- 1Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, China-Japan Friendship Hospital, Beijing, China
- 2National Center for Respiratory Medicine, Beijing, China
- 3Institute of Respiratory Medicine, Chinese Academy of Medical Sciences, Beijing, China
- 4National Clinical Research Center for Respiratory Disease, Beijing, China
- 5Department of Pulmonary and Critical Care Medicine, Beijing Hospital, Beijing, China
- 6Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Dalian Medical University, Dalian, China
- 7Department of Pulmonary and Critical Care Medicine, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China
- 8Department of Pulmonary and Critical Care Medicine, West China Hospital, West China School of Medicine, Sichuan University, Chengdu, China
- 9Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 10Department of Pulmonary and Critical Care Medicine, First Hospital of Shanxi Medical University, Taiyuan, China
- 11National Center for Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Science, Beijing, China
- 12Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Henan University of Science and Technology, Luoyang, China
- 13Department of Pulmonary and Critical Care Medicine, The Second Hospital of Jilin University, Changchun, China
- 14Department of Pulmonary and Critical Care Medicine, Peking Union Medical College Hospital, Beijing, China
- 15Data and Project Management Unit, Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, Beijing, China
- 16Department of Pulmonary and Critical Care Medicine, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 17Department of Traditional Chinese Medicine for Pulmonary Diseases, Center of Respiratory Medicine, China-Japan Friendship Hospital, Beijing, China
- 18Department of Pathophysiology, Peking Union Medical College, Beijing, China
- 19Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 20Department of Respiratory Medicine, Capital Medical University, Beijing, China
Objectives: There are conflicting data concerning the prognostic significance of syncope in acute pulmonary embolism (PE). This study aimed to investigate the impact of syncope on clinical outcomes of acute PE, and determine the clinical phenotypes of PE patients with syncope and their correlation with prognosis.
Methods: In the ongoing, national, multicenter, registry study, the China pUlmonary thromboembolism REgistry Study (CURES) enrolling consecutive patients with acute PE, patients with and without syncope were investigated. Principal component analysis (PCA) was performed using nine variables relevant to syncope and PE, including age, sex, body mass index, history of cardiovascular disease, recent surgery or trauma, malignancy, pulse, systolic blood pressure, and respiratory rate. Patient classification was performed using cluster analysis based on the PCA-transformed data. The clinical presentation, disease severity and outcomes were compared among the phenotypes.
Results: In 7,438 patients with acute PE, 777 (10.4%) had syncope, with younger age, more females and higher body mass index. Patients with syncope had higher frequency of precordial pain, palpitation, and elevated cardiac biomarkers, as well as higher D-Dimer level. In the syncope group, more patients had right ventricular/left ventricular ratio > 0.9 in ultrasonic cardiogram and these patients had higher estimated pulmonary arterial systolic pressure compared with patients without syncope. As the initial antithrombotic treatment, more patients with syncope received systemic thrombolysis. Despite a higher prevalence of hemodynamic instability (OR 7.626, 95% CI 2.960–19.644, P < 0.001), syncope did not increase in-hospital death. Principal component analysis revealed that four independent components accounted for 60.3% of variance. PE patients with syncope were classified into four phenotypes, in which patients with high pulse and respiratory rate had markedly higher all-cause mortality during admission.
Conclusion: Syncope was associated with hemodynamic instability and more application of thrombolysis, without increasing in-hospital deaths. Different clinical phenotypes existed in PE patients with syncope, which might be caused by various mechanisms and thus correlated with clinical outcomes.
Introduction
High morbidity and poor clinical outcomes associated with pulmonary embolism (PE) require accurate and rapid risk assessment for patients. So far, risk stratification is mainly based on the presence of hemodynamic instability, right ventricular dysfunction (RVD) and myocardial injury (1). Clinical presentation of PE varies widely from hemodynamic instability to clinically silent disease, incidentally discovered on computed tomography or found on autopsy of patients with unexpected sudden death (2, 3). Typical symptoms of acute PE had been reported to be associated with adverse outcome (4, 5). Syncope presents as one of the initial symptoms in 9–35% of acute PE cases (6–11). Conversely, the incidence of objectively confirmed acute PE was 1.4–17.3% in the patients hospitalized for a first episode of syncope (12–15).
There are conflicting data concerning the prognostic significance of syncope in patients with acute PE. Several studies suggested that syncope is associated with higher mortality (11, 16). In the ICOPER registry, the 3-month mortality of patients with syncope was 26.8%, significantly higher than the overall mortality of 17% (17). Syncope has been used in combination with cardiac biomarkers and tachycardia to develop a model for advanced risk stratification in PE (18, 19). However, many studies did not reproduce the association between syncope and higher mortality (6, 7, 9, 10, 20, 21). Syncope may occur in the presence or absence of hemodynamic instability, the mechanism of which is not clear. Different clinical phenotypes may exist in PE patients with syncope, with heterogeneous pathogenesis. Identification of the relatively higher-risk group among PE patients with syncope could improve the risk stratification and prognosis of these patients.
As an ongoing, national, multicenter, registry study, the China pUlmonary thromboembolism REgistry Study (CURES) enrolls consecutive patients diagnosed with acute symptomatic PE. We analyzed data in the CURES registry to explore the impact of syncope on the characteristics, therapeutic strategy and outcomes in patients with confirmed acute PE. Clinical phenotypes of PE patients with syncope were determined and their impact on clinical outcomes were further identified.
Materials and Methods
Patients Enrollment
The CURES registry is an ongoing national, multicenter, observational, prospective registry study, involving 100 medical centers across China. Consecutive patients greater than or equal to 18 years and diagnosed with acute symptomatic PE have been enrolled since 2009. In the patients with suspected PE, computed tomographic pulmonary angiography, ventilation-perfusion lung scintigraphy, magnetic resonance pulmonary angiography or pulmonary angiography were used to confirm the diagnosis. Patients were managed according to the clinical practice of each participating hospital center.
The registry complies with the Declaration of Helsinki and was approved by ethics committees in participating centers and hospital-based institutional review boards. The CURES registry is registered on ClinicalTrials.gov (NCT02943343). All patients provided written informed consent for their participation in the registry, in accordance with the requirements of the ethics committee in each hospital.
Data Collection
Data were recorded in a standardized case report form based on the original medical records at each participating center. Patients enrolled in CURES had data collected that included demographic information, comorbidities, risk factors for PE, symptoms and signs, physical and laboratory examinations, in addition to results of image testing, therapeutic management and clinical outcomes both in hospital and during the follow-up. Data quality was regularly monitored and documented electronically to identify inconsistencies or errors, which were resolved by the local coordinators at each participating center.
Variable Definition and Clinical Endpoint
Patients were allocated into two groups based on the presence of syncope, defined as a sudden transient loss of consciousness that has a rapid onset, short duration and spontaneous resolution (22). The primary endpoint was all-cause death and fatal PE during admission. For deaths confirmed by autopsy or those following a clinically severe PE, in the absence of any alternative diagnosis, the investigators were instructed to judge if the death was due to fatal PE. Major bleeding was defined as previously reported (23). Death, cause of death and major bleeding events were adjudicated by the registry coordinators.
Hemodynamic instability, also defined as high-risk, was defined according to the European Society of Cardiology Guidelines (1). Simplified pulmonary embolism severity index (sPESI) was calculated (24) for patients with hemodynamically stable PE. Cardiac biomarkers used in the risk assessment of PE include cardiac troponin I, cardiac troponin T, brain natriuretic peptide (BNP), and N terminal-pro BNP (NT-proBNP).
Statistical Analyses
Qualitative data were reported as n (%). Quantitative data were reported as mean (standard deviation, SD) or median (interquartile range, IQR). Independent T-tests and one-way ANOVA were used to compare mean of normally distributed data, while nonparametric tests were used to compare non-normally distributed or discrete data. The χ2 test was used to compare categorical data. A logistic regression model was used to identify the risk factors for syncope. Any variable achieving a P-value < 0.1 on univariate analysis was included in a multivariate logistic regression analysis. Odds ratio (OR) and the corresponding 95% confidence interval (CI) were reported. Nine variables were selected for their relevance to syncope and PE: age, sex, body mass index (BMI), history of cardiovascular disease (CVD), recent surgery or trauma, malignancy, pulse, systolic blood pressure (SBP), and respiratory rate (RR). On account of redundancy, principal component analysis (PCA) was performed on these variables to reduce interaction between variables. Then, cluster analysis was performed based on the main principal components to identify phenotype clusters of syncope in PE patients. The clinical characteristics and outcomes were compared among these phenotype clusters. A P-value of <0.05 was considered to be statistically significant. All the analyses were performed using the IBM SPSS software (Version 26.0).
Results
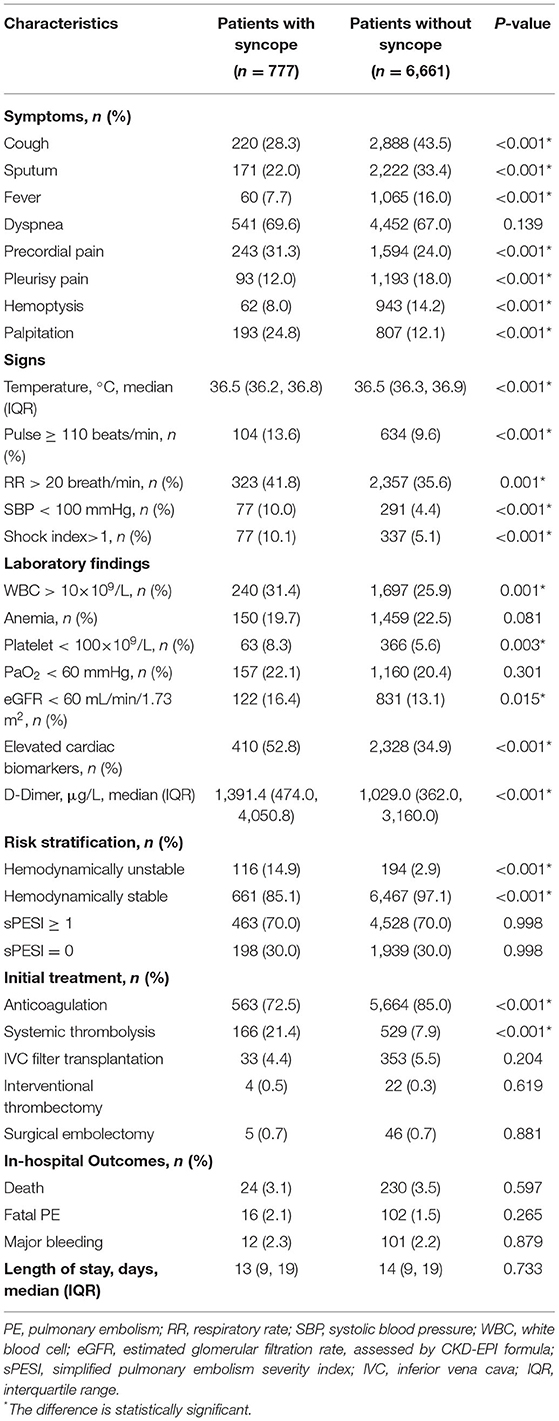
Between January 2009 and December 2015, a total of 7,438 consecutive adult patients with acute PE were included in the CURES registry. Of these patients 777 (10.4%) had syncope. A flowchart describing the main methodology and results of this study is shown in Figure 1.
Figure 1. The flowchart of the study. We enrolled 7,438 patients confirmed with acute PE, in which 777 patients had syncope as one of initial symptoms. Nine variables were selected for their relevance to syncope and PE: age, sex, body mass index, cardiovascular disease, recent surgery or trauma, malignancy, pulse, systolic blood pressure, and respiratory rate. Complete data for the nine variables were available for 725 subjects with syncope. In the system cluster analysis, they were classified into four phenotypes, with different outcomes during admission. *The difference is statistically significant compared with other phenotypes.
Demographic Characteristics and Comorbidities
Of all the patients, 3,939 (53.0%) are males, while there were fewer males in patients with syncope (43.2 vs. 54.1%, P < 0.001). The mean age of all patients was 61.3 ± 15.1 years and patients with syncope were younger than those without (60.1 ± 14.9 years vs. 61.4 ± 15.1 years, P = 0.023). Patients with syncope had higher BMI (24.4 ± 3.5 kg/m2 vs. 24.0 ± 3.6 kg/m2, P = 0.012) (Table 1).
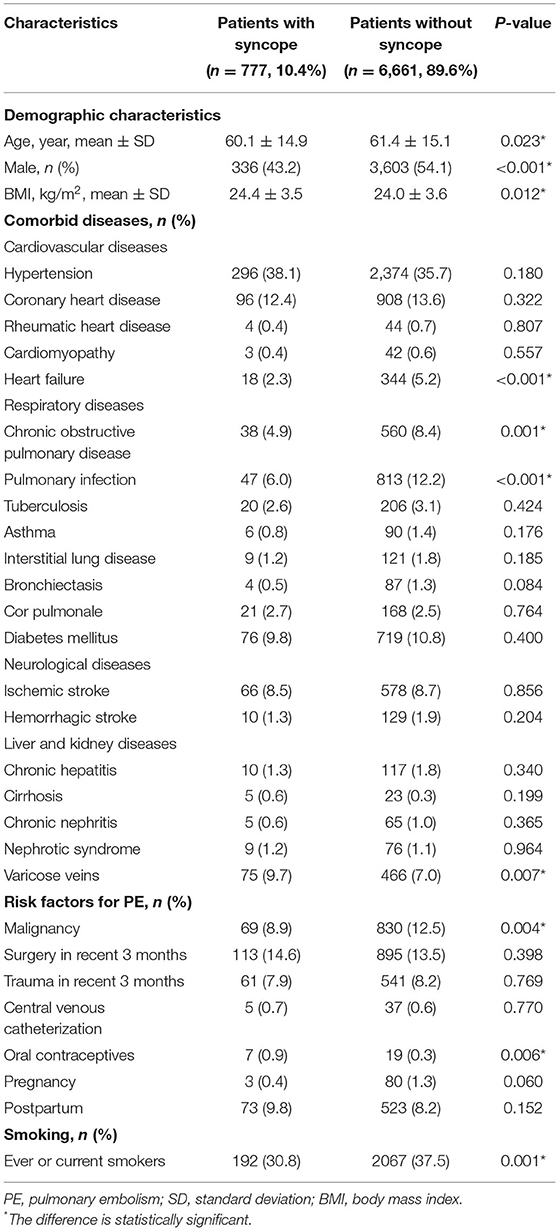
Table 1. Demographic characteristics, comorbid diseases, and risk factors of patients with acute PE.
There were significantly fewer patients with heart failure, chronic obstructive pulmonary disease and pulmonary infection in the syncope group. Compared with patients without syncope, more patients with syncope had varicose veins (9.7 vs. 7.0%, P = 0.007), while fewer patients had malignancy (8.9 vs. 12.5%, P = 0.004).
Clinical Presentation and Risk Stratification
Patients with syncope had higher frequency of precordial pain and palpitation, whereas cough, sputum, fever, pleurisy pain and hemoptysis were more common in patients without syncope. Compared with the non-syncope group, there were more patients with pulse ≥ 110 beats/min, RR > 20 breath/min and SBP < 100 mmHg in the syncope group (P < 0.001, P = 0.001, P < 0.001) (Table 2).
Patients with syncope were more likely to have white blood cell > 10×109/L (31.4 vs. 25.9%, P = 0.001) and platelet < 100×109/L (8.3 vs. 5.6%, P = 0.003). In the syncope group, more patients had elevated cardiac biomarkers, including cardiac troponin, BNP and NT-proBNP (52.8 vs. 34.9%, P < 0.001). There were also more patients with estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 in the syncope group (16.4 vs. 13.1%, P = 0.015). The D-Dimer level of patients with syncope was significantly higher compared with that of patients without syncope (1,391.4 vs. 1,029.0 μg/L, P < 0.001) (Table 2).
The electrocardiogram (ECG) and cardiac ultrasonography (UCG) findings were also presented in Supplementary Table 1, as well as thrombus location in CTPA. In the syncope group, there were more patients with SIQIIITIII in ECG, more patients with RV/LV ratio > 0.9 and RV free wall mobility ≤ 5 mm in UCG. These patients also had higher level of estimated pulmonary arterial systolic pressure compared with the non-syncope group. More patients had thrombus in central pulmonary arteries (including pulmonary trunk, right and left pulmonary artery) in the syncope group.
Of the 7,438 patients enrolled, 310 (4.2%) patients had hemodynamically unstable PE. The proportion of patients with hemodynamically unstable PE were markedly higher in the syncope group than that in the non-syncope group (14.9 vs. 2.9%, P < 0.001). In patients with hemodynamically stable PE, there was no significant difference in the sPESI classification between the two groups.
Further comparison of the demographic characteristics, comorbidities and clinical presentation was performed between patients with and without syncope in hemodynamic stable and unstable groups, and the results were listed in Supplementary Table 2.
Initial Anti-thrombotic Therapy
Regarding initial treatment, 6,227 (83.7%) patients received anticoagulation, while 695 (9.3%) patients received systemic thrombolysis. Compared to patients without syncope, more patients received thrombolysis (21.4 vs. 7.9%, P < 0.001) in the syncope group (Table 2).
In the further analysis of the hemodynamically unstable PE patients, 51.7% of syncope patients and 35.1% of non-syncope patients received systemic thrombolysis (P = 0.004). There were also more patients with syncope who received thrombolysis among the hemodynamically stable PE patients (16.0 vs. 7.1%, P < 0.001) (Supplementary Table 3).
In-hospital Outcomes
Of all the patients, 254 (3.4%) patients died and 118 (1.6%) patients had fatal PE, while 113 patients (2.2%) had major bleeding. Clinical outcomes in PE patients with and without syncope was shown in Table 2. There was no significant difference between patients with and without syncope with regard to the incidence of all-cause mortality, fatal PE, major bleeding, or length of stay.
Clinical Factors Related With Syncope
In the multivariate analysis, female (OR 1.567, 95% CI 1.345–1.825, P < 0.001), varicose veins (OR 1.386, 95% CI 1.069–1.797, P = 0.014) and platelet < 100×109/L (OR 1.677, 95% CI 1.265–2.223, P < 0.001) were independent risk factors related with syncope in PE, while history of heart failure (OR 0.482, 95% CI 0.293–0.793, P = 0.004) and pulmonary infection (OR 0.513, 95% CI 0.376–0.699, P < 0.001) were protective factors (Figure 2).
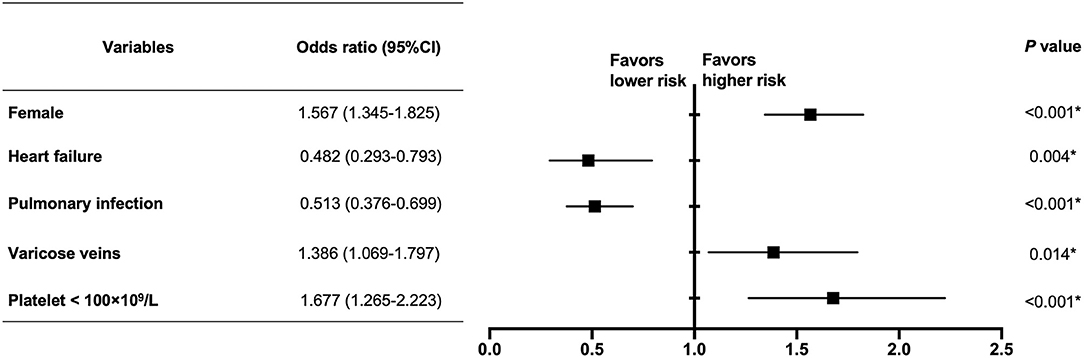
Figure 2. Multivariate analysis of clinical factors related with syncope in PE patients. In the multivariate analysis, female (OR 1.567, 95% CI 1.345–1.825, P < 0.001), varicose veins (OR 1.386, 95% CI 1.069–1.797, P = 0.014), and platelet < 100 × 109/L (OR 1.677, 95% CI 1.265–2.223, P < 0.001) were independent risk factors related with syncope in PE, while heart failure (OR 0.482, 95% CI 0.293–0.793, P = 0.004) and pulmonary infection (OR 0.513, 95% CI 0.376–0.699, P < 0.001) were protective factors. *The difference is statistically significant.
PCA of Clinical Variables
Complete data for the nine variables, necessary for principal component and cluster analyses, were available for 725 subjects with syncope. PCA was performed to transform data in the nine selected variables into four independent components, which contributed significantly to explaining the relationships among the selected variables (eigenvalues > 1) accounted for 60.3% of the variance. Correlations of the selected variables with these four independent components are shown in Table 3. Component 1 was correlated with age, CVD and SBP, and was inversely correlated with pulse. Component 2 was correlated with pulse and RR. Component 3 was correlated with recent surgery or trauma and malignancy, and was inversely correlated with sex. Component 4 was correlated with BMI and recent surgery or trauma.
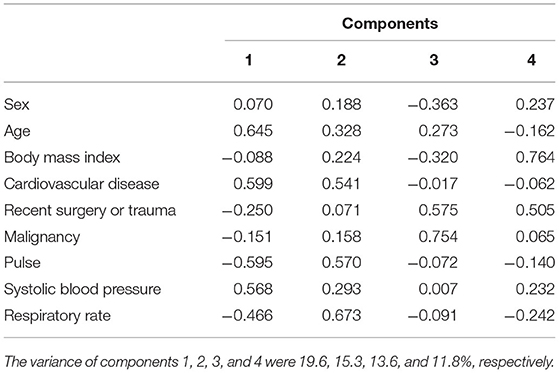
Table 3. Correlations of the nine original variables with the four main components derived from the principal component analysis in the 725 PE patients with syncope.
Clusters of Patients With Syncope
In order to classify of PE subjects with syncope, the four principal components identified above were used in a cluster analysis. Pseudo-F and pseudo-t2 statistics determined that the data could be optimally grouped into four clusters. Clinical characteristics of the 725 PE patients with syncope according to these four phenotypes clusters were presented in Table 4.
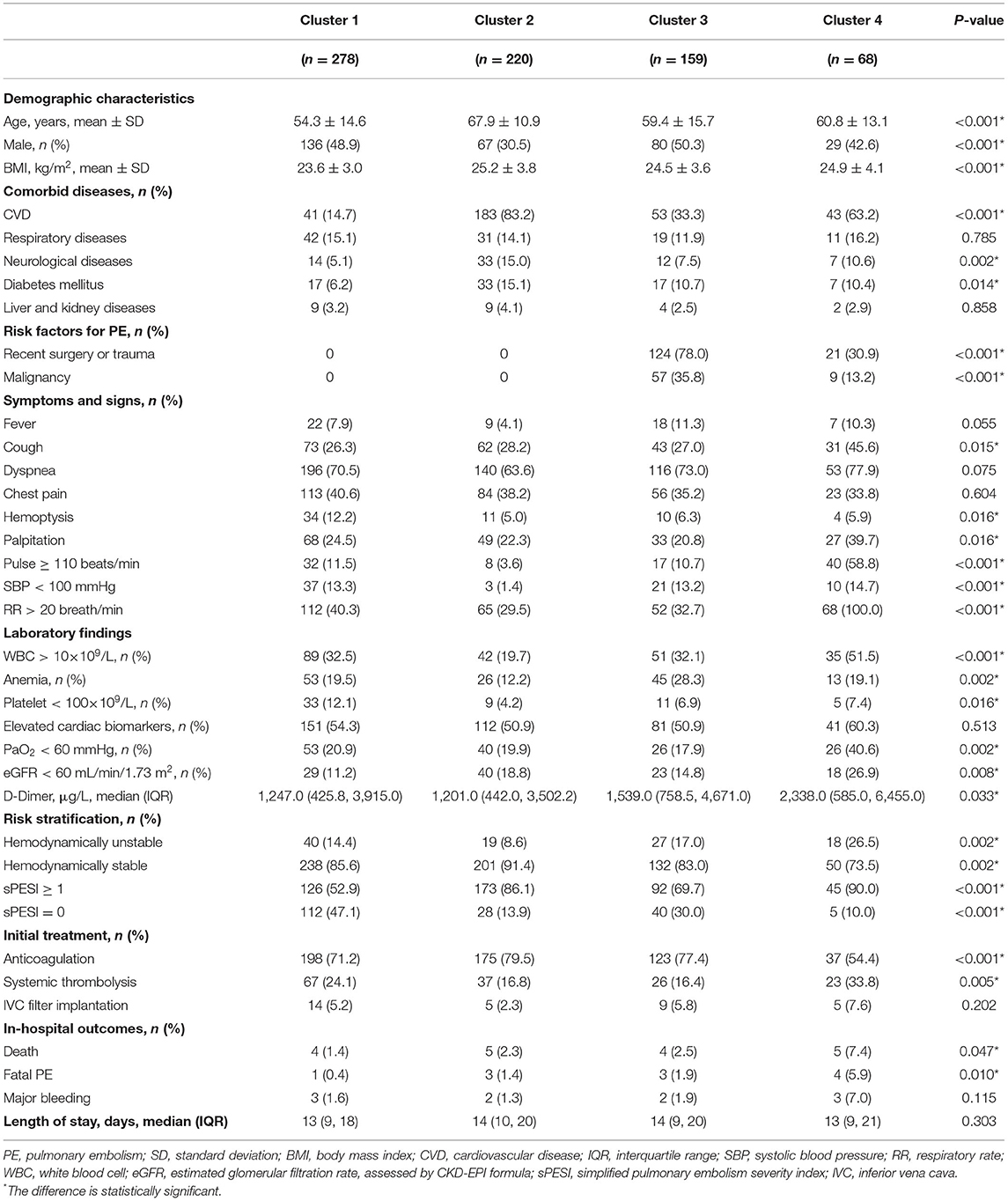
Table 4. Characteristics of the 725 PE patients with syncope according to the four phenotypes identified using principal component analysis-based cluster analysis.
We found marked differences among these groups. Phenotype B was composed of older subjects (n = 220, mean age 67.9 years) and more females (69.5%) with higher BMI and frequent CVD. No patients had recent surgery or trauma, or malignancy as risk factors for PE in this phenotype. Compared with the other three phenotypes, phenotype B was significantly less likely to have SBP < 100 mmHg, while no significant difference was found in the prevalence of low SBP (< 100 mmHg) among phenotypes A, C and D. Phenotype A had relatively young subjects (n = 278, mean age 54.3 years), with no patients with recent surgery or trauma, or malignancy, and CVD was infrequent. On the contrary, phenotype C had high prevalence of recent surgery or trauma (78.0%) and malignancy (35.8%). Phenotype D had significantly higher prevalence of pulse ≥ 110 beats/min and RR > 20 breath/min. Furthermore, the proportion of patients with PaO2 <60 mmHg and eGFR < 60 mL/min/1.73 m2 as well as the D-Dimer level were markedly higher in phenotype D, which also had more patients with hemodynamically unstable PE and sPESI ≥ 1. These four phenotypes were summarized in Table 5.
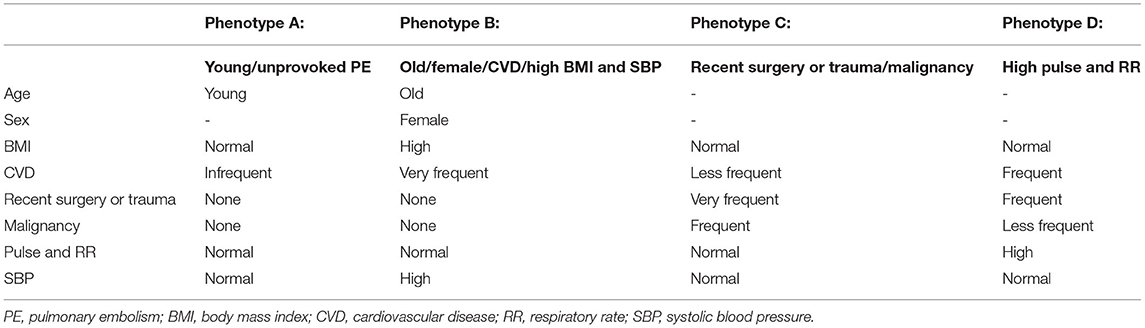
Table 5. Summary of syncope phenotypes identified using principal component analysis-based cluster analysis.
Impact of Phenotypes on Outcomes
The comparison of in-hospital outcomes and length of stay among these four phenotypes were presented in Table 4. Significant differences were found in the frequency of death (P = 0.047) and fatal PE (P = 0.010) among these phenotypes. The rates of all-cause death (7.4%), fatal PE (5.9%) and major bleeding (7.0%) were all highest in phenotype cluster D, although the difference in major bleeding was not significant. No significant difference was found in the length of stay among these phenotypes.
Discussion
This study revealed the heterogeneity in clinical presentation between PE patients with and without syncope, and found that various phenotypes existed in PE patients with syncope. To our knowledge, PCA and cluster analysis were applied for the first time to classify PE subjects with syncope. Four phenotypes were identified. In-hospital outcomes were markedly different among patients with similar SBP (phenotypes A, C, and D). In PE patients with syncope, those with high pulse and RR were at higher risk for adverse outcomes.
Differences in Clinical Presentation Between Patients With and Without Syncope
In our study, 10.4% patients had syncope as an initial symptom, that is more common than 5.5% in the EMPEROR registry (25). In a meta-analysis (26), the overall prevalence of syncope in PE was 16.9%, ranging between 6.8 and 29.9%. We found the rate of syncope in high-risk PE was 37.4%, comparable with 35% in the German registry (8). The difference of symptoms between patients with and without syncope might reflect different clinical phenotypes in PE. Symptoms, including fever, cough, sputum, hemoptysis and pleurisy pain, could be explained by distal thromboembolism and consequent pulmonary infarction. Other symptoms, like syncope, dyspnea, precordial pain and palpitation, might be caused by central and relative massive clot. In a study derived from RIETE registry, 3,391 PE patients without chronic lung disease or heart failure were divided into three groups: patients with pulmonary infarction, isolated dyspnea and circulatory collapse. Patients with pulmonary infarction had a significantly lower mortality rate (5).
In our study, PE patients with syncope exhibited female-predominance, similar with prior reports (16, 19, 27, 28). Sex difference in the function of the autonomic nervous system can be related to syncope (29). Moreover, smaller and stiffer left ventricular in females may lead to a relatively larger reduction in stroke volume and make females more vulnerable to syncope (30). Besides female gender, history of varicose veins was also found to be associated with syncope in patients with PE. In further analysis, we found patients with varicose veins had higher D-Dimer level, that suggests these patients had higher clot burden. Thrombocytopenia was associated with syncope in patients with PE for the same reason since platelet number might be consumptively reduced due to heavy thrombus burden.
We found that the patients with syncope had higher D-Dimer level, higher frequency of SIQIIITIII in ECG and RV/LV ratio > 0.9 in UCG, higher level of estimated pulmonary arterial systolic pressure in UCG, as well as higher frequency of central thrombus in CT pulmonary angiography (CTPA). These variables reflect clot burden directly or indirectly. We also found that more patients had cardiac injury in the syncope group. Similarly, previous studies have reported that PE patients with syncope showed significantly higher cardiac biomarker levels, as well as higher rates of central PE and RVD (21, 26). The presence of syncope might indicate more severe clot burden which causes cardiac injury and RVD, leading to reduced cardiac output and transient hypoperfusion of brain. This explains the higher prevalence of hemodynamical instability in patients with syncope. The present study shows more patients with SBP < 100 mmHg at admission in the syncope group, also supporting this mechanism for syncope.
Impact of Syncope on Disease Severity and Outcomes of Patients With PE
Consistent with previous studies (16, 26), we found more patients had hemodynamically unstable PE in the syncope group. Though syncope was not associated with in-hospital outcome, patients with syncope were more often treated with thrombolysis, even in normotensive PE. The all-cause mortality was significantly higher in hemodynamically unstable patients without syncope. We speculate less use of systemic thrombolysis is the main reason leading to higher rate of death in these patients. Similarly, a better 30-day survival was only identified in hemodynamically unstable PE patients with syncope (31). The presence of syncope might be regarded as a sign of severe PE leads to more intensive monitoring and more aggressive treatment. Nonetheless, the prognostic role of syncope in PE varies in different studies (9, 32). These inconsistent results might be attributable to the heterogeneity of mechanism for syncope in PE patients.
Clinical Phenotypes of PE Patients With Syncope
The underlying mechanisms for syncope during PE are not completely understood (7, 12). In acute PE, syncope may occur when the pulmonary vasculature is occluded more than 50%, which causes a sudden drop in cardiac output and temporary cerebral hypoperfusion (6, 33), or due to arrhythmia caused by right ventricular overload (11). The vasovagal reflex leading to neurogenic syncope is another possible reason (6). These different pathophysiological changes may lead to syncope with different outcomes. We notice that SBP < 100 mmHg is particularly rare in phenotype B with more female patients. Therefore, we speculate that the presence of syncope in phenotype B is probably caused by vasovagal reflex and the dysfunction of the autonomic nervous system, instead of sudden drop in cardiac output. Compared with neurogenic syncope, patients with syncope caused by RVD and drop in cardiac output might have more severe disease and poorer prognosis, that was indicated by the difference between phenotypes B and D. The D-Dimer level was markedly higher in phenotype D, that implied higher clot burden in patients with this phenotype. Higher pulse and RR in this phenotype suggested significant cardiopulmonary compensation caused by heavier clot burden.
Few studies have used cluster analysis to assess phenotypes in patients with PE. In a retrospective study, 551 PE patients were classified into five clusters based on 10 symptoms, using PCA and system clustering method (34). However, they did not compare the outcome among different phenotypes. We focused on the patients with syncope and tried to identify clinically based phenotypes that can be used in daily practice. In the four phenotypes identified, phenotype D consisted of patients with high pulse and RR had the poorest outcome. Although no significant difference was found in the prevalence of SBP < 100 mmHg among phenotypes A, C, and D, outcomes during admission were markedly different. Pulse and RR might be more sensitive than SBP in the assessment of disease severity in acute PE.
Limitations
There are some limitations in our study. Firstly, syncope remains a symptom difficult to define, frequently reported by relatives or bystanders. It may lead to imprecise estimates of its prevalence as well as the associated risks among patients with PE. Secondly, in this longstanding, multicenter registry study, ultrasonic or radiological parameters related to RVD are missing for some patients, which made further analysis difficult. In addition, our phenotyping was exclusively based on clinical variables, which could be improved with the inclusion of variables relevant to the pathogenesis of syncope, such as imaging-derived parameters associated with RVD and cardiac biomarkers. Further studies are needed to validate the prognostic value of these phenotypes and illustrate the underlying pathophysiological mechanisms leading to a particular phenotype.
Conclusion
In summary, we found differences in the clinical characteristics of PE patients with and without syncope. Syncope was associated with hemodynamic instability and more application of thrombolysis, whereas it did not impact the in-hospital outcomes. We identified four phenotypes with prognostic implications in PE patients with syncope. PE patients with syncope need to be managed more cautiously if they had higher pulse and RR, since they are at high risk for adverse prognosis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethic Committee of all participating centers (Approval No. 2012BJYYEC-050-02, 2017-24). The participants provided their written informed consent to participate in this study.
Author Contributions
ZheZ and CW had full access to all of the data in the study, take responsibility for the content of manuscript, and conceived and designed the study. SZ, XX, YJ, YY, QY, HC, XH, ZL, and YM analyzed the data and drafted the manuscript. JL, DW, ZhuZ, and SW integrated the data and take responsibility for the accuracy of the data analysis. YZ, MZ, and XS participated in data acquisition. JZ, JS, QG, XT, WX, JW, ZhoZ, BF, and PY contributed to the clinical inputs and interpretation of the data. All authors provided final approval of the version to be published.
Funding
This work was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2021-I2M-1-049), the National Key R&D Program of China, Ministry of Science and Technology of China (No. 2018YFC1315100), the Fund of the National Key Research and Development Program of China (No. 2016YFC0905600), and Elite Medical Professionals Project of China-Japan Friendship Hospital (No. ZRJY2021-QM11).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate the important contribution and continuous support from Jun An (The First Affiliated Hospital of Dalian Medical University), Jifeng Li (Beijing Chao-Yang Hospital, Capital Medical University), Lan Wang, Haixia Zhou, Maoyun Wang (West China Hospital, West China School of Medicine, Sichuan University), Xiaohui Wang (The First Affiliated Hospital of Chongqing Medical University), He Yang (Beijing Hospital), Qin Luo (Fuwai Hospital, Chinese Academy of Medical Science; National Center for Cardiovascular Diseases), Mian Zeng, Xia Li (The First Affiliated Hospital, Sun Yat-sen University), Ling Zhu, Yi Liu (Shandong Provincial Hospital), Kejing Ying, Guofeng Ma, Chao Yan (Sir Run Run Shaw Hospital, Zhejiang University School of Medicine), Lixia Dong, Wei Zhou (Tianjin Medical University General Hospital), Chong Bai, Wei Zhang (Changhai Hospital), Liangxing Wang, Yupeng Xie, Xiaoying Huang (The First Affiliated Hospital of Wenzhou Medical University), Chen Qiu, Yazhen Li, Yingyun Fu, Shengguo Liu (Shenzhen People's Hospital), Shengqing Li, Jian Zhang, Xinpeng Han (Xijing Hospital), Qixia Xu, Xiaoqing Li, Yingying Pang, Beilei Gong (The First Affiliated Hospital of Bengbu Medical College), Ping Huang, Yanwei Chen, Jiming Chen (Shenzhen Sixth People's Hospital (Nanshan Hospital) Huazhong University of Science and Technology Union Shenzhen Hospital), Guochao Shi, Yongjie Ding (Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine), Zhaozhong Cheng, Li Tong (The Affiliated Hospital of Qingdao Univer- sity), Zhuang Ma, Lei Liu (The General Hospital of Shenyang Military), Luning Jiang, Zhijun Liang (Affiliated Hospital of Jining Medical University), Chaosheng Deng, Minxia Yang, Dawen Wu (The First Affiliated Hospital of Fujian Medical University), Shudong Zhang, Lijun Kang (Yantaishan Hospital), Hong Chen, Fangfei Yu, Xuewei Chen (The Second Affiliated Hospital of Harbin Medical University), Dan Han, Shasha Shen (The First Affiliated Hospital of Kunming Medical University), Guohua Sun, Yutao Hou, Baoliang Liu (Zibo First Hospital), Xiaohong Fan, Wei Zhang (Nanjing Drum Tower Hospital), Ping Zhang, Ruhong Xu (Dongguan People's Hospital), Zaiyi Wang, Cunzi Yan (The First Affiliated Hospital of Xinjiang Medical University), Chunxiao Yu, Zhenfang Lu, Jing Hua (Beijing Jingmei Group General Hospital), Zhenyang Xu, Hongxia Zhang, Jinxiang Wang (Beijing Luhe Hospital, Capital Medical University), Xiaohong Yang, Ying Chen (People's Hospital of Xinjiang Uygur Autonomous Region), Yongjun Tang, Wei Yang (Xiangya Hospital Central South University), Nuofu Zhang, Linli Duan, Simin Qing, Chunli Liu (The First Affiliated Hospital of Guangzhou Medical University (Guangzhou Institute of Respiratory Health)), Junping Fan (Peking Union Medical College Hospital), Lian Jiang, Hongda Zhao, Chengying Liu (Jiangyin People's Hospital), Yadong Yuan, Xiaowei Gong (The Second Hospital of Hebei Medical University), Xinhong Zhang, Chunyang Zhang (The Sixth Medical Center of People's Liberation Army General Hospital), Shuyue Xia, Hui Jia, Yunxia Liu (Central Hospital Affiliated to Shenyang Medical College), Dongmei Zhang, Yuntian Ma (Tianjin Ninghe District Hospital), Lu Guo, Jing Zhang (Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital), Lina Han, Xiaomin Bai (Handan First Hospital), Guoru Yang, Guohua Yu (Weifang Respiratory Disease Hospital), Ruian Yang, Jingyuan Fan (The First People's Hospital of Yunnan Province), Aizhen Zhang, Rui Jiang, Xueshuang Li, Yuzhi Wu, Jun Han (Shanxi Provincial People's Hospital), Jingping Yang, Xiyuan Xu, Baoying Bu (The Third Affiliated Hospital of Inner Mongolia Medical University), Chaobo Cui, Ning Wang (Harrison International Peace Hospital), Yipeng Ding, Heping Xu, Dingwei Sun (Hainan General Hospital), Yonghai Zhang, Jie Duo, Yajun Tuo (Qinghai Provincial People's Hospital), Xiangyan Zhang, Weijia Liu (Guizhou Provincial People's Hospital), Hongyang Wang, Yuan Wang, Aishuang Fu (North China University of Science and Technology Affiliated Hospital), Songping Huang, Qinghua Xu (Quanzhou First Hospital), Wenshu Chai, Jing Li (The First Affiliated Hospital of Jinzhou Medical University), Yanping Ye, Wei Hu, Jin Chen (Fu Xing Hospital, Capital Medical University), Bo Liu, Lijun Suo (Linzi District People's Hospital), Changcheng Guo, Ping Wang (Taiyuan Central Hospital), Jinming Liu, Qinhua Zhao (Shanghai Pulmonary Hospital), Qin Luo, Le Kang (The Third Affiliated Hospital of Xinjiang Medical University), Jianying Xu, Lifen Zhao, Mengyu Cheng, Wei Duan (Shanxi Academy of Medical Sciences, Shanxi Dayi Hospital), Qi Wu, Li Li (Tianjin Haihe Hospital), Ping Wang, Xiuqing He, Yueyue Li (The 306th Hospital of People's Liberation Army), Gang Chen, Yunxia Zhao, Zixiao Liu (The Third Hospital of Hebei Medical University), Guoguang Xia, Tianshui Li, Nan Chen, Xiaoyang Liu (Beijing Jishuitan Hospital), Tao Bian, Yan Wu (Wuxi People's Hospital), Huiqin Yang, Xiaoli Tang (Xinjiang Uygur Autonomous Region Hospital of Traditional Chinese medicine), Yiwen Zhang (Anhui Chest Hospital), Faguang Jin, Ning Wang, Yanli Chen, Yanyan Li (Tangdu Hospital), Jing Li, Miaochan Lao (Guangdong Academy of Medical Sciences, Guangdong General Hospital), Shengqing Li, Liang Dong (Shanghai Huashan Hospital), Guangfa Zhu, Wenmei Zhang (Beijing Anzhen Hospital, Capital Medical University), Liangan Chen, Zhixin Liang (Chinese People's Liberation Army General Hospital (Medical School of Chinese People's Liberation Army), Liping Cui, Cenfeng Xia, Jin Zhang, Peng Zhang (General Hospital of Ningxia Medical University), Lianxiang Guo, Sha Niu, Sichong Yu (Jiaozuo Second People's Hospital), Guangjie Liu, Xinmao Wang (Beijing Tongren Hospitall, Capital Medical University), Yanhua Lv, Zhenyu Liang, Shaoxi Cai, Shuang Yang (Nanfang Hospital), Xinyi Zhang, Jiulong Kuang (The Second Affiliated Hospital of Nanchang University), Yanyan Ding, Yongxiang Zhang (People's Hospital of Beijing Daxing District), Xuejun Guo, Yanmin Wang (Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine), Jialie Wang, Ruimin Hu (Inner Mongolia People's Hospital), Lin Ma (The First Affiliated Hospital of Nanchang University), Yuan Gao, Rui Zheng (Shengjing Hospital of China Medical University), Zhihong Shi, Hong Li (The First Affiliated Hospital of Xi'an Jiaotong University), Yingqi Zhang, Guanli Su (The First Hospital of Hebei Medical University), Zhiqiang Qin, Guirong Chen (The People's Hospital of Guangxi Zhuang Autonomous Region), Xisheng Chen, Zhiwei Niu (The Hospital of Shunyi District Beijing), Jinjun Jiang, Shujing Chen (Zhongshan Hospital, Fudan University), Tiantuo Zhang, Hongtao Li, Jiaxin Zhu, Yuqi Zhou (The Third Affiliated Hospital, Sun Yat-sen University), Yinlou Yang, Jiangtao Cheng (Yue Bei People's Hospital), Jie Sun, Yanwen Jiang (Beijing Shijitan Hospitall, Capital Medical University), Jianhua Liu, Yujun Wang (Beijing Huairou Hospital of University of Chinese Academy of Sciences), Ju Yin, Lanqin Chen (Beijing Children's Hospital, Capital Medical University), Min Yang, Ping Jiang, Hongbo Liu (Tianjin First Central Hospital), Guohua Zhen, Kan Zhang (Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology), Yixin Wan, Hongyan Tao (Lanzhou University Second Hospital), Cuo Ping, Ci Ren De Ji (The Second People's Hospital of Tibet Autonomous Region), Yingqi Zhang (Tangshan Worker's Hospital).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.836850/full#supplementary-material
References
1. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. (2019) 54:1901647. doi: 10.1183/13993003.01647-2019
2. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. (1998) 158:585–93. doi: 10.1001/archinte.158.6.585
3. Khorana AA, O'Connell C, Agnelli G, Liebman HA, Lee AY. Subcommittee on H and Malignancy of the SSCotI. Incidental venous thromboembolism in oncology patients. J Thromb Haemost. (2012) 10:2602–4. doi: 10.1111/jth.12023
4. Keller K, Beule J, Balzer JO, Dippold W. Typical symptoms for prediction of outcome and risk stratification in acute pulmonary embolism. Int Angiol. (2016) 35:184–91. doi: 10.1016/j.artres.2016.05.002
5. Lobo JL, Zorrilla V, Aizpuru F, Uresandi F, Garcia-Bragado F, Conget F, et al. Clinical syndromes and clinical outcome in patients with pulmonary embolism: findings from the RIETE registry. Chest. (2006) 130:1817–22. doi: 10.1378/chest.130.6.1817
6. Castelli R, Tarsia P, Tantardini C, Pantaleo G, Guariglia A, Porro F. Syncope in patients with pulmonary embolism: comparison between patients with syncope as the presenting symptom of pulmonary embolism and patients with pulmonary embolism without syncope. Vasc Med. (2003) 8:257–61. doi: 10.1191/1358863x03vm510oa
7. Calvo-Romero JM, Perez-Miranda M, Bureo-Dacal P. Syncope in acute pulmonary embolism. Eur J Emerg Med. (2004) 11:208–9. doi: 10.1097/01.mej.0000136696.49343.8f
8. Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. (1997) 30:1165–71. doi: 10.1016/S0735-1097(97)00319-7
9. Duplyakov D, Kurakina E, Pavlova T, Khokhlunov S, Surkova E. Value of syncope in patients with high-to-intermediate risk pulmonary artery embolism. Eur Heart J Acute Cardiovasc Care. (2015) 4:353–8. doi: 10.1177/2048872614527837
10. Jiménez D, Díaz G, Valle M, Martí D, Escobar C, Vidal R, et al. Prognostic value of syncope in the presentation of pulmonary embolism. Arch Bronconeumol. (2005) 41:385–8. doi: 10.1016/S1579-2129(06)60246-2
11. Koutkia P, Wachtel TJ. Pulmonary embolism presenting as syncope: case report and review of the literature. Heart Lung. (1999) 28:342–7. doi: 10.1053/hl.1999.v28.a99733
12. Prandoni P, Lensing AW, Prins MH, Ciammaichella M, Perlati M, Mumoli N, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. (2016) 375:1524–31. doi: 10.1056/NEJMoa1602172
13. Verma AA, Masoom H, Rawal S, Guo Y, Razak F. Pulmonary embolism and deep venous thrombosis in patients hospitalized with syncope: a multicenter cross-sectional study in Toronto, Ontario, Canada. J Am Med Assoc Intern Med. (2017) 177:1046–8. doi: 10.1001/jamainternmed.2017.1246
14. Pop C, Ianos R, Matei C, Mercea D, Todea B, Dicu D, et al. Prospective study of pulmonary embolism presenting as syncope. Am J Ther. (2019) 26:e301–7. doi: 10.1097/MJT.0000000000000825
15. Badertscher P, du Fay de Lavallaz J, Hammerer-Lercher A, Nestelberger T, Zimmermann T, Geiger M, et al. Prevalence of pulmonary embolism in patients with syncope. J Am Coll Cardiol. (2019) 74:744–54. doi: 10.1016/j.jacc.2019.06.020
16. Iqbal U, Jameel A, Anwar H, Scribani MB, Bischof E, Chaudhary A. Does syncope predict mortality in patients with acute pulmonary embolism? A retrospective review. J Clin Med Res. (2017) 9:516–9. doi: 10.14740/jocmr3037w
17. Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. (1999) 353:1386–9. doi: 10.1016/S0140-6736(98)07534-5
18. Lankeit M, Friesen D, Schafer K, Hasenfuss G, Konstantinides S, Dellas C, et al. Simple score for rapid risk assessment of non-high-risk pulmonary embolism. Clin Res Cardiol. (2013) 102:73–80. doi: 10.1007/s00392-012-0498-1
19. Hobohm L, Hellenkamp K, Hasenfuss G, Munzel T, Konstantinides S, Lankeit M. Comparison of risk assessment strategies for not-high-risk pulmonary embolism. Eur Respir J. (2016) 47:1170–8. doi: 10.1183/13993003.01605-2015
20. Jenab Y, Lotfi-Tokaldany M, Alemzadeh-Ansari MJ, Seyyedi SR, Shirani S, Soudaee M, et al. Correlates of syncope in patients with acute pulmonary thromboembolism. Clin Appl Thromb Hemost. (2015) 21:772–6. doi: 10.1177/1076029614540037
21. Lee YH, Cha SI, Shin KM, Lim JK, Yoo SS, Lee SY, et al. Clinical relevance of syncope in patients with pulmonary embolism. Thromb Res. (2018) 164:85–9. doi: 10.1016/j.thromres.2018.02.147
22. Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. (2009) 30:2631–71. doi: 10.1093/eurheartj/ehp298
23. Schulman S, Kearon C. Subcommittee on control of anticoagulation of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. (2005) 3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x
24. Jiménez D, Aujesky D, Moores L, Gómez V, Lobo JL, Uresandi F, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. (2010) 170:1383–9. doi: 10.1001/archinternmed.2010.199
25. Pollack CV, Schreiber D, Goldhaber SZ, Slattery D, Fanikos J, O'Neil BJ, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J Am Coll Cardiol. (2011) 57:700–6. doi: 10.1016/j.jacc.2010.05.071
26. Barco S, Ende-Verhaar YM, Becattini C, Jimenez D, Lankeit M, Huisman MV, et al. Differential impact of syncope on the prognosis of patients with acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. (2018) 39:4186–95. doi: 10.1093/eurheartj/ehy631
27. Keller K, Beule J, Balzer JO, Dippold W. Syncope and collapse in acute pulmonary embolism. Am J Emerg Med. (2016) 34:1251–7. doi: 10.1016/j.ajem.2016.03.061
28. Barrios D, Morillo R, Guerassimova I, Barbero E, Escobar-Morreale H, Cohen AT, et al. Sex differences in the characteristics and short-term prognosis of patients presenting with acute symptomatic pulmonary embolism. PLoS ONE. (2017) 12:e0187648. doi: 10.1371/journal.pone.0187648
29. Baker SE, Limberg JK, Ranadive SM, Joyner MJ. Neurovascular control of blood pressure is influenced by aging, sex, and sex hormones. Am J Physiol Regul Integr Comp Physiol. (2016) 311:R1271–5. doi: 10.1152/ajpregu.00288.2016
30. Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol. (2004) 286:H449–57. doi: 10.1152/ajpheart.00735.2002
31. Roncon L, Zuin M, Casazza F, Becattini C, Bilato C, Zonzin P. Impact of syncope and pre-syncope on short-term mortality in patients with acute pulmonary embolism. Eur J Intern Med. (2018) 54:27–33. doi: 10.1016/j.ejim.2018.04.004
32. Ozyurt SA-O, Ozsu S, Erbay M, Oztuna F, Gumus A, Sahin U. Syncope as a subject of the risk assessment of pulmonary thromboembolism to be used for: a cross-sectional study. Clin Respir J. (2018) 12:2136–40. doi: 10.1111/crj.12784
33. Demircan A, Aygencel G, Keles A, Ozsoylar O, Bildik F. Pulmonary embolism presenting as syncope: a case report. J Med Case Rep. (2009) 3:7440. doi: 10.4076/1752-1947-3-7440
Keywords: pulmonary embolism, syncope, mortality, phenotype, cluster analysis
Citation: Zhang S, Xu X, Ji Y, Yang Y, Yi Q, Chen H, Hu X, Liu Z, Mao Y, Zhang J, Shi J, Lei J, Wang D, Zhang Z, Wu S, Gao Q, Tao X, Xie W, Wan J, Zhang Y, Zhang M, Shao X, Zhang Z, Fang B, Yang P, Zhai Z, Wang C and the China pUlmonary Thromboembolism REgistry Study (CURES) Investigators (2022) Clinical Phenotypes With Prognostic Implications in Pulmonary Embolism Patients With Syncope. Front. Cardiovasc. Med. 9:836850. doi: 10.3389/fcvm.2022.836850
Received: 16 December 2021; Accepted: 13 January 2022;
Published: 15 February 2022.
Edited by:
Luca Spiezia, University of Padua, ItalyReviewed by:
Giuseppe Pepe, Azienda Usl Toscana Nord Ovest, ItalyCarlos Jerjes-Sanchez, Tecnológico de Monterrey, Mexico
Liviu Macovei, Grigore T. Popa University of Medicine and Pharmacy, Romania
Copyright © 2022 Zhang, Xu, Ji, Yang, Yi, Chen, Hu, Liu, Mao, Zhang, Shi, Lei, Wang, Zhang, Wu, Gao, Tao, Xie, Wan, Zhang, Zhang, Shao, Zhang, Fang, Yang, Zhai, Wang and the China pUlmonary Thromboembolism REgistry Study (CURES) Investigators. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenguo Zhai, emhhaXpoZW5ndW8yMDExQDEyNi5jb20=; Chen Wang, d2FuZ2NoZW5AcHVtYy5lZHUuY24=
†These authors have contributed equally to this work
 Shuai Zhang
Shuai Zhang Xiaomao Xu5†
Xiaomao Xu5† Yuanhua Yang
Yuanhua Yang Jieping Lei
Jieping Lei Dingyi Wang
Dingyi Wang Yunxia Zhang
Yunxia Zhang Meng Zhang
Meng Zhang Peiran Yang
Peiran Yang Zhenguo Zhai
Zhenguo Zhai Chen Wang
Chen Wang