventricular systole. The prevalence of LAE in the young males was 4.85% (107/2206). The profiles of those with and without LAE are shown in Table 1 and compared by ANOVA, where a p < 0.05 was considered significant. The study design and protocol were approved by the Institutional Review Board of Mennonite Christian Hospital (No. 16-05-008) in Hualien City, Taiwan.
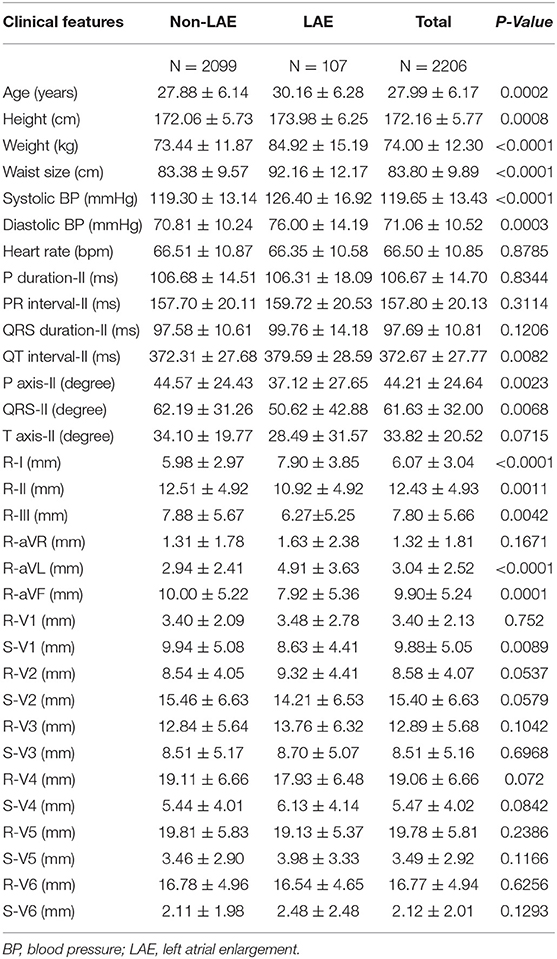
Table 1. Comparisons in electrocardiographic and biological features between participants with and without left atrial enlargement.
Three machine learning classifiers, including the multilayer perceptron (MLP) (37), logistic regression (LR) (38), and support vector machine (SVM) with a linear kernel (39), were used for 26 ECG features (heart rate; P wave duration in lead II; intervals of PR, QRS, and QT in lead II; axes of P, QRS, and T waves in lead II; voltages of the R wave in limb leads I, II, III, aVR, aVL, and aVF; voltages of both the R and S waves in precordial leads V1–V6) and with or without six biological features (age, body height, body weight, waist circumference, and systolic and diastolic blood pressure) training to identify the presence of LAE from military young males in Taiwan. The normalization of Min–Max scaling was used for the input data to execute a linear transformation (40). The original data of all 32 ECG and biological features were adjusted to a normalized value between 0 and 1. The MLP model includes an input layer, hidden layers, and an output layer (37). In hidden layers, the rectified linear unit (ReLU) activation function is utilized for each node, and the logistic regression function is used to determine the output layer. LR is a linear model that transforms its output using the logistic sigmoid function to return a probability value (38). The loss function includes the loss term and the regularization term. The loss term for learning the weight vector is negative log-likelihood, and the regularization term is used to avoid overfitting. In SVM (39), the maximum margin is constructed to maximize the distance from the hyperplane to the nearest subset of the training data points (support vectors) of the LAE or non-LAE class. The soft-margin SVM with regularization technique weighted by hyperparameter is adopted to allow the wide decision margin (39). The optimized hyperparameters for the three machine learning classifiers are chosen by grid search based on the average AUC of the ROC curves of the cross validation.
The data of the 2,206 participants were randomly grouped by a 3:1 ratio into a training/validation set (n = 1,654) and a test set (n = 552). Three subgroups of equal size were divided from the training/validation set. Two subgroups of the training/validation set were used for training, and the remaining subgroup was used for validation. The data numbers illustrated by the three folds are shown in Table 2. Because there was an imbalance in sample size between LAE and non-LAE cases, the synthetic minority oversampling technique (SMOTE) (41) was applied to artificially augment the LAE cases. Using the SMOTE to create sufficient new minority class cases, a near neighbor of the minority class of the index cases was randomly chosen for interpolation. The decision space for the LAE cases was magnified, and the SMOTE method could balance the number of each category. After data augmentation, the three subgroups were replaced to repeat the process: two for training and one for validation. An average of the three AUCs of the ROC curves from the 3-fold cross validations was treated as a single performance. The raw data that were not preprocessed by SMOTE for machine learning were used to confirm the validity of SMOTE. This study utilized scikit learn v0.20.2 software and Python programming language for the proposed methods. The flow chart for data preprocessing and machine learning is shown in Figure 1.
Table 3 demonstrates the optimized hyperparameters for the machine learning methods in the testing set. Regarding the MLP, LR, and SVM for 26 ECG features preprocessed by SMOTE, when the sensitivity was 73.68% for all, the specificity was 57.41, 69.79, and 72.42%, respectively, and the accuracy was 57.97, 69.93, and 72.46%, respectively, as shown in Table 4, which were superior to those without SMOTE in Supplementary Table 1. Regarding the MLP, LR, and SVM for all 32 ECG and biological features, when the sensitivity was 73.68% for all, the specificity was 81.05, 73.73, and 71.67%, and the accuracy was 80.80, 73.73, and 71.74%, respectively. While the traditional ECG criterion of the P wave duration in lead II was ≥ 106 ms for LAE, the sensitivity, specificity, and accuracy were 73.68, 48.78, and 49.64%, respectively. The AUCs of the ROC curves shown in Figure 2 were 72.93, 77.09, and 77.87% using the MLP, LR, and SVM, respectively, for 26 ECG features and 81.01, 78.99, and 76.74% utilizing the MLP, LR, and SVM, respectively, for 32 ECG and biological features, which were much >62.19% for the P wave duration in lead II.
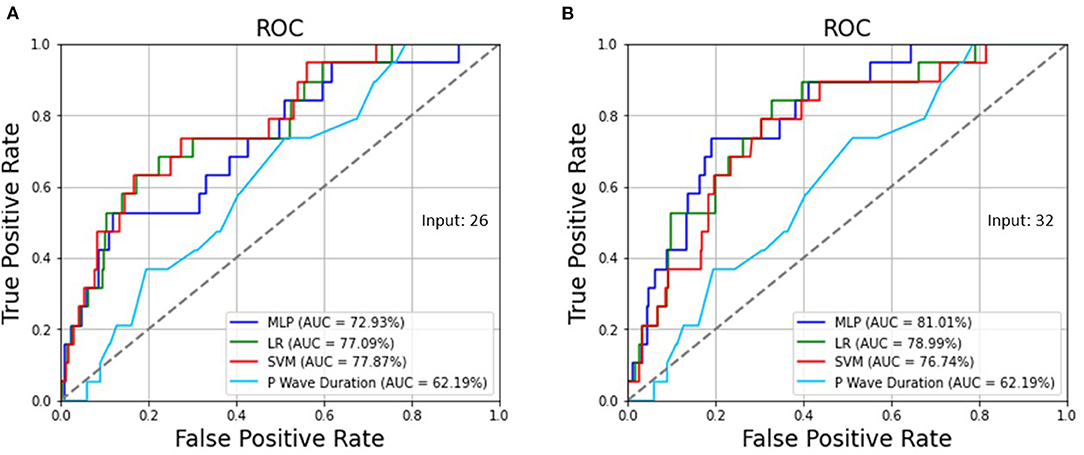
Figure 2. (A) The AUCs of the ROC curves were 72.93, 77.09, and 77.87% using the MLP, LR, and SVM, respectively, for 26 ECG features as well as (B) 81.01, 78.99, and 76.74% utilizing the MLP, LR, and SVM, respectively, for 32 ECG and biological features, which are >62.19% for the P wave duration in lead II.
Figure 3 shows the 26 ECG feature importance of the SVM classifier. A greater R wave voltage of leads aVL and I and QT interval of lead II were the most important factors of echocardiographic LAE, with a coefficient magnitude >2 in the SVM model. The other potent predictors of LAE with greater coefficient magnitude included greater S wave voltage of lead V1, P wave axis of lead II, and R wave voltage of lead aVR.
The study was the first report to show a better performance of machine learning to predict echocardiographic LAE compared to the traditional ECG criterion of P wave duration in young male adults who had a healthy status and without multiple comorbidities. Prior studies (29–31) have revealed that machine learning for ECG features could detect most of the LAE cases from hospitalized patients, probably due to those patients with LAE who were likely to have other cardiac comorbidities, such as heart failure, that were easily reflected by ECG features; thus, the results might not be appropriate for healthy individuals.
Some studies have shown that, in young adults, particularly physically fit people, an enlarged cardiac chamber is likely, and the typical ECG features for LAE might not be the same as those in middle-aged individuals and elderly individuals who had several cardiovascular comorbidities, i.e., hypertension. This study revealed that the P wave axis rather than the P wave duration was a strong indicator for LAE. In addition, a greater R wave in leads aVL and I and an S wave in lead V1 representing an enhanced left lateral electrical force in the heart (42) and a greater QT interval representing a longer diastolic phase of electrical repolarization and left ventricular relaxation were vital predictors of LAE. These findings emphasize the necessity of performing machine learning, specifically for physically young adults to identify LAE. The SVM was the best machine learning classifier for ECG features only to detect LAE in young males, achieving an AUC of 78% of the ROC. In contrast, the MLP was the best machine learning classifier, which could improve the performance from 73 to 81% after biological features were added to the MLP model. It was obvious that the addition of biological features did not improve the predictive performance of the SVM and LR classifiers.
The main strengths of this study included the following: First, military males were physically active, and the training program was conducted in Eastern Taiwan. In addition, since the living environment is a closed system, the participants have a similar daily schedule, and the unmeasured bias could be minimized. Third, this was the first study using machine learning for ECG and biological features to predict LAE early in young adults. In contrast, the data were only obtained from the males, and the results might not be the same for the females. Second, other feature learning methods, such as convolutional neural networks for ECG training to predict LAE, were not performed, which may be a focus of future works. Third, since LAE is highly associated with atrial fibrillation, follow-up studies allow a conclusion related to atrial fibrillation. Finally, oxidative stress was also related to the occurrence of atrial fibrillation (43), and this was not considered in this study.
This study suggests that it is reliable to use machine learning for ECG features and biological features to predict LAE in young adults. The proposed MLP, LR, and SVM methods could provide early detection of LAE in young adults in clinical settings and may be useful in screening for high-risk groups of young adults for cardiovascular diseases, i.e., atrial fibrillation, which has an important relationship with LAE.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Institutional Review Broad of Mennonite Christian Hospital (No. 16-05-008). The patients/participants provided their written informed consent to participate in this study.
C-YH wrote the paper. P-YL collected the data. S-HL, YK, and CL raised critical comments for the paper. G-ML analyzed data and edited the manuscript and was the principal investigator for the CHIEF study. All authors contributed to the article and approved the submitted version.
This study was supported by the Medical Affairs Bureau Ministry of National Defense and Hualien Armed Forces General Hospital, Taiwan, under the grants MND-MAB-110-148, MND-MAB-D-11115, HAFGH-D-110008, and HAFGH-D-111003.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.840585/full#supplementary-material
1. Siontis KC, Noseworthy PA, Attia ZI, Friedman PA. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat Rev Cardiol. (2021) 18:465–78. doi: 10.1038/s41569-020-00503-2
2. Lin GM, Nagamine M, Yang SN, Tai YM, Lin C, Sato H. Machine learning based suicide ideation prediction for military personnel. IEEE J Biomed Health Inform. (2020) 24:1907–16. doi: 10.1109/JBHI.2020.2988393
3. Lin GM, Lu HH. Electrocardiographic machine learning to predict left ventricular diastolic dysfunction in asian young male adults. IEEE Access. (2021) 9:49047–54 doi: 10.1109/ACCESS.2021.3069232
4. Lin GM, Zeng HC. Electrocardiographic machine learning to predict mitral valve prolapse in young adults. IEEE Access. (2021) 9:103132–40 doi: 10.1109/ACCESS.2021.3098039
5. Pao SI, Lin HZ, Chien KH, Tai MC, Chen JT, Lin GM. Detection of diabetic retinopathy using bichannel convolutional neural network. J Ophthalmol. (2020) 2020:9139713. doi: 10.1155/2020/9139713
6. Lin GM, Chen MJ, Yeh CH, Lin YY, Kuo HY, Lin MH, et al. Transforming retinal photographs to entropy images in deep learning to improve automated detection for diabetic retinopathy. J Ophthalmol. (2018) 2018:2159702. doi: 10.1155/2018/2159702
7. Chen MJ, Yang PH, Hsieh MT, Yeh CH, Huang CH, Yang CM, et al. Machine learning to relate PM2.5 and PM10 concentrations to outpatient visits for upper respiratory tract infections in Taiwan: a nationwide analysis. World J Clin Cases. (2018) 6:200–6. doi: 10.12998/wjcc.v6.i8.200
8. Sparapani R, Dabbouseh NM, Gutterman D, Zhang J, Chen H, Bluemke DA, et al. Detection of left ventricular hypertrophy using bayesian additive regression trees: the MESA. J Am Heart Assoc. (2019) 8:e009959. doi: 10.1161/JAHA.118.009959
9. Ambale-Venkatesh B, Yang X, Wu CO, Liu K, Hundley WG, McClelland R, et al. Cardiovascular event prediction by machine learning: the multi-ethnic study of atherosclerosis. Circ Res. (2017) 121:1092–101. doi: 10.1161/CIRCRESAHA.117.311312
10. Sengupta PP, Kulkarni H, Narula J. Prediction of abnormal myocardial relaxation from signal processed surface ECG. J Am CollCardiol. (2018) 71:1650–60. doi: 10.1016/j.jacc.2018.02.024
11. Lin GM, Lu HH. A 12-Lead ECG-based system with physiological parameters and machine learning to identify right ventricular hypertrophy in young adults. IEEE J TranslEng Health Med. (2020) 8:1900510. doi: 10.1109/JTEHM.2020.2996370
12. Lin GM, Liu K. An electrocardiographic system with anthropometrics via machine learning to screen left ventricular hypertrophy among young adults. IEEE J TranslEng Health Med. (2020) 8:1800111. doi: 10.1109/JTEHM.2020.2990073
13. Kim JH, Hollowed C, Liu C, Al-Badri A, Alkhoder A, Dommisse M, et al. Hypertension, and the emergence of a maladaptive cardiovascular phenotype among US Football Players. JAMA Cardiol. (2019) 4:1221–9. doi: 10.1001/jamacardio.2019.3909
14. Colan SD, Sanders SP, MacPherson D, Borow KM. Left ventricular diastolic function in elite athletes with physiologic cardiac hypertrophy. J Am CollCardiol. (1985) 6:545–9. doi: 10.1016/S0735-1097(85)80111-X
15. Teo SG, Yang H, Chai P, Yeo TC. Impact of left ventricular diastolic dysfunction on left atrial volume and function: a volumetric analysis. Eur J Echocardiogr. (2010) 11:38–43. doi: 10.1093/ejechocard/jep153
16. Vaziri SM, Larson MG, Lauer MS, Benjamin EJ, Levy D. Influence of blood pressure on left atrial size. The Framingham Heart Study. Hypertension. (1995) 25:1155–60. doi: 10.1161/01.HYP.25.6.1155
17. Gottdiener JS, Reda DJ, Williams DW, Materson BJ. Left atrial size in hypertensive men: influence of obesity, race and age. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am CollCardiol. (1997) 29:651–8. doi: 10.1016/S0735-1097(96)00554-2
18. Stritzke J, Markus MR, Duderstadt S, Lieb W, Luchner A, Döring A, et al. The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J Am Coll Cardiol. (2009) 54:1982–9. doi: 10.1016/j.jacc.2009.07.034
19. Laukkanen JA, Kurl S, Eränen J, Huttunen M, Salonen JT. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. (2005) 165:1788–93. doi: 10.1001/archinte.165.15.1788
20. Bouzas-Mosquera A, Broullón FJ, Álvarez-García N, Méndez E, Peteiro J, Gándara-Sambade T, et al. Left atrial size and risk for all-cause mortality and ischemic stroke. CMAJ. (2011) 183:E657–E64. doi: 10.1503/cmaj.091688
21. Fredgart MH, Lindholt JS, Brandes A, Steffensen FH, Frost L, Lambrechtsen J, et al. Prognostic importance of left atrial size measured by non-contrast cardiac computed tomography - A DANCAVAS study. Int J Cardiol. (2021) 328:220–6. doi: 10.1016/j.ijcard.2020.12.029
22. Liu PY, Tsai KZ, Lima JAC, Lavie CJ, Lin GM. Athlete's heart in asian military males: the CHIEF heart study. Front Cardiovasc Med. (2021) 8:725852. doi: 10.3389/fcvm.2021.725852
23. Engel DJ, Schwartz A, Homma S. Athletic cardiac remodeling in US professional basketball players. JAMA Cardiol. (2016) 1:80–7. doi: 10.1001/jamacardio.2015.0252
24. Diaz Babio G, Vera Janavel G, Constantin I, Masson G, Carrero C, Garcia Botta T, et al. Atrial size and sports. A great training for a greater left atrium: how much is too much? Int J Cardiovasc Imaging. (2021) 37:981–8. doi: 10.1007/s10554-020-02082-2
25. Armstrong AC, Liu K, Lewis CE, Sidney S, Colangelo LA, Kishi S, et al. Left atrial dimension and traditional cardiovascular risk factors predict 20-year clinical cardiovascular events in young healthy adults: the CARDIA study. Eur Heart J Cardiovasc Imaging. (2014) 15:893–9. doi: 10.1093/ehjci/jeu018
26. Waggoner AD, Adyanthaya AV, Quinones MA, Alexander JK. Left atrial enlargement. Echocardiographic assessment of electrocardiographic criteria. Circulation. (1976) 54:553–7. doi: 10.1161/01.CIR.54.4.553
27. Mozos I, Caraba A. Electrocardiographic predictors of cardiovascular mortality. Dis Markers. (2015) 2015:727401. doi: 10.1155/2015/727401
28. Mozos I, Gug C, Mozos C, Stoian D, Pricop M, Jianu D. Associations between intrinsic heart rate, P wave and QT interval durations and pulse wave analysis in patients with hypertension and high normal blood pressure. Int J Environ Res Public Health. (2020) 17:4350. doi: 10.3390/ijerph17124350
29. Tison GH, Zhang J, Delling FN, Deo RC. Automated and interpretable patient ECG profiles for disease detection, tracking, and discovery. Circ Cardiovasc Qual Outcomes. (2019) 12:e005289. doi: 10.1161/CIRCOUTCOMES.118.005289
30. Jiang J, Deng H, Xue Y, Liao H, Wu S. Detection of left atrial enlargement using a convolutional neural network-enabled electrocardiogram. Front Cardiovasc Med. (2020) 7:609976. doi: 10.3389/fcvm.2020.609976
31. Kashou AH, Ko WY, Attia ZI, Cohen MS, Friedman PA, Noseworthy PA. A comprehensive artificial intelligence-enabled electrocardiogram interpretation program. Cardiovasc Digit Health J. (2020) 1:62–70. doi: 10.1016/j.cvdhj.2020.08.005
32. Lin GM, Li YH, Lee CJ, Shiang JC, Lin KH, Chen KW, et al. Rationale and design of the cardiorespiratory fitness and hospitalization events in armed forces study in Eastern Taiwan. World J Cardiol. (2016) 8:464–71. doi: 10.4330/wjc.v8.i8.464
33. Chao WH, Su FY, Lin F, Yu YS, Lin GM. Association of electrocardiographic left and right ventricular hypertrophy with physical fitness of military males: the CHIEF study. Eur J Sport Sci. (2019) 19:1214–20. doi: 10.1080/17461391.2019.1595741
34. Lin YK, Tsai KZ, Han CL, Lin YP, Lee JT, Lin GM. Obesity phenotypes and electrocardiographic characteristics in physically active males: CHIEF study. Front Cardiovasc Med. (2021) 8:738575. doi: 10.3389/fcvm.2021.738575
35. Su FY, Lin YP, Lin F, Yu YS, Kwon Y, Lu HH, et al. Comparisons of traditional electrocardiographic criteria for left and right ventricular hypertrophy in young Asian women: the CHIEF heart study. Medicine (Baltimore). (2020) 99:e22836. doi: 10.1097/MD.0000000000022836
36. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am SocEchocardiogr. (2005) 18:1440–63. doi: 10.1016/j.echo.2005.10.005
39. Scholkopf B. Learning With Kernels: Support Vector Machines, Regularization, Optimization, and Beyond. MIT Press (2001).
40. Jain YK Bhandare SK. Min max normalization-based data perturbation method for privacy protection. Int J ComputCommun Tech. (2014) 3:45–50.
41. Chawla NV, Hall LO, Bowyer KW, Kegelmeyer WP. SMOTE: synthetic minority oversampling technique. J ArtifIntell Res. (2002) 16:321–57. doi: 10.1613/jair.953
42. Verdecchia P, Angeli F, Cavallini C, Mazzotta G, Repaci S, Pede S, et al. The voltage of R wave in lead aVL improves risk stratification in hypertensive patients without ECG left ventricular hypertrophy. J Hypertens. (2009) 27:1697–704. doi: 10.1097/HJH.0b013e32832c0031
Keywords: echocardiography, electrocardiography, machine learning, left atrial enlargement, young adults
Citation: Hsu C-Y, Liu P-Y, Liu S-H, Kwon Y, Lavie CJ and Lin G-M (2022) Machine Learning for Electrocardiographic Features to Identify Left Atrial Enlargement in Young Adults: CHIEF Heart Study. Front. Cardiovasc. Med. 9:840585. doi: 10.3389/fcvm.2022.840585
Received: 21 December 2021; Accepted: 31 January 2022;
Published: 01 March 2022.
Edited by:
Chayakrit Krittanawong, NYU Grossman School of Medicine, United StatesReviewed by:
Ioana Mozos, Victor Babes University of Medicine and Pharmacy, RomaniaCopyright © 2022 Hsu, Liu, Liu, Kwon, Lavie and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pang-Yen Liu, bGl1cHlkckBnbWFpbC5jb20=; Gen-Min Lin, ZmFybWVyNTA3QHlhaG9vLmNvbS50dw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.